Variations in Leaf Functional Traits and Photosynthetic Parameters of Cunninghamia lanceolata Provenances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Measurements of Leaf Functional Traits
2.3. Measurements of Gas Exchange Characteristics
2.4. Data Analysis
3. Results
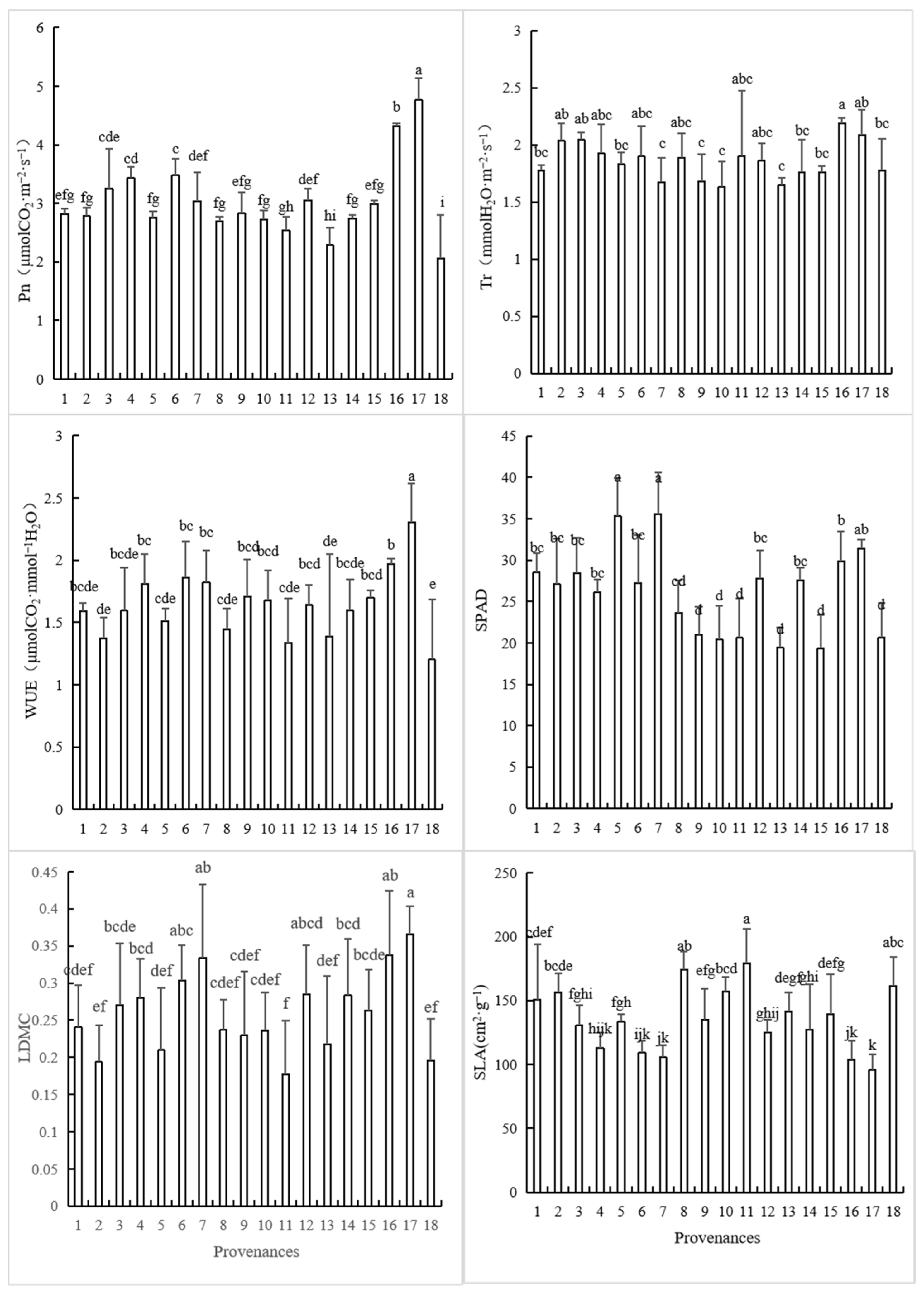
3.1. Variations in Leaf Functional Traits
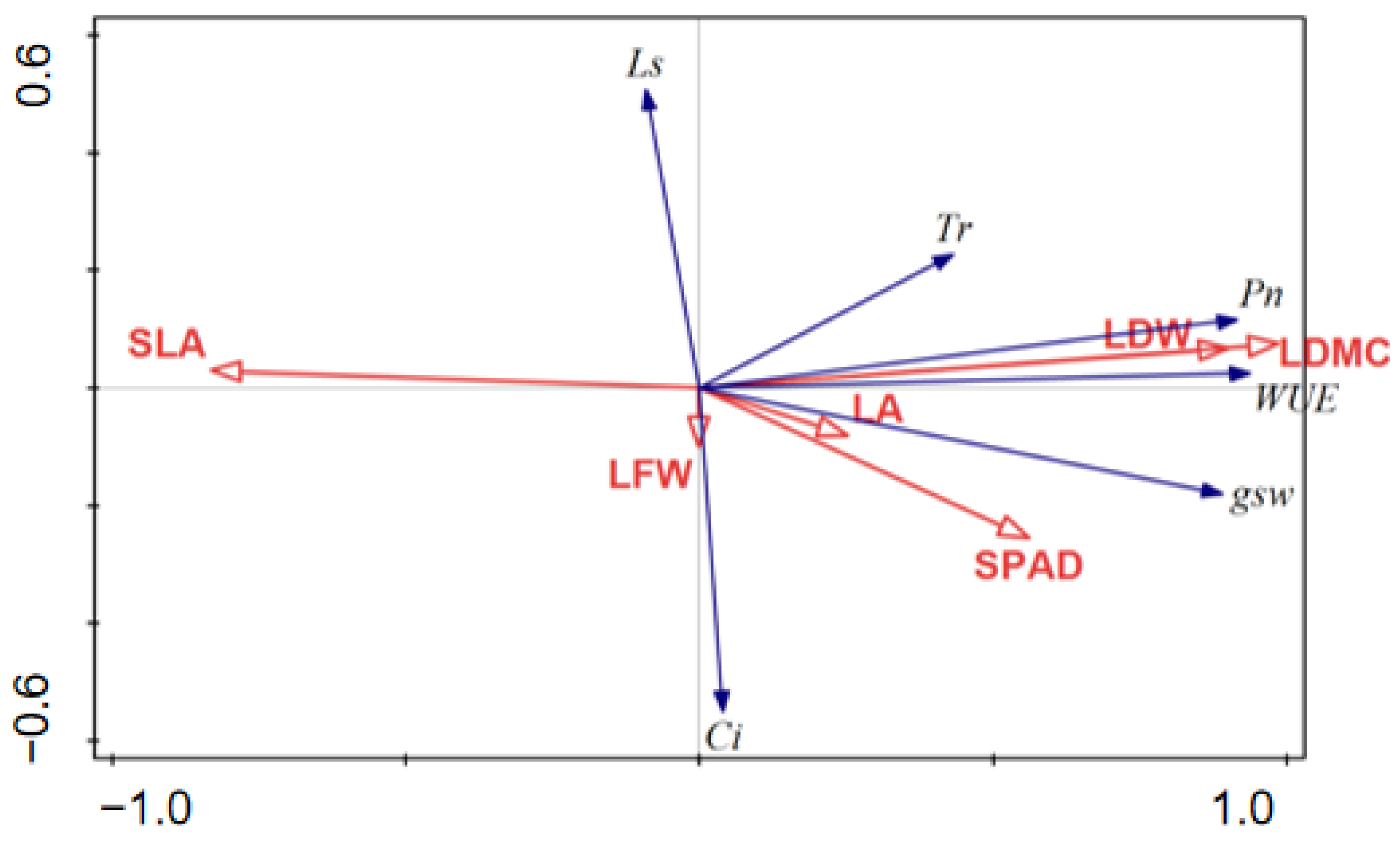
3.2. Correlations among Leaf Functional Traits
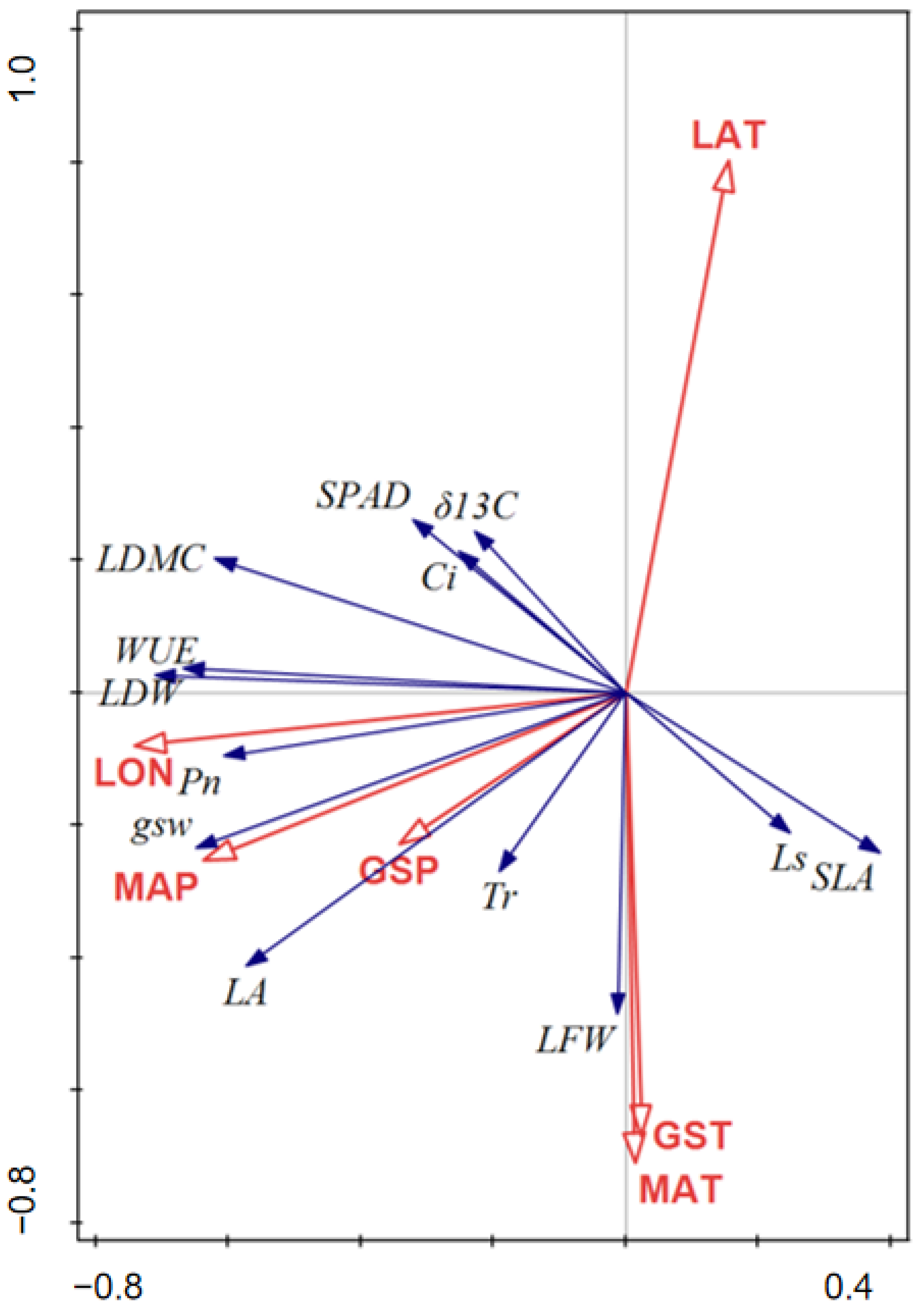
3.3. Relationship between Geo-Climatic Conditions of Provenances and Leaf Functional Traits of Provenances
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, W.H. Plant Physiology; Science Press: Beijing, China, 2008; p. 57. [Google Scholar]
- Wright, I.J.; Reich, P.B.; Westoby, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.; Garcia, S.; Grandis, A.; Nascimento, H.; Domingues, T.F.; Guedes, A.V.; Aleixo, I.; Camargo, P.; Campos, J.; Damasceno, A.; et al. Changes in leaf functional traits with leaf age: When do leaves decrease their photosynthetic capacity in Amazonian trees? Tree Physiol. 2022, 42, 922–938. [Google Scholar] [CrossRef] [PubMed]
- Thakur, D.; Singh, L.; Chawla, A. Reliability of leaf functional traits after delayed measurements. Aust. J. Bot. 2019, 68, 100–107. [Google Scholar] [CrossRef]
- Bruelheide, H.; Nadrowski, K.; Assmann, T.; Bauhus, J.; Both, S.; Buscot, F.; Chen, X.Y.; Ding, B.; Durka, W.; Erfmeier, A.; et al. Designing forest biodiversity experiments: General considerations illustrated by a new large experiment in subtropical China. Methods Ecol. Evol. 2014, 5, 74–89. [Google Scholar] [CrossRef]
- Roa-Fuentes, L.L.; Templer, P.H.; Campo, J. Effects of precipitation regime and soil nitrogen on leaf traits in seasonally dry tropical forests of the Yucatan Peninsula, Mexico. Oecologia 2012, 179, 585–597. [Google Scholar] [CrossRef]
