Abstract
Camellia oleifera, a woody plant indigenous to China, is primarily utilized for the production of cooking oil. However, it is frequently afflicted by anthracnose, a highly detrimental disease that leads to significant annual losses. Colletotrichum fructicola is the predominant etiological agent responsible for anthracnose in Ca. oleifera. Additionally, our investigation has revealed that a bZIP transcription factor CfHac1 in C. fructicola governs the pathogenicity and response to endoplasmic reticulum stress. In this study, we conducted an investigation of the role of the CfPDI1 gene in C. fructicola, which was significantly downregulated in ΔCfhac1 under endoplasmic reticulum stress. The CfPDI1 gene was deleted, resulting in reduced vegetative growth, conidiation, appressoria formation, and appressorium turgor generation. Furthermore, it was observed that the ΔCfpdi1 mutant exhibited impaired responsiveness to endoplasmic reticulum stresses, and the expression of UPR-related genes in C. fructicola was influenced by CfPdi1. Cytological investigations indicated that CfPdi1 is localized in the endoplasmic reticulum. Further analysis revealed that the ΔCfpdi1 mutant displays significantly reduced pathogenicity in Ca. oleifera. Taken together, this study illustrated crucial functions of CfPdi1 in development, response to ER stress, autophagy, and pathogenicity in C. fructicola.
1. Introduction
Camellia oleifera is a major tree species grown for producing edible oil, and it is commonly affected by anthracnose, which is one of the most destructive diseases [1]. Colletotrichum, a genus of plant pathogenic fungi, is among the top 10 causative agents of anthracnose, affecting almost every crop [2]. Our previous research has identified the Colletotrichum gloeosporioides complex as the primary pathogen responsible for anthracnose in Ca. oleifera, and Colletotrichum fructicola is the dominant pathogen [1]. We have demonstrated that the CfSet1 and CfVps35 genes were involved in pathogenicity in C. fructicola [3,4]. As reported by Li et al. [5], it was shown that CfVps39, a constituent of the homotypic fusion and vacuole protein sorting (HOPS) complex, exerts regulatory control over the endoplasmic reticulum (ER) pressure stress response and pathogenicity in C. fructicola. Additionally, Zhang et al. [6,7] demonstrated that the histone acetyltransferase CfGcn5 plays a pivotal role in modulating the responses to ER stress and pathogenicity. Notwithstanding this awareness, the fundamental pathogenic mechanisms of C. fructicola remain largely ambiguous, impeding significantly the prevention and control of Ca. oleifera anthracnose.
Previously, our team conducted an investigation of CfHac1, a bZIP transcription factor in C. fructicola, and discovered its involvement in regulating pathogenicity and the endoplasmic reticulum’s stress response [8]. Subsequently, we conducted a transcriptome analysis of a ΔCfhac1 mutant strain under ER stress induced by dithiothreitol, which revealed a significant decrease in the A04491 gene. It was an objective in this study to explore the function of the A04491 gene and identify a potential target for the development of novel fungicides. The results may also lay the foundation for future breeding to improve the disease resistance of Camellia.
2. Materials and Methods
2.1. Strains and Culture Conditions
The wild-type strain of C. fructicola utilized in this study was the CFLH16 strain, which was isolated from Ca. oleifera [1]. All strains were cultivated on either potato dextrose agar (PDA) or minimal medium (MM), at a temperature of 28 °C, under conditions devoid of light. For the extraction of conidia, DNA, RNA, and protein, the culture strains were subjected to liquid potato glucose broth medium (PDB).
2.2. Gene Deletion and Complementation
The coding region of CfPDI1 was replaced with the HPH gene according to the homologous recombination principle. The one-step replacement strategy of targeted gene deletion was employed, as previously described [9]. Complementation was achieved by introducing the constructed pYF11:: CfPDI1 vectors into protoplasts of specific mutant strains. The primers sequences are listed in Supplementary Table S1.
