Nutritional Status of Wood Melick (Melica uniflora Retz.) in a Natural Forest Stand in South-Western Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Characteristics
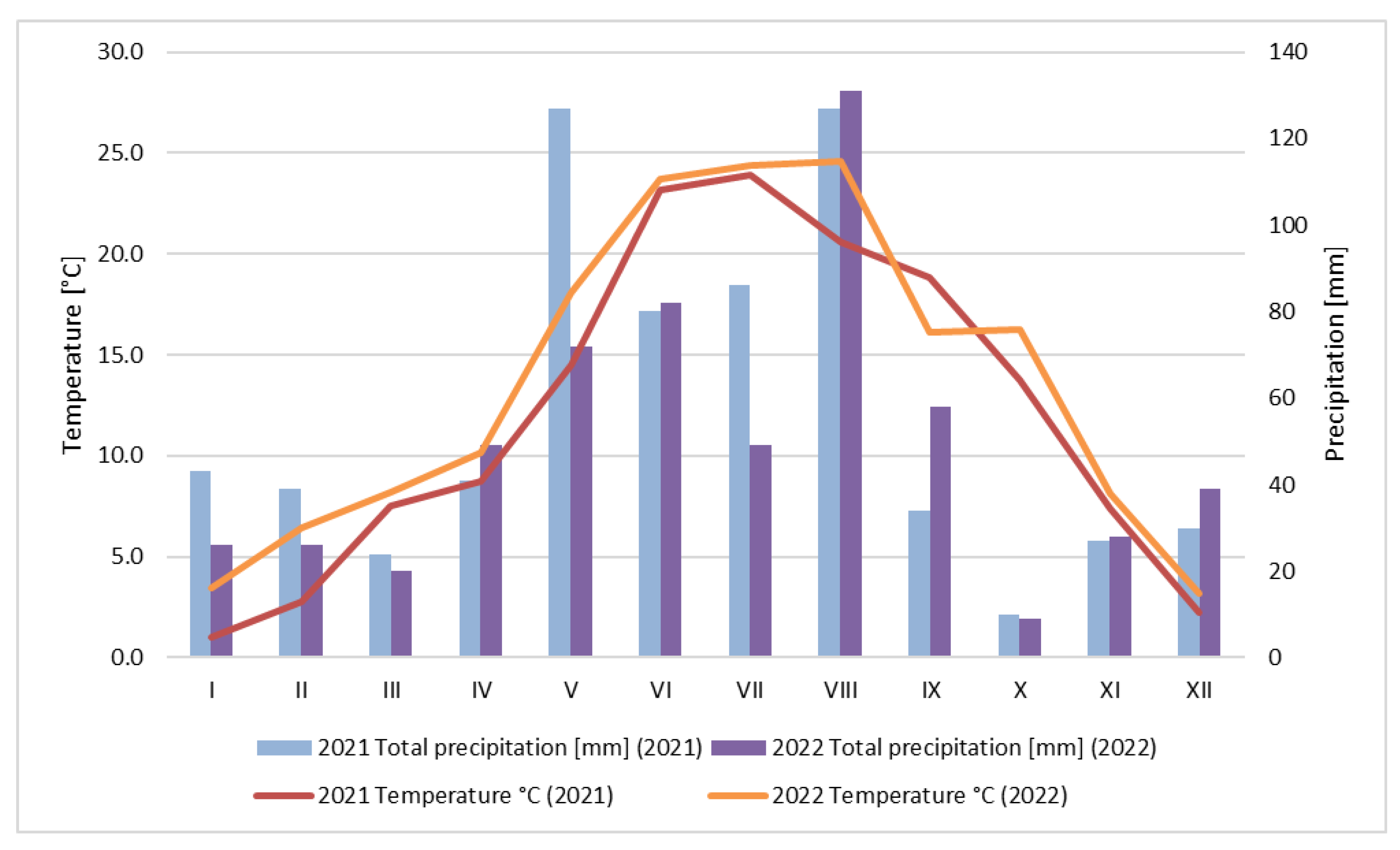
2.1.1. Weather Conditions
2.1.2. Soil Conditions
2.2. Collection and Analysis of Plant Material
2.3. Statistical Analysis
3. Results
3.1. Weather Conditions
3.2. Soil Conditions
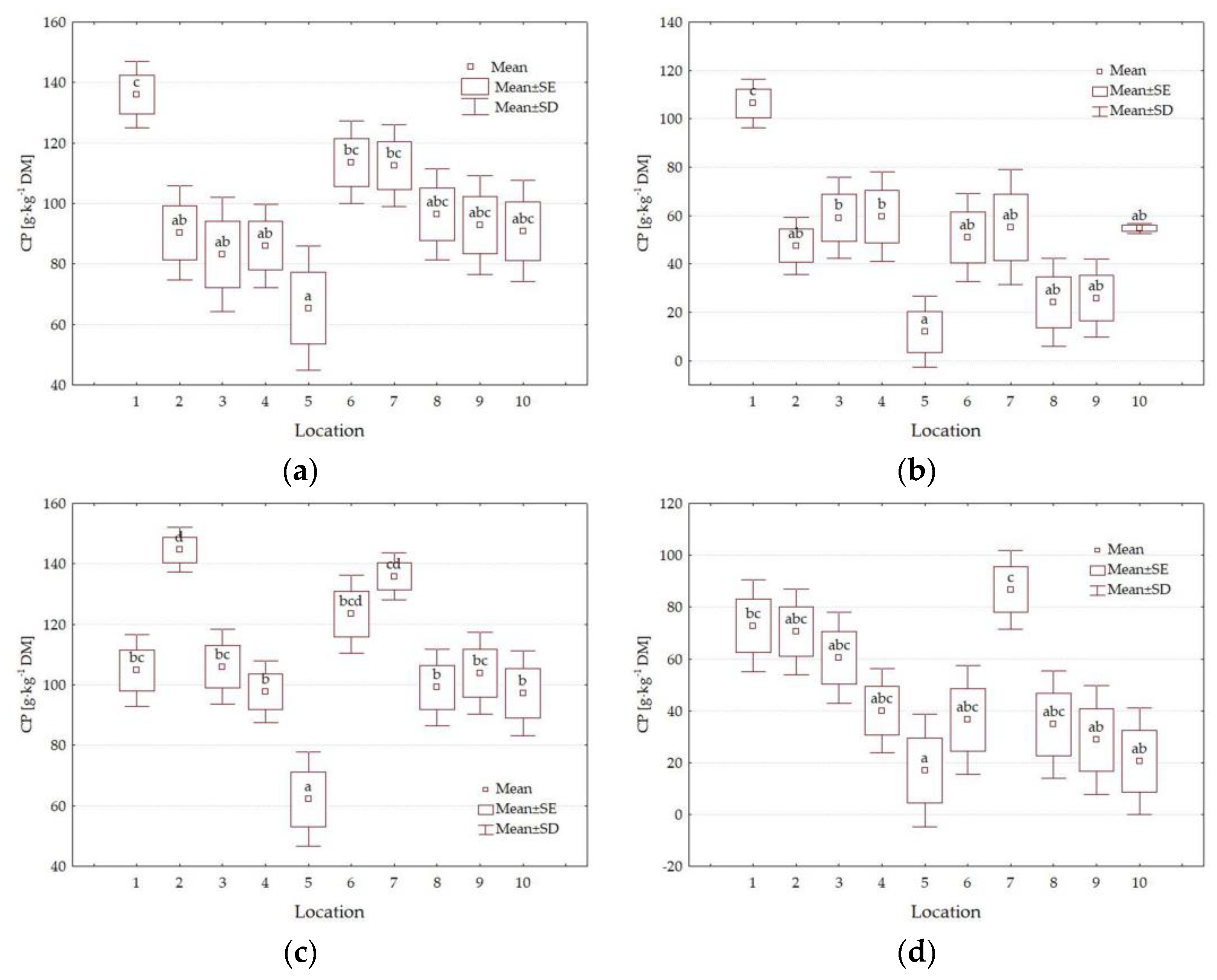
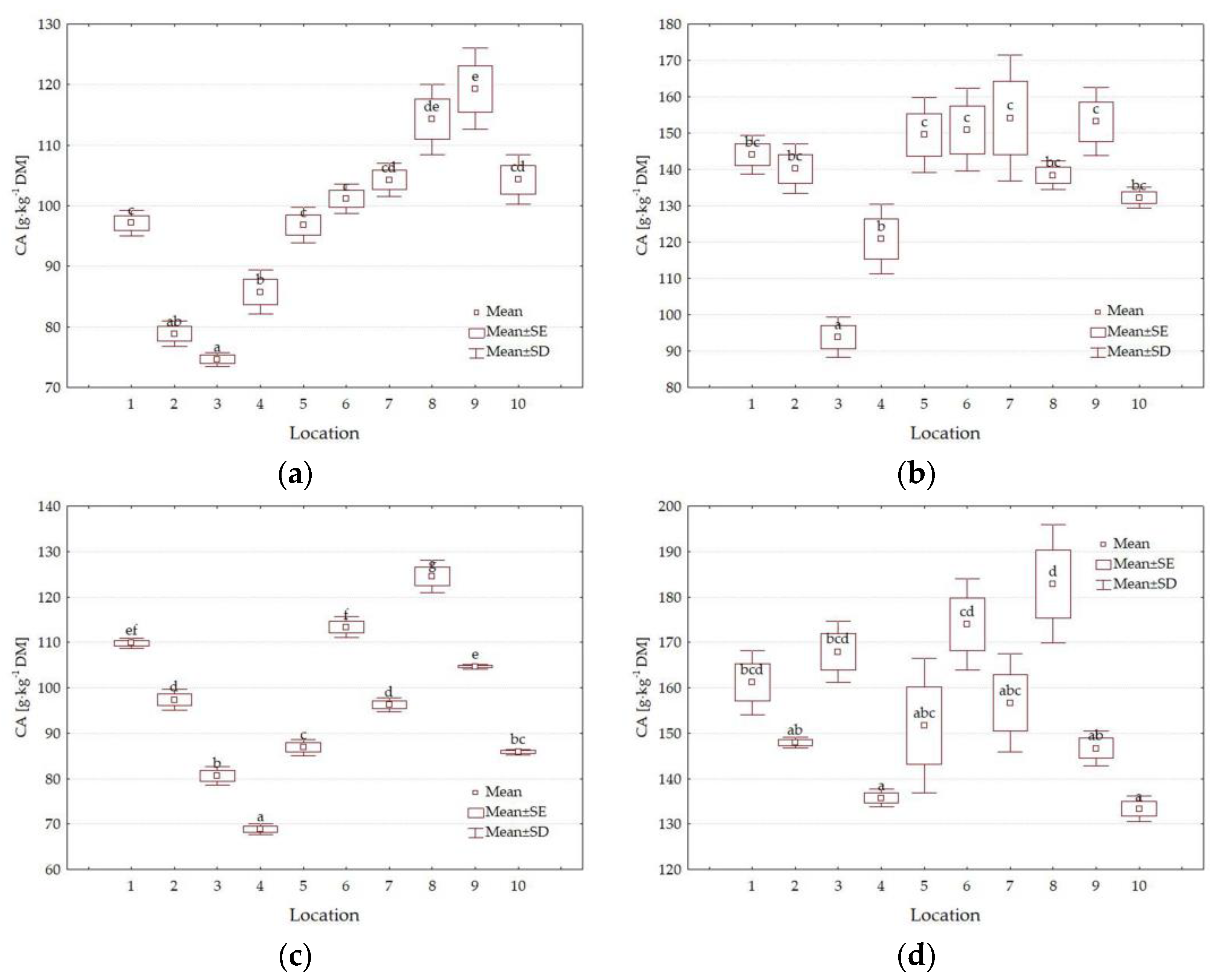
3.3. Chemical Composition
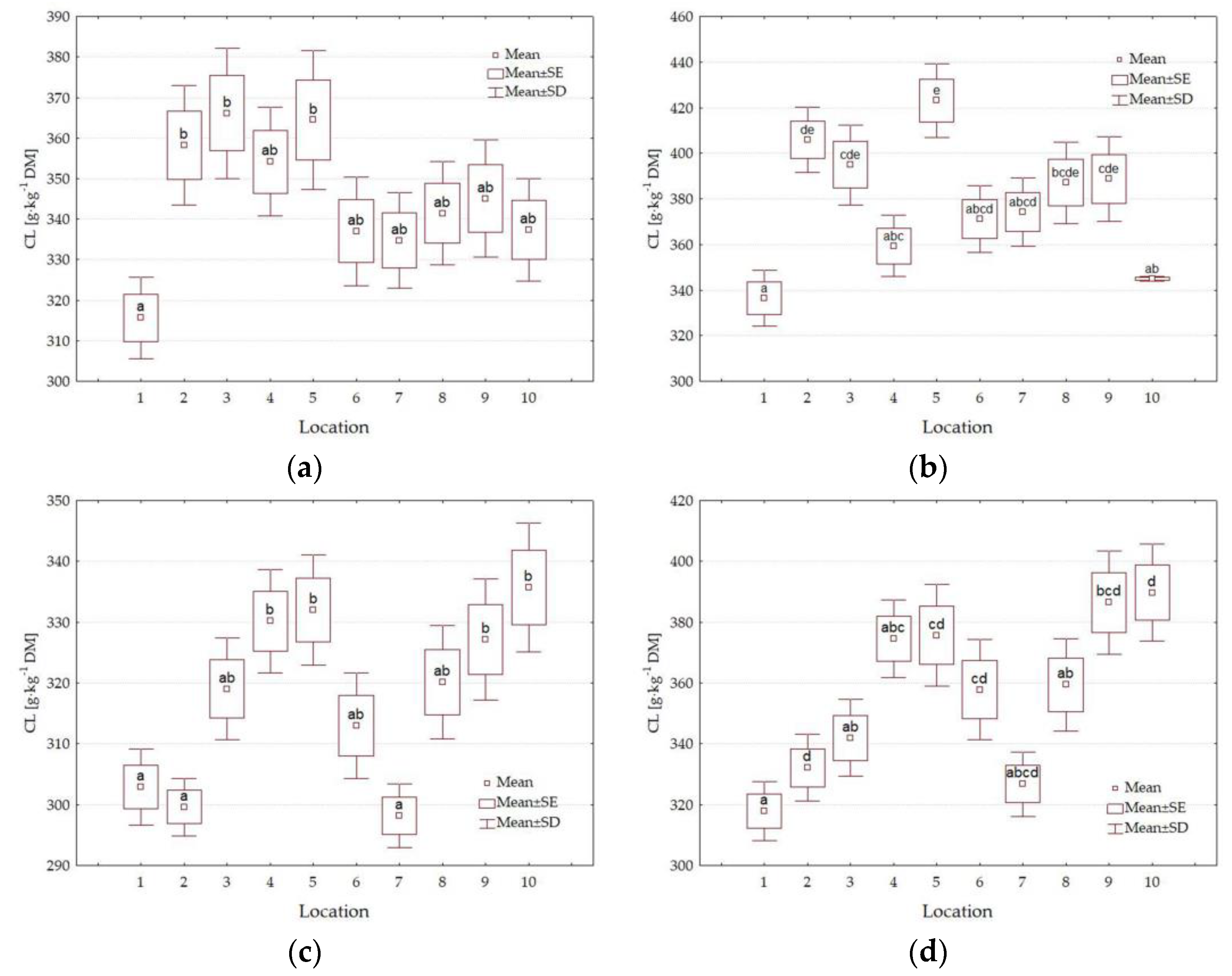
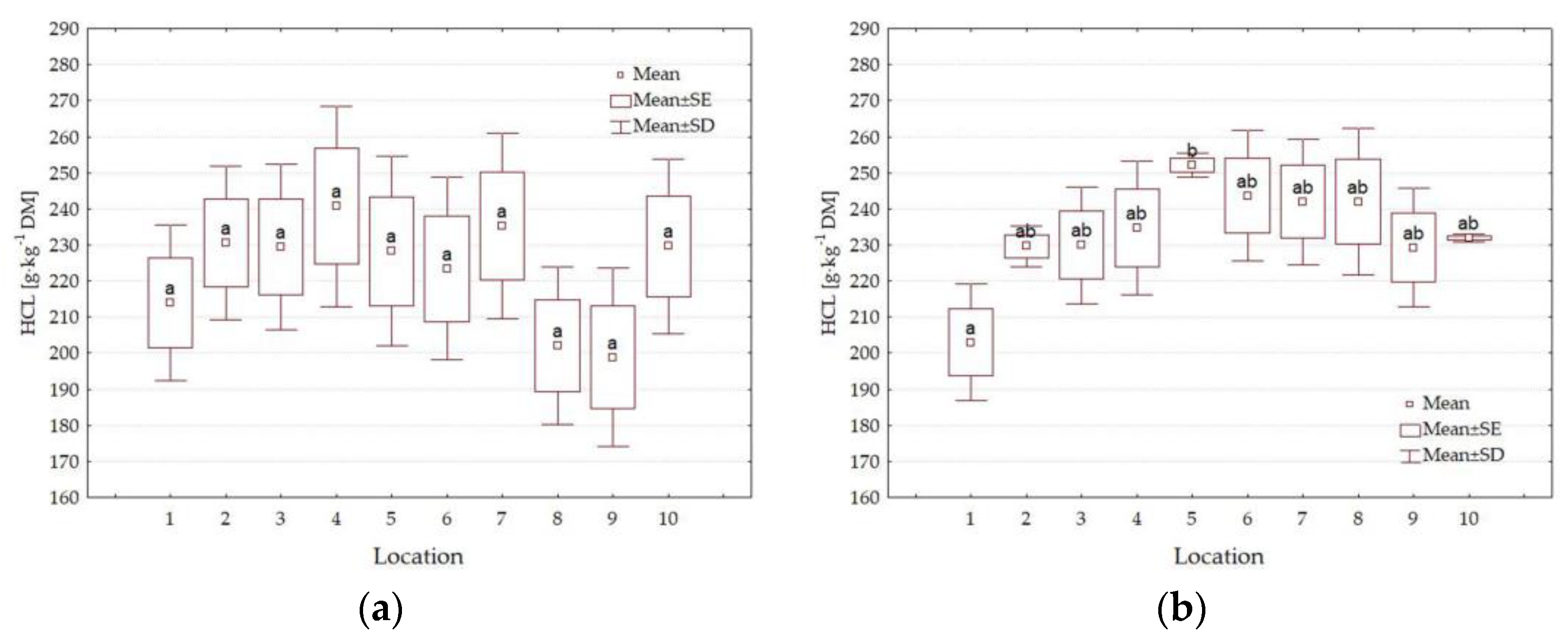
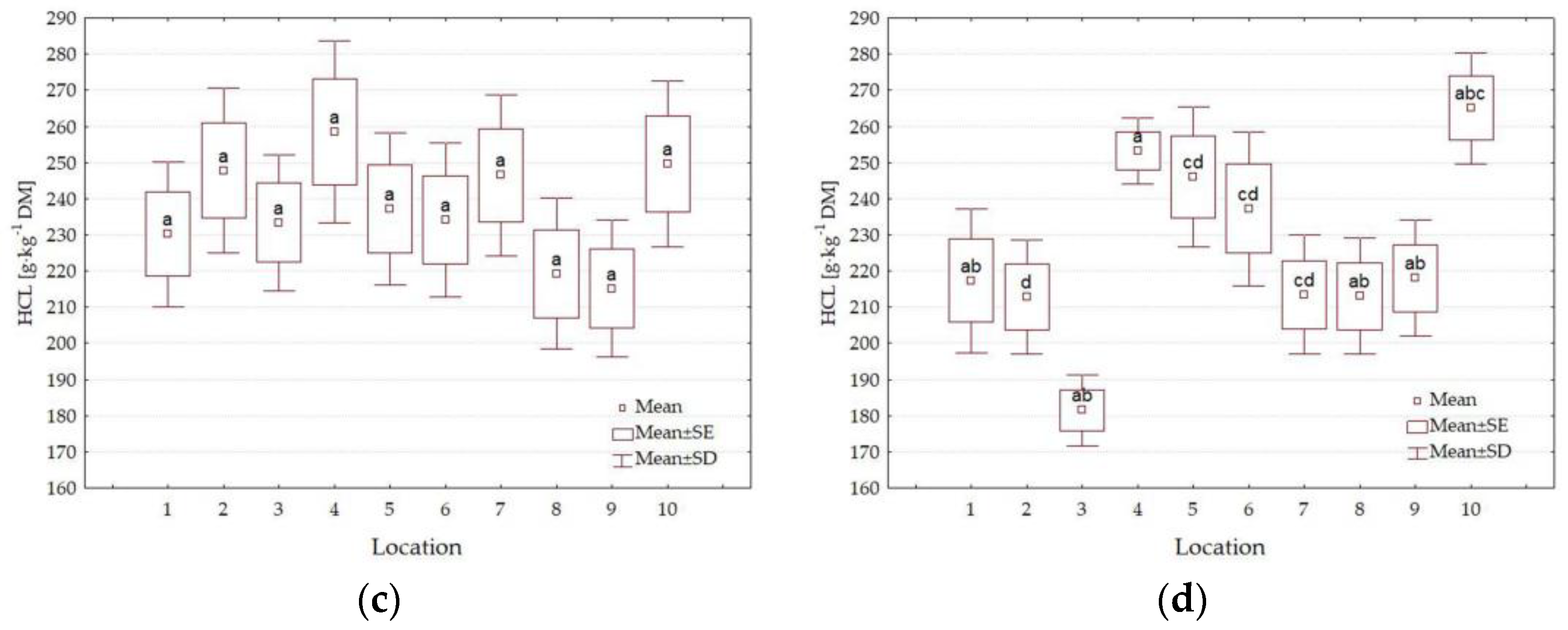
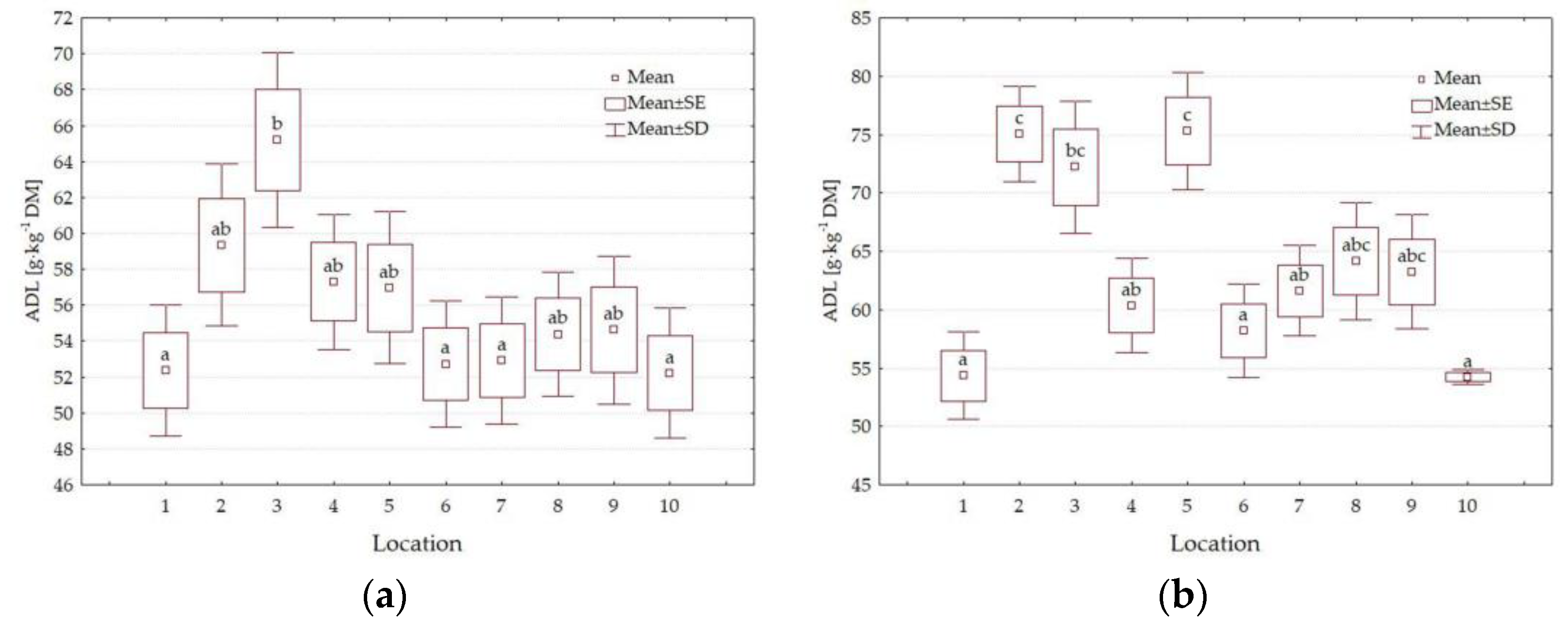
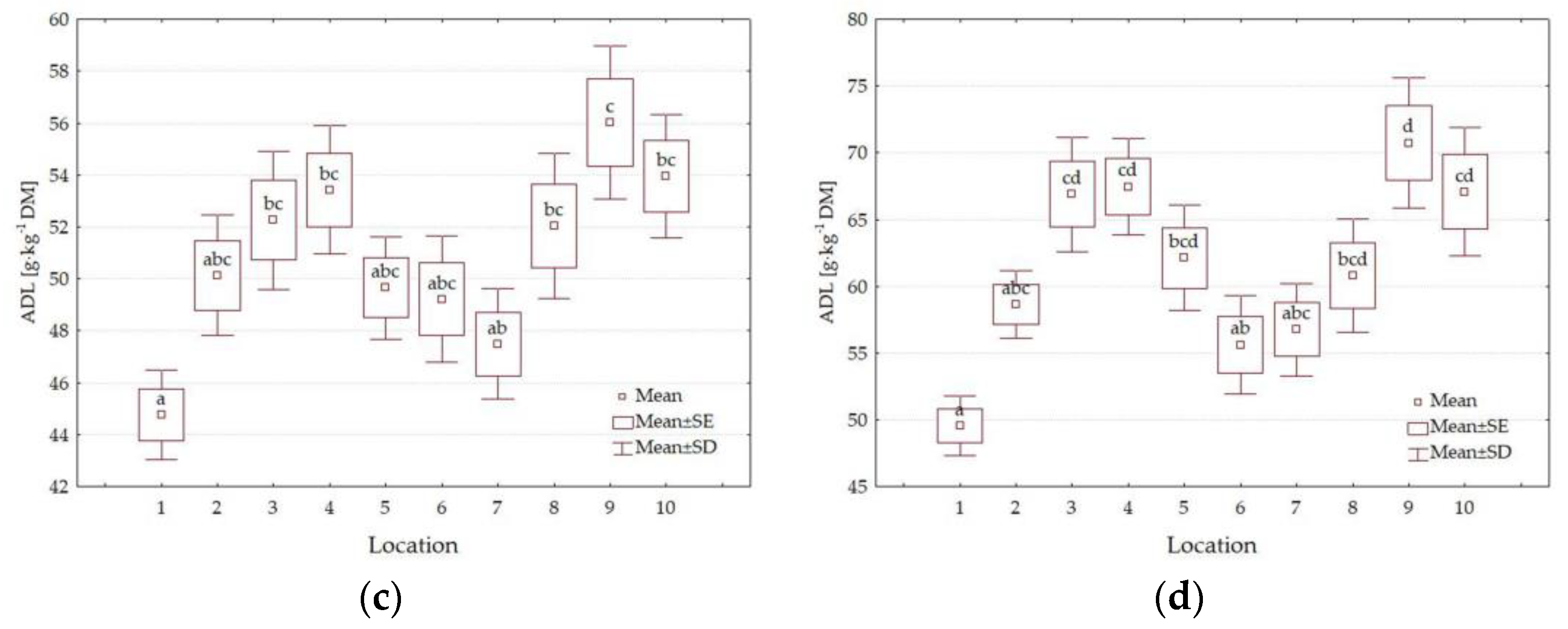
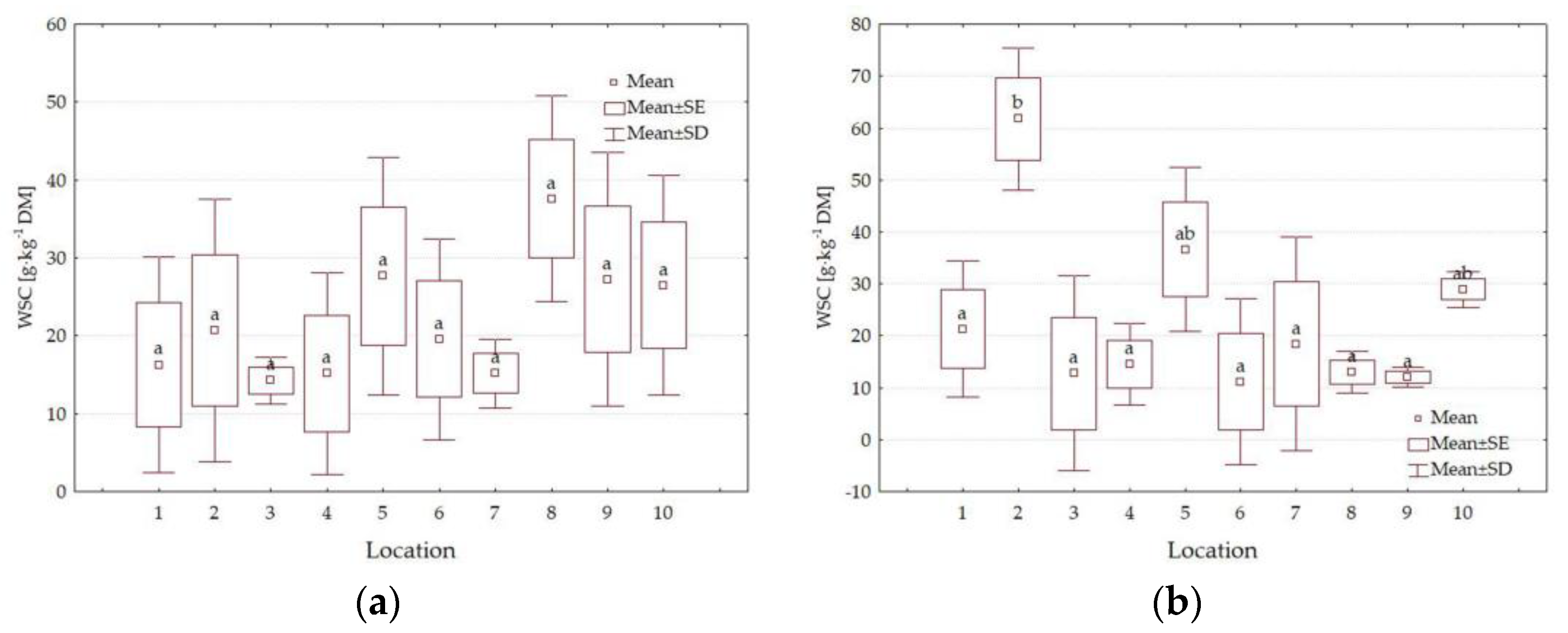
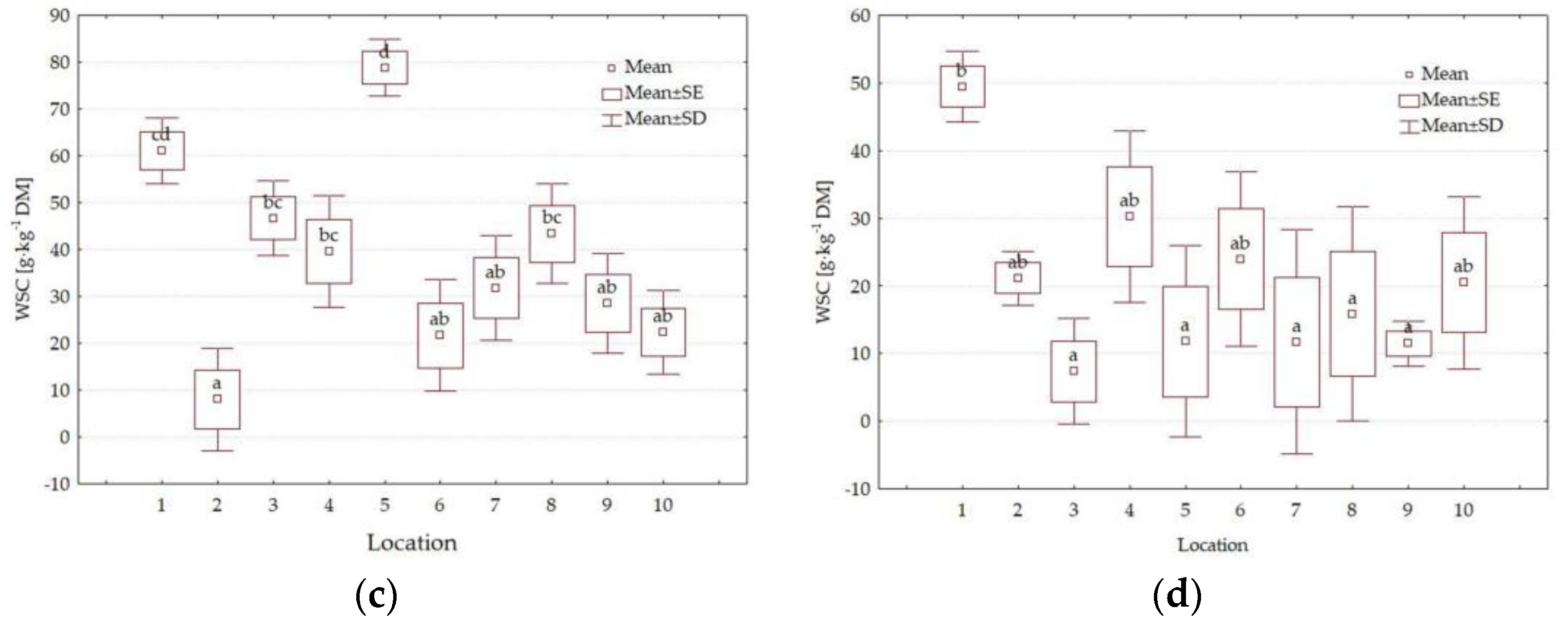
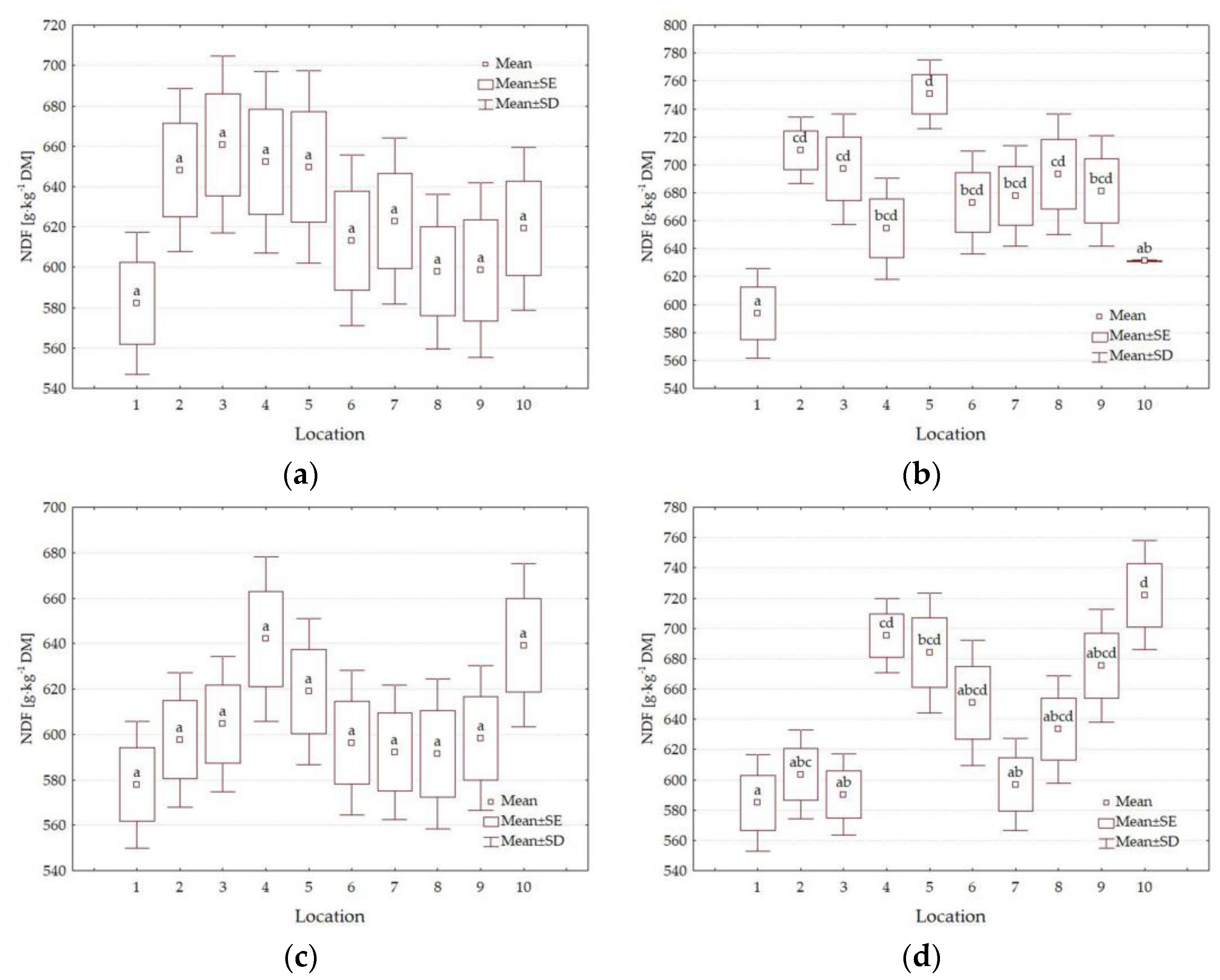
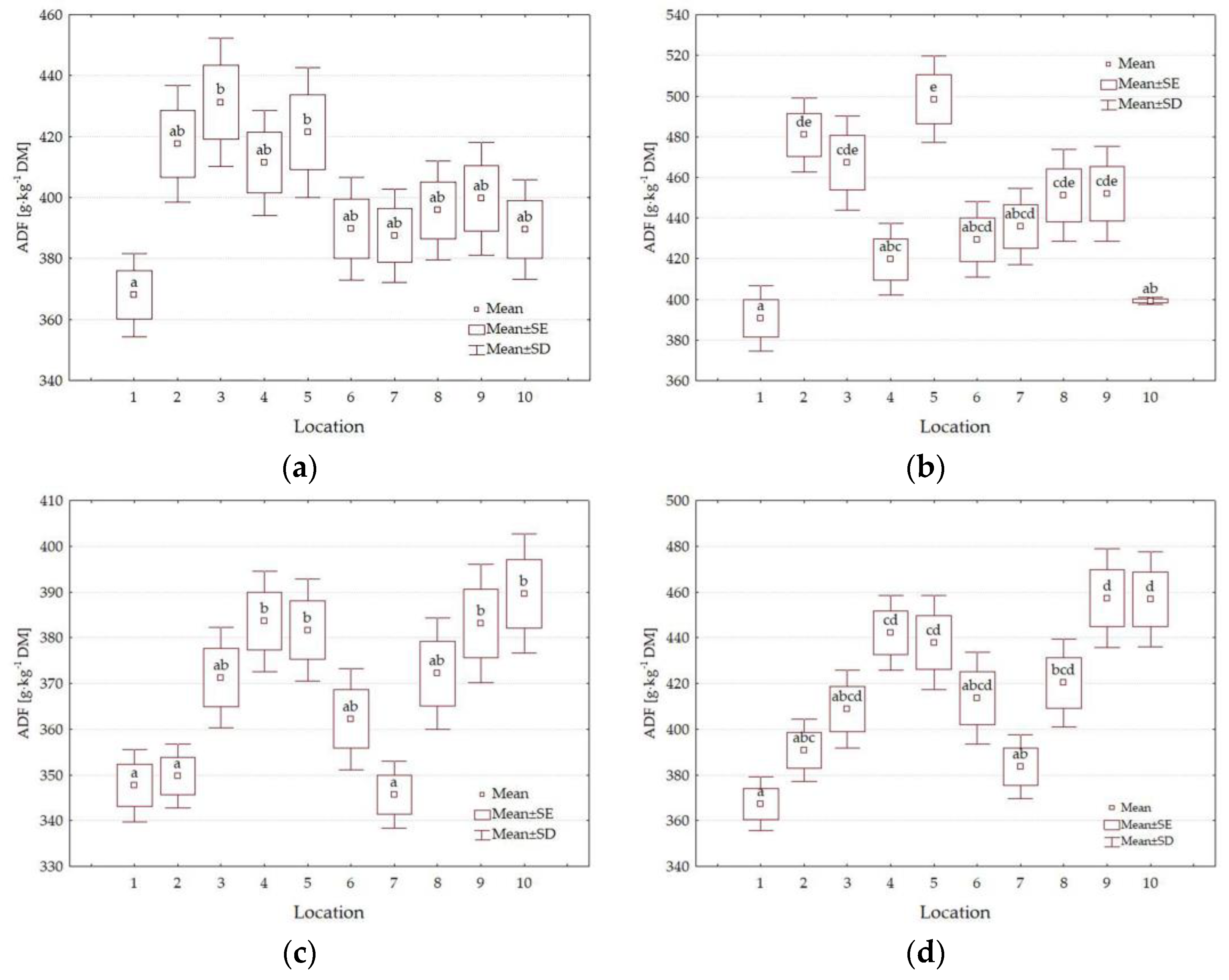
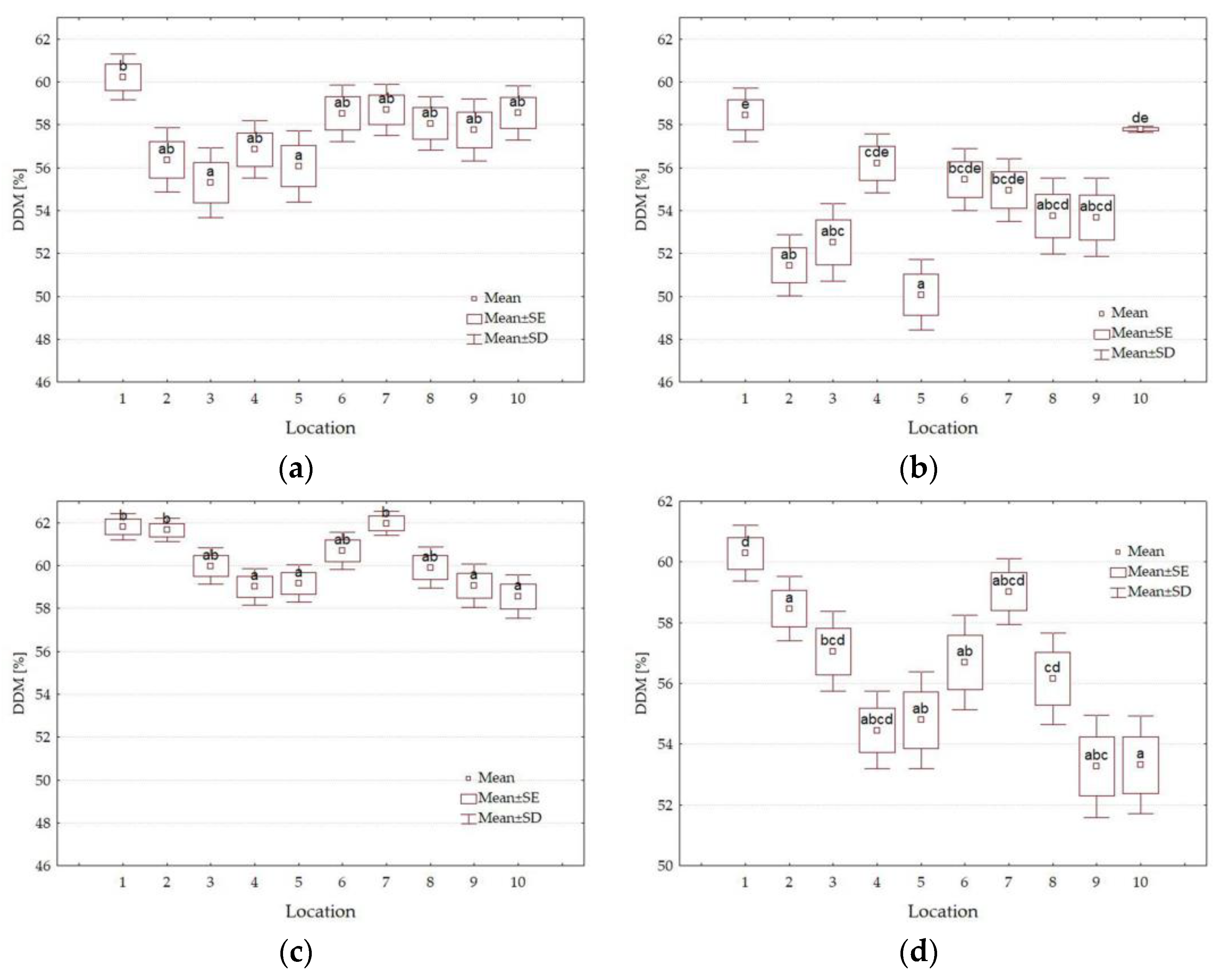
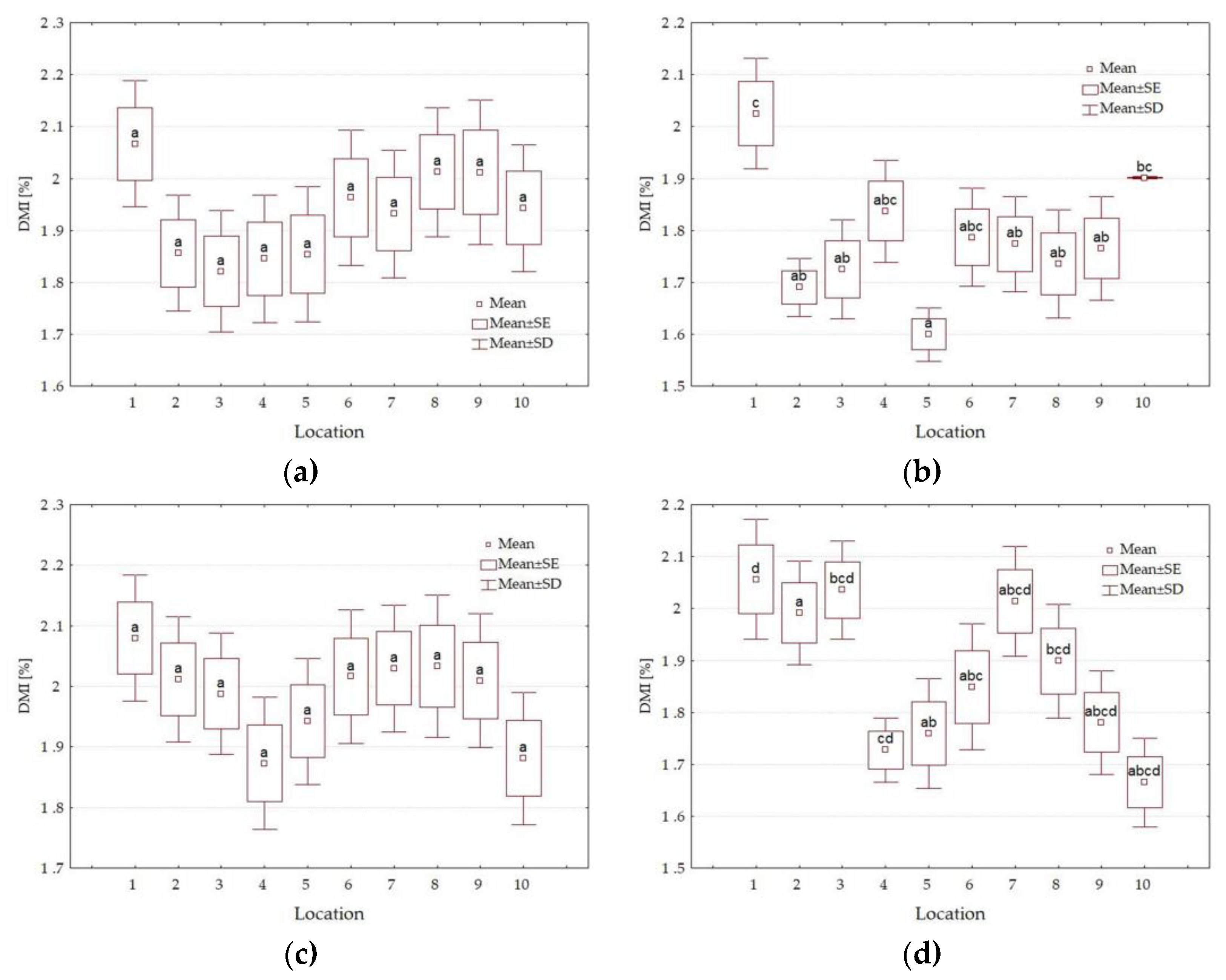
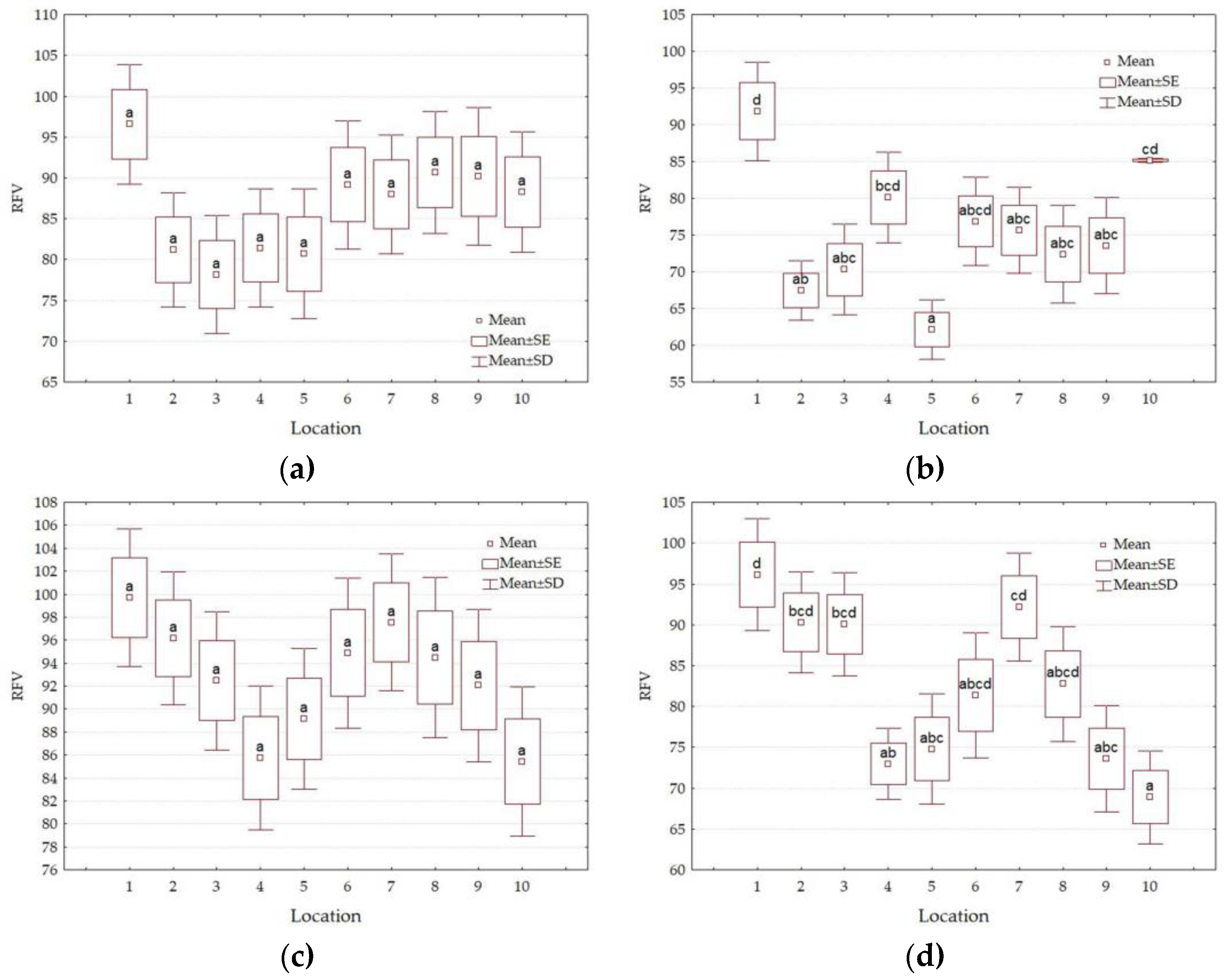
3.4. Forage Quality Parameters
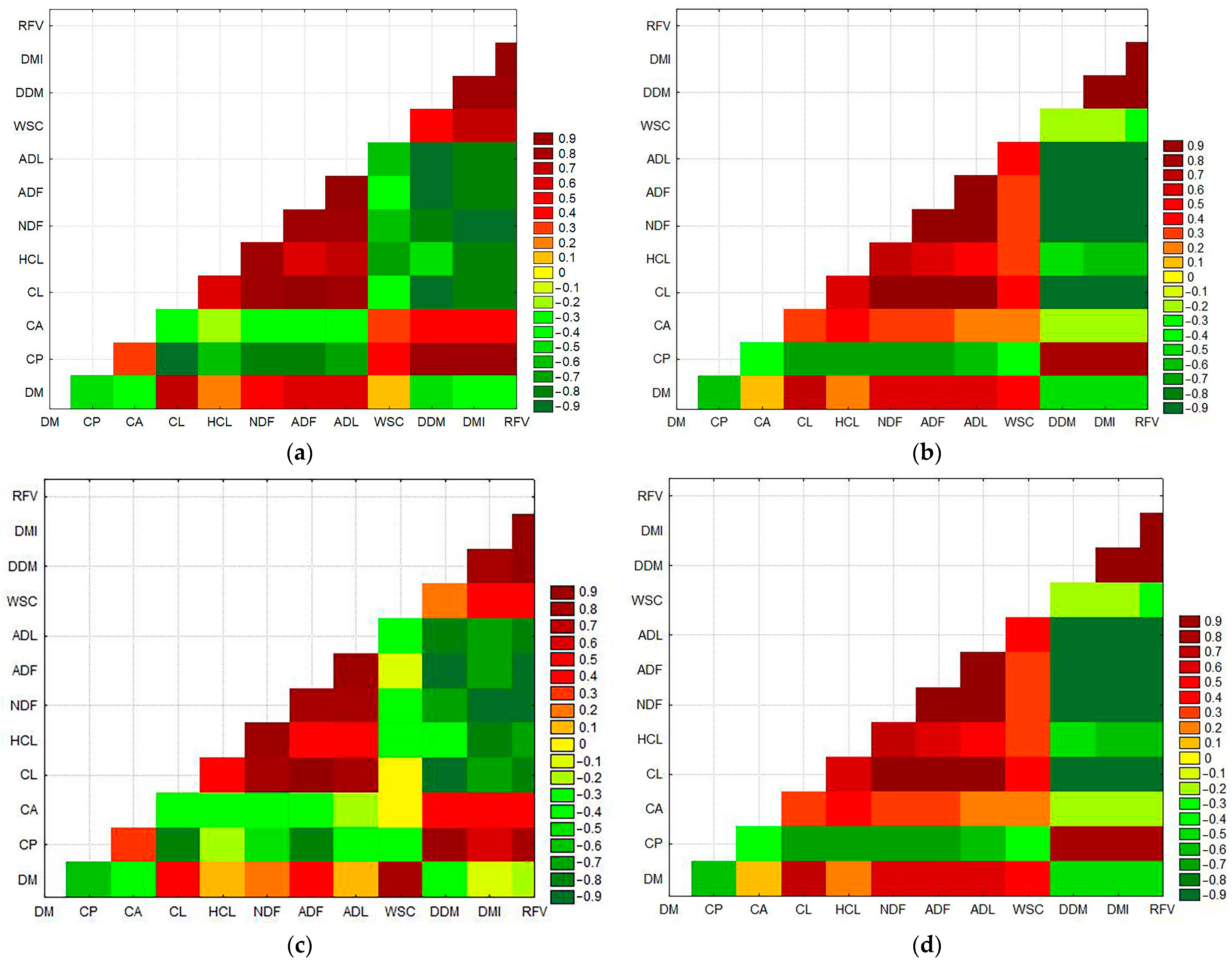
3.5. Correlation between Nutrients
4. Discussion
4.1. Impact of the Study Year
4.2. Impact of Harvest Date
4.3. Impact of Location
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabryszuk, M.; Barszczewski, J.; Wróbel, B. Characteristics of grasslands and their use in Poland. J. Water Land Dev. 2021, 51, 243–249. [Google Scholar]
