Effect of Temperature, Seed Size, Sowing Depth, and Position on Seed Germination and Seedling Growth of Bauhinia retusa Roxb. and Bauhinia variegata L.
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmid, R. Plant Systematics: A Phylogenetic Approach; Photo Gallery of Vascular Plants. Taxon 2007, 56, 1316. Available online: https://www.jstor.org/stable/25065934?origin=crossref (accessed on 9 August 2023).
- Khare, P.; Kamal, K.; Sharma, D.K. Historical aspects, medicinal uses, phytochemistry and pharmacological review of Bauhinia variegata. Asian J. Pharm. Pharmacol. 2018, 4, 546–562. [Google Scholar] [CrossRef]
- Mishra, A.; Nautiyal, S.; Nautiyal, D.P. Growth characteristics of some indigenous fuelwood and fodder tree species of sub-tropical Garhwal Himalayas. Indian For. 2009, 135, 373–379. [Google Scholar]
- Albert, S.; Sharma, B. Comparative foliar micromorphological studies of some Bauhinia (Leguminosae) species. Turk. J. Bot. 2013, 37, 5. [Google Scholar] [CrossRef]
- Nagar, A.; Khanduri, V.P.; Singh, B.; Riyal, M.K.; Singh, I. Altitudinal variation in morphometric traits of pod, seed, and seedling growth of Bauhinia variegata L. in Garhwal Himalaya. Ann. Silvic. Res. 2022, 47, 85–94. [Google Scholar]
- Gautam, S. Bauhinia variegata Linn: All-purpose utility and medicinal tree. ENVIS For. Bull. 2012, 12, 61–64. [Google Scholar]
- Al-Snafi, A.E. The pharmacological importance of Bauhinia variegata, a review. Int. J. Pharma Sci. Res. 2013, 4, 160–164. [Google Scholar]
- Pillai, P.K.C.; Viswanath, S.C.; Hrideek, T.K.; Jiji, A.H. Seed characteristics and germination behaviour of Bauhinia malabarica Roxb. In Horticultural Crops; Baimey, H.K., Hamamouch, N., Kolombia, Y.A., Eds.; IntechOpen-London: London, UK, 2020. [Google Scholar]
- Borchert, M.I.; Davis, F.W.; Michaelson, J.; Oyler, L.D. Interaction of factors affecting seedling recruitment of blue oak (Quercus douglassii) in California. Ecology 1989, 70, 389–404. [Google Scholar] [CrossRef]
- Armstrong, W.D.M. Root growth and metabolism under oxygen deficiency. In Plant Roots: The Hidden Half; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Mercel Dekker: New York, NY, USA, 2002; pp. 729–761. [Google Scholar]
- Guo, C.-R.; Wang, Z.L.; Lu, J.-Q. Seed germination and seedling development of Prunusarmeniaca under different burial depths in the soil. J. For. Res. 2010, 21, 492–496. [Google Scholar] [CrossRef]
- De Souza, F.H.D.; Marcos-Filho, J. The seed coat as a modulator of seed-environment relationships in Fabaceae. Revta Bras. Bot São Paulo. 2001, 24, 365–375. [Google Scholar] [CrossRef]
- Ertekin, M.; Kirdar, E.R. Effects of seed coat colour on seed characteristics of honeylocust (Gleditsia triacanthos). Afr. J. Agric. Res. 2010, 5, 2434–2438. [Google Scholar]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Plenum Press: New York, NY, USA, 1994; p. 221. [Google Scholar]
- Prasad, P.; Nautiyal, A.R. Influence of seed orientation on seedling emergence in Bauhinia retusa Ham. Ex. Roxb. Seed Sci. Technol. 1995, 20, 70–73. [Google Scholar]
