Habitat Suitability of Pine Wilt Disease in Northeast China under Climate Change Scenario
Abstract
:1. Introduction
2. Materials and Methods
2.1. Distribution Data
2.2. Climate Data
2.3. Data Analysis
3. Results
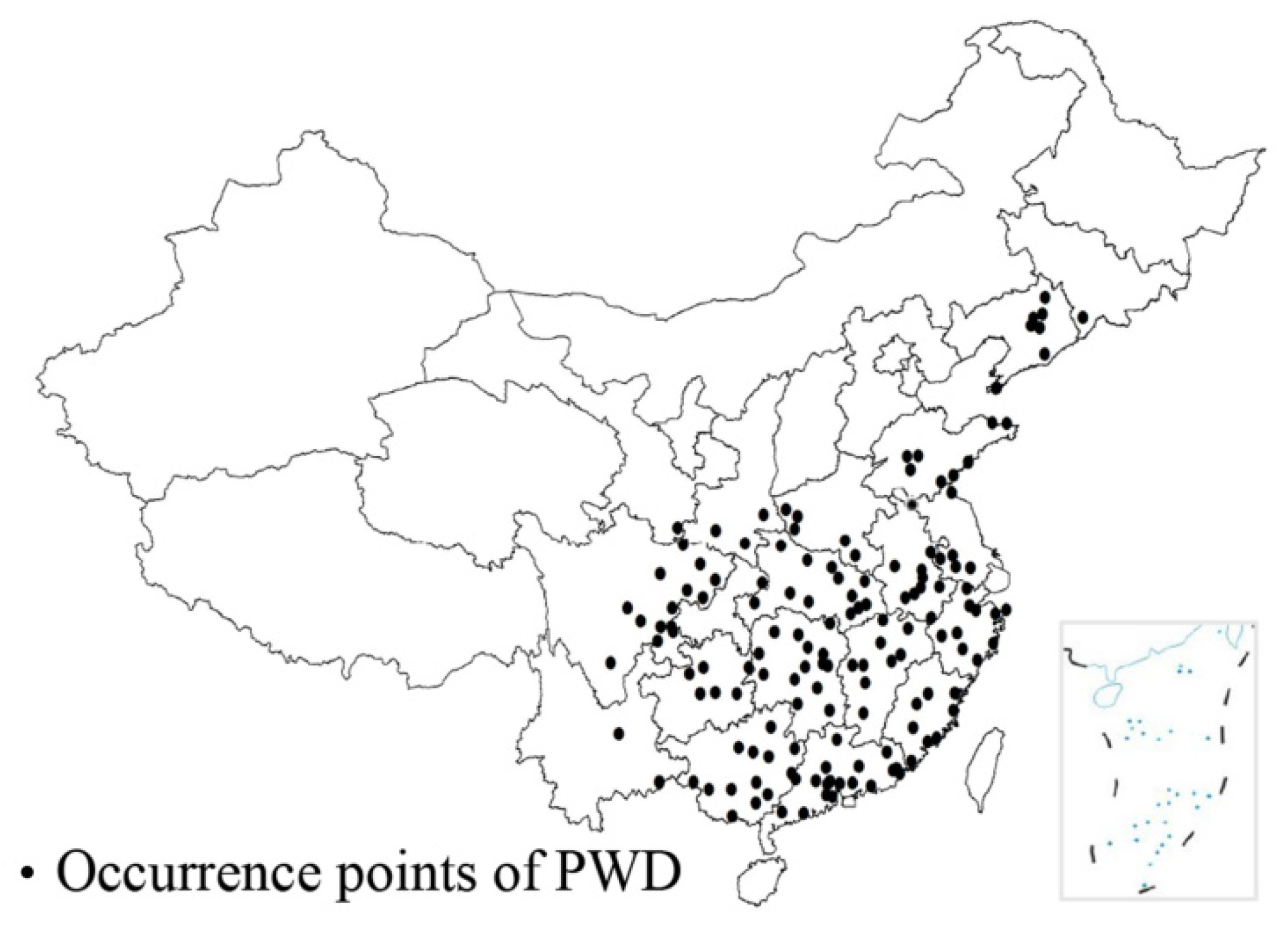
3.1. Distribution of PWD in China
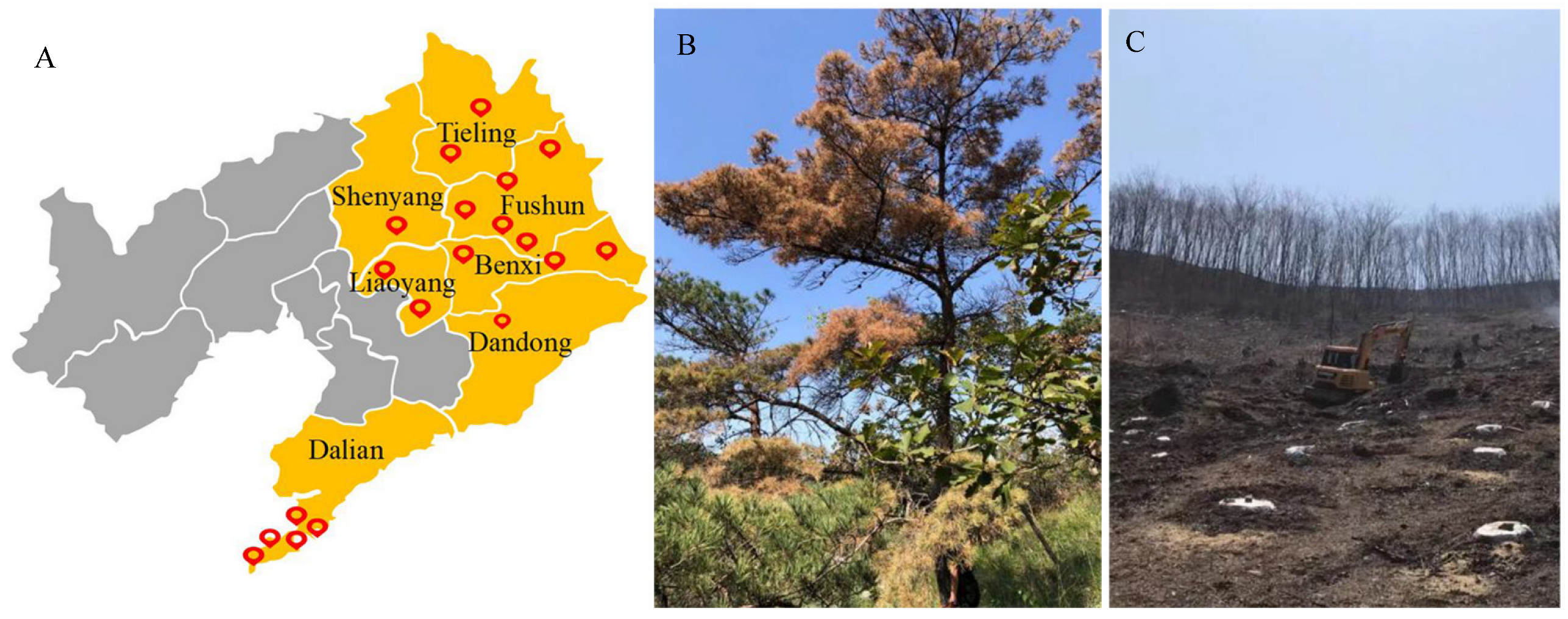
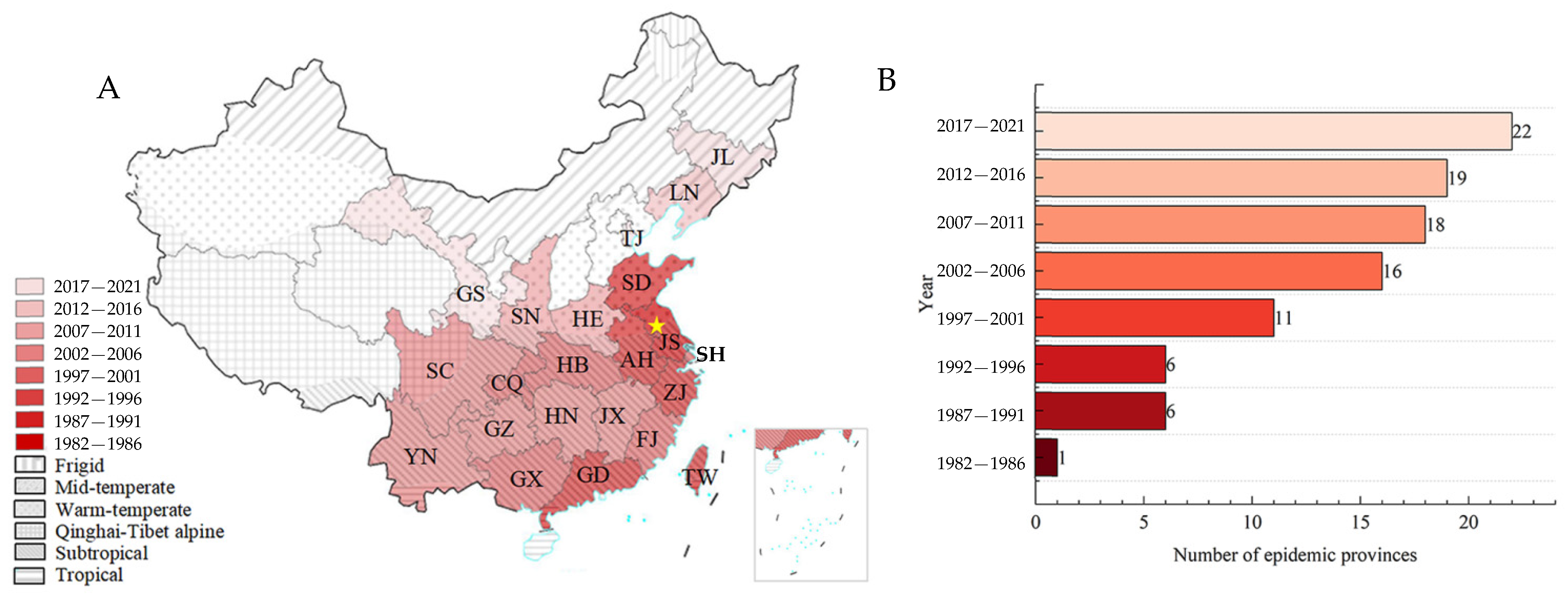
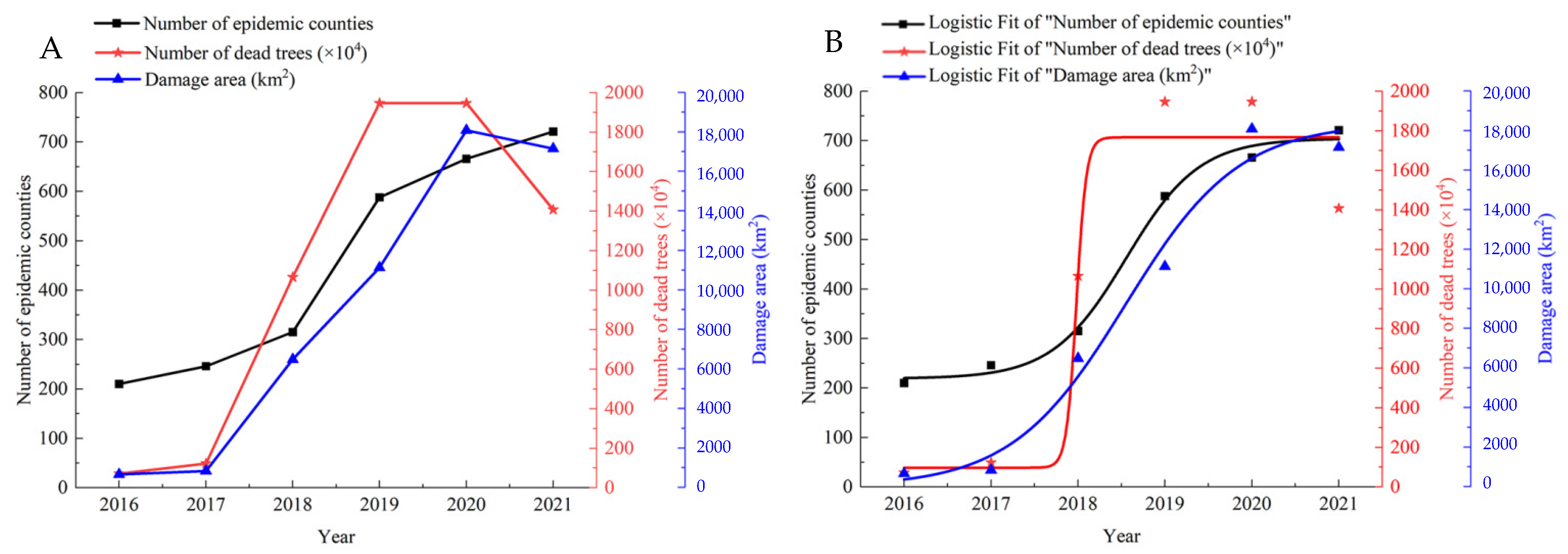
3.1.1. Spread Path and Spatiotemporal Distribution of PWD
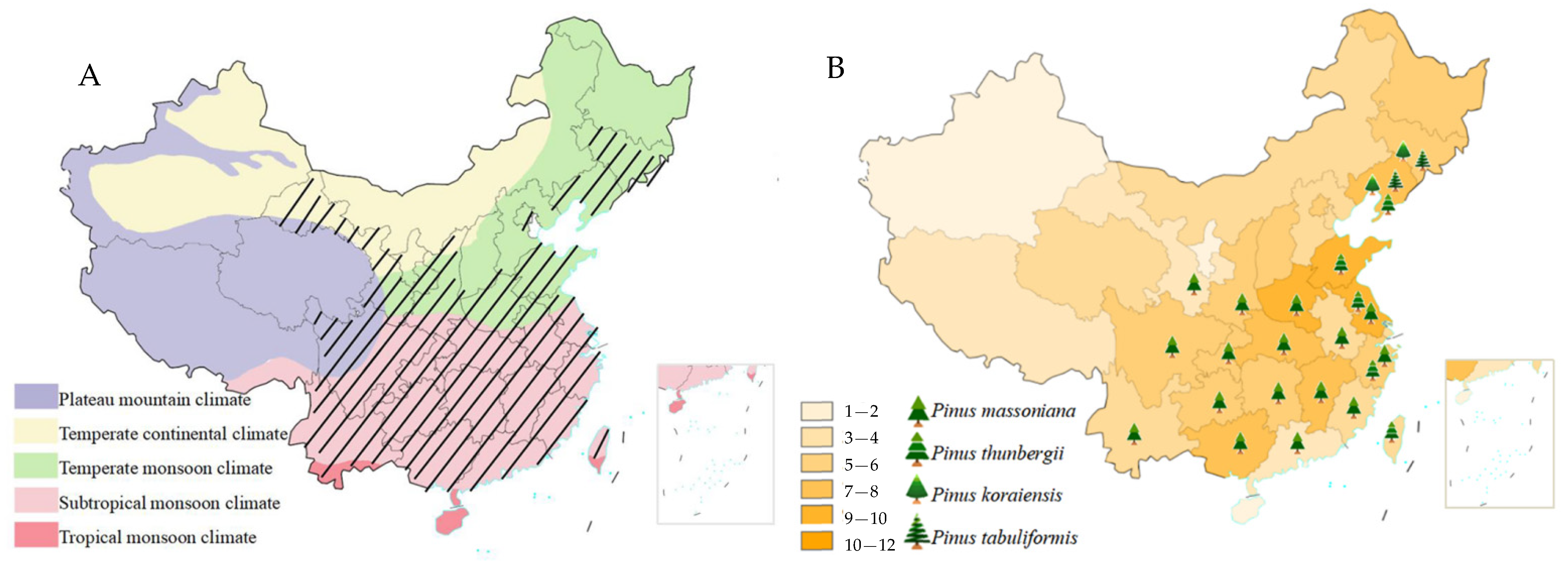
3.1.2. Climate Conditions and Host Tree Distribution in PWD Epidemic Areas
3.2. Relationship between PWD Epidemic Situation and Climate Factors
3.2.1. Damage Inflicted by PWD in China
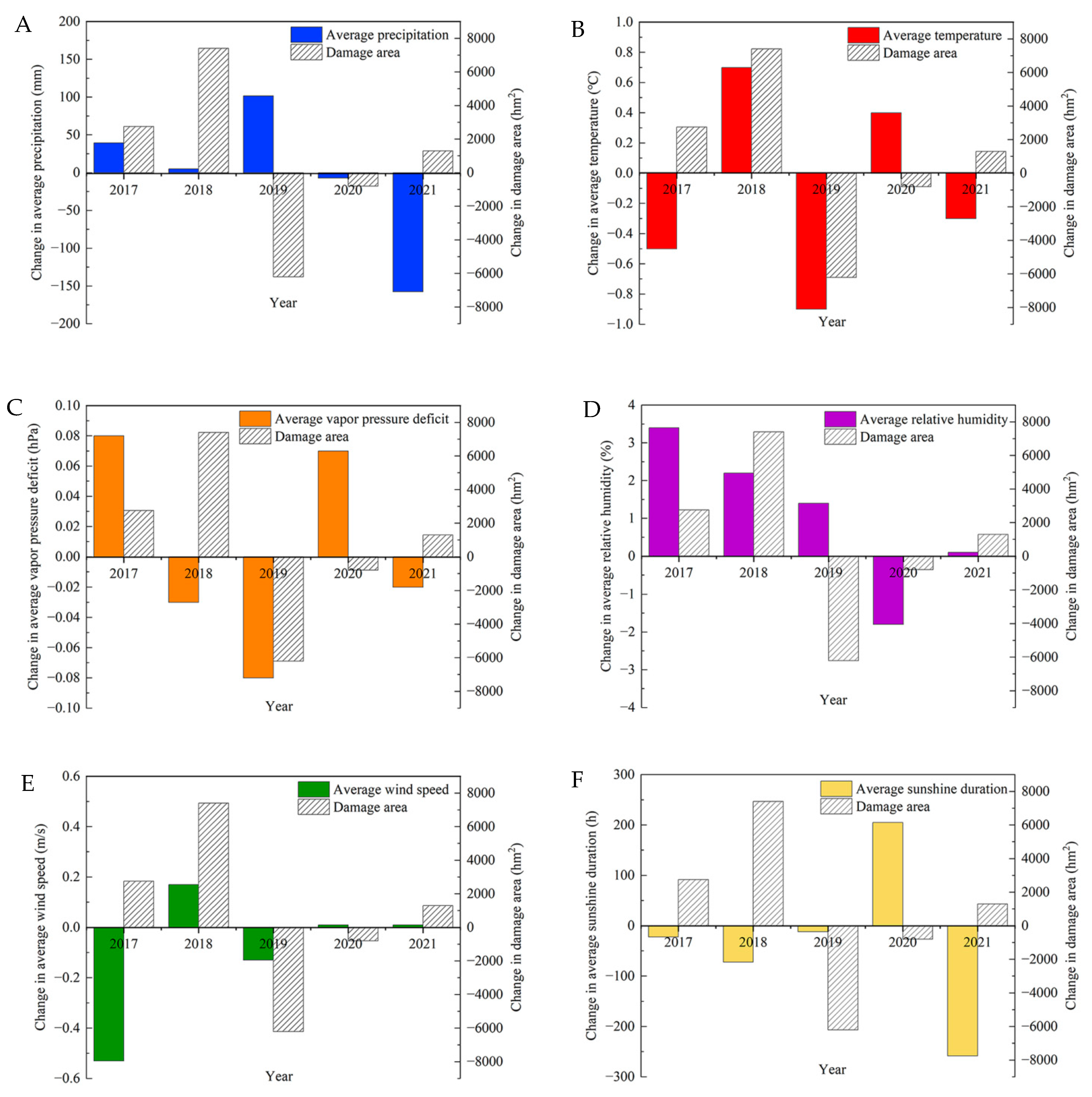
3.2.2. Relationship between the Epidemic Area and Climate Factors
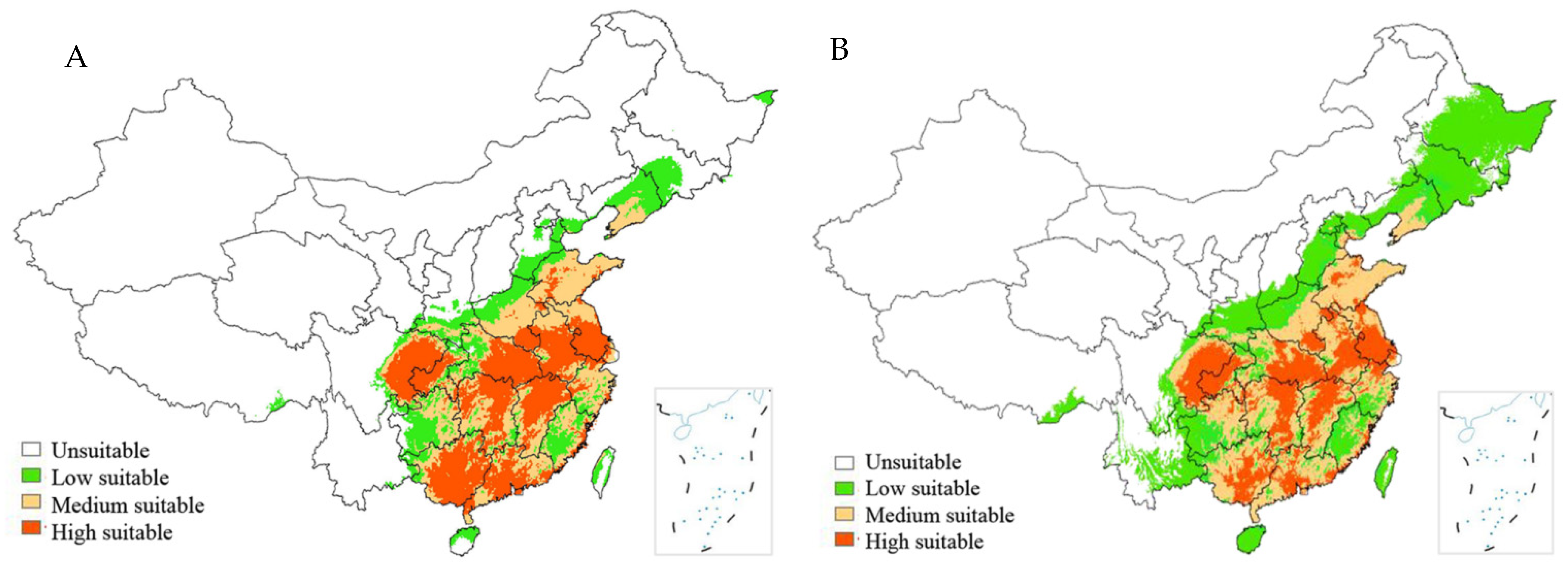
3.3. Predicted Geographical Distribution of PWD in China under Climate Warming
4. Discussion
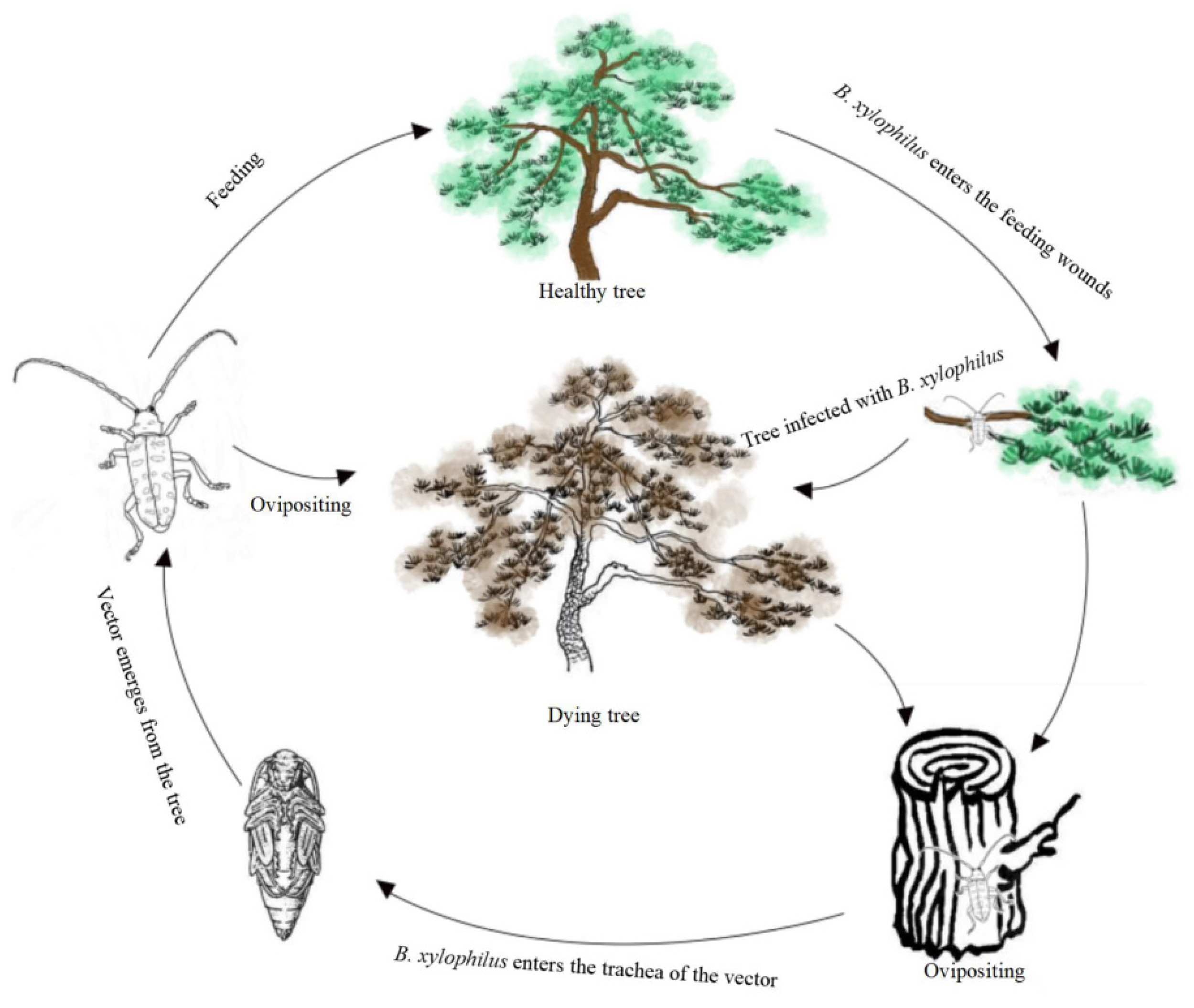
4.1. Spread and Hosts of PWD in China
4.2. Main Climate Factors Affecting the Ecological Suitability of PWD
4.3. The MaxEnt Model
4.4. Changes in PWD Distribution Areas under Future Climate Change
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mota, M.; Vieira, P. Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Springer: Berlin/Heidelberg, Germany, 2008; pp. 25–33. [Google Scholar]
- Ye, J.R. Epidemic status of pine wilt disease in China and its prevention and control techniques and counter measures. Sci. Silvae Sin. 2019, 55, 1–10. (In Chinese) [Google Scholar]
- Steiner, G.; Buhrer, E.M. Aphelenchoides xylophilus n. sp. A nemaode associated with blue stain and other fungi in timber. J. Agric. Res. 1934, 48, 949–951. [Google Scholar]
