Post-Fire Restoration and Deadwood Management: Microsite Dynamics and Their Impact on Natural Regeneration †
Abstract
:1. Introduction
2. Methods
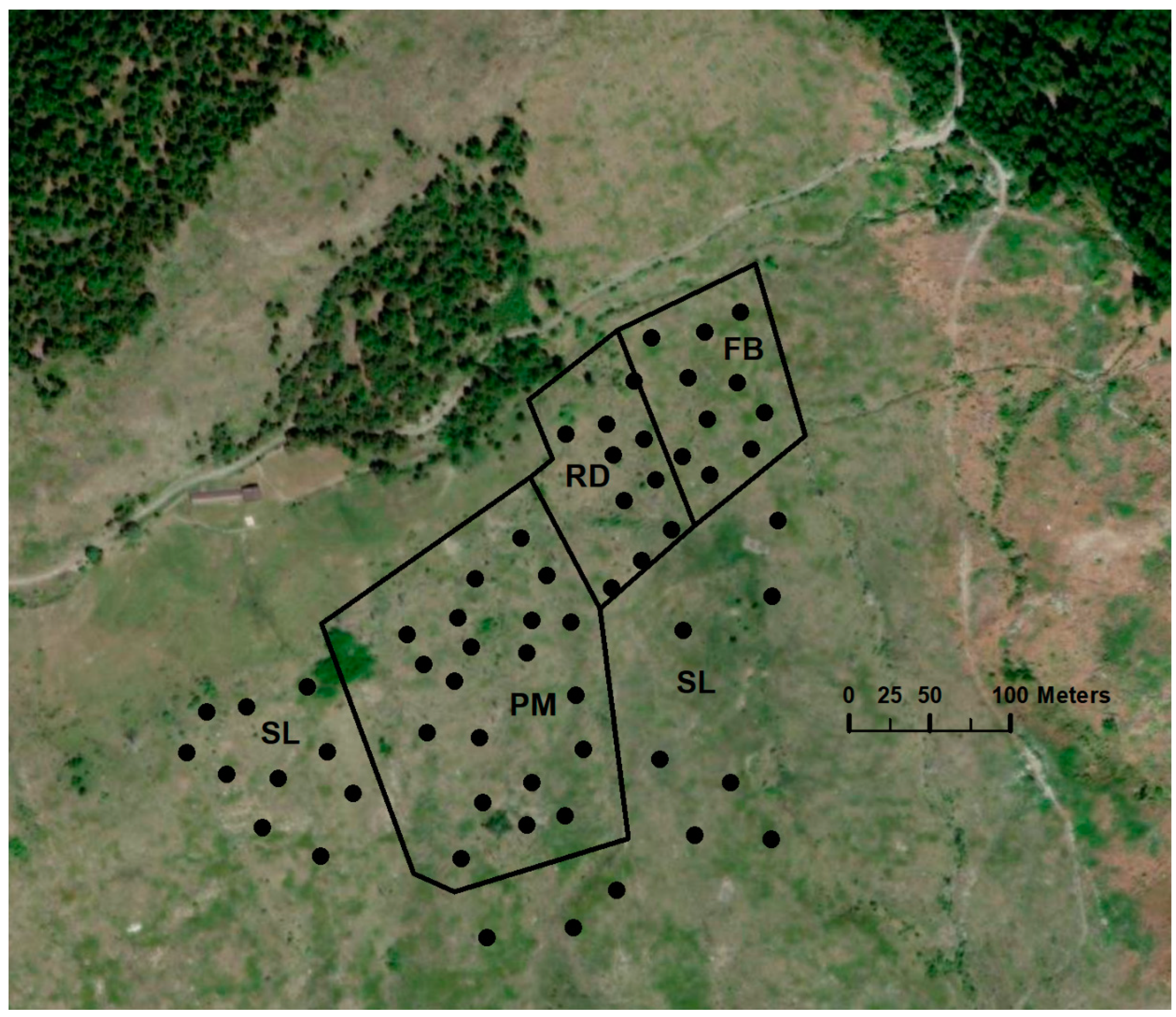
2.1. Study Site
2.2. Field Sampling
2.3. Data Analysis
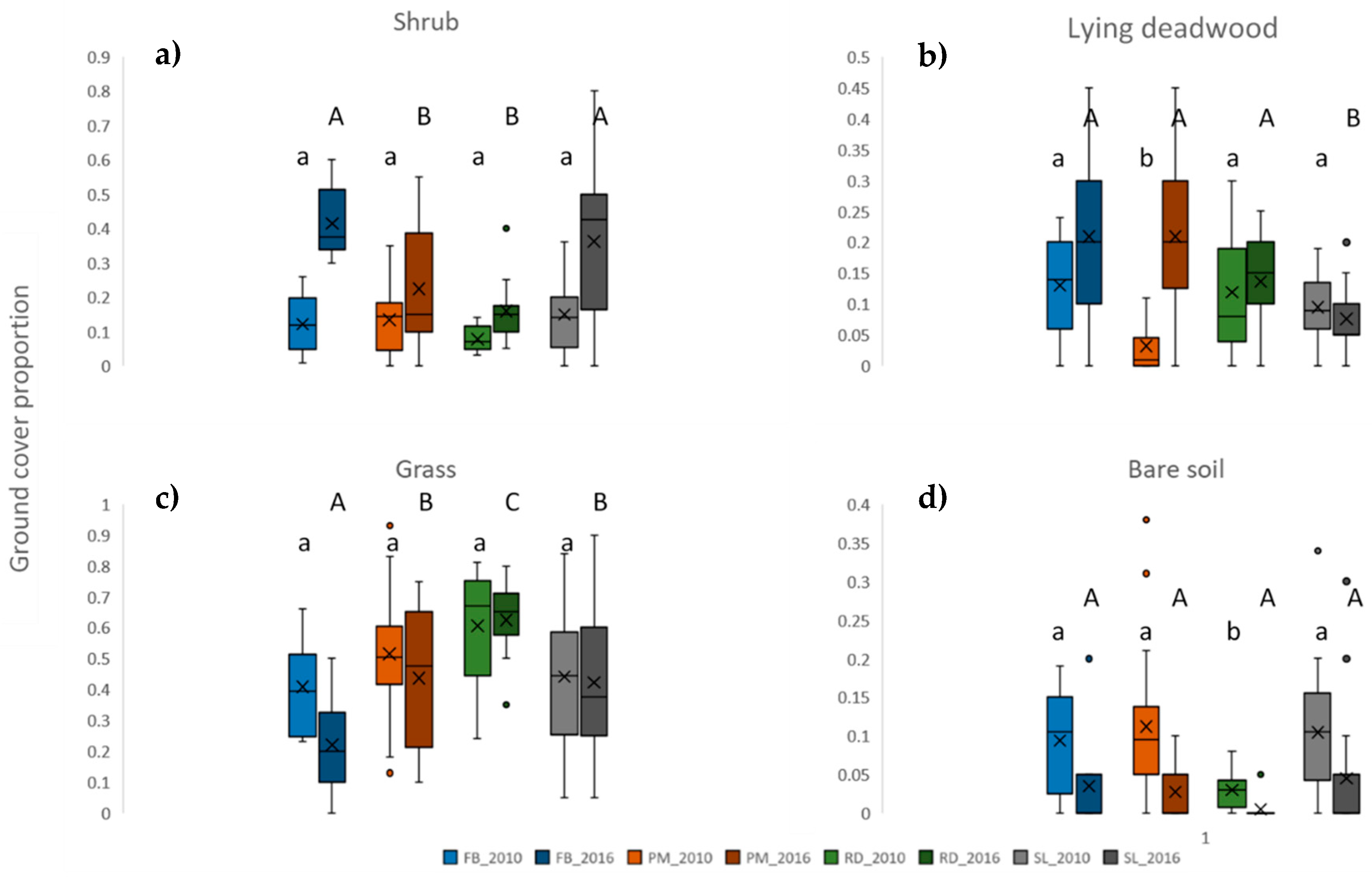
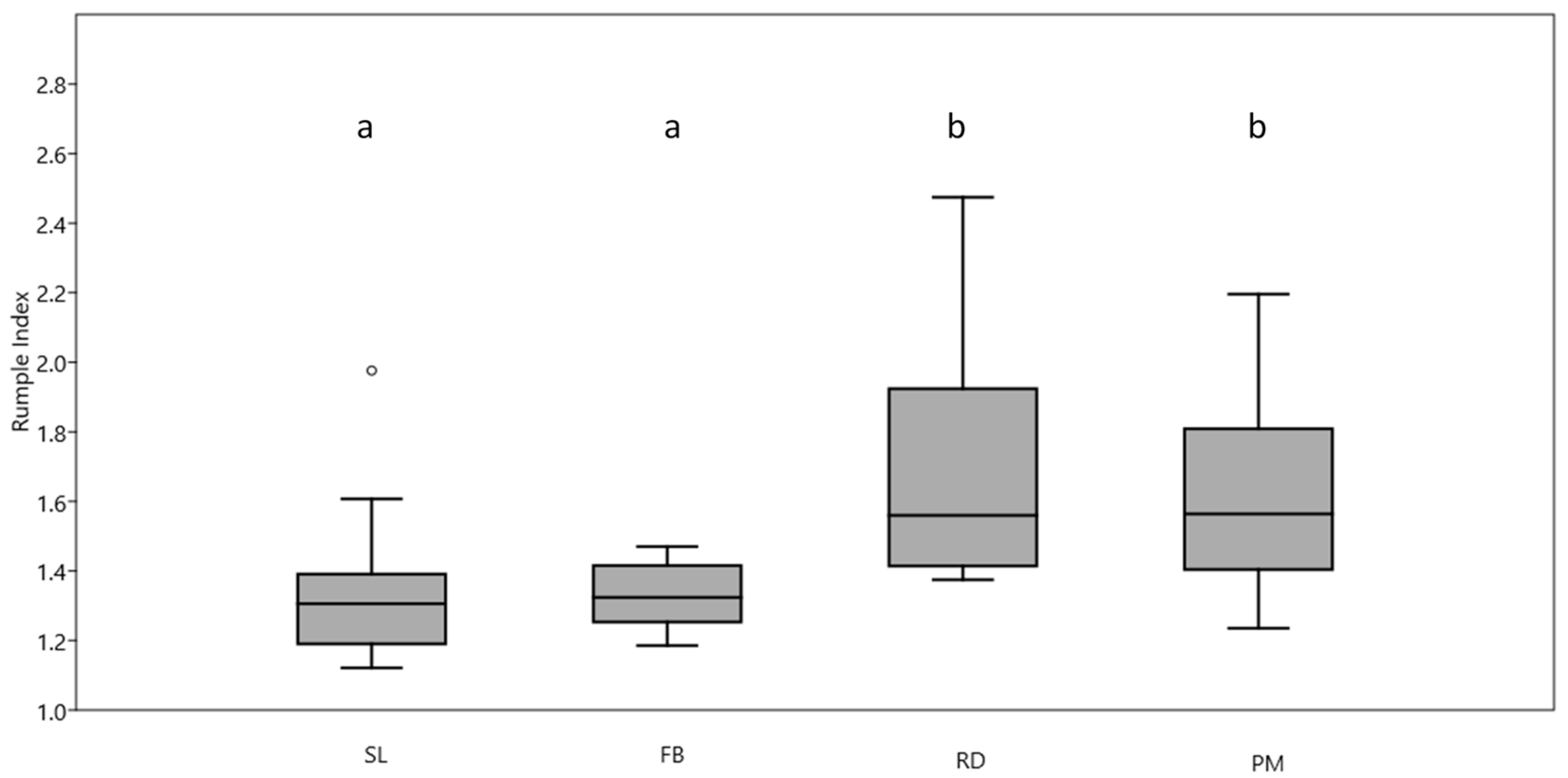
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilisson, T.; Chen, H.Y.H. The direct regeneration hypothesis in northern forests. J. Veg. Sci. 2009, 20, 735–744. [Google Scholar] [CrossRef]
- Münzbergová, Z.; Herben, T. Seed, dispersal, microsite, habitat and recruitment limitation: Identification of terms and concepts in studies of limitations. Oecologia 2005, 145, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Liu, Y.P.; Gong, J.; Fan, S.H.; Shen, G.C.; Zhou, Y.; Fang, Q.; Tang, Q.; Yang, Y.; Wang, R.; et al. Unraveling the roles of various ecological factors in seedling recruitment to facilitate plant regeneration. For. Ecol. Manag. 2021, 492, 119219. [Google Scholar] [CrossRef]
- Nathan, R.; Muller-Landau, H.C. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 2000, 15, 278–285. [Google Scholar] [CrossRef]
- Castro, J.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Seedling establishment of a boreal tree species (Pinus sylvestris) at its southernmost distribution limit: Consequences of being in a marginal Mediterranean habitat. J. Ecol. 2004, 92, 266–277. [Google Scholar] [CrossRef]
- Collins, S.L.; Good, R.E. The Seedling Regeneration Niche: Habitat Structure of Tree Seedlings in an Oak-Pine Forest. Oikos 1987, 48, 89. [Google Scholar] [CrossRef]
- Marzano, R.; Garbarino, M.; Marcolin, E.; Pividori, M.; Lingua, E. Deadwood anisotropic facilitation on seedling establishment after a stand-replacing wildfire in Aosta Valley (NW Italy). Ecol. Eng. 2013, 51, 117–122. [Google Scholar] [CrossRef]
- Vesk, P.A.; Westoby, M. Funding the bud bank: A review of the costs of buds. Oikos 2004, 106, 200–208. [Google Scholar] [CrossRef]
- Bell, D.M.; Bradford, J.B.; Lauenroth, W.K. Early indicators of change: Divergent climate envelopes between tree life stages imply range shifts in the western United States. Glob. Ecol. Biogeogr. 2014, 23, 168–180. [Google Scholar] [CrossRef]
- Pröll, G.; Darabant, A.; Gratzer, G.; Katzensteiner, K. Unfavourable microsites, competing vegetation and browsing restrict post-disturbance tree regeneration on extreme sites in the Northern Calcareous Alps. Eur. J. For. Res. 2015, 134, 293–308. [Google Scholar] [CrossRef]
- Rigling, A.; Bigler, C.; Eilmann, B.; Feldmeyer-Christe, E.; Gimmi, U.; Ginzler, C.; Graf, U.; Mayer, P.; Vacchiano, G.; Weber, P.; et al. Driving factors of a vegetation shift from Scots pine to pubescent oak in dry Alpine forests. Glob. Chang. Biol. 2013, 19, 229–240. [Google Scholar] [CrossRef]
- Tapias, R.; Climent, J.; Pardos, J.A.; Gil, L. Life histories of Mediterranean pines. Plant Ecol. 2004, 171, 53–68. [Google Scholar] [CrossRef]