- Gera, M.; Gera, N.; Ginwal, H.S. Seed source variation in germination and early growth among ten indigenous population of Dalbergia sissoo Roxb. India J. For. 1999, 125, 1190–1197. [Google Scholar]
- Uniyal, A.K.; Singh, B.; Todaria, N.P. Effects of seed size, sowing orientation and depth on germination and seedling growth in Neem, Azadirachta indica. Seed Technol. 2007, 29, 68–75. [Google Scholar]
- Singh, B.; Rawat, J.M.S.; Pandey, V. Influence of sowing depth and orientation on germination and seedling emergence of Cinnamomum tamala Nees. J. Environ. Biol. 2017, 38, 271. [Google Scholar] [CrossRef]
- Vander, W.S.B. A model of caching depth: Implication for scatter hoarders and plant dispersal. Am. Nat. 1993, 141, 217–232. [Google Scholar]
- Chen, H.; Maun, M.A. Effect of sand burial depth on seed germination and seedling emergence of Cirsium pitcher. Plant Ecol. 1999, 140, 53–60. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. The quantification of aging and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Kendrick, R.E.; Frankland, B. Photo control of germination in Amaranthus caudatus. Planta 1981, 85, 326–329. [Google Scholar] [CrossRef]
- Harrington, J.E. Problems of seed storage. In Seed Ecology; Heydecker, W., Ed.; Butterworths: London, UK, 1973; pp. 251–263. [Google Scholar]
- Simon, E.W.; Minchin, A.; Mcmenamin, M.M.; Smith, J.M. The low-temperature limit for seed germination. New Phytol. 1976, 77, 301–311. [Google Scholar] [CrossRef]
- Singh, S.P.; Phartyal, S.S.; Rosbakh, S. Tree seed traits’ response to monsoon climate and altitude in Indian subcontinent with particular reference to the Himalayas. Ecol. Evol. 2017, 7, 7408–7419. [Google Scholar] [CrossRef] [PubMed]
- Todaria, N.P.; Singh, B.; Kumar, R. Seed source variation and germination studies in Albizia lebbek from Garhwal Himalaya. J. Tree Sci. 2002, 21, 27–34. [Google Scholar]
- Singh, B.; Bhatt, B.P.; Prasad, P. Variation in seed and seedling traits of Celtis australis, a multipurpose tree, in Central Himalaya, India. Agrofor. Syst. 2006, 67, 115–122. [Google Scholar] [CrossRef]
- Singh, B.; Bhatt, B.P. Provenance variation in pod, seed and seedling traits of Dalbergia sissoo (Roxb. ex dc), Central Himalaya, India. Trop. Agric. Res. Ext. 2008, 11, 39–44. [Google Scholar] [CrossRef]
- Chauhan, S.; Bhatt, B.P.; Todaria, N.P. Germination behavior of Terminalia species influenced by temperature, light and seed maturity in Garhwal Himalaya. J. Plant Biol. 2002, 29, 208–209. [Google Scholar]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 17, 351. [Google Scholar]
- Zangoie, M.S.; Parsa, M.; Mahmoodi, M.; Jami, A.-A.; Sanjari, G. Effect of temperature regimes on seed germination asafoetida (Ferula assafoetida L.). Plant Breed. Seed Sci. 2014, 69, 59–67. [Google Scholar] [CrossRef]
- Teketay, D. Germination ecology of twelve indigenous and eight exotic multipurpose leguminous species from Ethiopia. For. Ecol. Manag. 1996, 80, 209–223. [Google Scholar] [CrossRef]
- López, U.J.; Mendoza, H.A.J.; Jasso Mata, J.; Gómez, G.A. Morphological variation of plants and the influence of pH of irrigation water on twelve population Pinusgregii. Madera Y Bosques 2000, 6, 81–94. [Google Scholar]
