The Impact of Natural Regeneration of Phoebe bournei in Anfu County, Jiangxi Province, on Community Diversity and Soil Nutrient Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sample Plot Setting
2.2. Soil Sampling and Testing
2.3. Diversity Measure
2.3.1. Species Diversity
2.3.2. Structural Diversity
2.4. Importance Value Calculation
2.5. Biomass Calculation
2.6. Data Analysis
3. Results
3.1. Community Diversity and Productivity Characteristics
3.2. Characteristics of Soil Nutrient and Microbial Diversity
3.3. The Relationship between Community Productivity, Diversity, and Soil Nutrients
4. Discussion
4.1. Changes in Community Diversity and Productivity during P. bournei Regeneration Process
4.2. Changes in Soil Nutrients and Microbial Diversity during the Regeneration Process of P. bournei
4.3. The Relationship between Community Productivity and Diversity and Soil Environment during the Regeneration Process of P. bournei
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, K.; Zhu, J.; Zhang, M.; Yan, Q.; Sun, O.J. Soil microbial biomass carbon and nitrogen in forest ecosystems of Northeast China: A comparison between natural secondary forest and larch plantation. J. Plant Ecol. 2010, 3, 175–182. [Google Scholar] [CrossRef]
- Wang, C.K.; Yang, J.Y. Rhizospheric and heterotrophic compo-nents of soil respiration in six Chinese temperate forests. Glob. Chang. Biol. 2007, 13, 123–131. [Google Scholar] [CrossRef]
- Burton, J.; Chen, C.R.; Xu, Z.H. Gross nitrogen transformations in adjacent native and plantation forests of subtropical Australia. Soil Biol. Biochem. 2007, 39, 426–433. [Google Scholar] [CrossRef]
- Chandra, L.R.; Gupta, S.; Pande, V.; Singh, N. Impact of forest vegetation on soil characteristics: A correlation between soil biological and physico-chemical properties. 3 Biotech. 2016, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Ma, Y.H.; Jin, X.; Wang, Z.; Ma, Y.; Fu, S.L.; Chen, H.Y.H. Soil enzyme activities increase following resto ration of degraded subtropical forests. Geoderma 2019, 351, 180–187. [Google Scholar] [CrossRef]
- Green, R. Ecology and Evolution of Communities.by Martin L. Cody; Jared M. Diamond. Biometrics 1976, 32, 701. [Google Scholar] [CrossRef]