- Li, M.; Li, H.; Ding, X.L.; Wang, L.C.; Wang, X.Y.; Chen, F.M. The detection of pine wilt disease: A literature review. Int. J. Mol. Sci. 2022, 23, 10797. [Google Scholar] [CrossRef]
- Mamiya, Y. Hietory of pine wilt disease in Japan. J. Nematol. 1988, 20, 219–226. [Google Scholar]
- Yi, C.K.; Byun, B.H.; Park, J.D.; Chang, K.H.; Yang, S. First finding of the pine wood nematode Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle and its insect vector in Korea. Res. Rep. For. Res. Inst. 1989, 38, 141–149. [Google Scholar]
- Sun, Y.C. Pine wood nematode found in Zhongshan Mausoleum Nanjing. J. Jiangsu For. Sci. Technol. 1982, 4, 27–47. (In Chinese) [Google Scholar]
- Yu, H.Y.; Wu, H. New host plants and new vector insects of pine wood nematode found in Liaoning. For. Pests Dis. 2018, 37, 61. (In Chinese) [Google Scholar]
- National Forestry and Grassland Administration. Pine Wilt Disease Epidemic Area in 2022. No.6. 2022. Available online: http://www.forestry.gov.cn/main/6206/20220406/151041215456755.html (accessed on 23 September 2022).
- National Forestry and Grassland Administration. Five-Year Action Plan for the Prevention and Control of Pine Wood Nematode Disease in China. 2022. Available online: http://www.forestry.gov.cn/main/216/20210707/084935642722530.html (accessed on 23 September 2022).
- Mamiya, Y.; Enda, N. Transmission of Bursaphelenchus Lignicolus (Nematoda: Aphelenchoididae) by Monochamus Alternatus (Coleoptera: Cerambycidae). Nematologica 1972, 18, 159–162. [Google Scholar] [CrossRef]