- Packialakshmi, M.; Palani Divya, M.; Baranidharan, K.; Geetha, S.; Nalliappan Ganesan, K.; Vijayabhama, M.; Manivasakan, S.; Hemalatha, P.; Radha, P.; Tilak, M.; et al. Exploring the Nutritional Potential of Wild Grass Fodder for Mega Herbivore (Elephas maximus) in the Foothills of Western Ghats. Animals 2022, 12, 2668. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.J.H.; Smith, G.; Lindborg, R. Grasslands—More important for ecosystem services than you might think. Ecosphere 2019, 10, e02582. [Google Scholar] [CrossRef]
- Obidziński, A.; Kiełtyk, P.; Borkowski, J.; Bolibok, L.; Remuszko, K. Autumn–winter diet overlap of fallow, red, and roe deer in forest ecosystems, Southern Poland. Open Life Sci. 2013, 8, 8–17. [Google Scholar] [CrossRef]
- Parker, K.L.; Barboza, P.S.; Gillingham, M.P. Nutrition integrates environmental responses of ungulates. Funct. Ecol. 2009, 23, 57–69. [Google Scholar] [CrossRef]
- Zielewicz, W.; Kozłowski, S. Występowanie barwników chlorofilowych i karotenowych w trawach leśnych. Grassl. Sci. Pol. 2011, 14, 161–170. [Google Scholar]
- Bakshi, M.P.S.; Singh, M.P.; Wadhwa, M.; Singh, B. Evaluation of forest grasses as livestock feed. Livest. Res. Rural. Dev. 2005, 17, 127. [Google Scholar]
- Kozłowski, S.; Zielewicz, W.; Swędrzyński, A.; Olejarnik, Ł. Właściwości chemiczne traw leśnych. Grassl. Sci. Pol. 2012, 15, 109–118. [Google Scholar]
- Pontes, L.D.S.; Carrere, P.; Andueza, D.; Louault, F.; Soussana, J.F. Seasonal productivity and nutritive value of temperate grasses found in semi-natural pastures in Europe: Responses to cutting frequency and N supply. Grass Forage Sci. 2007, 62, 485–496. [Google Scholar] [CrossRef]