- Vilà-Cabrera, A.; Rodrigo, A.; Martínez-Vilalta, J.; Retana, J. Lack of regeneration and climatic vulnerability to fire of Scots pine may induce vegetation shifts at the southern edge of its distribution. J. Biogeogr. 2012, 39, 488–496. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Wildfires and global change. Front. Ecol. Environ. 2021, 19, 387–395. [Google Scholar] [CrossRef]
- Pausas, J.G. Evolutionary fire ecology: Lessons learned from pines. Trends Plant Sci. 2015, 20, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Martín-Alcón, S.; Coll, L. Unraveling the relative importance of factors driving post-fire regeneration trajectories in non-serotinous Pinus nigra forests. For. Ecol. Manag. 2016, 361, 13–22. [Google Scholar] [CrossRef]
- Hille, M.; den Ouden, J. Improved recruitment and early growth of Scots pine (Pinus sylvestris L.) seedlings after fire and soil scarification. Eur. J. For. Res. 2004, 123, 213–218. [Google Scholar] [CrossRef]
- Parro, K.; Metslaid, M.; Renel, G.; Sims, A.; Stanturf, J.A.; Jõgiste, K.; Köster, K. Impact of postfire management on forest regeneration in a managed hemiboreal forest, Estonia. Can. J. For. Res. 2015, 45, 1192–1197. [Google Scholar] [CrossRef]
- Greene, D.F.; Gauthier, S.; Noë, J.; Rousseau, M.; Bergeron, Y. A field experiment to determine the effect of post-fire salvage on seedbeds and tree regeneration. Front. Ecol. Environ. 2006, 4, 69–74. [Google Scholar] [CrossRef]
- Marangon, D.; Marchi, N.; Lingua, E. Windthrown elements: A key point improving microsite amelioration and browsing protection to transplanted seedlings. For. Ecol. Manag. 2022, 508, 120050. [Google Scholar] [CrossRef]
- Callaway, R.M. Positive Interactions and Interdependence in Plant Communities; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; ISBN 9781402062247. [Google Scholar]
- Cavieres, L.A.; Brooker, R.W.; Butterfield, B.J.; Cook, B.J.; Kikvidze, Z.; Lortie, C.J.; Michalet, R.; Pugnaire, F.I.; Schöb, C.; Xiao, S.; et al. Facilitative plant interactions and climate simultaneously drive alpine plant diversity. Ecol. Lett. 2014, 17, 193–202. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Sun, J.; Zhang, Z.; Xu, X.; Zhou, H.; Wu, G.L.; Xu, M.; Tsunekawa, A.; Haregeweyn, N.; et al. Shift in nurse effect from facilitation to competition with increasing size of Salix cupularis canopy in a desertified alpine meadow on the Tibetan Plateau. Catena 2020, 195, 104757. [Google Scholar] [CrossRef]
- Olsen, S.L.; Töpper, J.P.; Skarpaas, O.; Vandvik, V.; Klanderud, K. From facilitation to competition: Temperature-driven shift in dominant plant interactions affects population dynamics in seminatural grasslands. Glob. Chang. Biol. 2016, 22, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.J.; Pugnaire, F.I.; Armas, C.; Rodríguez-Echeverría, S.; Schöb, C. The shift from plant–plant facilitation to competition under severe water deficit is spatially explicit. Ecol. Evol. 2017, 7, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Martelletti, S.; Lingua, E.; Meloni, F.; Freppaz, M.; Motta, R.; Nosenzo, A.; Marzano, R. Microsite manipulation in lowland oak forest restoration results in indirect effects on acorn predation. For. Ecol. Manag. 2018, 411, 27–34. [Google Scholar] [CrossRef]