2.3. Phenotypes Analysis
Growth rates were measured according to the method described by Zhang et al. [6]. To quantify sporulation, the CFLH16, ΔCfpdi1, and ΔCfpdi1/CfPDI1 were cultured in PDB at 28 °C and 160 r/min for three days. Sporulation was determined by using a hemocytometer. Appressorium formation was observed for 12 h post-inoculation (hpi), and appressorium turgor was evaluated using cytorrhysis assays, as previously described by Howard et al. [10]. Specifically, appressoria at 12 hpi were incubated in 2.0 M glycerol for 10 min, and the proportion of collapsed appressoria was recorded [11]. To assess stress sensitivity, the aforementioned strains were cultivated on PDA medium supplemented with an ER stress inducer (DTT, DL-Dithiothreitol, Solarbio: 3483-12-3) for a duration of three days. Conidia suspensions (1 × 105/mL) of strains were inoculated on leaves of Ca. oleifera. The experiments were performed in triplicates and repeated three times.
2.4. Observation of Fluorescence Fusion Proteins in C. fructicola
The gene encoding CfPdi1 was amplified with A04491-9F/A04491-10R primers from the wild-type genomic DNA and fused with GFP in a Xho1-linearized pYF11-GFP containing a bleomycin resistance gene. The ER-localized protein gene LHS1 was amplified through PCR using a primer set (LHSRFPF/LHSRFPR) and fused with RFP in a Xho1-linearized pYF11-RFP containing a hygromycin resistance gene. Two types of plasmids were co-transformed into ΔCfpdi1 protoplasts via PEG-mediated methods. Fluorescence signals in the hyphae tip were observed using a fluorescence microscope.
2.5. qRT-PCR and Identification of Target Gene
Total RNA was reverse-transcribed into cDNA using reverse transcription kit (Vazyme). A qRT-PCR was conducted on an ABI Quant Studio 3 with the application of primers, and relative gene expression was standardized by using ACTIN gene. All experiments were replicated at least thrice, with each replication consisting of three independent biological replicates.
2.6. Autophagy Induction and Western Blotting Assays
To elucidate the association between CfPdi1 and the autophagy process, CFLH16 and ΔCfpdi1 strains were subjected to genetic modification with the GFP-CfATG8 gene and subsequently cultivated in liquid CM medium for a duration of 48 h. The mycelia were washed with ddH2O and then treated with MM-N for 5 h. The western blotting analysis was conducted following the methodology described in our previous publication [7].
2.7. Statistical Analysis
The statistical data were presented as means ± standard deviation and subjected to a one-way analysis of variance (ANOVA) using Duncan’s new multiple range test, with a significance level of p < 0.01.
3. Results
3.1. Identification of CfPdi1 in C. fructicola
The analysis of transcripts demonstrated a significant decrease in the expression of the A04491 gene in the ΔCfhac1 mutant according to our previous expression data [12]. The qRT-PCR results indicated a significant increase in the expression of A04491 in the wild-type strain following exposure to DTT, while its expression was downregulated in the untreated or DTT-treated mutant strain ΔCfhac1 (Figure 1). The results demonstrated that the CfHac1 plays a role in governing the expression of the A04491 gene in C. fructicola.
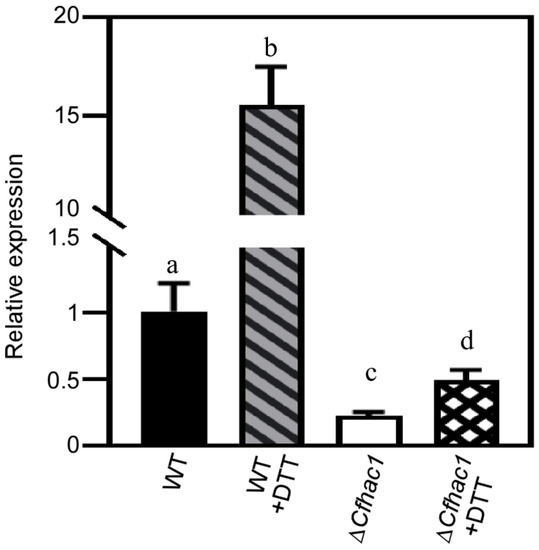
Figure 1.
The expression levels of the A04491 in ΔCfhac1 were analyzed using a qRT-PCR. Different lowercase letters (a–d) indicate significant difference (p < 0.01).