- Abu-Alrub, I.; Marcum, K.B.; Kabir, N.; Ahmed, A.; Al Hammadi, M. Productivity and nutritional value of four forage grass cultivars compared to Rhodes grass irrigated with saline water. Aust. J. Crop Sci. 2018, 12, 203–209. [Google Scholar] [CrossRef]
- Kuklová, M.; Hniličková, H.; Hnilička, F.; Pivková, I.; Kukla, J. Impact of expressway on physiology of plants and accumulation of risk elements in forest ecosystems. Plant Soil Environ. 2019, 65, 46–53. [Google Scholar] [CrossRef]
- Kuklová, M.; Kukla, J.; Hniličková, H.; Hnilička, F.; Pivková, I. Spatial variability of nutrients in soils and plants of forest ecosystems located near the highway. Environ. Monit. Assess. 2020, 192, 534. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, B.; Zielewicz, W.; Paszkiewicz-Jasińska, A.; Spychalski, B.; Jakubowska, Z. Effect of harvest date on structural carbohydrates and lignin content in meadow sward in different pluvio-thermal conditions. J. Water Land Dev. 2022, 55, 60–66. [Google Scholar]
- Andueza, D.; Picard, F.; Note, P.; Carrère, P. Relationship between the chemical composition, nutritive value and the maturity stage of six temperate perennial grasses during their first growth cycle along an altitude gradient. Eur. J. Agron. 2021, 130, 126364. [Google Scholar] [CrossRef]