- Vincent, A.G.; Schleucher, J.; Grbner, G.; Vestergren, J.; Persson, P.; Jansson, M.; Giesler, R. Changes in organic phosphorus composition in boreal forest humus soils: The role of iron and aluminium. Biogeochemistry 2012, 108, 485–499. [Google Scholar] [CrossRef]
- Gren, G.I.; Wetterstedt, J.M.; Billberger, M.F.K. Nutrient limitation on terrestrial plant growth–modeling the interaction between nitrogen and phosphorus. New Phytol. 2012, 194, 953–960. [Google Scholar]
- Gnankambary, Z.; Ilstedt, U.; Nyberg, G.; Hien, V.; Malmer, A. Nitrogen and phosphorus limitation of soil microbial respiration in two tropical agroforestry parklands in the South-Sudanese zone of Burkina Faso: The effects of tree canopy and fertilization. Soil Biol. Biochem. 2008, 40, 350–359. [Google Scholar] [CrossRef]
- Hu, B.; Yang, B.; Pang, X.Y.; Bao, W.K.; Tian, G.L. Responses of soil phosphorus fractions to gap size in a reforested spruce forest. Geoderma 2016, 279, 61–69. [Google Scholar] [CrossRef]
- Lewandowska, A.M.; Biermann, A.; Borer, E.T.; Cebrián-Piqueras, M.A.; Hillebrand, H. The influence of balanced and imbalanced resource supply on biodiversity-functioning relationship across ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 1–9. [Google Scholar] [CrossRef]
- Fridley, J.D. Resource availability do minates an dalters the relationship between species diversity an decosystem productivity in experimental plant communities. Oeclolgical 2002, 132, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Darwin, G.; Margot, V.; Lander, B.; Jan, V.; An, M.; Dries, B.; Luc, L.; Kris, V. Competition, tree age and size drivethe productivity of mixed forests of pedunculate oak, beech and red oak. For. Ecol. Manag. 2018, 430, 609–617. [Google Scholar]
- Lehman, C.L.; Tilman, D. Biodiversity stability, and productivity in com petitive communities. Am. Nat. 2000, 156, 534–552. [Google Scholar] [CrossRef] [PubMed]
- Pedro, M.S.; Ramme, R.W.; Seidl, R. Disentangling the effects of composi tional and structural diversity on forest productivity. J. Veg. Sci. 2017, 28, 649–658. [Google Scholar] [CrossRef]