- Dong, N.; Prentice, I.C.; Wright, I.J.; Evans, B.J.; Togashi, H.F.; Caddy-Retalic, S.; McInerney, F.A.; Sparrow, B.; Leitch, E.; Lowe, A.J. Components of leaf-trait variation along environmental gradients. New Phytol. 2020, 228, 82–94. [Google Scholar] [CrossRef]
- Qin, J.; Shangguan, Z.P. Effects of forest types on leaf functional traits and their interrelationships of Pinus massoniana coniferous and broad-leaved mixed forests in the subtropical mountain, Southeastern China. Ecol. Evol. 2019, 9, 6922–6932. [Google Scholar] [CrossRef]
- Liu, M.-C.; Dong, T.-F.; Feng, W.-W.; Qu, B.; Kong, D.-L.; van Kleunen, M.; Feng, Y.-L. Leaf trait differences between 97 pairs of invasive and native plants across China: Effects of identities of both the invasive and native species. NeoBiota 2022, 71, 1–22. [Google Scholar] [CrossRef]
- Zhou, J.H.; Cieraad, E.; Bodegom, P.M. Global Global analysis of trait-trait relationships within and between species. New Phytol. 2021, 233, 1643–1656. [Google Scholar] [CrossRef]
- Maire, V.; Wright, I.J.; Prentice, I.C.; Batjes, N.H.; Bhaskar, R.; van Bodegom, P.M.; Cornwell, W.K.; Ellsworth, D.; Niinemets, Ü.; Ordonez, A.; et al. Global effects of soil and climate on leaf photosynthetic traits and rates. Global Ecol. Biogeogr. 2015, 24, 706–717. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Valencia, R.; Ackerly, D.D. Functional traits and niche-based tree community assembly in an Amazonian forest. Science 2008, 322, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Klisz, M.; Buras, A.; Sass-Klaassen, U.; Puchalka, R.; Koprowski, M.; Ukalska, J. Limitations at the limit? Diminishing of genetic effects in Norway spruce provenance trials. Front. Plant Sci. 2019, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Mihai, G.; Teodosiu, M.; Birsan, M.-V.; Alexandru, A.-M.; Mirancea, I.; Apostol, E.-N.; Garbacea, P.; Ionita, L. Impact of Climate Change and Adaptive Genetic Potential of Norway Spruce at the South-eastern Range of Species Distribution. Agric. For. Meteorol. 2020, 291, 108040. [Google Scholar] [CrossRef]