- Khullar, P.; Thapliyal, R.C.; Beniwal, B.S.; Vakshasya, R.K.; Sharma, A. Forest Seed; FRI Publication: Dehradun, India, 1991; p. 412. [Google Scholar]
- Singh, B.G.; Mahadeevan, N.P.; Manimuthu, L. The effect of storage temperature on the viability of Casusarina equistifolia Forester and Forester. In Seed and Nursery Technology of Forest Trees; New Age Publications: New Delhi, India, 1999; pp. 183–187. [Google Scholar]
- Abdul-Banki, A.A.; Anderson, J.D. Relationship between decarboxylation of glutamic acid and vigour in soybean seeds. Crop Sci. 1973, 13, 222–226. [Google Scholar]
- Carrera-Castaño, G.; Calleja-Cabrera, J.; Pernas, M.; Gómez, L.; Oñate-Sánchez, L. An updated overview on the regulation of seed germination. Plants 2020, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Chand, G. Seed Storage and Viability of Toona Ciliata and Shorea robusta. Master’s Thesis, Parmar University of Horticulture, Solan, India, 1994; pp. 125–136. [Google Scholar]
- Prasad, P.; Nautiyal, A.R. Effect of orientation of seed placement in soil on seedling emergence in two Bauhinia species: Bauhinia vahlii WightetArn. and Bauhinia racemosa Lam. Seed Sci. Technol. 2003, 31, 183–186. [Google Scholar] [CrossRef]
- Rawat, D.C.S.; Nautiyal, A.R. Physiology of germination in Quercus. II. Effect of seed orientation in Quercus leucotrichophora. Seed Res. 1998, 26, 183–186. [Google Scholar]
- Thapiyal, R.C. Influence of seed orientation on seedling emergence in Chir plant Pinusroxburghii. Indian J. Exp. Biol. 1979, 17, 119–120. [Google Scholar]
- Sharma, M.M.; Purohit, A.N. Seedling survival and seed germination under natural and laboratory conditions in Shorea robusta. Seed Sci. Technol. 1980, 8, 283–287. [Google Scholar]
- Swaminathan, C.; Rai, V.; Suresh, R.S.; Sivaganam, K. Improving seed germination of Derris indica by vertical showing. J. Trop. For. Sci. 1993, 6, 152–158. [Google Scholar]
- Krishna, A.; Ganiger, B.S.; Rathod, R. Effect of seed weight and seed coat colour on germination and vigour of Forest tree Erythrina indica (Lam). Karnataka J. Agric. Sci. 2005, 18, 208–209. [Google Scholar]
- Liu, H.; Zhang, Z. Effect of mast seedling and rodent abundance on seed predation and dispersal by rodents in Prunus armeniaca (Rosaceae). For. Ecol. Manag. 2007, 242, 511–517. [Google Scholar] [CrossRef]
- Bonner, F.T.; Vozzo, J.A.; Elam, W.W.; Land, S.B.J. Tree Seed Technology Training Course; Instructional Manual, Southern Forest Experimental Station, U.S. Department of Agriculture: New Orleans, Louisiana, 1994; p. 160. [Google Scholar]
- Bonfil, C. The effects of seed size, cotyledon reserves, and herbivory on seedling survival and growth in Quercus rugosa and Q. laurina (Fagaceae). Am. J. Bot. 1998, 85, 79–87. [Google Scholar] [CrossRef]
- Tripathi, R.S.; Khan, M.L. Effects of seed weight and microsite characteristics on germination and seedling fitness in two species of Quercus in a subtropical wet hill forest. Oiko 1990, 57, 289–296. [Google Scholar] [CrossRef]
