Changes in Community Structure and Functional Characteristics of Soil Bacteria and Fungi along Karst Vegetation Succession
Abstract
1. Introduction
2. Materials and Methods
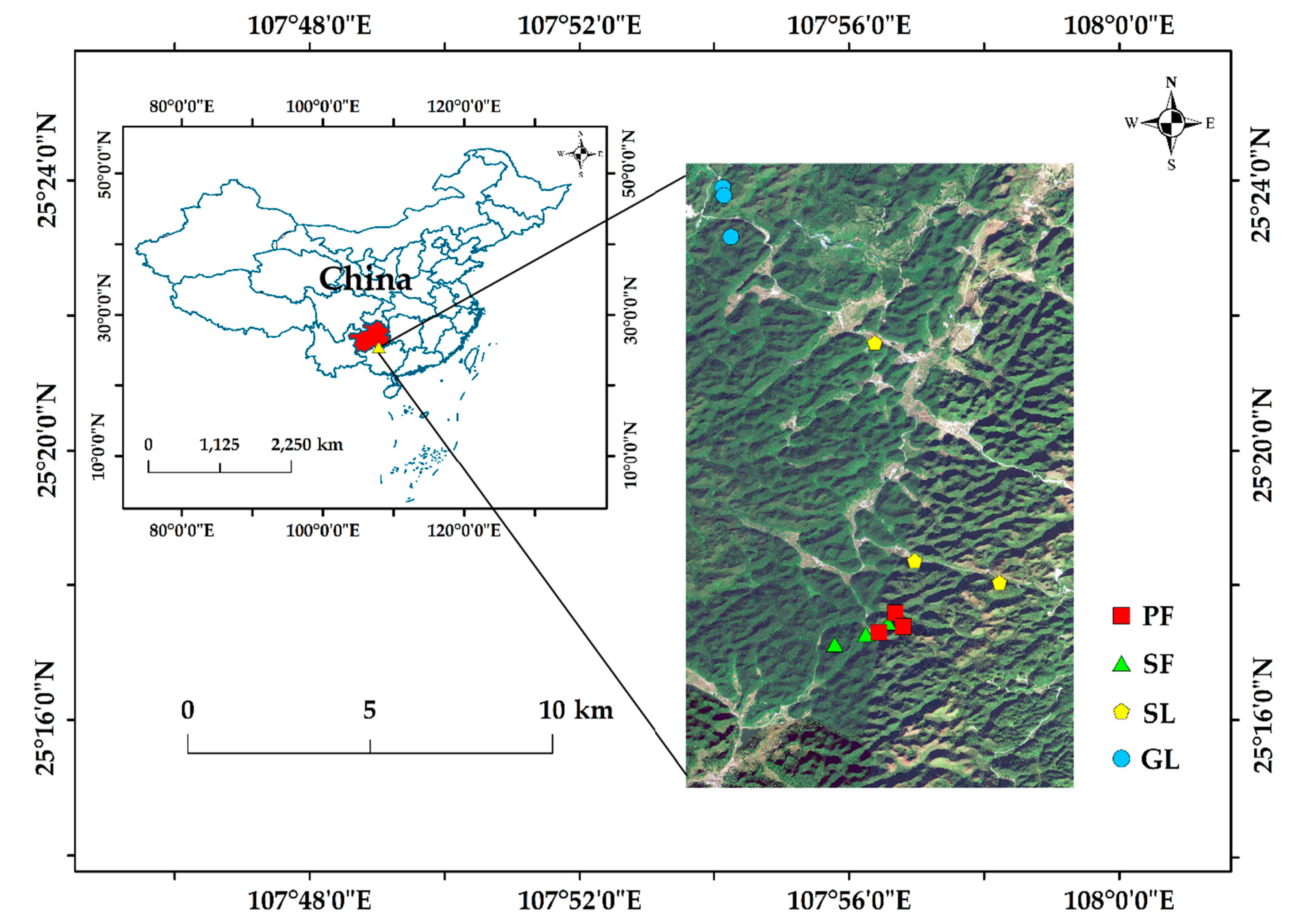
2.1. Study Region and Soil Sample Gathering
2.2. Soil Properties and Enzymatic Activity Determination
2.3. DNA Extraction and High-Throughput Sequencing