- Gorné, L.D.; Díaz, S.; Minden, V.; Onoda, Y.; Kramer, K.; Muir, C.; Michaletz, S.T.; Lavorel, S.; Sharpe, J.; Jansen, S.; et al. The acquisitive-conservative axis of leaf trait variation emerges even in homogeneous environments. Ann. Bot. 2021, 129, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Mao, K.; Yang, H.; Wang, Y.; Feng, Q.; Wang, S.; Miao, N. Stand characteristics and ecological benefits of Chinese Fir, Chinese Cedar, and mixed plantations in the mountainous areas of the Sichuan Basin. For. Ecol. Manag. 2023, 554, 121168. [Google Scholar] [CrossRef]
- Wu, H.; Lei, J.; Li, X.; Wang, H.; Duan, A.; Zhang, J. Aggregation distributions across stand age in provenances of Cunninghamia lanceolata (Lamb.) Hook. For. Ecol. Manag. 2021, 494, 119317. [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration. China Forest Resources Report 2014–2018; China Forestry Publishing Press: Beijing, China, 2019. (In Chinese) [Google Scholar]
- Hao, J.; Chen, N.; Yan, P.; Xu, K.; Zhang, L.; Zhang, H. Study on the variation in and selection of Fraxinus mandshurica provenances and families in northeast China. J. For. Res. 2023, 34, 519–529. [Google Scholar] [CrossRef]
- Hong, J.S. Excellent provenance selection of Cunninghamia lanceolata afforestation. For. Res. 1994, 7, 1–25. (In Chinese) [Google Scholar]
- Gao, S.; Cai, Z.-Y.; Yang, C.-C.; Luo, J.-X.; Zhang, S. Provenance-specific ecophysiological responses to drought in Cunninghamia lanceolata. J. Plant Ecol. 2021, 14, 1060–1072. [Google Scholar] [CrossRef]
- Akram, M.A.; Wang, X.; Shrestha, N.; Zhang, Y.; Sun, Y.; Yao, S.; Li, J.; Hou, Q.; Hu, W.; Ran, J.; et al. Variations and driving factors of leaf functional traits in the dominant desert plant species along an environmental gradient in the drylands of China. Sci. Total Environ. 2023, 897, 165394. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Liang, Y.C. A study on the selection of Chinese fir in the Dagangshan region. For. Res. 1992, 5, 1–6. (In Chinese) [Google Scholar]