The A04491 gene had a total length of 1578 bp and was responsible for encoding a protein consisting of 518 amino acids. Through domain prediction analysis, it was determined that the gene contained three thioredoxin domains (Figure 2A). A04491 produces amino acids that are orthologous to ScPdi1 in S. cerevisiae (NP_009887.1), hence being designated CfPDI1. The amino acid sequence of CfPdi1 was utilized to retrieve the amino acid sequences of homologous proteins from other species through the NCBI database and used to construct a bootstrap neighbor-joining phylogenetic tree (Figure 2B). The results of this study demonstrated a significant degree of similarity between the CfPdi1 protein of C. fructicola and the ChPdi1 protein of C. higginsianum while exhibiting a comparatively lower level of similarity with the ScPdi1 protein of S. cerevisiae. This result reveals that the PDI1 gene is relatively evolutionarily conserved among fungi.
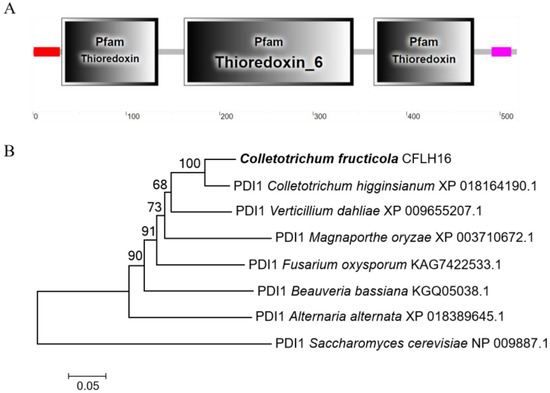
Figure 2.
Phylogenetic analysis and domain prediction of A04491. (A): The domain prediction of A04491. (B): Based on the amino acid sequence, the phylogenetic tree was constructed by MEGA 7.0 using N-J method with 1000 bootstrap replicates.
The gene knockout strategy for CfPDI1 is depicted in Supplementary Figure S1A. PCR verification findings indicate that the wild-type genome exhibits a single detectable band when utilizing A04491-7F/A04491-8R primers targeting the gene of interest while no band is observed in the mutant counterpart. The Pdi1-5F/H855R primers successfully identified a singular band that was amplified through PCR in the mutant strain. Conversely, no band was observed in both the CFLH16 and ΔCfpdi1/CfPDI1, suggesting the successful knockout of the CfPDI1 gene in C. fructicola (Supplementary Figure S1B). Consequently, we obtained the mutant ΔCfpdi1. In order to validate the mutant phenotype resulting from the deletion of CfPDI1, the ΔCfpdi1 was restored to its original state by introducing a native copy of CfPDI1 from the wild type, which encompassed a 1.5 kb promoter region.
3.2. CfPdi1 Is Localized in the ER
Lhs1 serves as a marker located in the endoplasmic reticulum (ER) [13]. To investigate the subcellular distribution of CfPdi1 in C. fructicola, we constructed two expression vectors containing fluorescence fusion proteins (PDI1-GFP and LHS1-RFP) and subsequently co-transformed them into the ΔCfpdi1 strain. The co-localization of green fluorescence signals from Pdi1-GFP and red fluorescence signals from Lhs1-RFP (Figure 3) suggests that CfPdi1 is localized within the ER.
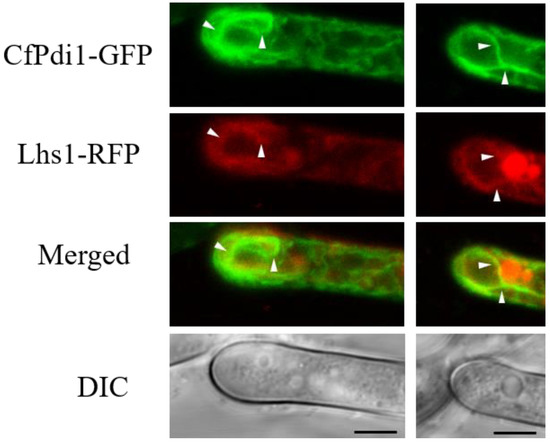
Figure 3.
Subcellular localization of Pdi1 and Lhs1 in Colletotrichum fructicola. The endoplasmic reticulum is indicated by white arrows. Bar = 5.0 μm.