- Zheng, Y.N.; Liu, P.X.; Shi, Y.; Wu, H.; Yu, H.Y.; Jiang, S.W. Difference analysis on pine wilt disease between Liaoning Province of northeastern China and other epidemic areas in China. J. Beijing For. Univ. 2021, 43, 155–160. (In Chinese) [Google Scholar]
- Linit, M.J.; Edwards, O.R. Transmission of Bursaphelenchus xylophilus through oviposition wounds of Monochamm carolinensis (Coleoptera: Cerambycidae). J. Nematol. 1992, 24, 133–139. [Google Scholar]
- Yang, B.J.; Pan, H.Y.; Tang, J.; Wang, Y.Y.; Wang, L.F. Bursaphelenchus xylophilus; China Forestry Publishing House: Beijing, China, 2003; pp. 12–125. [Google Scholar]
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.L.; Mota, M.; Vieira, P.; Butcher, R.A.; Sun, J. Interspecifific communication between pinewood nematode, its insect vector, and associated microbes. Trends Parasitol. 2014, 30, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, M.; Jenkins, T. Estimate global risks of a forest disease under current and future climates using species distribution model and simple thermal model-Pine Wilt disease as a model case. For. Ecol. Manag. 2018, 409, 343–352. [Google Scholar] [CrossRef]
- Roques, A.; Zhao, L.; Sun, J.; Robinet, C. Pine wood nematode, pine wilt disease, vector beetle and pine tree: How a multiplayer system could reply to climate change. In Climate Change and Insect Pests; CABI: Wallingford, UK, 2015; pp. 35–78. [Google Scholar]
- Zhao, L.L.; Wei, W.; Kang, L.; Sun, J.H. Chemotaxis of the pinewood nematode, Bursaphelenchus xylophilus, to volatiles associated with host pine, Pinus massoniana, and its vector Monochamus alternatus. J. Chem. Ecol. 2007, 33, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.E.A.; Kriticos, D.J.; Leriche, A. The current and future potential geographical distribution