Enhancing the Cryopreservation System of Larch Embryogenic Culture by Optimizing Pre-Culture, Osmoprotectants, and Rapid Thawing
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture Media
2.2. The Cryopreservation of Embryogenic Tissue
2.3. The Recovery of Embryogenic Tissue from Cryopreservation
2.4. Maturation of Somatic Embryos and Plantlet Regeneration
2.5. Experimental Design, Data Recording, and Statistical Analysis
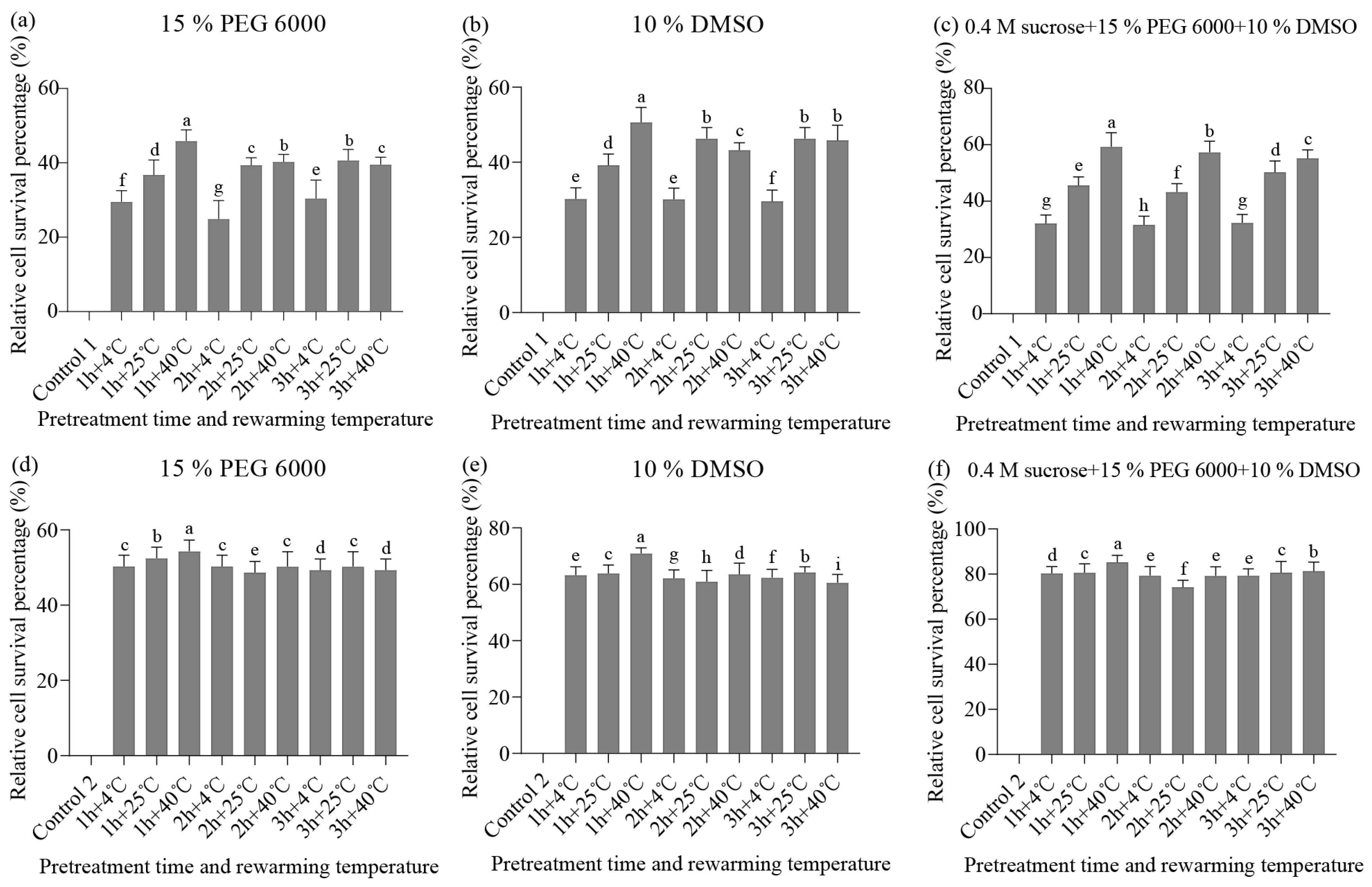
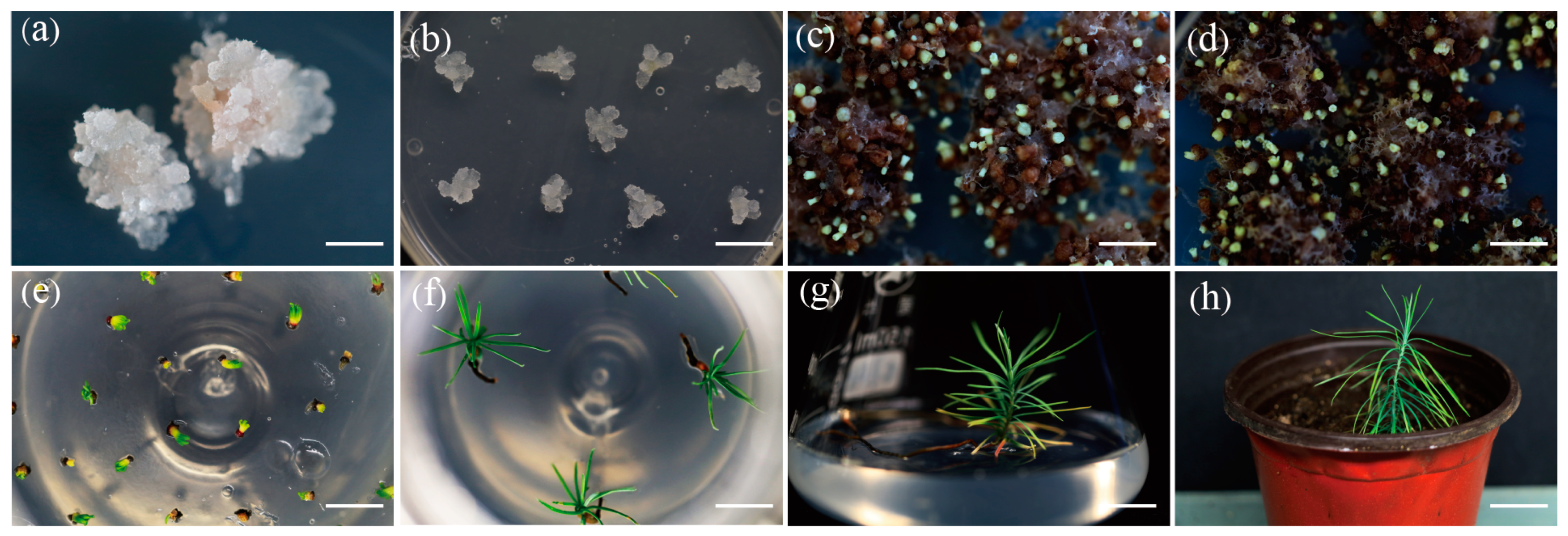
3. Results
3.1. Recovery of Cryopreserved Embryogenic Tissues
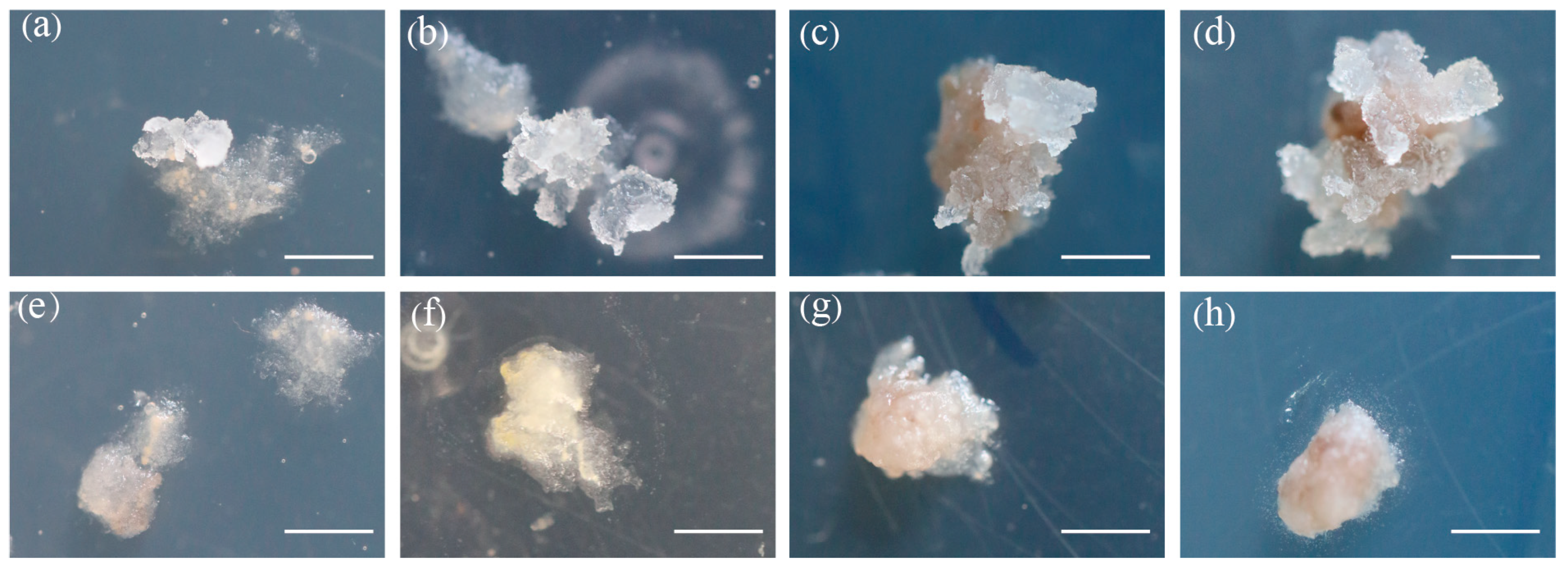
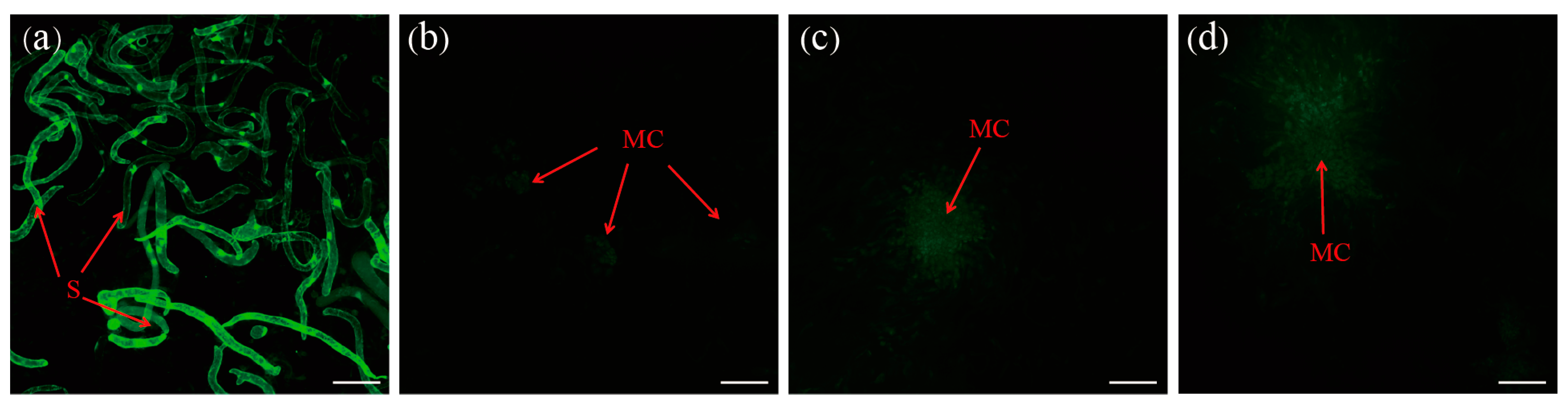
3.2. Morphological Characterization
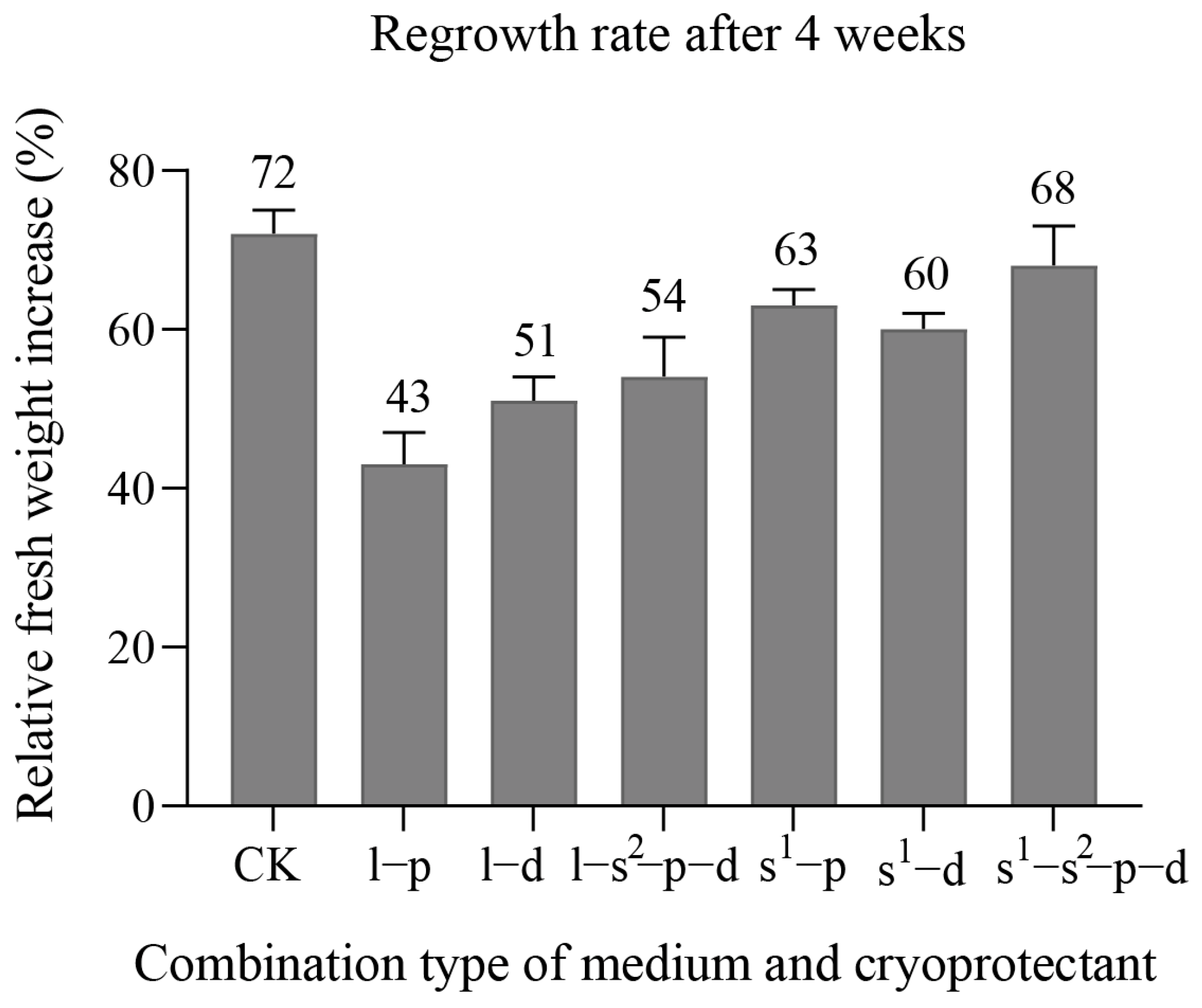
3.3. Maturation Ability and Plantlet Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Normah, M.N.; Sulong, N.; Reed, B.M. Cryopreservation of shoot tips of recalcitrant and tropical species: Advances and strategies. Cryobiology 2019, 87, 1–14. [Google Scholar] [CrossRef]
- Kartha, K.K.; Fowke, L.C.; Leung, N.L.; Caswell, K.L.; Hakman, I. Induction of somatic embryos and plantlets from cryopreserved cell cultures of white spruce (Picea glauca). J. Plant Physiol. 1988, 132, 529–539. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Org. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Wang, M.R.; Lambardi, M.; Engelmann, F.; Pathirana, R.; Panis, B.; Volk, G.M.; Wang, Q.C. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant sources. Plant Cell Tissue Org. Cult. 2021, 144, 7–20. [Google Scholar] [CrossRef]
- Ren, L.; Wang, M.R.; Wang, Q.C. ROS-induced oxidative stress in plant cryopreservation: Occurrence and alleviation. Planta 2021, 254, 124. [Google Scholar] [CrossRef] [PubMed]