- Castro, J.; Allen, C.D.; Molina-Morales, M.; Marañón-Jiménez, S.; Sánchez-Miranda, Á.; Zamora, R. Salvage Logging Versus the Use of Burnt Wood as a Nurse Object to Promote Post-Fire Tree Seedling Establishment. Restor. Ecol. 2011, 19, 537–544. [Google Scholar] [CrossRef]
- Heinemann, K.; Kitzberger, T. Effects of position, understorey vegetation and coarse woody debris on tree regeneration in two environmentally contrasting forests of north-western Patagonia: A manipulative approach. J. Biogeogr. 2006, 33, 1357–1367. [Google Scholar] [CrossRef]
- Garbarino, M.; Marzano, R.; Shaw, J.D.; Long, J.N. Environmental drivers of deadwood dynamics in woodlands and forests. Ecosphere 2015, 6, 1–24. [Google Scholar] [CrossRef]
- Franklin, J.F.; Lindenmayer, D.B.; MacMahon, J.A.; McKee, A.; Magnusson, J.; Perry, D.A.; Waide, R.; Foster, D.R. Threads of continuity: Ecosystem disturbances, biological legacies and ecosystem recovery. Conserv. Biol. Pract. 2000, 1, 8–16. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Burton, P.J.; Franklin, J.F. Salvage Logging and Its Ecological Consequences; Island Press: Washington, DC, USA, 2009; Volume 46, ISBN 978-1-59726-402-0. [Google Scholar]
- Kitenberga, M.; Elferts, D.; Adamovics, A.; Katrevics, J.; Donis, J.; Baders, E.; Jansons, A. Effect of salvage logging and forest type on the post-fire regeneration of Scots pine in hemiboreal forests. New For. 2020, 51, 1069–1085. [Google Scholar] [CrossRef]
- Leverkus, A.B.; Rey Benayas, J.M.; Castro, J.; Boucher, D.; Brewer, S.; Collins, B.M.; Donato, D.; Fraver, S.; Kishchuk, B.E.; Lee, E.J.; et al. Salvage logging effects on regulating and supporting ecosystem services—A systematic map. Can. J. For. Res. 2018, 48, 983–1000. [Google Scholar] [CrossRef]
- Thorn, S.; Bässler, C.; Brandl, R.; Burton, P.J.; Cahall, R.; Campbell, J.L.; Castro, J.; Choi, C.Y.; Cobb, T.; Donato, D.C.; et al. Impacts of salvage logging on biodiversity: A meta-analysis. J. Appl. Ecol. 2018, 55, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Mantero, G.; Morresi, D.; Negri, S.; Anselmetto, N.; Lingua, E.; Bonifacio, E.; Garbarino, M.; Marzano, R. Short-term drivers of post-fire forest regeneration in the Western Alps. Fire Ecol. 2023, 19, 23. [Google Scholar] [CrossRef]
- Leverkus, A.B.; Gustafsson, L.; Rey Benayas, J.M.; Castro, J. Does post-disturbance salvage logging affect the provision of ecosystem services? A systematic review protocol. Environ. Evid. 2015, 4, 16. [Google Scholar] [CrossRef]
- Marcolin, E.; Marzano, R.; Vitali, A.; Garbarino, M.; Lingua, E. Post-fire management impact on natural forest regeneration through altered microsite conditions. Forests 2019, 10, 1014. [Google Scholar] [CrossRef]
- Beghin, R.; Lingua, E.; Garbarino, M.; Lonati, M.; Bovio, G.; Motta, R.; Marzano, R. Pinus sylvestris forest regeneration under different post-fire restoration practices in the northwestern Italian Alps. Ecol. Eng. 2010, 36, 1365–1372. [Google Scholar] [CrossRef]