- Bussotti, F.; Pollastrini, M. Evaluation of leaf features in forest trees: Methods, techniques, obtainable information and limits. Ecol. Indic. 2015, 52, 219–230. [Google Scholar] [CrossRef]
- Xiao, W.F. A Study on photosynthesis: Modeling and scaling up from a leaf to canopy in a Chinese fir plantation. Acta. Ecol. Sin. 1998, 18, 621–628. [Google Scholar]
- Guo, Q.; Sun, Y.; Zhang, J.; Li, Y. Variation of phenotypic and physiological traits of Robinia pseudoacacia L. from 20 provenances. PLoS ONE 2022, 17, e0262278. [Google Scholar] [CrossRef]
- Hallik, L.; Niinemets, U.; Wright, I.J. Are species shade and drought tolerance reflected in leaf-level structural and functional differentiation in Northern Hemisphere temperate woody flora? New Phytol. 2009, 184, 257–274. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, H.; Liu, J. Differences in leaf functional traits between red and green leaves of two ever-green shrubs Photinia × fraseri and Osmanthus fragrans. J. For. Res. 2017, 28, 473–479. [Google Scholar] [CrossRef]
- Li, J.; Feng, Y.; Wang, X.; Peng, J.; Yang, D.; Xu, G.; Luo, Q.; Wang, L.; Ou, D.; Su, W. Stability and applicability of the leafvalue model for variable nitrogen application based on SPAD value in rice. PLoS ONE 2020, 15, e0233735. [Google Scholar]
- Baraloto, C.; Paine, C.E.T.; Poorter, L.; Beauchene, J.; Bonal, D.; Domenach, A.-M.; Hérault, B.; Patiño, S.; Roggy, J.-C.; Chave, J. Decoupled leaf and stem economics in rain forest trees. Ecol. Lett. 2010, 13, 1338–1347. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, N.; Zhang, H.; Zhao, M.; Ren, T.; Liu, C.; Westerband, A.; He, N. Divergent long-and short-term responses to environmental gradients in specific leaf area of grassland species. Ecol. Indic. 2021, 130, 108058. [Google Scholar] [CrossRef]