- Pretzsch, H. Canopy space filling and tree crown morphology in mixed-species stands compared with monocultures. For. Ecol. Manag. 2014, 327, 251–264. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Noguez, A.M.; Escalante, A.E.; Forney, L.J.; Mendoza, M.N.; Rosas, I.; Souza, V.; Oliva, F.G. Soil aggregates in a tropical deciduous forest: Effects on C and N dynamics, and microbial communities as determined by t-RFLPs. Biogeochemistry 2008, 89, 209–220. [Google Scholar] [CrossRef]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The influence of functional diversity and composition on ecosystem processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef]
- Tilman, D.; Reich, P.B.; Knops, J.; David, W.; Troy, M.; Lehman, C. Diversity and Productivity in a Long-Term Grassland Experiment. Science 2001, 294, 843–845. [Google Scholar] [CrossRef]
- Zhen, W.J. Chinese Tree Records; China Forestry Publishing House: Beijing, China, 1983; pp. 795–797. [Google Scholar]
- Li, T.; Min, X. Dormancy characteristics and germination requirements of Phoebe bournei seed. Sci. Hortic. 2020, 260, 108903. [Google Scholar] [CrossRef]
- Wu, D.R.; Zhu, Z.D. Preliminiary study on structure and spatial distribution pattern of Phoebe bournei in Luoboyan nature reserve in Fujian province. Sci. Silvae Sin. 2003, 39, 23–30, (In Chinese, with English Abstract). [Google Scholar]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Pielou, E.C. Ecological Diversity; John Wiley and Sons: New York, NY, USA, 1975; p. 165. [Google Scholar]
- Schnabel, F.; Schwarz, J.A.; Dnescu, A.; Fichtner, A.; Nock, C.A.; Jürgen, B.; Potvin, C. Drivers of productivity and its temporal stability in a tropical tree diversity experiment. Glob. Chang. Biol. 2019, 25, 4257–4272. [Google Scholar] [CrossRef]
- Luo, J.; Dai, C.D.; Tian, Y.X.; Peng, P.; Ma, F.F.; Zeng, Z.Q.; Zhou, X.L.; Zhang, M. Establishment of main constructive species biomass model for project forests of carbon sink in Hunan. Hunan For. Sci. Technol. 2016, 43, 12–16+21, (In Chinese, with English Abstract). [Google Scholar]
- Lasky, J.R.; Uriarte, M.; Boukili, V.K.; Erickson, D.L.; Kress, W.J.; Chazdon, R.L. The relationship between tree biodiversity and biomass dynamics changes with tropical forest succession. Ecol. Lett. 2014, 17, 1158–1167. [Google Scholar] [CrossRef]
- Lei, X.D.; Wang, W.F.; Peng, C.H. Relationships between stand growth and structural diversity in spruce-dominated forests in New Brunswick, Canada. Can. J. For. Res. 2009, 39, 1835–1847. [Google Scholar] [CrossRef]