- Aicardi, I.; Garbarino, M.; Lingua, A.; Lingua, E.; Marzano, R.; Piras, M. Monitoring Post-Fire Forest Recovery Using Multi-Temporal Digital Surface Models Generated From. EARSeL Eproceedings 2016, 15, 1–8. [Google Scholar]
- Marchi, N.; Pirotti, F.; Lingua, E. Airborne and terrestrial laser scanning data for the assessment of standing and lying deadwood: Current situation and new perspectives. Remote Sens. 2018, 10, 1356. [Google Scholar] [CrossRef]
- Wilson, M.F.J.; O’Connell, B.; Brown, C.; Guinan, J.C.; Grehan, A.J. Multiscale terrain analysis of multibeam bathymetry data for habitat mapping on the continental slope. Mar. Geod. 2007, 30, 3–35. [Google Scholar] [CrossRef]
- Jenness, J.S. Calculating landscape surface area from digital elevation models. Wildl. Soc. Bull. 2004, 32, 829–839. [Google Scholar] [CrossRef]
- McCune, B.; Keon, D. Equations for potential annual direct incident radiation and heat load. J. Veg. Sci. 2002, 13, 603–606. [Google Scholar] [CrossRef]
- Blair, D.P.; McBurney, L.M.; Blanchard, W.; Banks, S.C.; Lindenmayer, D.B. Disturbance gradient shows logging affects plant functional groups more than fire. Ecol. Appl. 2016, 26, 2280–2301. [Google Scholar] [CrossRef] [PubMed]
- Leverkus, A.B.; Gustafsson, L.; Lindenmayer, D.B.; Castro, J.; Rey Benayas, J.M.; Ranius, T.; Thorn, S. Salvage logging effects on regulating ecosystem services and fuel loads. Front. Ecol. Environ. 2020, 18, 391–400. [Google Scholar] [CrossRef]
- Eliasson, L. Growth Regulators in Populus tremula IV. Apical Dominance and Suckering in Young Plants. Physiol. Plant. 1971, 25, 263–267. [Google Scholar] [CrossRef]
- Palik, B.; Kastendick, D. Woody plant regeneration after blowdown, salvage logging, and prescribed fire in a northern Minnesota forest. For. Ecol. Manag. 2009, 258, 1323–1330. [Google Scholar] [CrossRef]
- Baeza, M.J.; Valdecantos, A.; Alloza, J.A.; Vallejo, V.R. Human disturbance and environmental factors as drivers of long-term post-fire regeneration patterns in Mediterranean forests. J. Veg. Sci. 2007, 18, 243. [Google Scholar] [CrossRef]
- Puerta-Piñero, C.; Espelta, J.M.; Sánchez-Humanes, B.; Rodrigo, A.; Coll, L.; Brotons, L. History matters: Previous land use changes determine post-fire vegetation recovery in forested Mediterranean landscapes. For. Ecol. Manag. 2012, 279, 121–127. [Google Scholar] [CrossRef]
- Serra-Diaz, J.M.; Maxwell, C.; Lucash, M.S.; Scheller, R.M.; Laflower, D.M.; Miller, A.D.; Tepley, A.J.; Epstein, H.E.; Anderson-Teixeira, K.J.; Thompson, J.R. Disequilibrium of fire-prone forests sets the stage for a rapid decline in conifer dominance during the 21st century. Sci. Rep. 2018, 8, 6749. [Google Scholar] [CrossRef]
- Harvey, B.J.; Donato, D.C.; Turner, M.G. High and dry: Post-fire tree seedling establishment in subalpine forests decreases with post-fire drought and large stand-replacing burn patches. Glob. Ecol. Biogeogr. 2016, 25, 655–669. [Google Scholar] [CrossRef]