- Ozudogru, E.A.; Lambardi, M. Cryotechniques for the long-term conservation of embryogenic cultures from woody plants. Methods Mol. Biol. 2016, 1359, 537–550. [Google Scholar]
- Shmakov, V.N.; Konstantinov, Y.M. Somatic embryogenesis in Larix: The state of art and perspectives. Vavilovskii Z. Genet. Sel. 2020, 24, 575–588. [Google Scholar] [CrossRef]
- Li, B.; Wyckoff, G.W. Breeding strategies for Larix decidua, L. leptolepis and their hybrids in the United States. For. Genet. 1994, 1, 65–72. [Google Scholar]
- Larkin, P.J.; Scowcroft, W.R. Somaclonal variation-a novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Ward, C.; Cheliak, W.M. Cryopreservation and plant regeneration from embryogenic cultures of larch (Larix×eurolepis) and black spruce (Picea mariana). J. Exp. Bot. 1992, 43, 73–79. [Google Scholar] [CrossRef]
- Yan, X.; Wang, K.; Zheng, K.; Zhang, L.; Ye, Y.; Qi, L.; Zhu, M. Efficient organogenesis and taxifolin production system from mature zygotic embryos and needles in larch. For. Res. 2023, 3, 4. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, J.; Hu, J.; Ling, J. Application of the somatic embryogenesis technique in conifer species. For. Res. 2022, 2, 18. [Google Scholar]
- Ford, C.S.; Jones, N.B.; Staden, J.V. Cryopreservation and plant regeneration from somatic embryos of Pinus patula. Plant Cell Rep. 2000, 19, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, C.; Grace, L.; Holden, D. Nurse culture for efficient recovery of cryopreserved Pinus radiata D. Don embryogenic cell lines. Plant Cell Rep. 2002, 21, 40–45. [Google Scholar]