- Hardiman, B.S.; Bohrer, G.; Gough, C.M.; Vogel, C.S.; Curtisi, P.S. The role of canopy structural complexity in wood net primary production of a maturing northern deciduous forest. Ecology 2011, 92, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.F.; Lei, X.D.; Ma, Z.H.; Kneeshaw, D.D.; Peng, C.H. Positive relationship between aboveground carbon stocks and structural diversity in spruce-dominated forest stands in New Brunswick, Canada. For. Sci. 2011, 57, 506–515. [Google Scholar]
- Forrester, D.I.; Pretzsch, H. Tamm review: On the strength of evidence when comparing ecosystem functions of mixtures with monocultures. For. Ecol. Manag. 2015, 356, 41–53. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J. A review of processes behind diver-Sity-productivity relationships in forests. Curr. For. Rep. 2016, 2, 45–61. [Google Scholar] [CrossRef]
- Dănescu, A.; Albrecht, A.T.; Bauhus, J. Structural diversity promotes productivity of mixed, uneven-aged forests in southwestern Germany. Oecologia 2016, 182, 319–333. [Google Scholar] [CrossRef]
- Fichtner, A.; Härdtle, W.; Bruelheide, H.; Kunz, M.; Li, Y.; Oheimb, G.V. Neighbourhood interactions drive overyielding in mixed-species tree communities. Nat. Commun. 2018, 9, 1144. [Google Scholar] [CrossRef]
- Zhu, D.H.; Peng, S.H.; Wang, J.Y.; Hui, D.F. Responses of nutrient resorption to human disturbances in Phoebe bournei forests. Forests 2022, 13, 905. [Google Scholar] [CrossRef]
- Wan, X.H.; Huang, Z.Q.; He, Z.M.; Yu, Z.P.; Wang, M.H.; Davis, M.R.; Yang, Y.S. Soil C:N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 2015, 387, 103–116. [Google Scholar] [CrossRef]
- Shen, F.F.; Wu, J.P.; Fan, H.B.; Liu, W.F.; Guo, X.M.; Duan, H.L.; Hu, L.; Lei, X.M.; Wei, X.H. Soil N/P and C/P ratio regulate the responses of soil microbial community composition and enzyme activities in a long-term nitrogen loaded Chinese fir forest. Plant Soil 2019, 436, 91–107. [Google Scholar] [CrossRef]
- Meetei, T.T.; Devi, Y.; Khaidem, J. Effect of various enzymes on mineralization of soil organic matter. Plant Arch. 2020, 20, 3392–3395. [Google Scholar]