- Littlefield, C.E.; Dobrowskia, S.Z.; Abatzoglouc, J.T.; Parksd, S.A.; Davise, K.T. A climatic dipole drives short- And long-term patterns of postfire forest recovery in the western United States. Proc. Natl. Acad. Sci. USA 2020, 117, 29730–29737. [Google Scholar] [CrossRef]
- Del Barrio, J.; Luis-Calabuig, E.; Tárrega, R. Vegetative response of Arctostaphylos uva-ursi to experimental cutting and burning. Plant Ecol. 1999, 145, 191–195. [Google Scholar] [CrossRef]
- Hobbs, R.J. Possible Chemical Interactions among Heathland Plants. Oikos 1984, 43, 23. [Google Scholar] [CrossRef]
- Hewitt, R.E.; Chapin, F.S.; Hollingsworth, T.N.; Taylor, D.L. The potential for mycobiont sharing between shrubs and seedlings to facilitate tree establishment after wildfire at Alaska arctic treeline. Mol. Ecol. 2017, 26, 3826–3838. [Google Scholar] [CrossRef]
- Karavani, A.; Boer, M.M.; Baudena, M.; Colinas, C.; Díaz-Sierra, R.; Pemán, J.; de Luis, M.; Enríquez-de-Salamanca, Á.; Resco de Dios, V. Fire-induced deforestation in drought-prone Mediterranean forests: Drivers and unknowns from leaves to communities. Ecol. Monogr. 2018, 88, 141–169. [Google Scholar] [CrossRef]
- Marañón-Jiménez, S.; Castro, J.; Querejeta, J.I.; Fernández-Ondoño, E.; Allen, C.D. Post-fire wood management alters water stress, growth, and performance of pine regeneration in a Mediterranean ecosystem. For. Ecol. Manag. 2013, 308, 231–239. [Google Scholar] [CrossRef]
- García-Orenes, F.; Arcenegui, V.; Chrenková, K.; Mataix-Solera, J.; Moltó, J.; Jara-Navarro, A.B.; Torres, M.P. Effects of salvage logging on soil properties and vegetation recovery in a fire-affected Mediterranean forest: A two year monitoring research. Sci. Total Environ. 2017, 586, 1057–1065. [Google Scholar] [CrossRef]
- Smit, C.; Den Ouden, J.; Díaz, M. Facilitation of Quercus ilex recruitment by shrubs in Mediterranean open woodlands. J. Veg. Sci. 2008, 19, 193–200. [Google Scholar] [CrossRef]
- Morán-Ordóñez, A.; Duane, A.; Gil-Tena, A.; De Cáceres, M.; Aquilué, N.; Guerra, C.A.; Geijzendorffer, I.R.; Fortin, M.; Brotons, L. Future impact of climate extremes in the Mediterranean: Soil erosion projections when fire and extreme rainfall meet. L. Degrad. Dev. 2020, 31, 3040–3054. [Google Scholar] [CrossRef]
- Prosser, I.P.; Williams, L. The effect of wildfire on runoff and erosion in native Eucalyptus forest. Hydrol. Process. 1998, 12, 251–265. [Google Scholar] [CrossRef]
- Shakesby, R.A. Post-wildfire soil erosion in the Mediterranean: Review and future research directions. Earth-Sci. Rev. 2011, 105, 71–100. [Google Scholar] [CrossRef]
- Molinas-González, C.R.; Leverkus, A.B.; Marañón-Jiménez, S.; Castro, J. Fall rate of burnt pines across an elevational gradient in a Mediterranean mountain. Eur. J. For. Res. 2017, 136, 401–409. [Google Scholar] [CrossRef]
- Taboada, A.; Fernández-García, V.; Marcos, E.; Calvo, L. Interactions between large high-severity fires and salvage logging on a short return interval reduce the regrowth of fire-prone serotinous forests. For. Ecol. Manag. 2018, 414, 54–63. [Google Scholar] [CrossRef]