2.4. Data Analyses
3. Results
3.1. Soil Properties in Different Vegetation Successional Stages
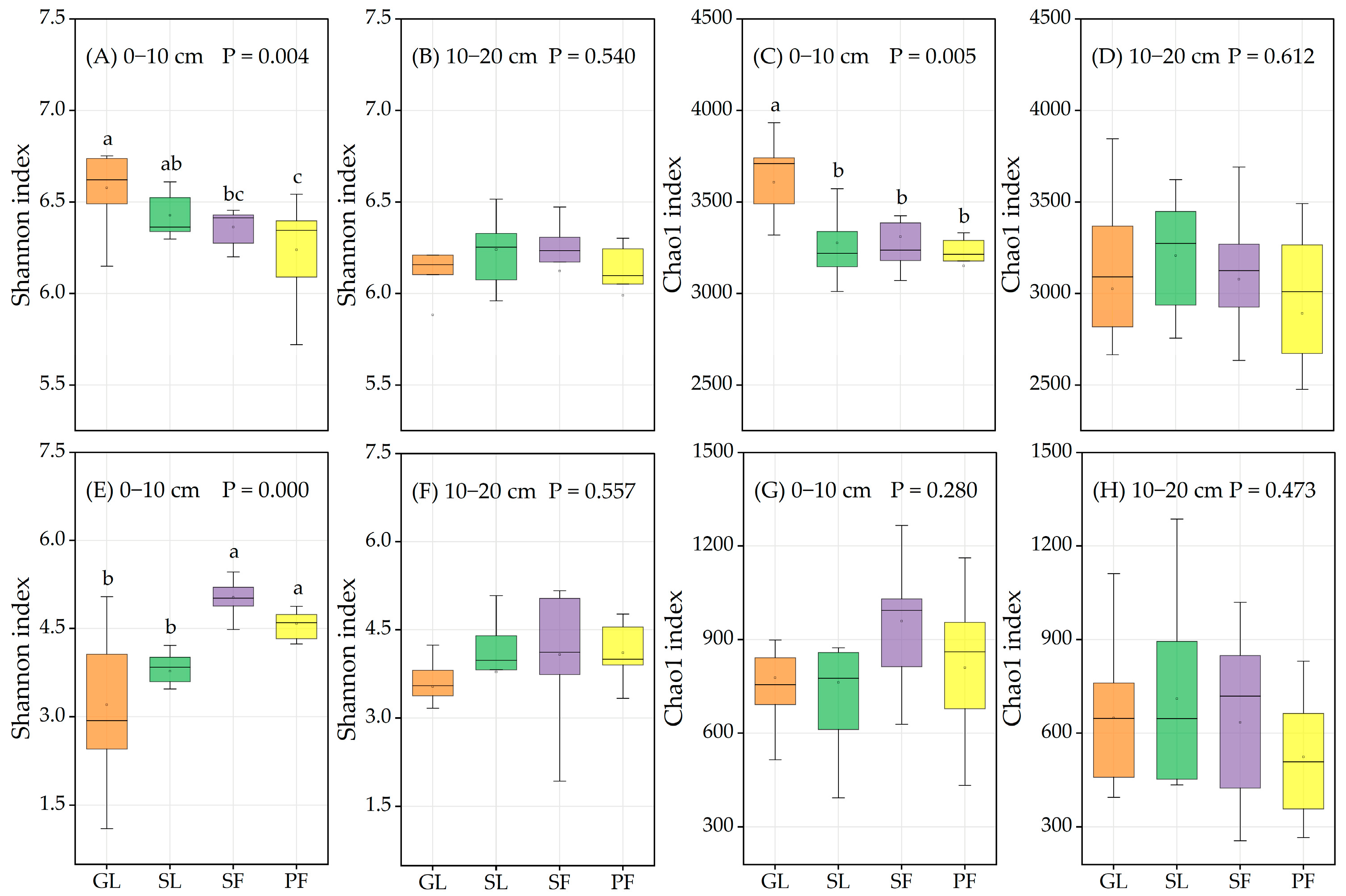