- Donovan, P.G.; Kathleen, R.B.M.; Madeleine, M.S.; Steven, D.A. The Michaelis-Menten kinetics of soil extracellular enzymes in response to temperature: A cross-latitudinal study. Glob. Chang. Biol. 2012, 18, 1468–1479. [Google Scholar]
- Ricklefs, R.E.; Latham, R.; Qian, H. Nordic society oikos global patterns of tree species richness in moist forests: Distinguishing ecological influences and historical contingency. Oikos 1999, 86, 369–373. [Google Scholar] [CrossRef]
- Kitayama, K.; Majalap-Lee, N.; Aiba, S. Soil phosphorus fractionation and phosphorus-use efficiencies of tropical rainforests along altitudinal gradients of Mount Kinabalu, Borneo. Oecologia 2000, 123, 342–349. [Google Scholar] [CrossRef]
- See, C.R.; Yanai, R.D.; Fisk, M.C.; Vadeboncoeur, M.A.; Quintero, B.A.; Faheyet, T.J. Soil nitrogen affects phosphorus recycling: Foliar resorption and plant-soil feedbacks in a northern hardwood forest. Ecology 2015, 96, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Barrufol, M.; Schmid, B.; Bruelheide, H.; Chi, X.; Hector, A.; Ma, K.; Michalski, S.; Tang, Z.; Niklaus, P.A. Correction: Biodiversity Promotes Tree Growth during Succession in Subtropical Forest. PLoS ONE 2013, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Bruelheide, H.; Nadrowski, K.; Assmann, T.; Bauhus, J.; Both, S.; Buscot, F.; Chen, X.Y.; Ding, B.Y.; Durka, W.; Erfmeier, A.; et al. Designing forest biodiversity experiments: General considerations illustrated by a new large experiment in subtropical China. Methods Ecol. Evol. 2014, 5, 74–89. [Google Scholar] [CrossRef]
- Cavanaugh, K.C.; Gosnell, J.S.; Davis, S.L.; Ahumada, J.; Boundja, P.; Clark, D.B.; Mugerwa, B.; Jansen, P.A.; O’Brien, T.G.; Rovero, F. Carbon storage in tropical forests correlates with taxonomic diver sity and functional dominance on a global scale. Glob. Ecol. Biogeogr. 2014, 23, 563–573. [Google Scholar] [CrossRef]
- Kaiser, J. Rift Over Biodiversity Divides Ecologists. Science 2000, 289, 1282–1283. [Google Scholar] [CrossRef] [PubMed]
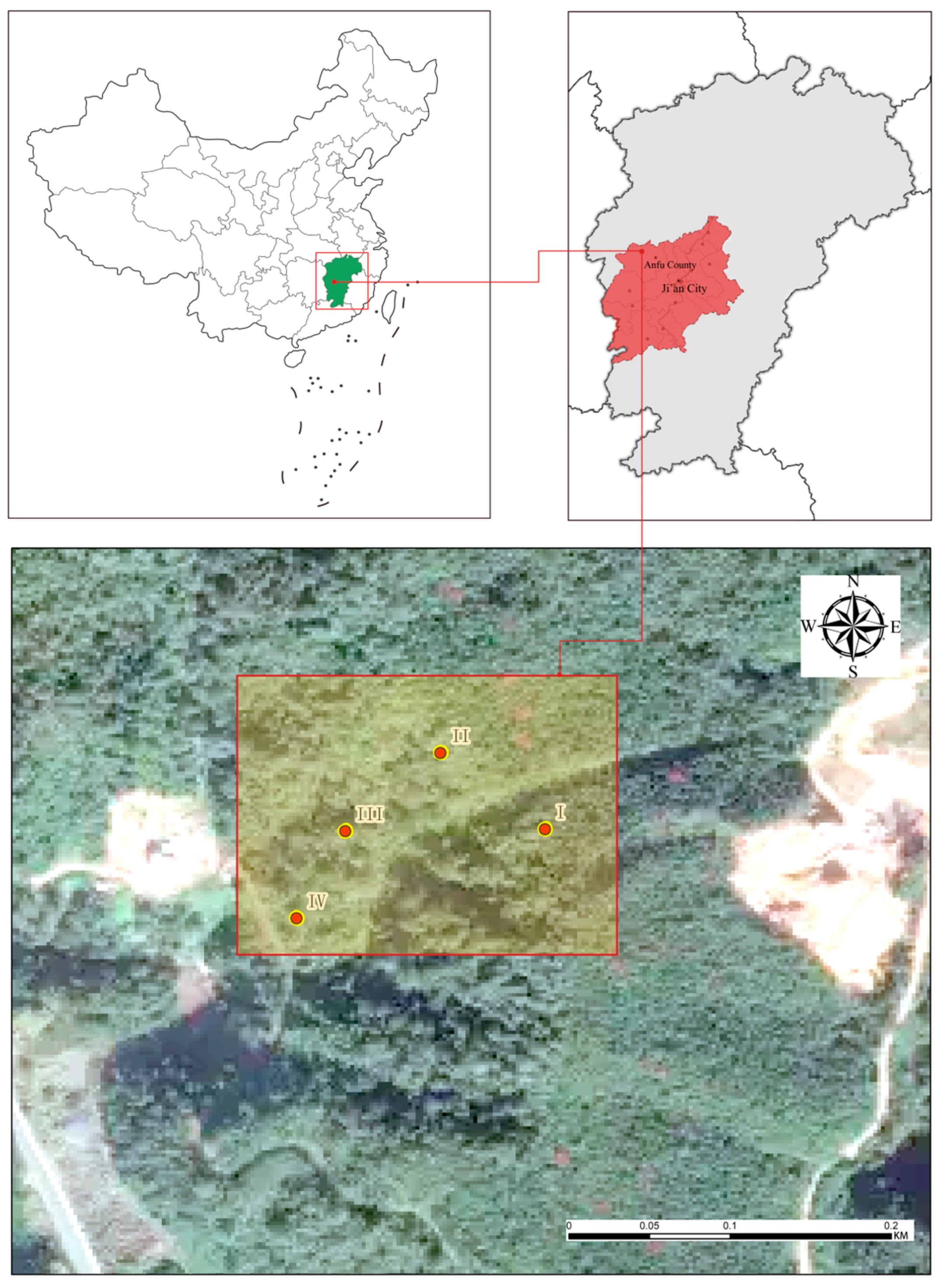
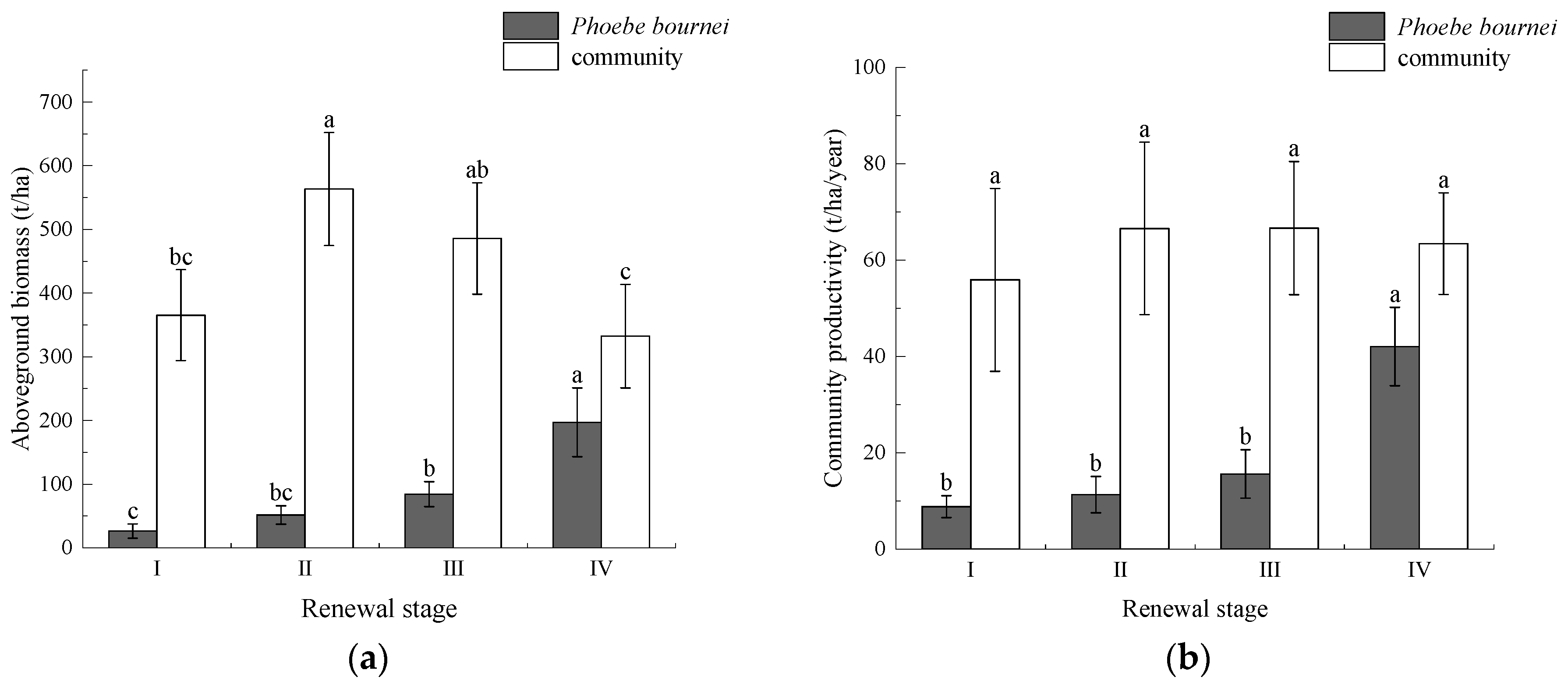


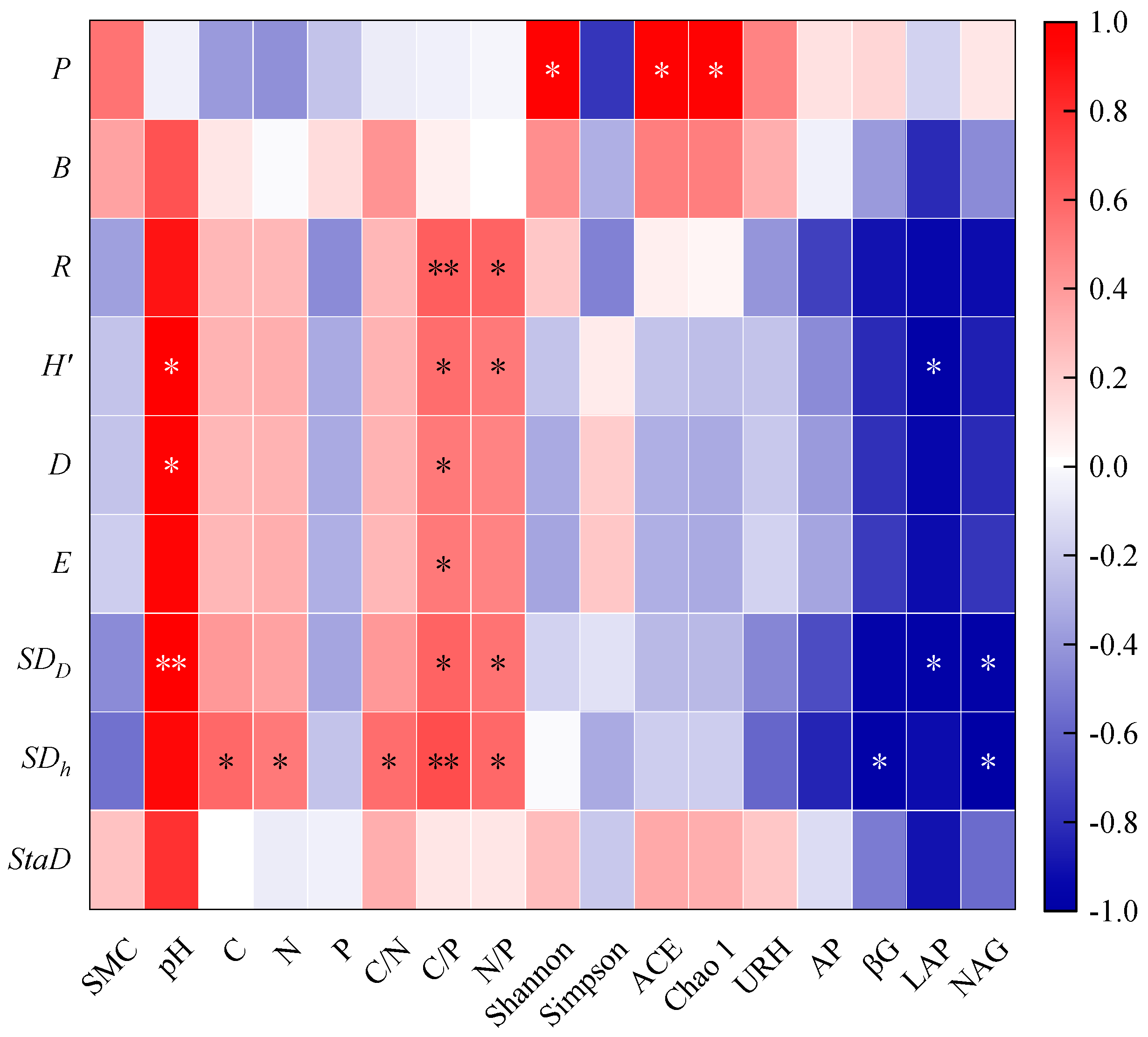
| Regeneration Stage | Year | Community Abundance (Stem/Plot) | P. bournei Abundance (Stem/Plot) | Proportion (%) | Maximum DBH of P. bournei (cm) | Maximum Age of P. bournei (a) |
|---|---|---|---|---|---|---|