of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Bull. Entomol. Res. 2007, 97, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.M.; Newman, J.A. Climate change scenarios and models yield conflicting predictions about the future risk of an invasive species in North America. Agric. For. Entomol. 2010, 12, 213–221. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Ahmadi, M. A comparison of absolute performance of different correlative and mechanistic species distribution models in an independent area. Ecol. Evol. 2016, 6, 5973–5986. [Google Scholar] [CrossRef]
- Xu, W.L.; Li, Q.K.; Yang, X.; Wang, J.S. Prediction of potential distribution of the invasive plant Tagetes minuta L. (Wild Marigold) in Tibet under climate change. Acta Ecol. Sin. 2022, 42, 7266–7277. (In Chinese) [Google Scholar]
- Moat, J.; Williams, J.; Baena, S.; Wilkinson, T.; Gole, T.W.; Challa, Z.K.; Demissew, S.; Davis, A.P. Resilience potential of the Ethiopian coffee sector under climate change. Nat. Plants 2017, 3, 17081. [Google Scholar] [CrossRef]
- Hao, Z.; Fang, G.; Huang, W.; Ye, H.; Zhang, B.; Li, X. Risk Prediction and Variable Analysis of Pine Wilt Disease by a Maximum Entropy Model. Forests 2022, 13, 342. [Google Scholar] [CrossRef]
- Tang, X.; Yuan, Y.; Li, X.; Zhang, J. Maximum entropy modeling to predict the impact of climate change on pine wilt disease in China. Front. Plant Sci. 2021, 12, 652500. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Peng, W.; Liu, X.; He, G.; Cai, Y. Spatiotemporal Dynamics and Factors Driving the Distributions of Pine Wilt Disease-Damaged Forests in China. Forests 2022, 13, 261. [Google Scholar] [CrossRef]