- Lampainen, J.; Kuuluvainen, T.; Wallenius, T.H.; Karjalainen, L.; Vanha-Majamaa, I. Long-term forest structure and regeneration after wildfire in Russian Karelia. J. Veg. Sci. 2004, 15, 245–256. [Google Scholar] [CrossRef]
- Moghimian, N.; Jalali, S.G.; Kooch, Y.; Rey, A. Downed logs improve soil properties in old-growth temperate forests of northern Iran. Pedosphere 2020, 30, 378–389. [Google Scholar] [CrossRef]
- Kuuluvainen, T. Gap disturbance, ground microtopography, and the regeneration dynamics of boreal coniferous forests in Finland: A review. Ann. Zool. Fennici 1994, 31, 35–51. [Google Scholar]
- Castro, J.; Gómez, J.M.; García, D.; Zamora, R.; Hódar, J.A. Seed predation and dispersal in relict Scots pine forests in southern Spain. Plant Ecol. 1999, 145, 115–123. [Google Scholar] [CrossRef]
- Smit, C.; Gusberti, M.; Müller-Schärer, H. Safe for saplings; safe for seeds? For. Ecol. Manag. 2006, 237, 471–477. [Google Scholar] [CrossRef]
- van Ginkel, H.A.L.; Kuijper, D.P.J.; Churski, M.; Zub, K.; Szafrańska, P.; Smit, C. Safe for saplings not safe for seeds: Quercus robur recruitment in relation to coarse woody debris in Białowieża Primeval Forest, Poland. For. Ecol. Manag. 2013, 304, 73–79. [Google Scholar] [CrossRef]
- Neilly, H.; Cale, P. ‘Branching’ with complex coarse woody debris reduces herbivory on recovering erosion scalds. Ecol. Manag. Restor. 2020, 21, 143–146. [Google Scholar] [CrossRef]
- Leverkus, A.B.; Lindenmayer, D.B.; Thorn, S.; Gustafsson, L. Salvage logging in the world’s forests: Interactions between natural disturbance and logging need recognition. Glob. Ecol. Biogeogr. 2018, 27, 1140–1154. [Google Scholar] [CrossRef]
- Kleinman, J.S.; Goode, J.D.; Fries, A.C.; Hart, J.L. Ecological consequences of compound disturbances in forest ecosystems: A systematic review. Ecosphere 2019, 10, e02962. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Westgate, M.J.; Scheele, B.C.; Foster, C.N.; Blair, D.P. Key perspectives on early successional forests subject to stand-replacing disturbances. For. Ecol. Manag. 2019, 454, 117656. [Google Scholar] [CrossRef]
- Leverkus, A.B.; Puerta-Piñero, C.; Guzmán-Álvarez, J.R.; Navarro, J.; Castro, J. Post-fire salvage logging increases restoration costs in a Mediterranean mountain ecosystem. New For. 2012, 43, 601–613. [Google Scholar] [CrossRef]
- Thom, D.; Sommerfeld, A.; Sebald, J.; Hagge, J.; Müller, J.; Seidl, R. Effects of disturbance patterns and deadwood on the microclimate in European beech forests. Agric. For. Meteorol. 2020, 291, 108066. [Google Scholar] [CrossRef] [PubMed]
- Urza, A.K.; Weisberg, P.J.; Chambers, J.C.; Sullivan, B.W. Shrub facilitation of tree establishment varies with ontogenetic stage across environmental gradients. New Phytol. 2019, 223, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- Bürzle, B.; Schickhoff, U.; Schwab, N.; Wernicke, L.M.; Müller, Y.K.; Böhner, J.; Chaudhary, R.P.; Scholten, T.; Oldeland, J. Seedling recruitment and facilitation dependence on safe site characteristics in a Himalayan treeline ecotone. Plant Ecol. 2018, 219, 115–132. [Google Scholar] [CrossRef]