- Mathur, G.; Alkutkar, V.A.; Nadgauda, R.S. Cryopreservation of embryogenic culture of Pinus roxburghii. Bio. Plant. 2003, 46, 205–210. [Google Scholar] [CrossRef]
- Carneros, E.; Hernández, I.; Díaz-Sala, C.; Toribio, M.; Celestino, C. Effect of different cryoprotectant procedures on the recovery and maturation ability of cryopreserved Pinus pinea embryogenic lines of different ages. Vitro Cell. Dev. Biol. Plant 2017, 53, 469–477. [Google Scholar] [CrossRef]
- Gupta, P.K.; Durzan, D.J. Biotechnology of somatic polyembryogenesis and plantlet regeneration in loblolly pine. Nat. Biotechnol. 1987, 5, 147–151. [Google Scholar] [CrossRef]
- Touchell, D.; Chiang, V.; Tsai, C.J. Cryopreservation of embryogenic cultures of Picea mariana (black spruce) using vitrification. Plant Cell Rep. 2002, 21, 118–124. [Google Scholar]
- Gale, S.; John, A.; Harding, K.; Benson, E.E. Developing cryopreservation for Picea sitchensis (sitka spruce) somatic embryos: A comparison of vitrification protocols. CryoLetters 2008, 29, 135–144. [Google Scholar]
- Hazubska-Przybył, T.; Chmielarz, P.; Michalak, M.; Bojarczuk, K. Cryopreservation of embryogenic tissues of Picea omorika (Serbian spruce). Plant Cell Tissue Org Cult. 2010, 102, 35–44. [Google Scholar] [CrossRef]
- Dutra, N.T.; Silveira, V.; de Azevedo, I.G.; Gomes-Neto, L.R.; Façanha, A.R.; Steiner, N.; Guerra, M.P.; Floh, E.I.; Santa-Catarina, C. Polyamines affect the cellular growth and structure of pro-embryogenic masses in Araucaria angustifolia embryogenic cultures through the modulation of proton pump activities and endogenous levels of polyamines. Physiol. Plant. 2013, 148, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Salaj, T.; Matušíková, I.; Fráterová, L.; Piršelová, B.; Salaj, J. Regrowth of embryogenic tissues of Pinus nigra following cryopreservation. Plant Cell Tissue Org. Cult. 2011, 106, 55–61. [Google Scholar] [CrossRef]