- Cyzman, W. Uwarunkowania siedliskowe i gospodarcze kształtowania naturalnych buczyn ze związku Fagion sylvaticae na granicy zasięgu buka zwyczajnego w Polsce północnej i środkowej. Zarządzanie Ochr. Przyr. Lasach 2013, 7, 38–65. [Google Scholar]
- Kazimierczakowa, R.; Zarzycki, K.; Mirek, Z. Polska Czerwona Księga Roślin: Paprotniki i rośliny Kwiatowe; Instytut Ochrony Przyrody Polskiej Akademii Nauk: Krakow, Poland, 2014. [Google Scholar]
- Szczesniak, E.; Krukowski, M.; Podlaska, M. Zanikanie perłówki siedmiogrodzkiej Melica transsilvanica Schur. w Sudetach polskich. Przyr. Sudet. 2018, 21, 9–22. [Google Scholar]
- Szczepaniak, M.; Cieslak, E. Genetic and morphological differentiation between Melica ciliata L. and M. transsilvanica Schur (Poaceae) in Europe reveals the non-presence of M. ciliata in the Polish flora. Acta Soc. Bot. Pol. 2011, 8, 301–313. [Google Scholar] [CrossRef]
- Biblioteka Cyfrowa UMSC (The Maria Curie-Skłodowska University). Available online: http://87.246.207.98/dlibra/docmetadata?id=16598&from=publication (accessed on 3 March 2023).
- Zielewicz, W.; Wróbel, B. Ocena wybranych cech morfologicznych perłówki zwisłej (Melica nutans) i perłówki orzęsionej (Melica ciliata). Grassl. Sci. Pol. 2019, 22, 107–121. [Google Scholar]
- Encyklopedia Leśna. Available online: https://www.encyklopedialesna.pl/haslo/perlowka/ (accessed on 3 March 2023).
- Mirek, Z.; Piękoś-Mirkowa, H. Trawy gór. In Księga Polskich Traw; Frey, L., Ed.; Instytut Botaniki im. W. Szaffera, PAN: Krakow, Poland, 2007; pp. 203–228. [Google Scholar]