- De Chantal, M.; Kuuluvainen, T.; Lindberg, H.; Vanha-Majamaa, I. Early regeneration of populus tremula from seed after forest restoration with fire. Scand. J. For. Res. 2005, 20, 33–42. [Google Scholar] [CrossRef]
- Seidl, R.; Turner, M.G. Post-disturbance reorganization of forest ecosystems in a changing world. Proc. Natl. Acad. Sci. USA 2022, 119, e2202190119. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.J.; Bicknell, J.E.; Baldwin-Cantello, W.; Struebig, M.J.; Davies, Z.G. Quantifying the impacts of defaunation on natural forest regeneration in a global meta-analysis. Nat. Commun. 2019, 10, 4590. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, X.; Shi, Y.S.; Li, Y.Y.; Wu, J.; He, F.; Chen, X.Y. Habitat fragmentation changes top-down and bottom-up controls of food webs. Ecology 2020, 101, e03062. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, K.; Kitzberger, T.; Veblen, T.T. Influences of gap microheterogeneity on the regeneration of Nothofagus pumilio in a xeric old-growth forest of northwestern Patagonia, Argentina. Can. J. For. Res. 2000, 30, 25–31. [Google Scholar] [CrossRef]
- Oreja, B.; Goberna, M.; Verdú, M.; Navarro-Cano, J.A. Constructed pine log piles facilitate plant establishment in mining drylands. J. Environ. Manag. 2020, 271, 111015. [Google Scholar] [CrossRef] [PubMed]
- Leverkus, A.B.; Buma, B.; Wagenbrenner, J.; Burton, P.J.; Lingua, E.; Marzano, R.; Thorn, S. Tamm review: Does salvage logging mitigate subsequent forest disturbances? For. Ecol. Manag. 2021, 481, 118721. [Google Scholar] [CrossRef]
- Dobor, L.; Hlásny, T.; Rammer, W.; Zimová, S.; Barka, I.; Seidl, R. Is salvage logging effectively dampening bark beetle outbreaks and preserving forest carbon stocks? J. Appl. Ecol. 2020, 57, 67–76. [Google Scholar] [CrossRef]
- Larson, A.J.; Jeronimo, S.M.A.; Hessburg, P.F.; Lutz, J.A.; Povak, N.A.; Cansler, C.A.; Kane, V.R.; Churchill, D.J. Tamm Review: Ecological principles to guide post-fire forest landscape management in the Inland Pacific and Northern Rocky Mountain regions. For. Ecol. Manag. 2022, 504, 119680. [Google Scholar] [CrossRef]
- Wohlgemuth, T.; Schwitter, R.; Bebi, P.; Sutter, F.; Brang, P. Post-windthrow management in protection forests of the Swiss Alps. Eur. J. For. Res. 2017, 136, 1029–1040. [Google Scholar] [CrossRef]
- Baggio, T.; Brožová, N.; Bast, A.; Bebi, P.; D’Agostino, V. Novel indices for snow avalanche protection assessment and monitoring of wind-disturbed forests. Ecol. Eng. 2022, 181, 106677. [Google Scholar] [CrossRef]
- Lingua, E.; Bettella, F.; Pividori, M.; Marzano, R.; Garbarino, M.; Piras, M.; Kobal, M.; Berger, F. The Protective Role of Forests to Reduce Rockfall Risks and Impacts in the Alps under a Climate Change Perspective. In Climate Change Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 333–347. [Google Scholar]
| Survey | Predictor Variable | df | F | p |
|---|---|---|---|---|
| 2010 | T | 3, 31 | 4.75 | 0.008 |
| R3 | 1, 31 | 1.45 | 0.239 | |
| HLI | 1, 31 | 2.22 | 0.147 | |
| DIST | 1, 31 | 2.94 | 0.097 | |
| T × R3 | 1, 31 | 4.81 | 0.008 | |