- Ma, X.; Bucalo, K.; Determann, R.O.; Cruse-Sanders, J.M.; Pullman, G.S. Somatic embryogenesis, plant regeneration, and cryopreservation for Torreya taxifolia, a highly endangered coniferous species. Vitro Cell. Dev. Biol. Plant 2012, 48, 324–334. [Google Scholar] [CrossRef]
- McLellan, M.R.; Day, J.G. Cryopreservation and freeze-drying protocols. Methods Mol. Biol. 1995, 38, 1–5. [Google Scholar]
- Varis, S.; Ahola, S.; Jaakola, L.; Aronen, T. Reliable and practical methods for cryopreservation of embryogenic cultures and cold storage of somatic embryos of norway spruce. Cryobiology 2017, 76, 8–17. [Google Scholar] [CrossRef]
- Zamecnik, J.; Faltus, M.; Bilavcik, A. Vitrification solutions for plant cryopreservation: Modification and properties. Plants 2021, 10, 2623. [Google Scholar] [CrossRef]
- Lineros, Y.; Balocchi, C.; Muñoz, X.; Sánchez, M.; Ríos, D. Cryopreservation of Pinus radiata embryogenic tissue: Effects of cryoprotective pretreatments on maturation ability. Plant Cell Tissue Org. Cult. 2018, 135, 357–366. [Google Scholar] [CrossRef]
- Peng, C.; Gao, F.; Wang, H.; Shen, H.; Yang, L. Optimization of maturation process for somatic embryo production and cryopreservation of embryogenic tissue in Pinus koraiensis. Plant Cell Tissue Org. Cult. 2021, 144, 185–194. [Google Scholar] [CrossRef]
- Marum, L.; Estêvão, C.; Oliveira, M.M.; Amâncio, S.; Rodrigues, L.; Miguel, C. Recovery of cryopreserved embryogenic cultures of maritime pine-effect of cryoprotectant and suspension density. CryoLetters 2004, 25, 363–374. [Google Scholar] [PubMed]