- Vera, M.L. Effects of altitude and seed size on germination and seedling survival of heathland plants in north Spain. Plant Ecol. 1997, 133, 101–106. [Google Scholar] [CrossRef]
- Abiyu, A.; Teketay, D.; Glatzel, G.; Gratzer, G. Seed production, seed dispersal and seedling establishment of two Afromontane tree species in and around a church forest: Implications for forest restoration. For. Ecosyst. 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Baraloto, C.; Forget, P.M.; Goldberg, D.E. Seed mass, seedling size, and neotropical tree seedling establishment. J. Ecol. 2005, 93, 1156–1166. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Tjoelker, M.G. Seed mass effects on germination and growth of diverse European Scots pine populations. Can. J. For. Res. 1994, 24, 306–320. [Google Scholar] [CrossRef]
- Michael, P.D.; Paul, B.C. Effects of wetting and drying on seed germination and seedling emergence of bull thistle, Cirsium vulgare (Savi) Ten. Can. J. Bot. 2000, 78, 1545–1551. [Google Scholar]
- Kos, M.; Poschlod, P. Correlation of inter-specific variation in germination response to water stress in a semi-arid savannah. Basic Appl. Ecol. 2008, 9, 645–652. [Google Scholar] [CrossRef]
- Li, T.S. Effect of seedling depth and soil texture on seedling emergence and root shape of American ginseng. Korean J. Ginseng Sci. 1997, 21, 115–118. [Google Scholar]
- Ahirwar, R.K. Effect of sowing depth on seed germination of Bute afrondosa(Roxb.). Int. Res. J. Biol. Sci. 2015, 4, 45–47. [Google Scholar]
- Ali, S.A.; Idris, A.Y. Effect of seed size and sowing depth on germination and some growth parameters of faba bean (Vicia faba L.). Agric. Biol. Sci. J. 2015, 1, 1–5. [Google Scholar]
- Guo, K.; Li, R.; Werger, M.J.A. Effect of acorn burying depth on germination, seedling emergence and development of Quercus aliena var. acuteserrata. Acta Bot. Sin. 2001, 43, 974–978. [Google Scholar]
- Guo, C.; Yang, J.; Lu, D.; Zhao, L. Impacts of burial and insect infection on germination and seedling growth of acorns of Quercus variabilis. For. Ecol. Man. 2009, 258, 1497–1502. [Google Scholar] [CrossRef]
- Proctor, J.T.; Sullivan, J.A. Effect of seeding depth on seedling growth and dry matter partitioning in American ginseng. J. Ginseng Res. 2013, 37, 254. [Google Scholar] [CrossRef]
- Masilamani, P.; Singh, G.B.; Chinnusamy, C.; Annadurai, K. Influence of seed orientation and depth of sowing on germination and vigour of Anjan (HardwickiabinataRoxb.). Trop. Agric. Res. Extension 1999, 2, 76–78. [Google Scholar]
- Mahgoub, S. The influence of positioning of seed during sowing on seed germination of some tree species in relation to germination type and seed shape. In Proceedings of the IUFRO Symposium on Innovations in Tropical Tree Seed Technology, Tampere, Finland, 6–12 August; 1995; pp. 149–159. [Google Scholar]
- BennetClark, T.A.; Younis, A.F.; Esnault, R. Geotropic behavior of roots. J. Exp. Bot. 1959, 10, 69–86. [Google Scholar] [CrossRef]