3.3. CfPdi1 Is Involved in the Development of C. fructicola
The findings of this study demonstrate a significant reduction in the colony diameters of ΔCfpdi1 compared to CFLH16 and ΔCfpdi1/CfPDI1 (p < 0.01), suggesting that the CfPDI1 gene plays a role in the regulation of growth (Figure 4A). Additionally, the ΔCfpdi1 exhibited a significantly lower production of conidia compared to the CFLH16 and ΔCfpdi1/CfPDI1 (Figure 4B). The rate of appressoria formation of ΔCfpdi1 was less than 50%, which was significantly lower compared to those of the CFLH16 and ΔCfpdi1/CfPDI1 (above 70%, p < 0.01) (Figure 4C).
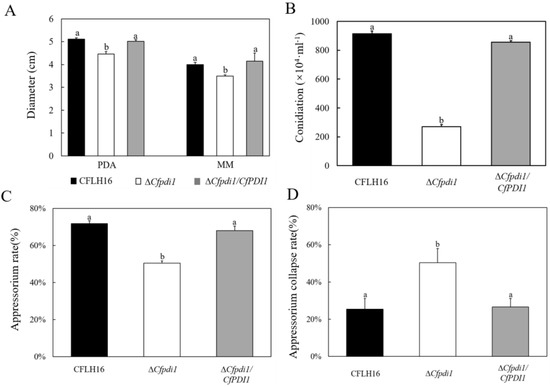
Figure 4.
CfPdi1 is involved in growth, sporulation, appressoria formation, and appressorium turgor generation. The figure shows the statistical analysis of the mycelial growth rate (A), conidia formation rate (B), appressorium rate (C), and appressorium collapse rate (D). Error bars are ±standard deviation; different lowercase letters (a,b) indicate significant difference (p < 0.01).
At 2 M glycerol, the appressorium collapse rate was around 50% in the ΔCfpdi1 compared to 25% in the WT and ΔCfpdi1/CfPDI1 (Figure 4D). These results illustrate the importance of CfPdi1 in vegetative growth, conidiation, appressoria formation, and appressorium turgor generation.
3.4. CfPdi1 Is Essential for Pathogenicity
The results of pathogenicity testing showed that the disease areas of the ΔCfpdi1 mutant were significantly smaller than those of the CFLH16 and ΔCfpdi1/CfPDI1 strains on both wounded and unwounded leaves (Figure 5A–C). These outcomes suggest that CfPdi1 plays a crucial role in the pathogenicity of C. fructicola.
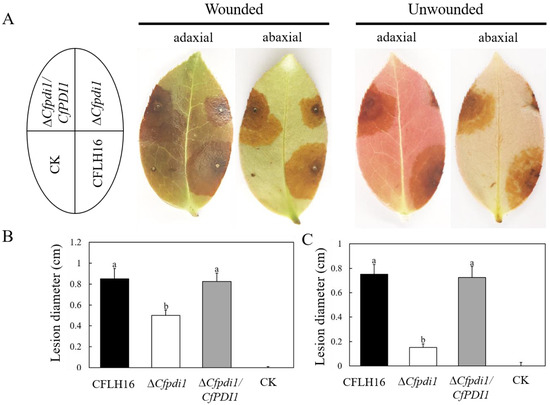
Figure 5.
Pathogenicity test. (A): Uninjured and injured leaves of the tea-oil tree were subjected to inoculation with conidial suspensions containing WT, ΔCfpdi1, and ΔCfpdi1/CfPDI1. (B,C): Statistical analysis of the disease lesion areas. CK: ddH2O. Bars represent standard errors from three independent experiments, each performed in replicates. Different lowercase letters indicate significant difference (p < 0.01).
3.5. CfPdi1 Participates in the Responses to ER Stresses
Recent research has demonstrated that pathogens are also subjected to endoplasmic reticulum (ER) stress originating from the host during the course of infection [14,15]. In order to ascertain the UPR function of CfPdi1 in response to ER stress, we conducted measurements of the growth of ΔCfpdi1 on media inducing ER stress (7.5 mmol/L or 10.0 mmol/L DTT). The inhibition rate of ΔCfpdi1 exhibited a significant increase when subjected to treatment with 7.5 mM DTT in comparison to both the CFLH16 and ΔCfpdi1/CfPDI1 (Figure 6A,B). A similar outcome was observed upon elevating the DTT concentration to 10.0 mM. These results suggest that CfPdi1 participates in the responses to ER stress.