| I | 2018 | 69 | 34 | 49.28 | 7.4 | 5–10 |
| 2019 | 93 | 57 | 61.29 | 7.8 | ||
| 2020 | 93 | 57 | 61.29 | 8.0 | ||
| 2021 | 97 | 61 | 62.89 | 8.2 | ||
| II | 2018 | 104 | 54 | 51.92 | 12.7 | 10–20 |
| 2019 | 121 | 72 | 59.50 | 14.0 | ||
| 2020 | 122 | 72 | 59.02 | 14.5 | ||
| 2021 | 122 | 73 | 59.84 | 15.1 | ||
| III | 2018 | 107 | 68 | 63.55 | 14.8 | 20–30 |
| 2019 | 125 | 84 | 67.20 | 16.2 | ||
| 2020 | 123 | 84 | 68.29 | 16.8 | ||
| 2021 | 124 | 85 | 68.55 | 16.9 | ||
| IV | 2018 | 114 | 104 | 91.23 | 32.0 | 30–40 |
| 2019 | 124 | 114 | 91.94 | 33.9 | ||
| 2020 | 125 | 115 | 92.00 | 35.3 | ||
| 2021 | 126 | 116 | 92.06 | 35.5 |
| Regeneration Stage | No. | Tree Species | Relative Density/% | Important Value/% | Mean DBH /cm | Mean Tree Height/m |
|---|---|---|---|---|---|---|
| I | 1 | Phoebe bournei | 62.89 | 35.34 | 4.81 ± 1.43 | 6.70 ± 1.61 |
| 2 | Castanopsis fargesii | 12.37 | 28.07 | 22.95 ± 14.51 | 22.95 ± 14.51 | |
| 3 | Cunninghamia lanceolata | 5.15 | 7.40 | 19.02 ± 4.87 | 18.90 ± 6.10 | |
| 4 | Cyclobalanopsis glauca | 5.15 | 5.79 | 14.38 ± 7.67 | 14.6 ± 7.51 | |
| 5 | Liquidambar formosana | 2.06 | 5.62 | 30.00 ± 3.39 | 24.00 ± 5.65 | |
| II | 1 | Phoebe bournei | 59.84 | 34.43 | 5.35 ± 2.26 | 8.30 ± 2.48 |
| 2 | Cyclobalanopsis glauca | 7.38 | 12.91 | 20.67 ± 12.19 | 18.55 ± 7.42 | |
| 3 | Castanopsis fargesii | 8.20 | 12.87 | 20.39 ± 8.40 | 17.40 ± 6.83 | |
| 4 | Photinia davidsoniae | 3.28 | 8.34 | 28.37 ± 12.08 | 26.75 ± 0.95 | |
| 5 | Vernicia montana | 3.28 | 7.12 | 26.75 ± 6.63 | 25.75 ± 3.30 | |
| 6 | Cinnamomum camphora | 1.64 | 5.89 | 37.20 ± 1.13 | 22.50 ± 0.70 | |
| III | 1 | Phoebe bournei | 68.55 | 42.45 | 6.27 ± 2.86 | 8.89 ± 3.11 |
| 2 | Schima superba | 10.48 | 21.35 | 20.70 ± 14.04 | 20.27 ± 8.70 | |
| 3 | Cunninghamia lanceolata | 11.29 | 9.40 | 10.64 ± 4.51 | 11.37 ± 3.21 | |
| 4 | Michelia figo | 1.61 | 7.40 | 39.15 ± 13.50 | 39.15 ± 13.50 | |
| 5 | Castanopsis fargesii | 0.81 | 5.22 | 48.70 ± 0.00 | 30.00 ± 0.00 | |
| IV | 1 | Phoebe bournei | 92.06 | 75.11 | 7.95 ± 4.85 | 11.19 ± 4.38 |
| 2 | Photinia davidsoniae | 3.97 | 10.67 | 24.04 ± 5.30 | 18.9 ± 2.92 | |
| 3 | Cyclobalanopsis glauca | 0.79 | 5.99 | 44.00 ± 0.00 | 18.00 ± 0.00 | |
| 4 | Quercus fabri | 0.79 | 5.79 | 43.20 ± 0.00 | 13.50 ± 0.00 |