- Uchendu, E.E.; Muminova, M.; Gupta, S.; Reed, B.M. Antioxidant and anti-stress compounds improve regrowth of cryopreserved rubus shoot tips. Vitro Cell. Dev. Biol. Plant 2010, 46, 386–393. [Google Scholar] [CrossRef]
- Mathew, L.; McLachlan, A.; Jibran, R.; Burritt, D.J.; Pathirana, R. Cold, antioxidant and osmotic pre-treatments maintain the structural integrity of meristematic cells and improve plant regeneration in cryopreserved kiwifruit shoot tips. Protoplasma 2018, 255, 1065–1077. [Google Scholar] [CrossRef]
- Volk, G.M.; Shepherd, A.N.; Bonnart, R. Successful cryopreservation of vitis shoot tips: Novel pre-treatment combinations applied to nine species. CryoLetters 2018, 39, 322–330. [Google Scholar]
- Wang, Q.; Laamanen, J.; Uosukainen, M.; Valkonen, J.P. Cryopreservation of in vitro-grown shoot tips of raspberry (Rubus idaeus L.) by encapsulation–vitrification and encapsulation–dehydration. Plant Cell Rep. 2005, 24, 280–288. [Google Scholar] [CrossRef]
- Pathirana, R.; McLachlan, A.; Hedderley, D.; Panis, B.; Carimi, F. Pre-treatment with salicylic acid improves plant regeneration after cryopreservation of grapevine (Vitis spp.) by droplet vitrification. Acta. Physiol. Plant. 2016, 38, 12. [Google Scholar] [CrossRef]
- Varis, S.A.; Virta, S.; Montalbán, I.A.; Aronen, T. Reducing pre- and post-treatments in cryopreservation protocol and testing storage at −80 °C for norway spruce embryogenic cultures. Int. J. Mol. Sci. 2022, 23, 15516. [Google Scholar] [CrossRef] [PubMed]
- Laine, E.; Bade, P.; David, A. Recovery of plants from cryopreserved embryogenic cell suspensions of Pinus caribaea. Plant Cell Rep. 1992, 11, 295–298. [Google Scholar] [CrossRef]