- Volney, W.J.A.; Fleming, R.A. Climate change and impacts of boreal forest insects. Agric. Ecosyst. Environ. 2000, 82, 283–294. [Google Scholar] [CrossRef]
- Walther, G.R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukát, Z.; Bugmann, H.; et al. Alien species in a warmer world: Risks and opportunities. Trends. Ecol. Evol. 2009, 1146, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.C. Pine Wilt Disease in Korea. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008. [Google Scholar]
- Wang, Y.Y. Pine-wilting nematode disease. Plant Quar. 1986, 3, 46–51. (In Chinese) [Google Scholar]
- Cheng, H.R.; Ge, J.J.; Zhou, G.L.; Sun, J.L.; Zhou, Y.S.; Ge, M.H.; Sun, Y.C. Survey on the occurrence and distribution of Bursaphelenchus xylophilus in China. Jiangsu For. Sci. Technol. 1988, 2, 28–30. (In Chinese) [Google Scholar]
- Ren, W.; Zhou, D.Q.; Jin, H. Preliminary study of Bursaphelenchus xylophilus on the branch of Pinus Yunnanensis. Yunnan For. Sci. Technol. 1991, 3, 59–63. (In Chinese) [Google Scholar]
- Wu, S.L.; Ma, L.; Lu, Q.; Liang, J.; Liu, Z.Y.; Zhang, X.Y. Identification of nematode isolated from Pinus armandii and its pathogenicity on P. massoniana in Tianzhu Mountain in Shaanxi Province. For. Pest Dis. 2013, 32, 5–9. (In Chinese) [Google Scholar]
- Yu, Y.; Chi, Z.; Zhang, J.; Sun, P.; Wang, C.; Pan, X. Assessing the invasive risk of bark beetles (Curculionidae: Scolytinae and Platypodinae). Ann. Entomol. Soc. Am. 2019, 112, 451–457. [Google Scholar] [CrossRef]
- Matsuhashi, S.; Hirata, A.; Akiba, M.; Songji, T.; Oguro, M.; Takano, K.T.; Nakao, K.; Hijioka, Y.; Matsui, T. Developing a point process model for ecological risk assessment of pine wilt disease at multiple scales. For. Ecol. Manag. 2020, 463, 118010. [Google Scholar] [CrossRef]