| Variables | Regeneration Stage I | Regeneration Stage II | Regeneration Stage III | Regeneration Stage IV | p |
|---|---|---|---|---|---|
| Species richness (R) | 14 | 17 | 12 | 7 | - |
| Shannon–Wiener diversity index (H′) | 1.55 ± 0.14 ab | 1.72 ± 0.09 a | 1.37 ± 0.28 b | 0.4 ± 0.01 c | <0.001 |
| Simpson diversity index (D) | 0.62 ± 0.05 a | 0.64 ± 0.03 a | 0.58 ± 0.11 a | 0.15 ± 0.01 b | <0.001 |
| Pielou evenness index (E) | 0.59 ± 0.05 a | 0.61 ± 0.03 a | 0.55 ± 0.11 a | 0.15 ± 0.01 b | <0.001 |
| Standard deviation of DBH (SDD, cm) | 9.59 ± 0.23 ab | 9.80 ± 0.11 a | 9.29 ± 0.34 b | 6.94 ± 0.22 c | <0.001 |
| Height standard deviation (SDh, m) | 6.74 ± 0.50 a | 6.82 ± 0.54 a | 6.04 ± 0.47 a | 3.81 ± 0.91 b | <0.001 |
| Stand density (StaD, m2/ha) | 35.16 ± 3.46 b | 50.02 ± 3.10 a | 44.72 ± 4.40 a | 29.94 ± 4.08 b | <0.001 |
| Index | Regeneration Stage I | Regeneration Stage II | Regeneration Stage III | Regeneration Stage IV | p |
|---|---|---|---|---|---|
| SMC (%) | 9.43 ± 1.75 b | 15.43 ± 3.19 a | 16.25 ± 4.26 a | 16.60 ± 3.69 a | <0.001 |
| pH | 4.58 ± 0.13 ab | 4.60 ± 0.11 a | 4.59 ± 0.23 ab | 4.39 ± 0.18 b | 0.052 |
| C (g·kg−1) | 31.47 ± 4.63 a | 21.14 ± 7.39 b | 12.28 ± 6.42 c | 21.34 ± 4.55 b | <0.001 |
| N (g·kg−1) | 2.18 ± 0.47 a | 1.37 ± 0.48 b | 1.12 ± 0.42 b | 1.30 ± 0.29 b | <0.001 |
| P (g·kg−1) | 0.26 ± 0.03 d | 0.47 ± 0.04 c | 0.58 ± 0.05 b | 0.72 ± 0.06 a | <0.001 |
| C/N | 11.22 ± 7.32 | 15.46 ± 1.82 | 12.26 ± 10.19 | 16.38 ± 0.89 | 0.276 |
| C/P | 97.83 ± 60.67 a | 47.05 ± 17.09 ab | 21.28 ± 10.79 b | 30.88 ± 5.98 b | 0.002 |
| N/P | 8.51 ± 2.29 a | 3.08 ± 1.13 b | 1.86 ± 0.75 b | 1.96 ± 0.41 b | <0.001 |
| URH (u·g−1) | 677.21 ± 83.88 a | 671.97 ± 113.56 a | 611.59 ± 117.34 a | 375.41 ± 88.77 b | <0.001 |
| AcP (u·g−1) | 10387.79 ± 132.28 a | 9676.03 ± 491.31 a | 7093.41 ± 271.95 b | 5186.03 ± 2129.45 c | 0.001 |
| βG (u·g−1) | 11.74 ± 3.22 a | 7.62 ± 2.19 b | 6.08 ± 1.82 b | 4.81 ± 1.69 b | <0.001 |
| LAP (u·g−1) | 1.02 ± 0.27 a | 0.38 ± 0.30 b | 0.23 ± 0.24 b | 0.48 ± 0.49 b | 0.004 |
| NAG (u·g−1) | 13.79 ± 8.12 a | 7.31 ± 3.71 ab | 4.75 ± 2.53 b | 3.56 ± 1.37 b | 0.006 |
| Class | Component | Eigenvalue | Contribution Rate of Variance (%) | Cumulative Contribution Rate of Variance (%) |
|---|---|---|---|---|