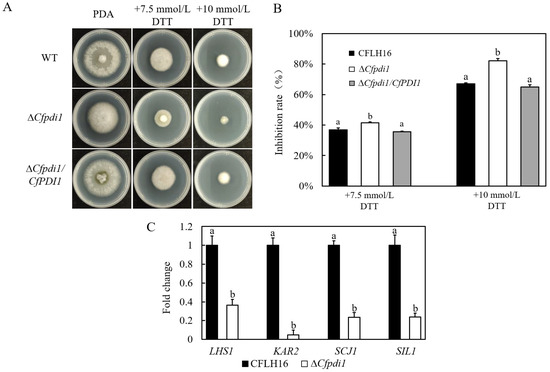
Figure 6.
ER stress sensitivity testing of CfPDI1 gene-deletion mutants and expression level analysis of four UPR-related genes in C. fructicola. (A): The growth of the wild-type strain (CFLH16) and CfPDI1 deletion mutant (ΔCfpdi1) and complemented strain (ΔCfpdi1/CfPDI1) on PDA plates containing DTT. (B): Statistical analysis of the growth inhibition rate of three strains. (C): qRT-PCR verification of the expression levels of four UPR-related genes in ΔCfpdi1. Bars represent standard errors from three independent experiments, each performed in replicates. Different lowercase letters indicate significant difference (p < 0.01).
3.6. The Expression of UPR-Related Genes Was Influenced by CfPdi1
Considering that CfPdi1 participates in the responses to ER stress, we measured the expression levels of four genes coding for unfolded protein response components [16] in the aerial hypha of C. fructicola. The expression levels of LHS1 (a heat shock protein 70), KAR2/BIP (an ER molecular chaperone), SCJ1 (a chaperone dnaJ 2), and SIL1 (a nucleotide exchange factor) were significantly downregulated in ΔCfpdi1 (Figure 6C). The findings of this study indicate that CfPdi1 has a significant impact on the regulation of UPR-related genes in C. fructicola.
3.7. The CfPdi1 Positively Regulates Autophagy Process
Western blotting results showed that the ΔCfpdi1 mutant exhibited a notably diminished relative ratio of free GFP compared to the WT in both non-induction and after 5 h of MM-N treatment (Figure 7), thereby corroborating the decreased autophagy level in the ΔCfpdi1 mutant. The findings of this study indicate that CfPdi1 plays a crucial role in the process of autophagy.
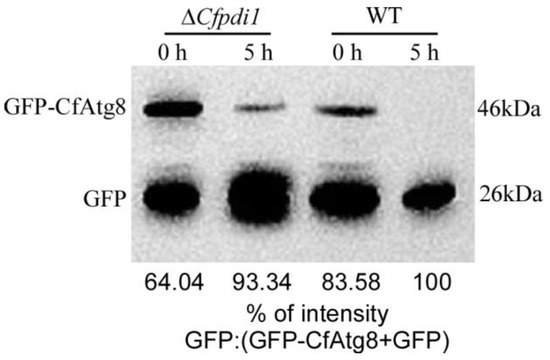
Figure 7.
CfPdi1 positively regulates autophagy. The proteolysis of GFP-CfAtg8 was analyzed using Western blotting. The upper lanes indicate the presence of full GFP-CfAtg8 (46 kDa) while the lower lanes indicate the presence of free GFP (26 kDa). The extent of autophagy was determined by quantifying the proportion of free GFP in relation to the combined amount of intact GFP-CfAtg8 and free GFP.
4. Discussion
The endoplasmic reticulum is an essential organelle that performs critical functions in protein folding and processing, which are indispensable for the virulence and development of fungi. The accumulation of misfolded or unfolded proteins, as well as the dysregulation of sterols or lipids, can lead to ER stress and affect the expression of specific genes, resulting in unfolded protein responses (UPR) [16]. The CfVAM7 gene plays a crucial role in regulating the responses of C. fructicola to ER stress and virulence [17]. Specifically, in P. oryzae, the involvement of four ER proteins, namely PoSpf1, PoPmt4, PoLhs1, and PoErr1, has been observed in the penetration of plants and the branching of invasive hyphae [14,18,19,20]. The absence of the ER molecular chaperone PoLHS1 led to a shortage in sporulation and appressorium-mediated penetration. Likewise, the deletion of the ER retention receptor PoERR1 resulted in the mutant’s incapacity to penetrate plants and its inability to generate disease lesions on wounded leaves. In P. oryzae, Spf1, a P5 type ATPase localized in the ER, is essential for fungal development and virulence due to its participation in ER functions. The indispensability of ScPdi1 for cellular growth and the oxidation process of secretory proteins cannot be overstated [21]. Furthermore, in Ustilago maydis, the pivotal role of Pdi1 in regulating fungal colonization within host tissues is crucial for virulence [22]. In the present study, we characterized the essential roles of an ER-localized protein disulfide isomerase, Pdi1, in fungal development, response to ER stress, and virulence in C. fructicola.