| T × HLI | 3, 31 | 4.15 | 0.015 | |
| T × DIST | 3, 31 | 3.34 | 0.335 | |
| R3 × HLI | 1, 31 | 3.58 | 0.069 | |
| R3 × DIST | 1, 31 | 5.55 | 0.026 | |
| DIST × HLI | 1, 31 | 3.81 | 0.061 | |
| T × R3 × HLI | 3, 31 | 4.23 | 0.014 | |
| T × HLI × DIST | 3, 31 | 3.77 | 0.022 | |
| T × R3 × DIST | 3, 31 | 3.74 | 0.022 | |
| R3 × HLI × DIST | 1, 31 | 5.40 | 0.028 | |
| T × R3 × HLI × DIST | 3, 31 | 4.12 | 0.015 | |
| 2016 | T | 3, 31 | 3.86 | 0.019 |
| R3 | 1, 31 | 5.25 | 0.029 | |
| T × R3 | 3, 31 | 4.19 | 0.013 | |
| T × HLI | 3, 31 | 3.91 | 0.018 | |
| T × DIST | 3, 31 | 3.81 | 0.019 | |
| R3 × HLI | 1, 31 | 7.63 | 0.009 | |
| R3 × DIST | 1, 31 | 6.39 | 0.017 | |
| T × R3 × HLI | 3, 31 | 4.10 | 0.015 | |
| T × HLI × DIST | 3, 31 | 3.90 | 0.018 | |
| T × R3 × DIST | 3, 31 | 4.15 | 0.014 | |
| R3 × HLI × DIST | 1, 31 | 5.09 | 0.031 | |
| T × R3 × HLI × DIST | 3, 31 | 4.15 | 0.014 |
| Explanatory Variable | Beta | S.E. | p-Value | Odds Ratio | 95% Confidence Interval for Odds Ratio | ||
|---|---|---|---|---|---|---|---|
| Proximity to: | Lower | Upper | |||||
| Regeneration 2010 | Rocks | 0.707 | 0.548 | 0.197 | 2.027 | 0.692 | 5.934 |
| n = 268 | Deadwood | 1.320 | 0.274 | <0.001 | 3.743 | 2.188 | 6.404 |
| Deadwood_N | 0.865 | 0.318 | 0.007 | 2.376 | 1.2727 | 4.435 | |
| Deadwood_E | 1.394 | 0.354 | <0.001 | 4.029 | 2.0147 | 8.058 | |
| Deadwood_S | 1.311 | 0.335 | <0.001 | 3.709 | 1.9244 | 7.148 | |
| Deadwood_W | 0.037 | 0.402 | 0.926 | 1.038 | 0.4722 | 2.282 | |
| Regeneration 2016 | Rocks | 0.511 | 0.516 | 0.323 | 1.667 | 0.606 | 4.586 |
| n = 147 | Deadwood | 1.299 | 0.651 | 0.046 | 3.667 | 1.023 | 13.143 |
| Deadwood_N | 1.485 | 0.650 | 0.022 | 4.417 | 1.236 | 15.785 | |
| Deadwood_E | 1.343 | 0.657 | 0.041 | 3.829 | 1.057 | 13.875 | |
| Deadwood_S | 0.803 | 0.633 | 0.204 | 2.233 | 0.646 | 7.716 | |
| Deadwood_W | 0.861 | 0.624 | 0.168 | 2.366 | 0.696 | 8.045 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lingua, E.; Marques, G.; Marchi, N.; Garbarino, M.; Marangon, D.; Taccaliti, F.; Marzano, R. Post-Fire Restoration and Deadwood Management: Microsite Dynamics and Their Impact on Natural Regeneration. Forests 2023, 14, 1820. https://doi.org/10.3390/f14091820
Lingua E, Marques G, Marchi N, Garbarino M, Marangon D, Taccaliti F, Marzano R. Post-Fire Restoration and Deadwood Management: Microsite Dynamics and Their Impact on Natural Regeneration. Forests. 2023; 14(9):1820. https://doi.org/10.3390/f14091820
Chicago/Turabian StyleLingua, Emanuele, Gonçalo Marques, Niccolò Marchi, Matteo Garbarino, Davide Marangon, Flavio Taccaliti, and Raffaella Marzano. 2023. "Post-Fire Restoration and Deadwood Management: Microsite Dynamics and Their Impact on Natural Regeneration" Forests 14, no. 9: 1820. https://doi.org/10.3390/f14091820
APA StyleLingua, E., Marques, G., Marchi, N., Garbarino, M., Marangon, D., Taccaliti, F., & Marzano, R. (2023). Post-Fire Restoration and Deadwood Management: Microsite Dynamics and Their Impact on Natural Regeneration. Forests, 14(9), 1820. https://doi.org/10.3390/f14091820