- Tong, R.; Yang, X.; Wang, Q.; Li, L.; Li, Y.; Shi, Y.; Mu, C.; Wang, J. The shift in key functional traits caused by precipitation under nitrogen and phosphorus deposition drives biomass change in Leymus chinensis. Plants 2023, 12, 1781. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, M.; Zhang, H.; Ren, T.; Liu, C.; He, N. Divergent response and adaptation of specific leaf area to environmental change at different spatio-temporal scales jointly improve plant survival. Glob. Chang Biol. 2023, 29, 1144–1159. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Camille, M.S.; Asaph, B.C. Leaf temperature impacts canopy water use efficiency independent of changes in leaf level water use efficiency. J. Plant Physiol. 2021, 258–259, 153357. [Google Scholar]
- Kwon, K.J.; Choi, J.; Kim, S.Y. Growth and Physiological Responses of Three Landscape Plants to Calcium Chloride. Sustainability 2021, 13, 5429. [Google Scholar] [CrossRef]
- Acosta-Rangel, A.; Ávila-Lovera, E.; De Guzman, M.E.; Torres, L.; Haro, R.; Arpaia, M.L.; Focht, E.; Santiago, L.S. Evaluation of leaf carbon isotopes and functional traits in avocado reveals water-use efficient cultivars. Agric. Ecosyst. Environ. 2018, 263, 60–66. [Google Scholar] [CrossRef]
- Aalap, D.; Thomas, K.; Owen, B. Trade-off between growth rate and water use efficiency in southwestern ponderosa pine provenances. For. Ecol. Manag. 2022, 515, 120239. [Google Scholar]
- Zhao, N.; Meng, P.; He, Y.; Yu, X. Interaction of CO2 concentrations and water stress in semiarid plants causes diverging response in instantaneous water use efficiency and carbon isotope composition. Biogeosciences 2017, 14, 3431–3444. [Google Scholar] [CrossRef]
- Xu, R.; Cheng, S.; Zhou, J.; Tigabu, M.; Ma, X.; Li, M. Intraspecific variations in leaf functional traits of Cunninghamia lanceolata provenances. BMC Plant Biol. 2023, 23, 92. [Google Scholar] [CrossRef]
- Cui, E.; Weng, E.; Yan, E.; Xia, J. Robust leaf trait relationships across species under global environmental changes. Nat. Commun. 2020, 11, 2999. [Google Scholar] [CrossRef]