- Rebetez, M.; Dobbertin, M. Climate change may already threaten Scots pine stands in the Swiss Alps. Theor. Appl. Climatol. 2004, 79, 1–9. [Google Scholar] [CrossRef]
- Kanzaki, N.; Giblin-Davis, R.M. Diversity and plant pathogenicity of Bursaphelenchus and related nematodes in relation to their vector bionomics. Curr. For. Rep. 2018, 4, 85–100. [Google Scholar] [CrossRef]
- Gao, H.; Gao, R.X. Review on the Impacts of Meteorological Factors on the Gymnosperms Growth Survey. For. Eng. 2014, 2, 6–9. (In Chinese) [Google Scholar]
- Zhou, Y.; Lei, Z.; Zhou, F.; Han, Y.; Yu, D.; Zhang, Y. Impact of climate factors on height growth of Pinus sylvestris var. mongolica. PLoS ONE 2019, 14, e0213509. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.M.; Weiss, R.M.; Olfert, O.; Hallett, R.H.; Newman, J.A. Will climate change be beneficial or detrimental to the invasive swede midge in North America? Contrasting predictions using climate projections from different general circulation models. Glob Chang. Biol. 2008, 14, 1721–1733. [Google Scholar] [CrossRef]
- Ni, W.L.; Chen, H.J.; Qu, W.W.; Wan, F.H.; A.M.; Pu, C.; Li, Z.H. The potential geographical distribution of the Monacrostichus citricola Bezzi based on the CLIMEX, in China. Plant Quar. 2010, 24, 20–25. (In Chinese) [Google Scholar]
- Zhou, Y.T.; Ge, X.Z.; Zou, Y.; Guo, S.W.; Wang, T.; Tao, J.; Zong, S.X. Prediction of the potential geographical distribution of Hylurgus ligniperda at the global scale and in China using the MaxEnt model. J. Beijing For. Univ. 2022, 44, 90–99. (In Chinese) [Google Scholar]