The UPR is orchestrated to mitigate ER stress through the upregulation of ER chaperone protein transcription. In the present study, it was observed that the expression levels of the molecular chaperones KAR2, LHS1, SCJ1, and SIL1 were markedly reduced in the ΔCfpdi1 strain, indicating that the elimination of the CfPDI1 gene resulted in an impaired UPR. The findings of our study suggest that the PDI1 gene plays a crucial role in the modulation of UPR-associated genes, and additional research is required to elucidate the underlying regulatory mechanisms. In addition, we found that the CfPDI1 gene exerts regulatory control over autophagy. However, additional investigation is required to elucidate the association between autophagy and endoplasmic reticulum stress.
5. Conclusions
In brief, the functions of Pdi1, a protein disulfide isomerase localized in the endoplasmic reticulum, were investigated in C. fructicola through functional genomics assays. Our findings indicate that CfPdi1 plays a crucial role in fungal development, response to ER stress, autophagy, and pathogenicity. The findings of our study offer a promising avenue for the advancement of novel fungicides that specifically target Pdi1.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14081597/s1, Table S1: The primers sequences used in this study; Figure S1: Generation of the CfPDI1 gene deletion mutant in Colletotrichum fructicola. A. Schematic diagram of the deletion gene strategy for the CfPDI1. B. M: DL2000 marker; −: H2O negative control; +: WT positive control; Δ: Mutant; C: Complemented strain.
Author Contributions
Conceptualization, S.L., J.C. and H.L.; Methodology, S.L. and H.L.; Investigation, S.L.; Writing—Original Draft, S.L. and H.L.; Writing—Review and Editing, J.C. and H.L.; Funding Acquisition, H.L.; Resources, J.C. and H.L.; Supervision, J.C. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32071765) and the Hunan Provincial Innovation Foundation for Postgraduate (QL20220175). The Science Fund of the Jiangsu Vocational College of Agriculture and Forestry (2020kj003) also contributed funds to this research.
Data Availability Statement
All data supporting the findings of this study are available within the paper and within its Supplementary Materials (published online).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, H.; Zhou, G.Y.; Liu, J.A.; Xu, J. Population genetic analyses of the fungal pathogen Colletotrichum fructicola on tea-oil trees in China. PLoS ONE 2016, 11, e0156841. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, S.; Li, H. H3K4 Methyltransferase CfSet1 Is Required for Development and Pathogenesis in Colletotrichum fructicola. J. Fungi 2022, 8, 363. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Li, H. Retromer subunit, CfVps35 is required for growth development and pathogenicity of Colletotrichum fructicola. BMC Genom. Data 2022, 23, 68. [Google Scholar] [CrossRef]
- Li, S.Z.; Zhang, S.P.; Li, H. A HOPS protein, CfVps39, is required for appressorium formation, environmental stress response and vacuolar fusion of Colletotrichum fructicola. For. Pathol. 2021, 51, e12692. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, Y.; Chen, S.; Li, H. The Histone Acetyltransferase CfGcn5 Regulates Growth, Development, and Pathogenicity in the Anthracnose Fungus Colletotrichum fructicola on the Tea-Oil Tree. Front. Microbiol. 2021, 12, e680415. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, Y.; Li, S.; Li, H. Histone Acetyltransferase CfGcn5-Mediated Autophagy Governs the Pathogenicity of Colletotrichum fructicola. mBio 2022, 13, e0195622. [Google Scholar] [CrossRef]