| Species diversity | 1 | 3.83 | 95.85 | 95.85 |
| 2 | 0.16 | 4.07 | 99.91 | |
| 3 | 0.00 | 0.08 | 100.00 | |
| Structural diversity | 1 | 2.61 | 87.10 | 87.10 |
| 2 | 0.36 | 11.87 | 98.97 | |
| 3 | 0.03 | 1.03 | 100.00 | |
| Soil nutrients | 1 | 6.16 | 76.99 | 76.99 |
| 2 | 1.47 | 18.38 | 95.37 | |
| 3 | 0.37 | 4.63 | 100.00 | |
| Soil enzyme activity | 1 | 3.36 | 84.10 | 84.10 |
| 2 | 0.63 | 15.80 | 99.90 | |
| 3 | 0.00 | 0.10 | 100.00 | |
| Microbial diversity | 1 | 4.06 | 81.18 | 81.18 |
| 2 | 0.90 | 17.94 | 99.12 | |
| 3 | 0.04 | 0.88 | 100.00 |
| Biomass | Productivity | Species Diversity | Structural Diversity | Microbial Diversity | Soil Nutrient | Enzyme Activity | |
|---|---|---|---|---|---|---|---|
| Biomass | 1 | ||||||
| Productivity | 0.023 | 1 | |||||
| Species diversity | 0.536 * | −0.123 | 1 | ||||
| Structural diversity | 0.793 ** | −0.065 | 0.917 | 1 | |||
| Soil nutrients | 0.447 | 0.982 * | −0.157 | 0.052 | 1 | ||
| Soil enzyme activity | 0.121 | −0.270 | 0.514 * | 0.506 * | −0.247 | 1 | |
| Microbial diversity | −0.308 | 0.209 | −0.772 | −0.827 | 0.167 | −0.665 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Z.; Sun, J.; Zhong, P.; Liang, L.; Dang, H.; Wang, G. The Impact of Natural Regeneration of Phoebe bournei in Anfu County, Jiangxi Province, on Community Diversity and Soil Nutrient Characteristics. Forests 2023, 14, 1783. https://doi.org/10.3390/f14091783
Xiong Z, Sun J, Zhong P, Liang L, Dang H, Wang G. The Impact of Natural Regeneration of Phoebe bournei in Anfu County, Jiangxi Province, on Community Diversity and Soil Nutrient Characteristics. Forests. 2023; 14(9):1783. https://doi.org/10.3390/f14091783
Chicago/Turabian StyleXiong, Ziqian, Jiawei Sun, Ping Zhong, Lixin Liang, Haoxuan Dang, and Guangjun Wang. 2023. "The Impact of Natural Regeneration of Phoebe bournei in Anfu County, Jiangxi Province, on Community Diversity and Soil Nutrient Characteristics" Forests 14, no. 9: 1783. https://doi.org/10.3390/f14091783
APA StyleXiong, Z., Sun, J., Zhong, P., Liang, L., Dang, H., & Wang, G. (2023). The Impact of Natural Regeneration of Phoebe bournei in Anfu County, Jiangxi Province, on Community Diversity and Soil Nutrient Characteristics. Forests, 14(9), 1783. https://doi.org/10.3390/f14091783