- Yu, H.Y.; Wu, H.; Zhang, X.D.; Wang, L.M.; Zhang, X.F.; Song, Y.S. Preliminary study on Larix spp. Infected by Bursaphelenchus xylophilus in natural environment. For. Pests Dis. 2019, 38, 7–10. (In Chinese) [Google Scholar]
- Morey, A.C.; Venette, R.C. Minimizing risk and maximizing spatial transferability: Challenges in constructing a useful model of potential suitability for an invasive insect. Ann. Entomol. Soc. Am. 2020, 113, 100–113. [Google Scholar] [CrossRef]
- Li, C.; Liao, J.H.; Ya, Y.K.; Liu, J.; Li, J.; Yu, G.W. Analysis of potential distribution of Spodoptera frugiperda in western China. J. Asia-Pac. Entomol. 2022, 25, 101985. [Google Scholar] [CrossRef]
- Ouyang, X.H.; Chen, A.L.; Li, Y.; Han, X.X.; Lin, H.P. Predicting the Potential Distribution of Pine Wilt Disease in China under Climate Change. Forests 2022, 13, 1147. [Google Scholar] [CrossRef]
- Mamiya, Y. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 1983, 21, 201–220. [Google Scholar] [CrossRef]
- Song, Y.S.; Zang, X.Q. Fitness analysis and quarantine countermeasures of pine wood nematode in China. For. Pest Dis. 1989, 4, 38–41. (In Chinese) [Google Scholar]
- Huang, R.F.; Gao, R.H.; Shi, J.; Song, D.W.; Li, Z.M. Cold tolerance of Bursaphelenchus xylophilus in the Three Gorges Region of Hubei Province. J. Northeast For. Univ. 2014, 42, 138–141. (In Chinese) [Google Scholar]
| Climate Variables | Mean | Unit | Contribution (%) |
|---|---|---|---|
| Bio 1 | Annual mean temperature | °C | 0.38 |
| Bio 2 | Mean diurnal range | °C | 3.87 |
| Bio 3 | Isothermality (Bio2/Bio7) (* 100) | % | 0.51 |
| Bio 4 | Temperature seasonality (standard deviation * 100) | °C | 19.10 |
| Bio 5 | Max temperature of warmest month | °C | 0.27 |