- Ádám, R.; Ódor, P.; Bölöni, J. The effects of stand characteristics on the understory vegetation in Quercus petraea and Q. cerris dominated forests. Community Ecol. 2013, 14, 101–109. [Google Scholar] [CrossRef]
- Matuszkiewicz, W. Przewodnik do oznaczania zbiorowisk roślinnych Polski. In Vademecum Geobotanicum; Faliński, J.B., Ed.; Wyd. Nauk. PWN: Warszawa, Poland, 2007; Volume 3. [Google Scholar]
- Falkowski, M. Trawy Polskie; Państwowe Wydawnictwo Rolnicze i Leśne (PWRiL): Warszawa, Poland, 1982. [Google Scholar]
- Badridze, G.; Kacharava, N.; Chkhubianishvili, E.; Rapava, L.; Kikvidze, M.; Chigladze, L.; Chanishvili, S. Content of antioxidants in leaves of some plants of Tbilisi environs. Bull. Georg. Natl. Acad. Sci. 2013, 7, 105–111. [Google Scholar]
- Zielewicz, W.; Wróbel, B.; Niedbała, G. Quantification of chlorophyll and carotene pigments content in mountain melick (Melica nutans L.) in relation to edaphic variables. Forests 2020, 11, 1197. [Google Scholar] [CrossRef]
- Elif, Ç.İ.L.; Türkiş, S.; Ayvaz, M.Ç.; Kasurka, C.B. In vitro biological activity evaluation of ethanolic extract of Melica uniflora leaves. Eskişehir Tek. Üniversitesi Bilim Teknol. Derg.-C Yaşam Bilim. Biyoteknoloji 2021, 10, 17–26. [Google Scholar]
- GUS (Central Office of Statistics). Statistical Yearbook of Forestry. Warszawa, Poland, 2021. Available online: https://stat.gov.pl/files/gfx/portalinformacyjny/pl/defaultaktualnosci/5515/13/4/1/rocznik_sstatystyczn_lesnictwa_2021.pdf (accessed on 4 March 2023).