- Yao, Q.; Guo, Y.; Wei, F.Y.; Li, S.Z.; Zhang, S.P.; Li, H. The bZIP transcription factor CfHac1 is involved in regulating the growth, development, and pathogenicity of Colletotrichum fructicola. Mycosystema 2019, 38, 1643–1652. [Google Scholar]
- Zhang, S.; Guo, Y.; Li, S.; Zhou, G.; Liu, J.; Xu, J.; Li, H. Functional analysis of CfSnf1 in the development and pathogenicity of anthracnose fungus Colletotrichum fructicola on tea-oil tree. BMC Genet. 2019, 20, 94. [Google Scholar] [CrossRef]
- Howard, R.J.; Ferrari, M.A.; Roach, D.H.; Money, N.P. Penetration of hard substrates by a fungus employing enormous turgor pressures. Proc. Natl. Acad. Sci. USA 1991, 88, 11281–11284. [Google Scholar] [CrossRef]
- Dixon, K.P.; Xu, J.R.; Smirnoff, N.; Talbot, N.J. Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium-mediated plant infection by Magnaporthe grisea. Plant Cell 1999, 11, 2045–2058. [Google Scholar] [CrossRef]
- Li, S.; Li, H. Genome-wide transcriptome analysis of Colletotrichum fructicola CfHAC1 regulation of the response to dithiothreitol stress. Mycosystema 2020, 39, 1886–1896. [Google Scholar]
- Yi, M.; Chi, M.H.; Khang, C.H.; Park, S.Y.; Kang, S.; Valent, B.; Lee, Y.H. The ER chaperone LHS1 is involved in asexual development and rice infection by the blast fungus Magnaporthe oryzae. Plant Cell 2009, 21, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Jiang, H.; Aron, O.; Wang, M.; Wang, X.; Chen, J. Endoplasmic reticulum-associated degradation mediated by MoHrd1 and MoDer1 is pivotal for appressorium development and pathogenicity of Magnaporthe oryzae. Environ. Microbiol. 2020, 22, 4953–4973. [Google Scholar] [CrossRef]
- Yin, Z.; Feng, W.; Chen, C.; Xu, J.; Li, Y.; Yang, L.; Wang, J.; Liu, X.; Wang, W.; Gao, C.; et al. Shedding light on autophagy coordinating with cell wall integrity signaling to govern pathogenicity of Magnaporthe oryzae. Autophagy 2020, 16, 900–916. [Google Scholar] [CrossRef]
- Tang, W.; Ru, Y.; Hong, L.; Zhu, Q.; Zuo, R.; Guo, X.; Wang, J.; Zhang, H.; Zheng, X.; Wang, P.; et al. System-wide characterization of bZIP transcription factor proteins involved in infection-related morphogenesis of Magnaporthe oryzae. Environ. Microbiol. 2015, 17, 1377–1396. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Li, B.; Li, H. The SNARE Protein CfVam7 is Required for Growth, Endoplasmic Reticulum Stress Response, and Pathogenicity of Colletotrichum fructicola. Front. Microbiol. 2021, 12, 736066. [Google Scholar] [CrossRef]
- Goh, J.; Jeon, J.; Lee, Y.H. ER retention receptor, MoERR1 is required for fungal development and pathogenicity in the rice blast fungus, Magnaporthe oryzae. Sci. Rep. 2017, 7, 1259. [Google Scholar] [CrossRef]
- Pan, Y.; Pan, R.; Tan, L.; Zhang, Z.; Guo, M. Pleiotropic roles of O-mannosyltransferase MoPmt4 in development and pathogenicity of Magnaporthe oryzae. Curr. Genet. 2019, 65, 223–239. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, J.; Zhu, X.; Dong, B.; Liu, X.; Lu, J.; Lin, F. The P5-type ATPase Spf1 is required for development and virulence of the rice blast fungus Pyricularia oryzae. Curr. Genet. 2020, 66, 385–395. [Google Scholar] [CrossRef]
- Nakanishi, H.; Nakayama, K.; Yokota, A.; Tachikawa, H.; Takahashi, N.; Jigami, Y. Hut1 proteins identified in Saccharomyces cerevisiae and Schizosaccharomyces pombe are functional homologues involved in the protein-folding process at the endoplasmic reticulum. Yeast 2001, 18, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Marin-Menguiano, M.; Moreno-Sanchez, I.; Barrales, R.R.; Fernandez-Alvarez, A.; Ibeas, J.I. Nglycosylation of the protein disulfide isomerase Pdi1 ensures full Ustilago maydis virulence. PLoS Pathog. 2019, 15, e1007687. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).