- Vondráková, Z.; Cvikrová, M.; Eliásová, K.; Martincová, O.; Vágner, M. Cryotolerance in norway spruce and its association with growth rates, anatomical features and polyamines of embryogenic cultures. Tree Physiol. 2010, 30, 1335–1348. [Google Scholar] [CrossRef] [PubMed]
- Kaviani, B.; Kulus, D. Cryopreservation of endangered ornamental plants and fruit crops from tropical and subtropical regions. Biology 2022, 11, 847. [Google Scholar] [CrossRef] [PubMed]
- Dinato, N.B.; Santos, I.R.I.; Vigna, B.B.Z.; Ferreira de Paula, A.; Favero, A.P. Perspective: Pollen cryopreservation for plant breeding and genetic resources conservation. CryoLetters 2020, 41, 115–127. [Google Scholar]
- Wang, M.R.; Chen, L.; Teixeira da Silva, J.A.; Volk, G.M.; Wang, Q.C. Cryobiotechnology of apple (Malus spp.): Development, progress and future prospects. Plant Cell Rep. 2018, 37, 689–709. [Google Scholar] [CrossRef]
- Serrano-Martinez, F.; Casas, J.L. Cryopreservation of Tetraclinis articulata (vahl.) masters. CryoLetters 2011, 32, 248–255. [Google Scholar]
- Bi, W.L.; Pan, C.; Hao, X.Y.; Cui, Z.H.; Kher, M.M.; Marković, Z.; Teixeira da Silva, J.A. Cryopreservation of grapevine (Vitis spp.)—A review. Vitro Cell. Dev. Biol.-Plant 2017, 53, 449–460. [Google Scholar] [CrossRef]
- Castander-Olarieta, A.; Moncaleán, P.; Montalbán, I.A. Somatic embryogenesis in pines. Methods Mol. Biol. 2022, 2527, 41–56. [Google Scholar] [PubMed]
- Volk, G.M. Application of functional genomics and proteomics to plant cryopreservation. Curr. Genom. 2010, 11, 24–29. [Google Scholar] [CrossRef]