- Kondracki, J. Geografia Fizyczna Polski; Wyd. Nauk. PWN: Warszawa, Poland, 2000. [Google Scholar]
- Majerowicz, A. Textural features and symptoms of ocean floor metamorphism in the top part of the Sleza ophiolite (SW Poland). Arch. Mineral. 1994, 50, 97–140. [Google Scholar]
- Majerowicz, A. Krótki Przewodnik Terenowy po Skałach Ofiolitowego Zespołu Ślęży Oraz Ich Petrologicznej i Geologicznej Historii; Wydawnictwo Uniwersytetu Wrocławskiego: Wrocław, Poland, 2006. [Google Scholar]
- Majerowicz, A.; Pin, C. The main petrological problems of the Mt. Sleza ophiolite complex, Sudetes, Poland. Zentralblatt Geol. Paläontologie 1992, 1, 898–1018. [Google Scholar]
- Pin, C.; Majerowicz, A.; Wojciechowska, I. Upper Paleozoic oceanic crust in the Polish Sudetes: Nd Sr isotope and trace element evidence. Lithos 1988, 21, 195–209. [Google Scholar] [CrossRef]
- Badach, B.; Orczewska, A.; Cabala, S. Struktura i regeneracja drzewostanów w zespole przyrodniczo-krajobrazowym Uroczysko Buczyna w Chorzowie [Gorny Slask]. Rocz. Dendrol. 2004, 52, 55–73. [Google Scholar]
- Kubiak, D.; Szczepkowski, A. Lichens of the Rogów Forests of Warsaw University of Life Sciences (2): Bukowiec and Kwaśna Buczyna reserves. Ann. WULS–SGGW For. Wood Technol. 2009, 67, 168–175. [Google Scholar]
- Świerkosz, K. Siedliska wymagające tworzenia specjalnych obszarów ochrony na Dolnym Śląsku. In Opracowanie Ekofizjograficzne dla Województwa Dolnośląskiego; Wojewódzkie Biuro Urbanistyczne we Wrocławiu: Wrocław, Poland, 2005; pp. 183–194. [Google Scholar]
- OpenWeather. Available online: https://openweathermap.org/ (accessed on 6 March 2023).
- Selyaninov, G.T. Methods of Agricultural Climatology. Agric. Meteorol. 1930, 22L, 4–20. [Google Scholar]
- PN-EN ISO 10390:2022-09; PolishStandard: Soil, Treated Biowaste and Sludge—Determination of pH. Polish Committee for Standardizations: Warsaw, Poland, 2022. (In English)
- Küchler, A.W. Prosta metoda kartowania roślinności. In Annals of the Association of American Geographers; IGSO PAS: Warsaw, Poland, 1955. [Google Scholar]
- Linn, J.G.; Martin, N.P. Forage Quality Tests and Interpretations (Revised 1989); University Minnesota, Agricultural Extension Service: Minneapolis, MN, USA, 1989; Available online: https://conservancy.umn.edu/handle/11299/207442 (accessed on 10 March 2023).
- Kozłowski, S.; Goliński, P.; Golińska, B. Barwniki chlorofilowe jako wskaźniki wartości użytkowej gatunków i odmian traw. Zesz. Probl. Post. Nauk. Rol. 2001, 474, 215–223. [Google Scholar]
- Schlegel, P.; Wyss, U.; Arrigo, Y.; Hess, H.D. Mineral concentrations of fresh herbage from mixed grassland as influenced by botanical composition, harvest time and growth stage. Anim. Feed. Sci. Technol. 2016, 219, 226–233. [Google Scholar] [CrossRef]
- Reiné, R.; Ascaso, J.; Barrantes, O. Nutritional Quality of Plant Species in Pyrenean Hay Meadows of High Diversity. Agronomy 2020, 10, 883. [Google Scholar] [CrossRef]
- Kagan, I.A. Water- and ethanol-soluble carbohydrates of temperate grass pastures: A review of factors affecting concentration and composition. J. Equine Vet. Sci. 2022, 110, 103866. [Google Scholar] [CrossRef] [PubMed]
- Niyigena, V.; Ashworth, A.J.; Nieman, C.; Acharya, M.; Coffey, K.P.; Philipp, D.; Meadors, L.; Sauer, T.J. Factors Affecting Sugar Accumulation and Fluxes in Warm- and Cool-Season Forages Grown in a Silvopastoral System. Agronomy 2021, 11, 354. [Google Scholar] [CrossRef]
- Jafari, A.A. Environmental and genetic variation for water soluble carbohydrate content in cool season forage grasses. In Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology; IntechOpen: Rijeka, Croatia, 2012; pp. 373–388. [Google Scholar]
- Dobrowolska, D. Normy Żywienia Bydła, Owiec i Kóz. Wartość Pokarmowa Pasz dla Przeżuwaczy; Wydanie II; Instytut Zootechniki: Krakow, Poland, 1997. [Google Scholar]
- Buxton, D.R. Quality-related characteristics of forages as influenced by plant environment and agronomic factors. Anim. Feed. Sci. Technol. 1996, 59, 37–49. [Google Scholar] [CrossRef]
- Rinne, M.; Nykanen, A. Timing of primary growth harvest affects the yield and nutritive value of timothy-red clover mixtures. Agric. Food Sci. Finl. 2000, 9, 121–134. [Google Scholar] [CrossRef]
- Buxton, D.R.; Brasche, M.R. Digestibility of structural carbohydrates in cool-season grass and legume forages. Crop Sci. 1991, 31, 1338–1345. [Google Scholar] [CrossRef]
- Kuoppala, K.; Ahvenjarvi, S.; Rinne, M.; Vanhatalo, A. Effects of feeding grass or red clover silage cut at two maturity stages in dairy cows. P. 2. Dry matter intake and cell wall digestion kinetics. J. Dairy Sci. 2009, 92, 5634–5644. [Google Scholar] [CrossRef]
- Stopa, W.; Wróbel, B.; Paszkiewicz-Jasińska, A.; Zielewicz, W. The effect of the timing of harvesting the first regrowth of the swardon relative feed value. J. Res. Appl. Agric. Eng. 2022, 67, 39–44. [Google Scholar]
- Jankowska-Huflejt, H.; Wróbel, B. Ocena przydatności pasz z użytków zielonych do produkcji zwierzęcej w badanych gospodarstwach ekologicznych. J. Res. Appl. Agric. Eng. 2008, 54, 103–108. [Google Scholar]
- Truba, M.; Sosnowski, J. The effect of Tytanit on fibre fraction content in Medicago x varia T. Martyn and Trifolium pratense L. cell walls. Agriculture 2022, 12, 191. [Google Scholar] [CrossRef]
- Truba, M.; Jankowski, K.; Wiśniewska-Kadżajan, B.; Sosnowski, J.; Malinowska, E. The effect of soil conditioners on the quality of selected forage grasses. Appl. Ecol. Environ. Res. 2020, 18, 5123–5133. [Google Scholar] [CrossRef]
- McDonald, I.; Baral, R.; Min, D. Effects of alfalfa and alfalfa-grass mixtures with nitrogen fertilization on dry matter yield and forage nutritive value. J. Anim. Sci. Technol. 2021, 63, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Dumont, B.; Andueza, D.; Niderkorn, V.; Luscher, A.; Porqueddu, C.; Picon-Cochard, C. A meta-analysis of climate change effects on forage quality in grasslands: Specificities of mountain and Mediterranean areas. Grass Forage Sci. 2015, 70, 239–254. [Google Scholar] [CrossRef]
- Lascano, C.E.; Schmidt, A.; Barahona, R. Forage Quality and the Environment; University of Kentucky: Lexington, KY, USA, 2021. [Google Scholar]
- Peterson, P.R.; Sheaffer, C.C.; Hall, M.H. Drought effects on perennial forage legume yield and quality. Agron. J. 1992, 84, 774–779. [Google Scholar] [CrossRef]
- Catunda, K.L.; Churchill, A.C.; Power, S.A.; Zhang, H.; Fuller, K.J.; Moore, B.D. Plant structural and nutritional responses to drought differ among common pasture species. bioRxiv 2021. [Google Scholar] [CrossRef]
- Halim, R.A.; Buxton, D.R.; Hattendorf, M.J.; Carlson, R.E. Water-deficit effects on alfalfa at various growth stages. Agron. J. 1989, 81, 765–770. [Google Scholar] [CrossRef]
- Borawska-Jarmułowicz, B.; Mastalerczuk, G.; Janicka, M.; Wróbel, B. Effect of Silicon-Containing Fertilizers on the Nutritional Value of Grass–Legume Mixtures on Temporary Grasslands. Agriculture 2022, 12, 145. [Google Scholar] [CrossRef]
- Fariaszewska, A.; Aper, J.; Van Huylenbroeck, J.; De Swaef, T.; Baert, J.; Pecio, Ł. Physiological and Biochemical Responses of Forage Grass Varieties to Mild Drought Stress Under Field Conditions. Int. J. Plant Prod. 2020, 14, 335–353. [Google Scholar] [CrossRef]
- Küchenmeister, K.; Küchenmeister, F.; Kayser, M.; Wrange-Monning, N.; Isselstein, J. Influence of drought stress on nutritive value of perennial forage legumes. Int. J. Plant Prod. 2013, 7, 693–710. [Google Scholar]
- Turner, L.R.; Holloway-Phillips, M.M.; Rawnsley, R.P.; Donaghy, D.J.; Pembleton, K.G. The morphological and physiological responses of perennial ryegrass (Lolium perenne L.), cocksfoot (Dactylis glomerata L.) and tall fescue (Festuca arundinacea Schreb. syn. Schedonorus phoenix Scop.) to variable water availability. Grass Forage Sci. 2012, 67, 507–518. [Google Scholar]