| Bio 6 | Min temperature of coldest month | °C | 0.15 |
| Bio 7 | Temperature annual range (Bio5-Bio6) | °C | 0.20 |
| Bio 8 | Mean temperature of wettest quarter | °C | 0.05 |
| Bio 9 | Mean temperature of driest quarter | °C | 0.09 |
| Bio 10 | Mean temperature of warmest quarter | °C | 5.76 |
| Bio 11 | Mean temperature of coldest quarter | °C | 0.32 |
| Bio 12 | Annual precipitation | mm | 0.01 |
| Bio 13 | Precipitation of wettest month | mm | 8.61 |
| Bio 14 | Precipitation of driest month | mm | 0.23 |
| Bio 15 | Precipitation seasonality (coefficient of variation) | mm | 2.50 |
| Bio 16 | Precipitation of wettest quarter | mm | 0.62 |
| Bio 17 | Precipitation of driest quarter | mm | 0.20 |
| Bio 18 | Precipitation of warmest quarter | mm | 56.88 |
| Bio 19 | Precipitation of coldest quarter | mm | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Deng, J.; Yan, W.; Zheng, Y. Habitat Suitability of Pine Wilt Disease in Northeast China under Climate Change Scenario. Forests 2023, 14, 1687. https://doi.org/10.3390/f14081687
Wang J, Deng J, Yan W, Zheng Y. Habitat Suitability of Pine Wilt Disease in Northeast China under Climate Change Scenario. Forests. 2023; 14(8):1687. https://doi.org/10.3390/f14081687
Chicago/Turabian StyleWang, Jue, Jifeng Deng, Wenfeng Yan, and Yanan Zheng. 2023. "Habitat Suitability of Pine Wilt Disease in Northeast China under Climate Change Scenario" Forests 14, no. 8: 1687. https://doi.org/10.3390/f14081687
APA StyleWang, J., Deng, J., Yan, W., & Zheng, Y. (2023). Habitat Suitability of Pine Wilt Disease in Northeast China under Climate Change Scenario. Forests, 14(8), 1687. https://doi.org/10.3390/f14081687