- Gregorio, N.O.; Herbohn, J.L.; Harrison, S.R. The potential role of nurseries in improving access to high quality planting stock and promoting appropriate silvicultural systems to improve the productivity of smallholder tree farms in Leyte, Philippines. In ACIAR Smallholder Forestry Project–Redevelopment of a Timber Industry Following Extensive Land Clearing: Proceedings from the End-of-Project Workshop; Harrison, S.R., Herbohn, J.L., Suh, J., Mangaoang, E., Vanclay, J., Eds.; ACIAR Smallholder Forestry Project: Ormoc, Phillipines, 2004; pp. 269–278. [Google Scholar]
- Allen, K.S.; Harper, R.W.; Bayer, A.; Brazee, N.J. A review of nursery production systems and their influence on urban tree survival. Urban For. Urban Green 2017, 21, 183–191. [Google Scholar] [CrossRef]
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.J.; Atkinson, C.J.; Bengough, A.G.; Else, M.A.; Fernandez-Fernandez, F.; Harrison, R.J.; Schmidt, S. Contributions of roots and rootstocks to sustainable, intensified crop production. J. Exp. Bot. 2013, 64, 1209–1222. [Google Scholar] [CrossRef]
- Seidel, S.J.; Gaiser, T.; Kautz, T.; Bauke, S.L.; Amelung, W.; Barfus, K.; Ewert, F.; Athmann, M. Estimation of the impact of precrops and climate variability on soil depth differentiated spring wheat growth and water, nitrogen and phosphorus uptake. Soil Till. Res. 2019, 195, 104427. [Google Scholar] [CrossRef]
- Chaichi, M.; Nemati, A.; Dadrasi, A.; Heydari, M.; Hassanisaadi, M.; Yousefi, A.R.; Baldwin, T.C.; Mastinu, A. Germination of Triticum aestivum L.: Effects of Soil–Seed Interaction on the Growth of Seedlings. Soil Syst. 2022, 6, 37. [Google Scholar] [CrossRef]
- Adams, C.A.; Rinne, R.W. Moisture Content as a Controlling Factor in Seed Development and Germination. In International Review of Cytology; Bourne, G.H., Danielli, J.F., Jeon, K.W., Eds.; Academic Press, 1980; Volume 68, pp. 1–8. [Google Scholar] [CrossRef]
- Rizzardi, M.; Luiz, A.R.; Roman, E.S.; Vargas, L. Effect of Cardinal Temperature and Water Potential on Morning Glory (Ipomoea triloba) Seed Germination. Planta Daninha 2009, 27, 13–21. [Google Scholar] [CrossRef]
- Ahn, J.; Oh, S.; Kang, Y.J.; Kim, K.; Moon, S.-K.; Moon, B.; Myung, S.; Kim, M.-S.; Lee, Y.K.; Ko, K. Effect of Oak Tree Sawdust Fermentation Period on Peanut Seed Germination, Seedling Biomass, and Morphology. Horticulturae 2021, 7, 182. [Google Scholar] [CrossRef]
- Wang, X.; Wu, R.; Shen, T.; Li, Z.; Li, C.; Wu, B.; Jiang, H.; Zhao, G. An R2R3-MYB Transcription Factor OsMYBAS1 Promotes Seed Germination under Different Sowing Depths in Transgenic Rice. Plants 2022, 11, 139. [Google Scholar] [CrossRef] [PubMed]

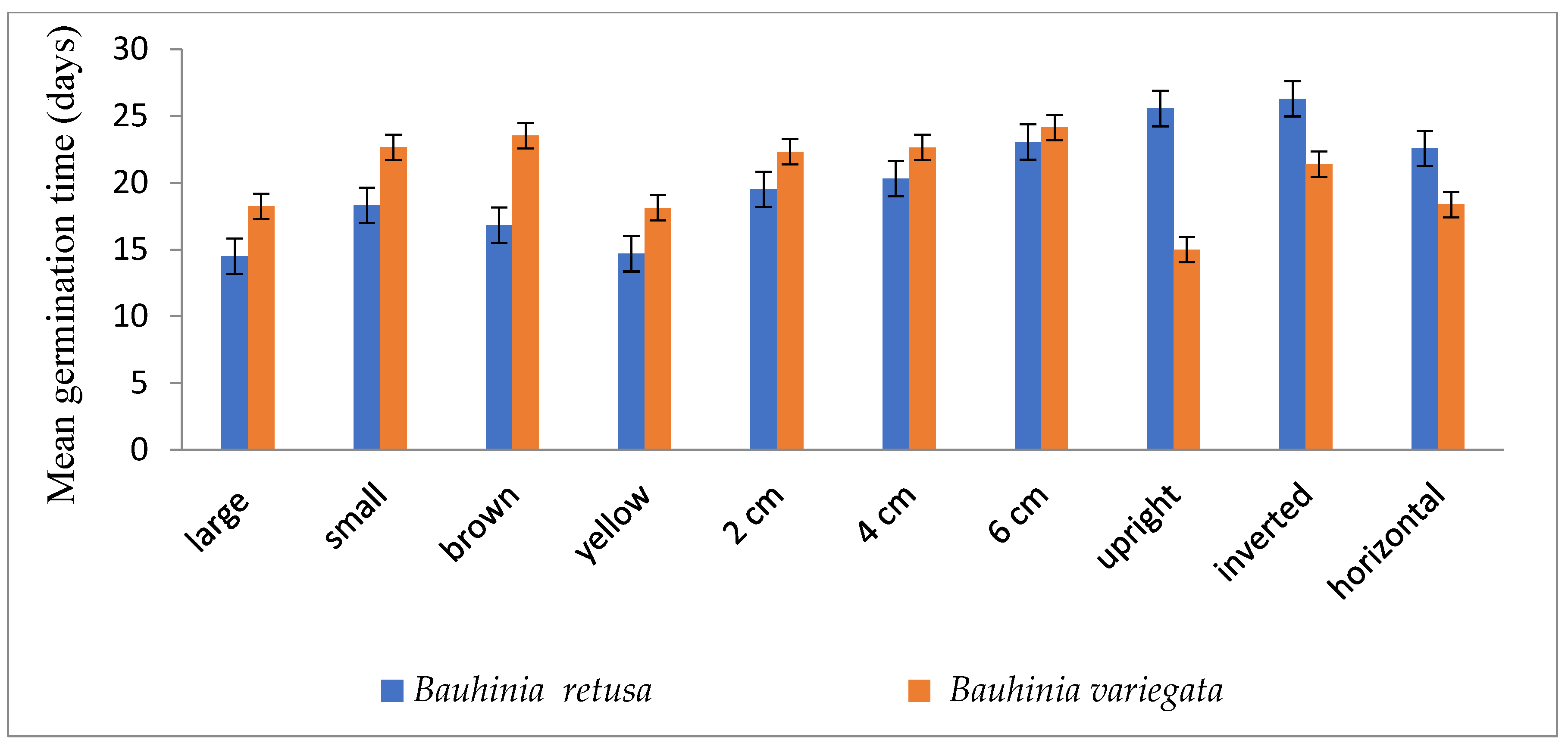
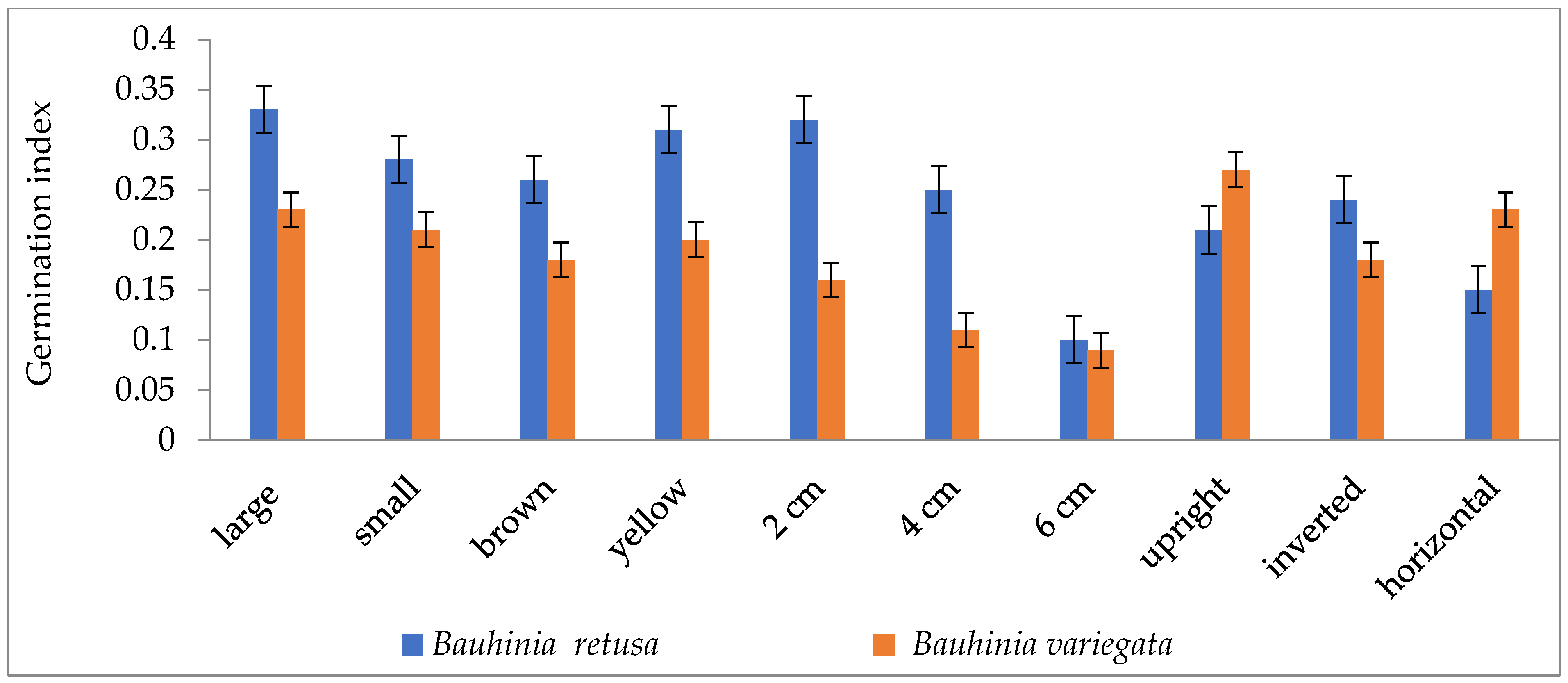
| Species | Temp. (°C) | Germination (%) | Radicle (cm) | Plumule (cm) |
|---|---|---|---|---|
| 25 °C | 83.33 ± 5.77 a | 8.98 ± 0.57 a | 3.28 ± 0.20 a | |
| Bauhinia retusa | 20 °C | 66.67 ± 11.55 b | 6.74 ± 0.96 ab | 2.81 ± 0.62 ab |
| 15 °C | 60 ± 10.0 b | 5.89 ± 0.68 b | 1.36 ± 0.22 b | |
| 25 °C | 86.67 ± 5.77 a | 9.40 ± 0.52 a | 3.52 ± 0.11 a | |
| Bauhinia variegata | 20 °C | 73.33 ± 5.77 ab | 6.94 ± 0.62 b | 2.81 ± 0.62 ab |
| 15 °C | 70.0 ± 10.0 b | 5.81 ± 0.29 b | 1.49 ± 0.13 b |
| Species | Containers | |||
|---|---|---|---|---|