- Popova, E.; Kulichenko, I.; Kim, H.H. Critical role of regrowth conditions in post-cryopreservation of in vitro plant germplasm. Biology 2023, 12, 542. [Google Scholar] [CrossRef]
- Schumacher, H.M.; Westphal, M.; Heine-Dobbernack, E. Cryopreservation of plant cell lines using alginate encapsulation. Methods. Mol. Biol. 2021, 2180, 639–645. [Google Scholar]
- Niino, T.; Yamamoto, S.I.; Fukui, K.; Martínez, C.R.C.; Arizaga, M.V.; Matsumoto, T.; Engelmann, F. Dehydration improves cryopreservation of mat rush (Juncus decipiens Nakai) basal stem buds on cryo-plates. CryoLetters 2013, 34, 549–560. [Google Scholar] [PubMed]
- Yamamoto, S.I.; Rafique, T.; Priyantha, W.S.; Fukui, K.; Matsumoto, T.; Niino, T. Development of a cryopreservation procedure using aluminium cryo-plates. CryoLetters 2011, 32, 256–265. [Google Scholar]
- Bradai, F.; Sanchez-Romero, C. Effect of cryopreservation on the ex vitro establishment of olive plants regenerated via somatic embryogenesis. Plants 2021, 10, 396. [Google Scholar] [CrossRef]
- Salaj, T.; Klubicová, K.; Matusova, R.; Salaj, J. Somatic embryogenesis in selected conifer trees Pinus nigra Arn. and Abies Hybrids. Front. Plant Sci. 2019, 10, 13. [Google Scholar] [CrossRef]
- Li, X.Y.; Huang, F.H.; Gbur, E.E., Jr. Effect of basal medium, growth regulators and phytagel concentration on initiation of embryogenic cultures from immature zygotic embryos of loblolly pine (Pinus taeda L.). Plant Cell Rep. 1998, 17, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Filonova, L.H.; Bozhkov, P.V.; Arnold, S.V. Developmental pathway of somatic embryogenesis in Picea abies as revealed by time-lapse tracking. J. Exp. Bot. 2000, 51, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Miguel, C.; Gonçalves, S.; Tereso, S.; Marum, L.; Maroco, J.; Margarida Oliveira, M. Somatic embryogenesis from 20 open-pollinated families of portuguese plus trees of maritime pine. Plant Cell Tissue Org. Cult. 2004, 76, 121–130. [Google Scholar] [CrossRef]


| Medium Type | Function | PGRs |
|---|---|---|
| BM1 | Basal proliferation medium | 0.2 µM 6-BA, 0.4 µM 2,4-D |
| BM2 | Recover proliferation medium | 2.0 µM 6-BA, 4.0 µM 2,4-D |
| BM3 | Regulator-free proliferation medium | none |
| BM4 | Maturated medium | 30 mg/L ABA |
| 1/2MS | Rooting medium | none |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Wang, X.; Zhang, C.; Pak, S.; Wu, H.; Yang, J.; Li, C. Enhancing the Cryopreservation System of Larch Embryogenic Culture by Optimizing Pre-Culture, Osmoprotectants, and Rapid Thawing. Forests 2023, 14, 1621. https://doi.org/10.3390/f14081621
Ma M, Wang X, Zhang C, Pak S, Wu H, Yang J, Li C. Enhancing the Cryopreservation System of Larch Embryogenic Culture by Optimizing Pre-Culture, Osmoprotectants, and Rapid Thawing. Forests. 2023; 14(8):1621. https://doi.org/10.3390/f14081621
Chicago/Turabian StyleMa, Miaomiao, Xuhui Wang, Chunyan Zhang, Solme Pak, Hongran Wu, Jingli Yang, and Chenghao Li. 2023. "Enhancing the Cryopreservation System of Larch Embryogenic Culture by Optimizing Pre-Culture, Osmoprotectants, and Rapid Thawing" Forests 14, no. 8: 1621. https://doi.org/10.3390/f14081621
APA StyleMa, M., Wang, X., Zhang, C., Pak, S., Wu, H., Yang, J., & Li, C. (2023). Enhancing the Cryopreservation System of Larch Embryogenic Culture by Optimizing Pre-Culture, Osmoprotectants, and Rapid Thawing. Forests, 14(8), 1621. https://doi.org/10.3390/f14081621