- Salazar, P.C.; Navarro-Cerrillo, R.M.; Cruz, G.; Villar, R. Intraspecific leaf functional trait variability of eight Prosopis pallida tree populations along a climatic gradient of the dry forests of northern Peru. J. Arid Environ. 2018, 152, 12–20. [Google Scholar] [CrossRef]

| Provenance | Longitude | Latitude | Mean Annual Temperature (°C) | Mean Annual Precipitation (mm) | Growing Season Mean Temperature (°C) | Growing Season Precipitation (mm) | |
|---|---|---|---|---|---|---|---|
| 1 | Zhaoping, Guangxi | 110°50′ E | 24°11′ N | 20.1 | 1992.6 | 23.96 | 1613.8 |
| 2 | Mengshan, Guangxi | 110°32′ E | 24°14′ N | 19.9 | 1741.2 | 23.84 | 1443.9 |
| 3 | Changting, Fujian | 116°22′ E | 25°41′ N | 18.5 | 1712.1 | 22.51 | 1279.9 |
| 4 | Yangxin, Hubei | 115°13′ E | 29°15′ N | 17.5 | 1454.3 | 22.53 | 1138.7 |
| 5 | Wufeng, Hubei | 110°20′ E | 25°24′ N | 14.9 | 1307.4 | 19.15 | 1161.2 |
| 6 | Rongshuisirong, Guangxi | 117°30′ E | 30°13′ N | 19.1 | 1992.6 | 23.38 | 1612.6 |
| 7 | Shitai, Anhui | 119°22′ E | 29°14′ N | 16.1 | 1797.6 | 20.88 | 1388.1 |
| 8 | Yongchang, Zhejiang | 119°50′ E | 24°11′ N | 16.1 | 1423.1 | 21.1 | 1099.7 |
| 9 | Yunhe, Zhejiang | 119°35′ E | 28°07′ N | 17.9 | 1631.5 | 22.32 | 1259.5 |
| 10 | Longquan, Zhejiang | 119°08′ E | 28°05′ N | 18 | 1649.2 | 22.29 | 1249.7 |
| 11 | Shanghang, Fujian | 116°25′ E | 25°04′ N | 20.3 | 1666.9 | 24 | 1304.5 |
| 12 | Fenyi, Jiangxi | 114°43′ E | 27°49′ N | 17.9 | 1640.9 | 22.64 | 1216.7 |
| 13 | Lingchuan, Guangxi | 109°13′ E | 25°13′ N | 20.98 | 1431.1 | 25.03 | 1184.7 |
| 14 | Yongfu, Guangxi | 110°00′ E | 24°60′ N | 19.1 | 1963.9 | 23.29 | 1590.1 |
| 15 | Dehua, Fujian | 117°48′ E | 26°24′ N | 18.8 | 1739.7 | 23.5 | 1239.8 |
| 16 | Sanming, Fujian | 117°39′ E | 26°46′ N | 19.6 | 1665.2 | 23.39 | 1238 |
| 17 | Shaxian, Fujian | 118°15′ E | 25°30′ N | 18.3 | 1823.3 | 21.75 | 1488.6 |
| 18 | Hongya, Sichuan | 103°22′ E | 29°55′ N | 16.8 | 1385 | 20.68 | 1282.8 |
| δ13C | Tr | Pn | WUE | Ci | Ls | gsw | SPAD | LFW | LDW | LA | SLA | LDMC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δ13C | 1 | ||||||||||||