| Months | Cotton Bag | Plastic Cane | Polybags | |
| Bauhinia retusa | 3 | 76.67 ± 11.56 a | 86.57 ± 5.77 b | 86.67 ± 5.77 b |
| 6 | 56.67 ± 5.77 b | 66.67 ± 5.77 ab | 70.0 ± 10.0 a | |
| 9 | 50.0 ± 10.0 b | 53.33 ± 5.77 ab | 66.67 ± 5.77 a | |
| 12 | 48.0 ± 6.58 b | 50.0 ± 10.0 ab | 56.67 ± 11.55 a | |
| Bauhinia variegata | 3 | 80.0 ± 10.0 b | 86.67 ± 5.77 a | 83.33 ± 5.77 ab |
| 6 | 76.67 ± 5.77 b | 80.0 ± 10.00 ab | 83.33 ± 11.55 a | |
| 9 | 66.67 ± 5.77 b | 73.33 ± 5.77 a | 80.0 ± 10.0 a | |
| 12 | 60.00 ± 10.00 b | 65.00 ± 10.0 a | 75.0 ± 11.56 a | |
| Containers | B. retusa | B. variegata | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 Month | 6 Month | 9 Month | 12 Month | 3 Month | 6 Month | 9 Month | 12 Month | |||||||||
| Radicle (cm) | Plumule (cm) | Radicle (cm) | Plumule (cm) | Radicle (cm) | Plumule (cm) | Radicle (cm) | Plumule (cm) | Radicle (cm) | Plumule (cm) | Radicle (cm) | Plumule (cm) | Radicle (cm) | Plumule (cm) | Radicle (cm) | Plumule (cm) | |
| Polythene Bag | 11.97 a ± 1.22 | 4.11 a ± 0.50 | 8.52 a ± 1.51 | 3.11 a ± 0.11 | 6.94 b ± 0.52 | 1.57 b ± 0.32 | 5.64 ab ± 0.42 | 1.45 b ±0.61 | 12.60 ab ± 1.11 | 5.16 ab ± 0.29 | 12.59 a ± 1.10 | 5.62 a ± 0.72 | 10.70 a ± 0.75 | 5.45 a ± 0.55 | 9.78 a ±0.20 | 5.12 a ±0.31 |
| Plastic cane | 9.77 b ± 0.48 | 3.50 ab ± 0.32 | 6.88 b ± 0.84 | 2.47 b ± 0.57 | 5.13 b ± 0.83 | 2.03 a ± 0.37 | 5.11 b ± 0.25 | 1.84 a ± 0.64 | 10.14 b ± 0.30 | 4.75 b ± 0.64 | 10.86 b ± 0.98 | 5.50 b ± 0.59 | 9.93 b ± 0.50 | 4.52 b ± 0.28 | 8.84 b ±0.27 | 4.24 b ±0.54 |
| Cotton Bag | 10.33 ab ± 0.87 | 3.26 b ± 0.26 | 7.45 ab ± 0.99 | 2.55 b ± 0.10 | 7.14 a ± 0.22 | 1.70 ab ± 0.50 | 6.84 a ± 0.78 | 1.67 ab ± 0.27 | 13.52 a ± 1.0 | 5.21 a ± 0.39 | 11.74 ab ± 0.82 | 5.52 ab ± 0.38 | 10.01 b ± 0.49 | 5.01 ab ± 0.20 | 9.14 ab ± 0.42 | 4.94 ab ± 0.18 |
| 12 Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sowing Conditions | Shoot Length (cm) | Collar Diameter (mm) | Number of Leaves | Root Length (cm) | Shoot Dry Weight (g) | Root Dry Weight (g) | Leaf Dry Weight (g) | Total Dry Weight (g) | Root/Shoot Dry Ratio |
| Seed size | |||||||||
| Large | 20.24 a ± 1.53 | 3.31 a ± 0.21 | 2.60 a ± 0.14 | 29.56 a ± 2.18 | 0.54 a ± 0.07 | 1.78 a ± 0.21 | 0.50 a ± 0.08 | 2.83 a ± 0.36 | 3.29 a |
| Small | 17.77 a ± 1.73 | 2.75 a ± 0.66 | 2.28 b ± 0.18 | 15.75 b ± 3.89 | 0.43 b ± 0.11 | 1.36 b ± 0.11 | 0.47 a ± 0.08 | 2.26 b ± 0.31 | 3.16 b |
| Seed Orientation | |||||||||
| Upright | 20.68 a ± 2.93 | 3.29 a ± 0.09 | 2.40 a ± 0.14 | 29.61 ab ± 3.96 | 0.56 a ± 0.07 | 1.79 a ± 0.20 | 0.54 a ± 0.09 | 2.90 a ± 0.37 | 3.19 b |
| Inverted | 17.27 b ± 1.26 | 3.08 b ± 0.26 | 2.28 b ± 0.11 | 21.04 b ± 4.27 | 0.40 b ± 0.12 | 1.34 b ± 0.09 | 0.42 b ± 0.08 | 2.17 b ± 0.29 | 3.35 a |
| Horizontal | 18.74 ab ± 1.10 | 3.11 ab ± 0.27 | 2.32 ab ± 0.18 | 30.36 a ± 2.53 | 0.48 ab ± 0.12 | 1.57 ab ± 0.21 | 0.53 ab ± 0.10 | 2.58 ab ± 0.43 | 3.27 ab |
| Seed Colour | |||||||||
| Brown | 15.91 b ± 1.63 | 2.60 a ± 0.41 | 2.28 a ± 0.11 | 14.48 b ± 2.10 | 0.43 b ± 0.10 | 1.36 b ± 0.11 | 0.46 a ± 0.08 | 2.25 b ± 0.29 | 3.0 b |