- Elgersma, A.; Søegaard, K. Changes in nutritive value and herbage yield during extended growth intervals in grass legume mixtures: Effects of species, maturity at harvest, and relationships between productivity and components of feed quality. Grass Forage Sci. 2018, 73, 78–93. [Google Scholar] [CrossRef]
- Fychan, R.; Sanderson, R.; Marley, C. Effects of harvesting red clover/ryegrass at different stage of maturity on forage yield and quality. Grassl. Sci. Eur. 2016, 21, 323–325. [Google Scholar]
- Barnes, R.F.; Nelson, C.J.; Collins, M.; Moore, K.J. (Eds.) Forages, Volume 1: An Introduction to Grassland Agriculture, 6th ed.; Wiley-Blackwell: Ames, IA, USA, 2003. [Google Scholar]
- Minson, D.J. Forage in Ruminant Nutrition; Academic Press Inc.: New York, NY, USA, 1990; pp. 150–161. [Google Scholar]
- Schjoerring, J.K.; Cakmak, I.; White, P.J. Plant nutrition and soil fertility: Synergies for acquiring global green growth and sustainable development. Plant Soil 2019, 434, 1–6. [Google Scholar] [CrossRef]
- Da Silveira Pontes, L.; Maire, V.; Schellberg, J.; Louault, F. Grass strategies and grassland community responses to environmental drivers: A review. Agron. Sustain. Dev. 2015, 35, 1297–1318. [Google Scholar] [CrossRef]
- Gommers, C.M.M.; Visser, E.J.W.; Onge, K.R.S.; Voesenek, L.A.C.J.; Ronald, P. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 1360–1385. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef] [PubMed]
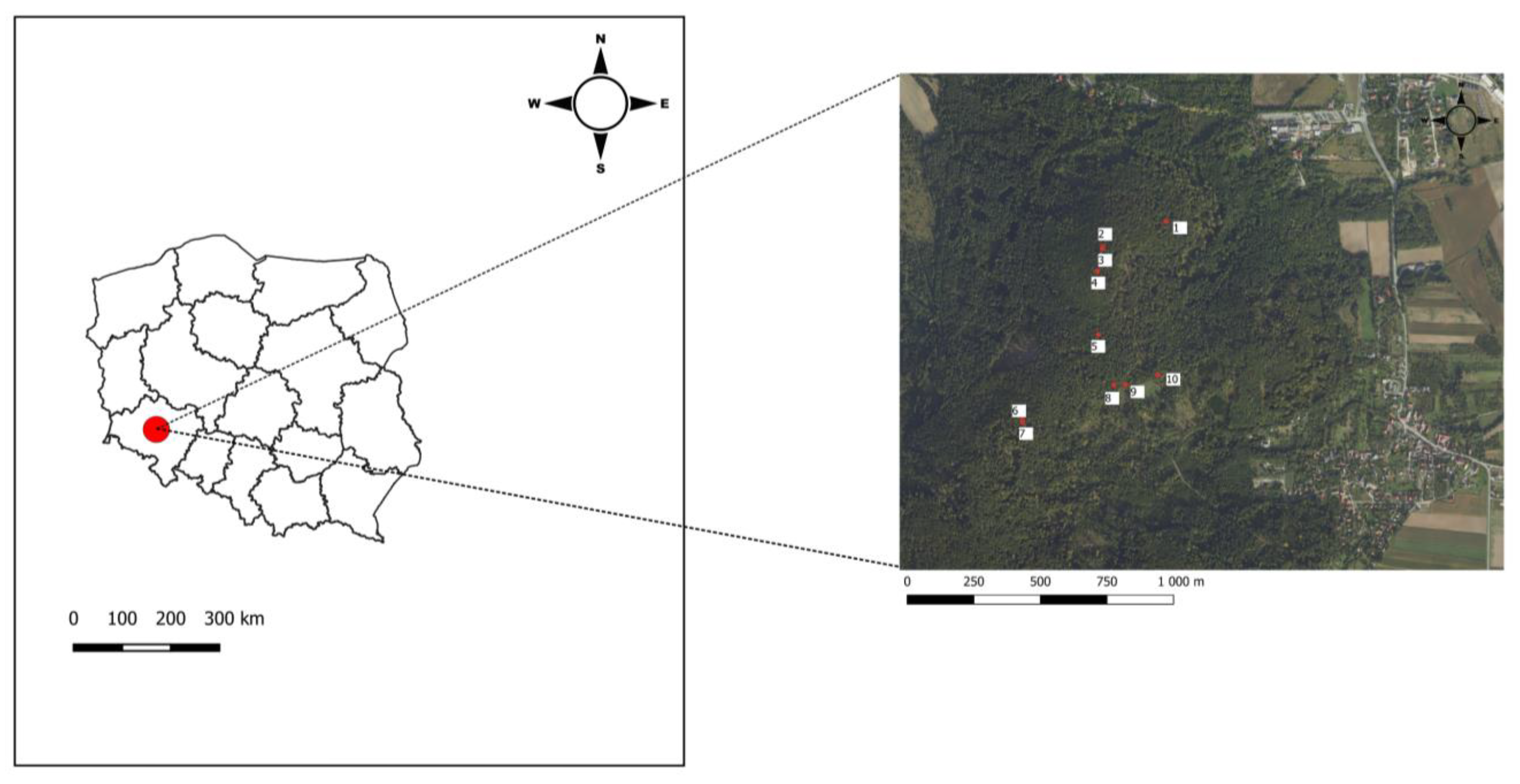
| Site No. | Geographical Coordinates | Altitude | Exposition | Location |
|---|---|---|---|---|
| M | ||||
| 1 | 50°53′16.7″ N 16°43′56.5″ E | 335 | N-E | Mount Wieżyca (another name for Mount Kościuszko) |
| 2 | 50°53′13.3″ N 16°43′44.4″ E | 411 | N | Mount Wieżyca |
| 3 | 50°53′13.1″ N 16°43′44.4″ E | 413 | N | Mount Wieżyca |
| 4 | 50°53′10.3″ N 16°43′43.5″ E | 414 | S | Mount Wieżyca |
| 5 | 50°53′02.5″ N 16°43′44.1″ E | 381 | N | Mount Bartoszek |
| 6 | 50°52′51.7″ N 16°43′30.0″ E | 389 | S-W | Mount Bartoszek |
| 7 | 50°52′51.4″ N 16°43′30.1″ E | 390 | S-W | Mount Bartoszek |
| 8 | 50°52′56.3″ N 16°43′47.4″ E | 368 | S-E | Mount Bartoszek |
| 9 | 50°52′′56.5″ N 16°43′49.6″ E | 367 | S-E | Mount Bartoszek |
| 10 | 50°52′57.7″ N 16°43′55.8″ E | 358 | S-E | Mount Bartoszek |
| Year/Month | IV | V | VI | VII | VIII | IX | X |
|---|---|---|---|---|---|---|---|
| 2021 | 1.6 | 2.8 | 1.1 | 1.2 | 2.0 | 0.6 | 0.2 |
| 2022 | 1.6 | 1.3 | 1.2 | 0.6 | 1.7 | 1.2 | 0.2 |
| Site No. | Soil Reaction | N | P | K | Mg | Ca |
|---|---|---|---|---|---|---|
| pH | g·100 g−1 Soil | g·100 g−1 Soil | g·100 g−1 Soil | g·100 g−1 Soil | g·100 g−1 Soil | |
| 1 | 3.6 | 0.507 | 0.016 | 0.005 | 0.013 | 0.071 |
| 2 | 4.9 | 0.678 | 0.030 | 0.005 | 0.047 | 0.334 |
| 3 | 3.8 | 0.971 | 0.010 | 0.004 | 0.016 | 0.305 |
| 4 | 4.1 | 0.332 | 0.021 | 0.003 | 0.017 | 0.044 |
| 5 | 3.9 | 0.635 | 0.020 | 0.004 | 0.020 | 0.049 |
| 6 | 2.4 | 0.727 | 0.027 | 0.005 | 0.007 | 0.019 |
| 7 | 3.5 | 0.724 | 0.038 | 0.004 | 0.011 | 0.085 |
| 8 | 5.8 | 0.764 | 0.014 | 0.004 | 0.033 | 0.379 |
| 9 | 6.1 | 0.501 | 0.014 | 0.003 | 0.037 | 0.430 |
| 10 | 3.9 | 0.209 | 0.011 | 0.003 | 0.016 | 0.019 |
| Factors | |||||||
|---|---|---|---|---|---|---|---|
| Item | Y | HT | L | Y*HT | HT*L | Y*L | Y*HT*L |
| p-value | |||||||
| DM g·kg−1 FM | 0.043 | <0.001 | 0.054 | <0.001 | <0.001 | 0.153 | <0.001 |
| CP g·kg−1 DM | 0.563 | <0.001 | <0.001 | <0.001 | <0.001 | 0.005 | <0.001 |
| CA g·kg−1 DM | 0.124 | <0.001 | 0.026 | <0.001 | <0.001 | 0.054 | <0.001 |
| CL g·kg−1 DM | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 |
| HCL g·kg−1 DM | 0.495 | 0.926 | <0.001 | 0.069 | 0.001 | 0.002 | <0.001 |
| ADL g·kg−1 DM | 0.014 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| WSC g·kg−1 DM | 0.048 | 0.013 | 0.048 | <0.001 | <0.001 | <0.001 | <0.001 |
| NDF g·kg−1 DM | 0.007 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 |
| ADF g·kg−1 DM | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 |
| DDM % of DM | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 |
| DMI % of body weight | 0.007 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 |
| RFV | 0.001 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 |
| Factors | Level of Factors | DM | CP | CA | CL | HCL | ADL | WSC |
|---|---|---|---|---|---|---|---|---|
| g·kg−1 FM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | ||
| Y | 2021 | 482.1 b | 73.2 a | 117.7 a | 362.0 b | 228.5 a | 56.2 a | 22.5 a |
| 2022 | 465.4 a | 77.2 a | 126.3 a | 337.0 a | 231.5 a | 59.8 b | 29.3 b | |
| HT | I | 380.8 a | 102.1 b | 97.2 a | 331.6 a | 230.2 a | 53.3 a | 30.1 b |
| II | 566.7 b | 48.2 a | 146.8 b | 367.4 b | 229.8 a | 62.7 b | 21.7 a | |
| L | 1 | 389.8 a | 105.0 f | 128.1 cd | 318.2 a | 216.2 ab | 50.2 a | 37.0 b |
| 2 | 472.8 a | 88.2 cde | 116.1 b | 349.0 abc | 230.2 abc | 60.8 cd | 27.9 ab | |
| 3 | 506.8 a | 77.2 bcd | 104.3 a | 355.5 abc | 218.6 abc | 64.1 d | 20.3 a | |
| 4 | 507.5 a | 70.9 bc | 102.8 a | 354.6 abc | 246.8 c | 59.6 cd | 24.9 ab | |
| 5 | 491.0 a | 39.2 a | 121.2 bc | 373.8 c | 240.9 abc | 61.0 cd | 38.7 b | |
| 6 | 409.6 a | 81.1 bcd | 134.8 de | 344.8 abc | 234.6 abc | 53.9 ab | 19.1 a | |
| 7 | 439.6 a | 97.6 ef | 127.8 cd | 333.5 ab | 234.3 abc | 54.7 ab | 19.3 a | |
| 8 | 499.9 a | 63.6 b | 140.0 e | 352.0 abc | 219.1 a | 57.8 bc | 27.4 ab | |
| 9 | 512.2 a | 62.9 b | 131.0 d | 361.9 bc | 215.3 a | 61.2 cd | 19.8 a | |
| 10 | 508.4 a | 65.9 b | 113.9 b | 352.0 abc | 244.1 bc | 56.9 bc | 24.6 ab | |
| Average | 473.8 | 75.2 | 122.0 | 349.5 | 230.0 | 58.0 | 25.9 |
| Y | HT | DM | CP | CA | CL | HCL | ADL | WSC |
|---|---|---|---|---|---|---|---|---|
| g·kg−1 FM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | ||
| 2021 | I | 393.0 a | 96.8 b | 97.7 a | 345.4 b | 223.2 a | 55.8 b | 2.20 a |
| II | 571.2 b | 49.6 a | 137.8 b | 378.6 c | 233.8 a | 63.9 d | 2.30 a | |
| 2022 | I | 368.7 a | 107.5 b | 96.8 a | 317.8 a | 237.2 a | 50.9 a | 3.82 b |
| II | 562.1 b | 46.8 a | 155.8 c | 356.3 b | 225.8 a | 61.6 c | 2.03 a |
| HT | L | DM | CP | CA | CL | HCL | ADL | WSC |
|---|---|---|---|---|---|---|---|---|
| g·kg−1 FM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | ||
| I | 1 | 337.0 a | 120.4 e | 103.5 abcde | 309.3 a | 222.1 ab | 48.5 a | 38.7 ab |
| 2 | 379.0 ab | 117.5 e | 88.1 ab | 328.9 abc | 239.2 ab | 54.7 abcd | 14.4 a | |
| 3 | 454.0 bc | 94.6 de | 77.6 a | 342.6 abcd | 231.4 ab | 58.7 abcdef | 30.5 ab | |
| 4 | 455.5 bc | 91.9 de | 77.3 a | 342.2 abcd | 249.6 b | 55.4 abcd | 27.4 ab | |
| 5 | 376.5 ab | 63.8 bcd | 91.9 abc | 348.2 abcd | 232.8 ab | 53.3 abcd | 53.2 b | |
| 6 | 317.5 a | 118.5 e | 107.3 bcdef | 325.0 abc | 228.8 ab | 51.0 ab | 20.7 ab | |
| 7 | 340.5 a | 124.2 e | 100.3 abcd | 316.4 ab | 240.9 ab | 50.2 ab | 23.5 ab | |
| 8 | 400.0 ab | 97.8 de | 119.4 cdefg | 330.8 abc | 210.6 ab | 53.2 abcd | 40.5 ab | |
| 9 | 362.2 ab | 98.4 de | 112.0 bcdef | 336.1 abc | 207.0 ab | 55.3 abcd | 27.9 ab | |
| 10 | 386.2 ab | 94.1 de | 95.1 abc | 336.5 abc | 239.7 ab | 53.1 abcd | 24.4 ab | |
| II | 1 | 443.0 bc | 89.6 de | 152.6 hij | 327.1 abc | 210.2 ab | 51.9 abc | 35.4 ab |
| 2 | 566.5 efg | 59.0 bcd | 144.1 ghij | 369.0 cde | 221.2 ab | 66.8 ef | 41.4 ab | |
| 3 | 559.3 de | 59.8 bcd | 130.9 efgh | 368.4 cde | 205.7 a | 69.6 f | 10.1 a | |
| 4 | 559.8 de | 49.8 abc | 128.3 defgh | 367.0 cde | 243.9 ab | 63.9 def | 22.4 ab | |
| 5 | 605.3 fg | 14.5 a | 150.6 hij | 399.4 e | 249.1 ab | 68.7 f | 24.2 ab | |
| 6 | 501.5 cd | 43.8 abc | 162.4 j | 364.5 bcde | 240.5 ab | 56.9 abcde | 17.5 a | |