| Tr | −0.084 | 1 | |||||||||||
| Pn | −0.006 | 0.689 ** | 1 | ||||||||||
| WUE | 0.014 | 0.35 | 0.918 ** | 1 | |||||||||
| Ci | 0.1 | −0.015 | −0.011 | 0.027 | 1 | ||||||||
| Ls | −0.098 | 0.015 | 0.013 | −0.026 | −0.999 ** | 1 | |||||||
| gsw | −0.168 | 0.508 * | 0.873 ** | 0.871 ** | 0.124 | −0.123 | 1 | ||||||
| SPAD | −0.171 | 0.367 | 0.493 * | 0.456 | 0.227 | −0.229 | 0.484 * | 1 | |||||
| LFW | −0.27 | 0.119 | −0.046 | −0.101 | 0.069 | −0.071 | 0.047 | −0.082 | 1 | ||||
| LDW | −0.109 | 0.417 | 0.903 ** | 0.949 ** | 0.034 | −0.033 | 0.860 ** | 0.545 * | −0.091 | 1 | |||
| LA | 0.127 | 0.084 | 0.156 | 0.184 | 0.04 | −0.039 | 0.308 | −0.167 | 0.365 | 0.187 | 1 | ||
| SLA | 0.186 | −0.302 | −0.767 ** | −0.828 ** | −0.081 | 0.080 | −0.706 ** | −0.614 ** | 0.258 | −0.876 ** | 0.279 | 1 | |
| LDMC | −0.004 | 0.346 | 0.836 ** | 0.893 ** | 0.029 | −0.027 | 0.777 ** | 0.547 * | −0.429 | 0.936 ** | 0.043 | −0.881 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Niu, X.; Wang, B.; Qiu, X.; Shou, Y.; Luo, J.; Guo, Y. Variations in Leaf Functional Traits and Photosynthetic Parameters of Cunninghamia lanceolata Provenances. Forests 2023, 14, 1708. https://doi.org/10.3390/f14091708
Xu T, Niu X, Wang B, Qiu X, Shou Y, Luo J, Guo Y. Variations in Leaf Functional Traits and Photosynthetic Parameters of Cunninghamia lanceolata Provenances. Forests. 2023; 14(9):1708. https://doi.org/10.3390/f14091708
Chicago/Turabian StyleXu, Tingyu, Xiang Niu, Bing Wang, Xiaohan Qiu, Ye Shou, Jiani Luo, and Yajun Guo. 2023. "Variations in Leaf Functional Traits and Photosynthetic Parameters of Cunninghamia lanceolata Provenances" Forests 14, no. 9: 1708. https://doi.org/10.3390/f14091708
APA StyleXu, T., Niu, X., Wang, B., Qiu, X., Shou, Y., Luo, J., & Guo, Y. (2023). Variations in Leaf Functional Traits and Photosynthetic Parameters of Cunninghamia lanceolata Provenances. Forests, 14(9), 1708. https://doi.org/10.3390/f14091708