| Yellow | 20.13 a ± 2.79 | 2.98 a ± 0.56 | 2.20 b ± 0.24 | 30.59 a ± 2.35 | 0.57 a ± 0.06 | 1.71 a ± 0.30 | 0.50 a ± 0.07 | 2.78 a ± 0.43 | 3.16 a |
| Sowing Depth | |||||||||
| 2 cm | 17.38 a ± 1.11 | 2.87 ab ± 0.35 | 2.44 ab ± 0.40 | 30.10 a ± 1.39 | 0.59 a ± 0.04 | 1.65 a ± 0.24 | 0.53 a ± 0.10 | 2.74 a ± 0.43 | 2.77 b |
| 4 cm | 13.63 ab ± 1.91 | 2.98 a ± 0.14 | 2.55 a ± 0.32 | 29.09 ab ± 2.48 | 0.49 ab ± 0.09 | 1.64 ab ± 0.33 | 0.51 ab ± 0.07 | 2.68 ab ± 0.43 | 3.36 a |
| 6 cm | 9.44 b ± 0.60 | 2.39 b ± 0.03 | 2.32 b ± 0.23 | 21.80 b ± 0.98 | 0.44 b ± 0.11 | 1.39 b ± 0.09 | 0.45 b ± 0.09 | 2.28 b ± 0.30 | 3.15 ab |
| 12 Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sowing Condition | Shoot Length (cm) | Collar Diameter (mm) | Number of Leaves | Root Length (cm) | Shoot Dry Weight (g) | Root Dry Weight (g) | Leaf Dry Weight(g) | Total Dry Weight(g) | Root/Shoo Dry Ratio |
| Seed size | |||||||||
| Large | 32.09 a ±1.72 | 3.71 a ± 0.19 | 13.43 a ± 0.43 | 31.46 a ± 2.26 | 0.44 a ± 0.08 | 0.30 a ± 0.06 | 0.30 ± 0.04 a | 1.04 a ± 0.16 | 0.68 b |
| Small | 13.21 b ±1.94 | 2.64 b ± 0.35 | 5.17 b ± 0.67 | 24.54 b ± 4.67 | 0.24 ± 0.06 b | 0.19 ± 0.05 b | 0.20 ± 0.04 b | 0.60 b ± 0.11 | 0.79 a |
| Seed Orientation | |||||||||
| Upright | 34.52 ab ± 4.27 | 3.16 ± 0.21 ab | 8.10 ± 1.17 a | 30.68 ± 3.73 a | 0.47 ± 0.08 ab | 0.33 ± 0.06 b | 0.30 ± 0.04 b | 1.10 ± 0.15 b | 0.70 b |
| Inverted | 21.53 b ± 1.25 | 2.98 ± 0.16 b | 7.60 ± 0.67 b | 24.07 ± 3.07 b | 0.42 ± 0.08 b | 0.31 ± 0.05 b | 0.30 ± 0.04 b | 1.04 ± 0.13 b | 0.73 a |
| Horizontal | 41.92 a ± 2.94 | 3.43 ± 0.15 a | 8.18 ± 0.69 a | 31.63 ± 2.78 a | 0.51 ± 0.08 a | 0.37 ± 0.06 a | 0.35 ± 0.04 a | 1.23 ± 0.16 a | 0.72 a |
| Seed Colour | |||||||||
| Brown | 36.17 b ± 1.48 | 3.21 a ± 0.20 | 9.32 a ± 1.52 | 24.86 b ± 5.01 | 0.45 b ± 0.06 | 0.30 b ± 0.05 | 0.29 b ± 0.03 | 1.04 b ± 0.11 | 0.66 b |
| yellow | 40.07 a ± 1.70 | 3.58 a ± 0.21 | 10.22 a ± 0.63 | 33.72 a ± 3.09 | 0.50 a ± 0.07 | 0.36 a ± 0.06 | 0.33 a ± 0.04 | 1.20 a ± 0.12 | 0.72 a |
| Sowing Depth | |||||||||
| 2 cm | 31.70 a ± 4.71 | 3.33 ab ± 0.23 | 7.68 a ± 0.62 | 31.36 a ± 1.92 | 0.38 a ± 0.06 | 0.30 a ± 0.06 | 0.29 a ± 0.05 | 0.97 a ± 0.10 | 0.78 b |
| 4 cm | 18.48 b ± 1.99 | 3.37 a ± 0.15 | 7.66 ab ± 0.87 | 29.47 ab ± 1.71 | 0.35 ab ± 0.06 | 0.28 ab ± 0.04 | 0.27 ab ± 0.06 | 0.89 ab ± 0.09 | 0.80 ab |
| 6 cm | 17.62 b ± 2.54 | 3.28 b ± 0.12 | 7.48 b ± 0.66 | 20.39 b ± 3.38 | 0.27 b ± 0.08 | 0.23 b ± 0.07 | 0.26 b ± 0.05 | 0.75 b ± 0.15 | 0.85 a |
| Source of Variation | DF | Germination (%) | Shoot Length (cm) | Collar Diameter (mm) | Number of Leaves | Root Length (cm) | Shoot Dry Wt (g) | Root Dry Wt (g) | Leaves Dry Wt (g) | Total Dry Wt (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Seed size | 1 | 3.13.60 ** | 15.25 NS | 0.80 NS | 0.25 * | 476.93 ** | 0.23 * | 0.44 ** | 0.00 NS | 0.79 ** |
| Replication | 4 | 16.25 NS | 2.58 NS | 0.22 NS | 0.04 NS | 9.39 NS | 0.002 NS | 0.01 NS | 0.004 NS | 0.006 NS |
| Seed coat color | 1 | 640.0 ** | 44.44 * | 0.08 NS | 0.02 NS | 650.28 ** | 0.05 ** | 0.306 ** | 0.004 NS | 0.72 ** |