| 7 | 538.7 cde | 71.0 cd | 155.4 hij | 350.5 abcd | 227.7 ab | 59.2 abcdef | 15.1 a | |
| 8 | 599.5 efg | 29.4 ab | 160.7 ij | 373.2 cde | 227.6 ab | 62.5 cdef | 14.4 a | |
| 9 | 662.5 g | 27.4 ab | 149.9 hij | 387.7 de | 223.7 ab | 67.0 ef | 11.7 a | |
| 10 | 630.5 fg | 37.7 abc | 132.8 fghi | 367.4 cde | 248.5 ab | 60.7 bcdef | 24.7 ab |
| Y | L | DM | CP | CA | CL | HCL | ADL | WSC |
|---|---|---|---|---|---|---|---|---|
| g·kg−1 FM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | g·kg−1 DM | ||
| 2021 | 1 | 374.5 a | 121.2 c | 120.6 ab | 326.0 abcd | 208.5 a | 53.3 abcd | 18.8 a |
| 2 | 535.5 a | 68.9 abc | 109.5 ab | 382.1 fg | 230.1 ab | 67.2 de | 41.2 ab | |
| 3 | 552.0 a | 71.1 abc | 84.3 a | 380.5 efg | 229.7 ab | 68.7 e | 13.5 a | |
| 4 | 522.5 a | 72.9 abc | 103.3 ab | 356.8 abcdefg | 237.8 ab | 58.8 abcde | 14.9 a | |
| 5 | 517.5 a | 38.7 a | 123.2 ab | 393.8 g | 240.2 ab | 66.2 cde | 32.1 ab | |
| 6 | 370.0 a | 82.3 abc | 126.0 ab | 354.1 abcdefg | 233.5 ab | 55.5 abcde | 15.4 a | |
| 7 | 436.5 a | 83.9 abc | 129.2 ab | 354.4 abcdefg | 238.6 ab | 57.3 abcde | 16.8 a | |
| 8 | 518.5 a | 60.3 abc | 126.4 ab | 364.2 cdefg | 222.0 ab | 59.3 abcde | 25.3 ab | |
| 9 | 505.0 a | 59.4 abc | 136.3 ab | 366.9 defg | 214.1 ab | 58.9 abcde | 19.6 a | |
| 10 | 488.8 a | 72.8 abc | 118.3 ab | 341.2 abcdef | 230.8 ab | 53.2 abc | 27.7 ab | |
| 2022 | 1 | 405.5 a | 88.8 abc | 135.5 ab | 310.4 a | 223.8 ab | 47.2 a | 55.3 b |
| 2 | 410.0 a | 107.6 abc | 122.7 ab | 315.9 abc | 230.3 ab | 54.4 abcd | 14.6 a | |
| 3 | 461.3 a | 83.3 abc | 124.3 ab | 330.5 abcde | 207.5 a | 59.6 abcde | 27.0 ab | |
| 4 | 492.8 a | 68.9 abc | 102.3 ab | 352.4 abcdefg | 255.8 b | 60.4 abcde | 34.9 ab | |
| 5 | 464.3 a | 39.6 ab | 119.3 ab | 353.9 abcdefg | 241.7 ab | 55.9 abcde | 45.3 ab | |
| 6 | 449.0 a | 80.0 abc | 143.7 ab | 335.4 abcdef | 235.7 ab | 52.4 abc | 22.8 ab | |
| 7 | 442.7 a | 111.3 bc | 126.5 ab | 312.5 ab | 230.0 ab | 52.1 ab | 21.7 ab | |
| 8 | 481.0 a | 66.9 abc | 153.7 b | 339.8 abcdef | 216.2 ab | 56.4 abcde | 29.6 ab | |
| 9 | 519.6 a | 66.3 abc | 125.7 ab | 356.8 abcdefg | 216.6 ab | 63.4 bcde | 20.0 a | |
| 10 | 527.8 a | 58.9 abc | 109.6 ab | 362.7 bcdefg | 257.3 b | 60.5 abcde | 21.4 ab |
| Factors | Level of Factors | NDF g·kg−1 DM | ADF g·kg−1 DM | DDM % | DMI % of Body Weight | RFV | Quality Class |
|---|---|---|---|---|---|---|---|
| Y | 2021 | 650.4 b | 421.8 b | 55.5 a | 1.86 a | 80 a | 4th |
| 2022 | 624.7 a | 393.3 a | 57.7 b | 1.93 b | 87 b | 3rd | |
| HT | I | 615.2 a | 385.0 a | 58.4 b | 1.96 b | 89 b | 3rd |
| II | 659.9 b | 430.1 b | 54.9 a | 1.83 a | 78 a | 4th | |
| L | 1 | 584.6 a | 368.4 a | 59.6 b | 2.06 b | 95 b | 3rd |
| 2 | 640.0 bc | 409.7 cde | 56.4 ab | 1.89 ab | 83 ab | 4th | |
| 3 | 638.2 bc | 419.6 def | 55.7 ab | 1.89 ab | 82 a | 4th | |
| 4 | 661.0 bc | 414.2 def | 56.1 ab | 1.82 a | 79 a | 4th | |
| 5 | 675.8 c | 434.9 f | 54.5 a | 1.79 a | 76 a | 4th | |
| 6 | 633.3 bc | 398.7 bc | 57.3 ab | 1.90 ab | 85 ab | 4th | |
| 7 | 622.4 ab | 388.2 ab | 58.1 ab | 1.94 ab | 87 ab | 3rd | |
| 8 | 629.0 abc | 409.9 cde | 56.4 ab | 1.92 ab | 84 ab | 4th | |
| 9 | 638.4 bc | 423.0 ef | 55.4 a | 1.89 ab | 82 a | 4th | |
| 10 | 652.9 bc | 408.8 cde | 56.5 ab | 1.85 a | 81 a | 4th | |
| Average | 637.6 | 407.5 | 56.6 | 1.89 | 83 | 4th |
| Y | HT | NDF | ADF | DDM | DMI | RFV |
|---|---|---|---|---|---|---|
| g·kg−1 DM | g·kg−1 DM | % | % of Body Weight | |||
| 2021 | I | 624.5 ab | 401.2 b | 57.6 b | 1.93 bc | 86 a |
| II | 676.3 c | 442.5 c | 54.4 a | 1.78 a | 76 b | |
| 2022 | I | 605.9 a | 368.7 a | 60.2 c | 1.99 c | 93 c |
| II | 643.6 b | 417.8 b | 56.4 b | 1.88 b | 82 a |
| HT | L | NDF | ADF | DDM | DMI | RFV |
|---|---|---|---|---|---|---|
| g·kg−1 DM | g·kg−1 DM | % | % of Body Weight | |||
| I | 1 | 580.0 a | 357.8 a | 61.0 f | 2.07 d | 98.1 d |
| 2 | 622.9 ab | 383.7 abcd | 59.0 cdef | 1.93 abcd | 88.7 bcd | |
| 3 | 632.7 abc | 401.3 abcde | 57.6 bcdef | 1.90 abcd | 85.3 abcd | |
| 4 | 647.1 abc | 397.5 abcde | 57.9 bcdef | 1.86 abcd | 83.6 abcd | |
| 5 | 634.3 abc | 401.5 abcde | 57.6 bcdef | 1.90 abcd | 84.9 abcd | |
| 6 | 604.8 ab | 376.0 abc | 59.6 def | 1.99 bcd | 92.0 cd | |
| 7 | 607.6 ab | 366.6 ab | 60.3 ef | 1.98 bcd | 92.8 cd | |
| 8 | 594.6 ab | 384.0 abcd | 59.0 cdef | 2.02 bcd | 92.6 cd | |
| 9 | 598.4 ab | 391.4 abcd | 58.4 cdef | 2.01 bcd | 91.1 bcd | |
| 10 | 629.3 abc | 389.6 abcd | 58.5 cdef | 1.91 abcd | 86.9 bcd | |
| II | 1 | 589.2 ab | 379.1 abcd | 59.4 cdef | 2.04 cd | 94.0 cd |
| 2 | 657.0 abc | 435.8 def | 55.0 abc | 1.84 abcd | 78.9 abc | |
| 3 | 643.7 abc | 438.0 def | 54.8 abc | 1.88 abcd | 80.2 abc | |
| 4 | 674.9 bc | 430.9 cdef | 55.3 abcd | 1.78 abc | 76.5 abc | |
| 5 | 717.3 c | 468.2 f | 52.4 a | 1.68 a | 68.5 a | |
| 6 | 661.9 abc | 421.4 bcdef | 56.1 abcde | 1.82 abcd | 79.1 abc | |
| 7 | 637.3 abc | 409.7 abcdef | 57.0 abcdef | 1.89 abcd | 83.9 abcd | |
| 8 | 663.3 abc | 435.7 def | 55.0 abc | 1.82 abcd | 77.6 abc | |
| 9 | 678.3 bc | 454.7 ef | 53.5 ab | 1.77 ab | 73.6 ab | |
| 10 | 676.5 bc | 428.1 cdef | 55.6 abcd | 1.78 abc | 77.0 abc |
| Y | L | NDF | ADF | DDM | DMI | RFV |
|---|---|---|---|---|---|---|
| g·kg−1 DM | g·kg−1 DM | % | % of Body Weight | |||
| 2021 | 1 | 587.9 ab | 379.4 ab | 59.3 cd | 2.05 c | 94.2 cd |
| 2 | 679.3 bc | 449.2 cd | 53.9 ab | 1.77 ab | 74.3 ab | |
| 3 | 678.9 bc | 449.2 cd | 53.9 ab | 1.77 ab | 74.2 ab | |
| 4 | 653.3 abc | 415.6 abcd | 56.5 abcd | 1.84 abc | 80.8 abcd | |
| 5 | 700.2 c | 460.0 d | 53.1 a | 1.73 a | 71.4 a | |
| 6 | 643.1 abc | 409.6 abcd | 57.0 abcd | 1.87 abc | 83.0 abcd | |
| 7 | 650.3 abc | 411.7 abcd | 56.8 abcd | 1.85 abc | 81.8 abcd | |
| 8 | 645.6 abc | 423.5 bcd | 55.9 abc | 1.87 abc | 81.5 abcd | |
| 9 | 639.9 abc | 425.9 bcd | 55.7 abc | 1.89 abc | 81.9 abcd | |
| 10 | 625.2 abc | 394.4 abc | 58.2 bcd | 1.92 abc | 86.7 abcd | |
| 2022 | 1 | 581.3 a | 357.5 a | 61.0 d | 2.07 c | 97.9 d |
| 2 | 600.6 ab | 370.3 ab | 60.1 cd | 2.00 bc | 93.2 cd | |
| 3 | 597.5 ab | 390.0 abc | 58.5 bcd | 2.01 bc | 91.3 bcd | |
| 4 | 668.7 abc | 412.9 abcd | 56.7 abcd | 1.80 abc | 79.4 abc | |
| 5 | 651.4 abc | 409.8 abcd | 57.0 abcd | 1.85 abc | 82.0 abcd | |
| 6 | 623.6 abc | 387.8 abc | 58.7 bcd | 1.93 abc | 88.1 abcd | |
| 7 | 594.6 ab | 364.6 ab | 60.5 cd | 2.02 bc | 94.9 cd | |
| 8 | 612.4 abc | 396.2 abcd | 58.0 abcd | 1.97 abc | 88.6 abcd | |
| 9 | 636.8 abc | 420.2 abcd | 56.2 abcd | 1.90 abc | 82.8 abcd | |
| 10 | 680.6 bc | 423.3 bcd | 55.9 abc | 1.77 ab | 77.2 abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paszkiewicz-Jasińska, A.; Wróbel, B.; Stopa, W.; Jakubowska, Z.; Steinhoff-Wrześniewska, A.; Zielewicz, W. Nutritional Status of Wood Melick (Melica uniflora Retz.) in a Natural Forest Stand in South-Western Poland. Forests 2023, 14, 1605. https://doi.org/10.3390/f14081605
Paszkiewicz-Jasińska A, Wróbel B, Stopa W, Jakubowska Z, Steinhoff-Wrześniewska A, Zielewicz W. Nutritional Status of Wood Melick (Melica uniflora Retz.) in a Natural Forest Stand in South-Western Poland. Forests. 2023; 14(8):1605. https://doi.org/10.3390/f14081605
Chicago/Turabian StylePaszkiewicz-Jasińska, Anna, Barbara Wróbel, Wojciech Stopa, Zuzanna Jakubowska, Aleksandra Steinhoff-Wrześniewska, and Waldemar Zielewicz. 2023. "Nutritional Status of Wood Melick (Melica uniflora Retz.) in a Natural Forest Stand in South-Western Poland" Forests 14, no. 8: 1605. https://doi.org/10.3390/f14081605
APA StylePaszkiewicz-Jasińska, A., Wróbel, B., Stopa, W., Jakubowska, Z., Steinhoff-Wrześniewska, A., & Zielewicz, W. (2023). Nutritional Status of Wood Melick (Melica uniflora Retz.) in a Natural Forest Stand in South-Western Poland. Forests, 14(8), 1605. https://doi.org/10.3390/f14081605