| Replication | 4 | 3.6 NS | 7.2 NS | 0.12 NS | 0.04 NS | 5.24 NS | 0.002 NS | 0.008 NS | 0.002 NS | 0.007 NS |
| Sowing depths | 2 | 2211.47 ** | 78.92 ** | 0.49 ** | 0.02 ** | 116.49 * | 0.32 ** | 0.11 * | 0.009 * | 0.31 ** |
| Replication | 4 | 7.07 NS | 3.31 NS | 0.17 NS | 0.04 NS | 3.69 NS | 0.005 NS | 0.04 NS | 0.004 NS | 0.06 NS |
| Seed orientation | 2 | 1198.87 ** | 14.62 * | 0.06 NS | 0.02 NS | 41.22 NS | 0.03 ** | 0.25 ** | 0.02 * | 0.66 ** |
| Replication | 4 | 1.27 NS | 5.93 NS | 0.05 NS | 0.03 NS | 9.67 NS | 0.002 NS | 0.005 NS | 0.006 NS | 0.006 NS |
| Source of Variation | DF | Germination (%) | Shoot Length (cm) | Collar Diameter (mm) | Number of Leaves | Root Length (cm) | Shoot Dry Wt (g) | Root Dry Wt (g) | Leaves Dry Wt (g) | Total Dry Wt (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Seed size | 1 | 435.60 * | 891.70 ** | 2.89 ** | 170.40 ** | 695.89 ** | 0.10 ** | 0.02 ** | 0.03 ** | 0.45 ** |
| Replication | 4 | 51.40 NS | 1.04 NS | 0.14 NS | 1.09 NS | 2.17 NS | 0.001 NS | 0.00 NS | 0.001 NS | 0.002 NS |
| Seed coat color | 1 | 409.60 ** | 37.95 ** | 0.33 * | 2.03 NS | 196.43 ** | 0.007 ** | 0.01 ** | 0.004 * | 0.07 ** |
| Replication | 4 | 38.40 NS | 4.23 NS | 0.05 NS | 1.45 NS | 2.84 NS | 0.001 NS | 0.00 NS | 0.001 NS | 0.006 NS |
| Sowing depths | 2 | 2211.47 ** | 311.42 ** | 0.01 NS | 0.06 NS | 172.05 ** | 0.02 NS | 0.006 NS | 0.03 ** | 17.73 ** |
| Replication | 4 | 7.07 NS | 6.84 NS | 0.03 NS | 0.62 NS | 5.25 NS | 0.005 NS | 0.009 NS | 0.000 NS | 0.01 NS |
| Seed orientations | 2 | 1198.87 ** | 532.95 ** | 0.25 ** | 0.49 NS | 84.77 ** | 0.009 ** | 0.005 ** | 0.004 ** | 0.05 ** |
| Replication | 4 | 1.27 NS | 16.52 NS | 0.07 NS | 1.06 NS | 12.68 NS | 0.007 NS | 0.001 NS | 0.001 NS | 0.01 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, N.; Khanduri, V.P.; Singh, B.; Dhanai, C.S.; Riyal, M.K.; Rawat, D.; Ahmad, T.; Kumar, M. Effect of Temperature, Seed Size, Sowing Depth, and Position on Seed Germination and Seedling Growth of Bauhinia retusa Roxb. and Bauhinia variegata L. Forests 2023, 14, 1664. https://doi.org/10.3390/f14081664
Yadav N, Khanduri VP, Singh B, Dhanai CS, Riyal MK, Rawat D, Ahmad T, Kumar M. Effect of Temperature, Seed Size, Sowing Depth, and Position on Seed Germination and Seedling Growth of Bauhinia retusa Roxb. and Bauhinia variegata L. Forests. 2023; 14(8):1664. https://doi.org/10.3390/f14081664
Chicago/Turabian StyleYadav, Neeraj, Vinod Prasad Khanduri, Bhupendra Singh, Chatar Singh Dhanai, Manoj Kumar Riyal, Deepa Rawat, Taufiq Ahmad, and Munesh Kumar. 2023. "Effect of Temperature, Seed Size, Sowing Depth, and Position on Seed Germination and Seedling Growth of Bauhinia retusa Roxb. and Bauhinia variegata L." Forests 14, no. 8: 1664. https://doi.org/10.3390/f14081664
APA StyleYadav, N., Khanduri, V. P., Singh, B., Dhanai, C. S., Riyal, M. K., Rawat, D., Ahmad, T., & Kumar, M. (2023). Effect of Temperature, Seed Size, Sowing Depth, and Position on Seed Germination and Seedling Growth of Bauhinia retusa Roxb. and Bauhinia variegata L. Forests, 14(8), 1664. https://doi.org/10.3390/f14081664