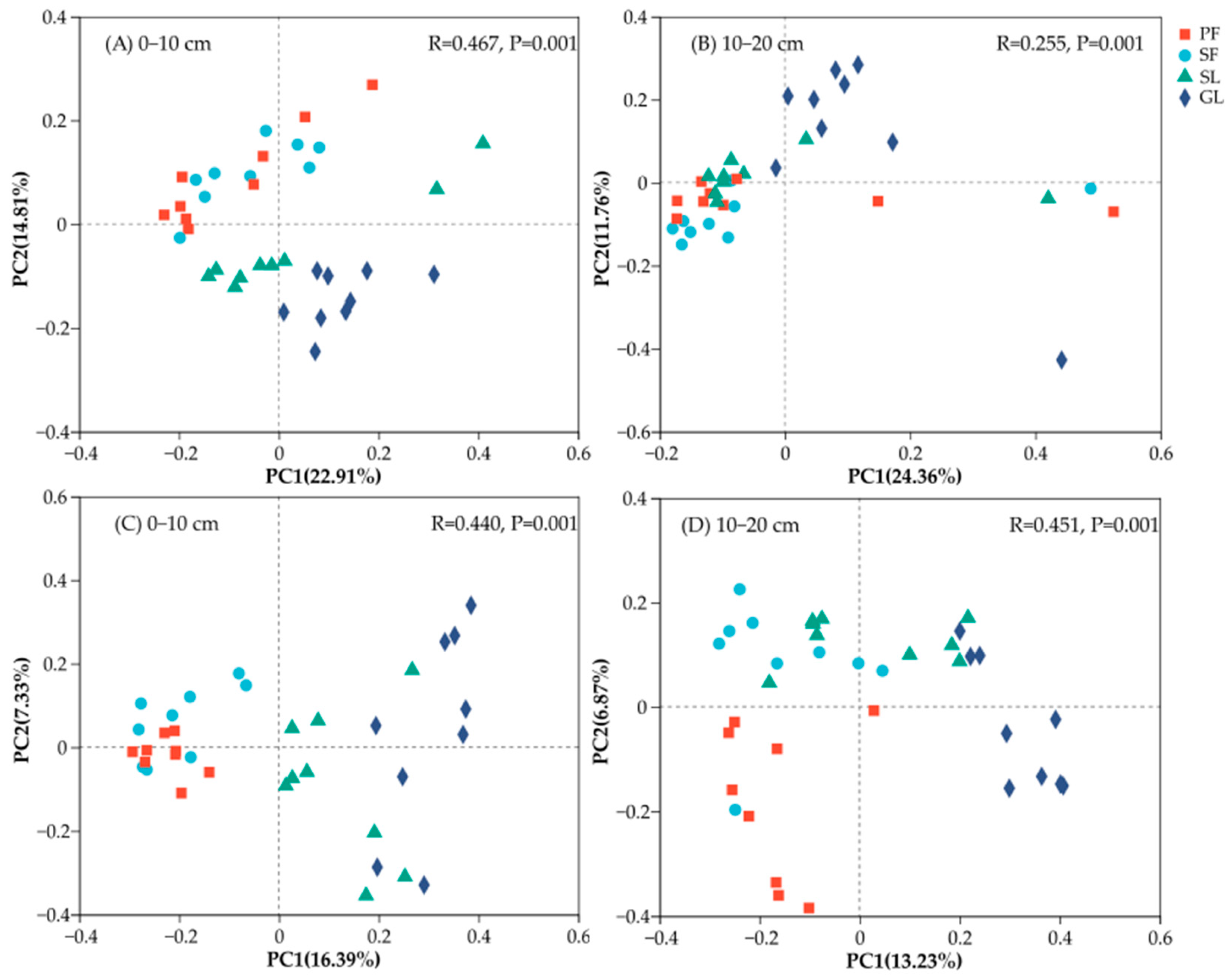
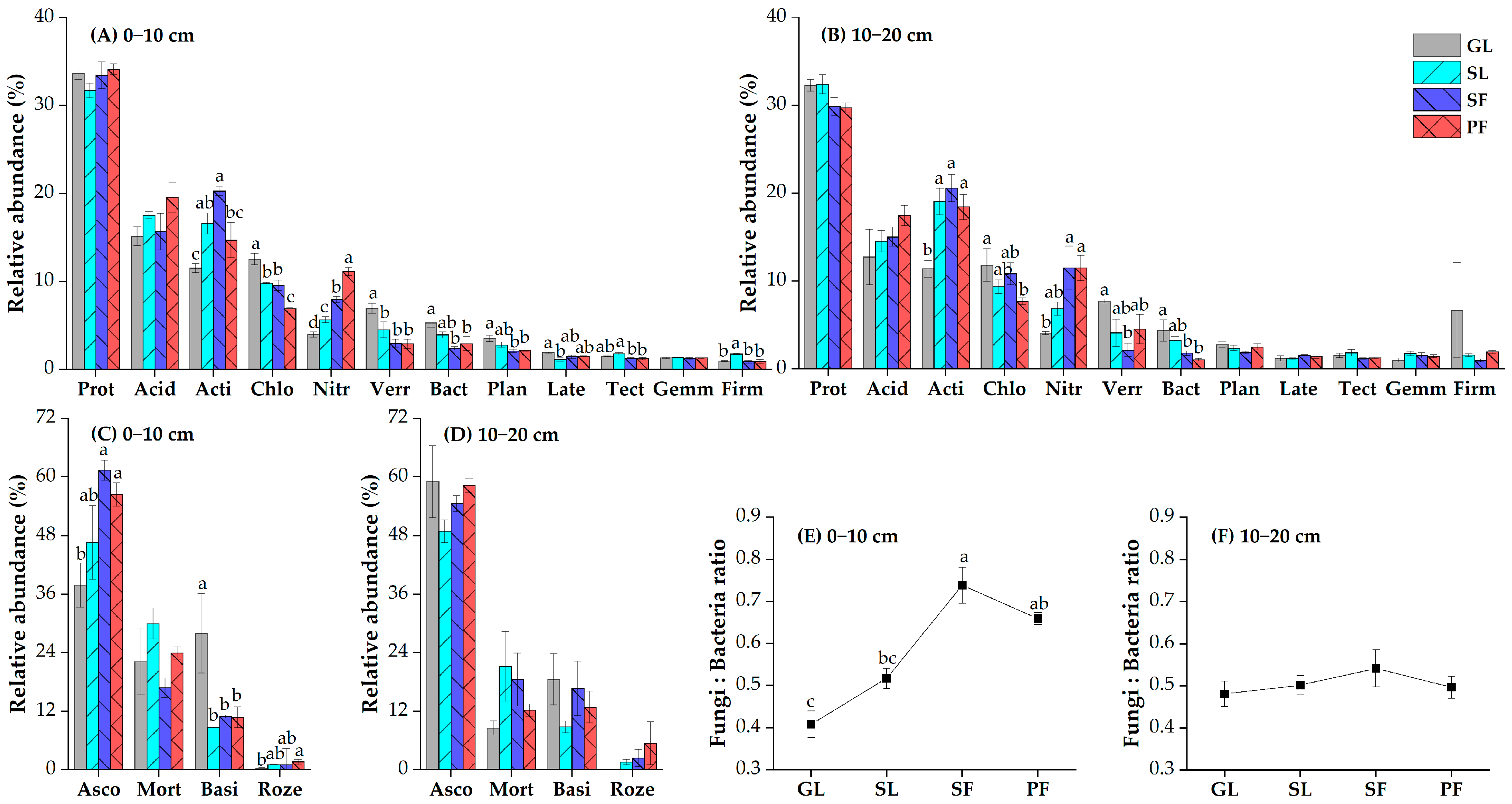
3.2. Soil Microbial Community Diversity and Composition in Different Vegetation Successional Stages
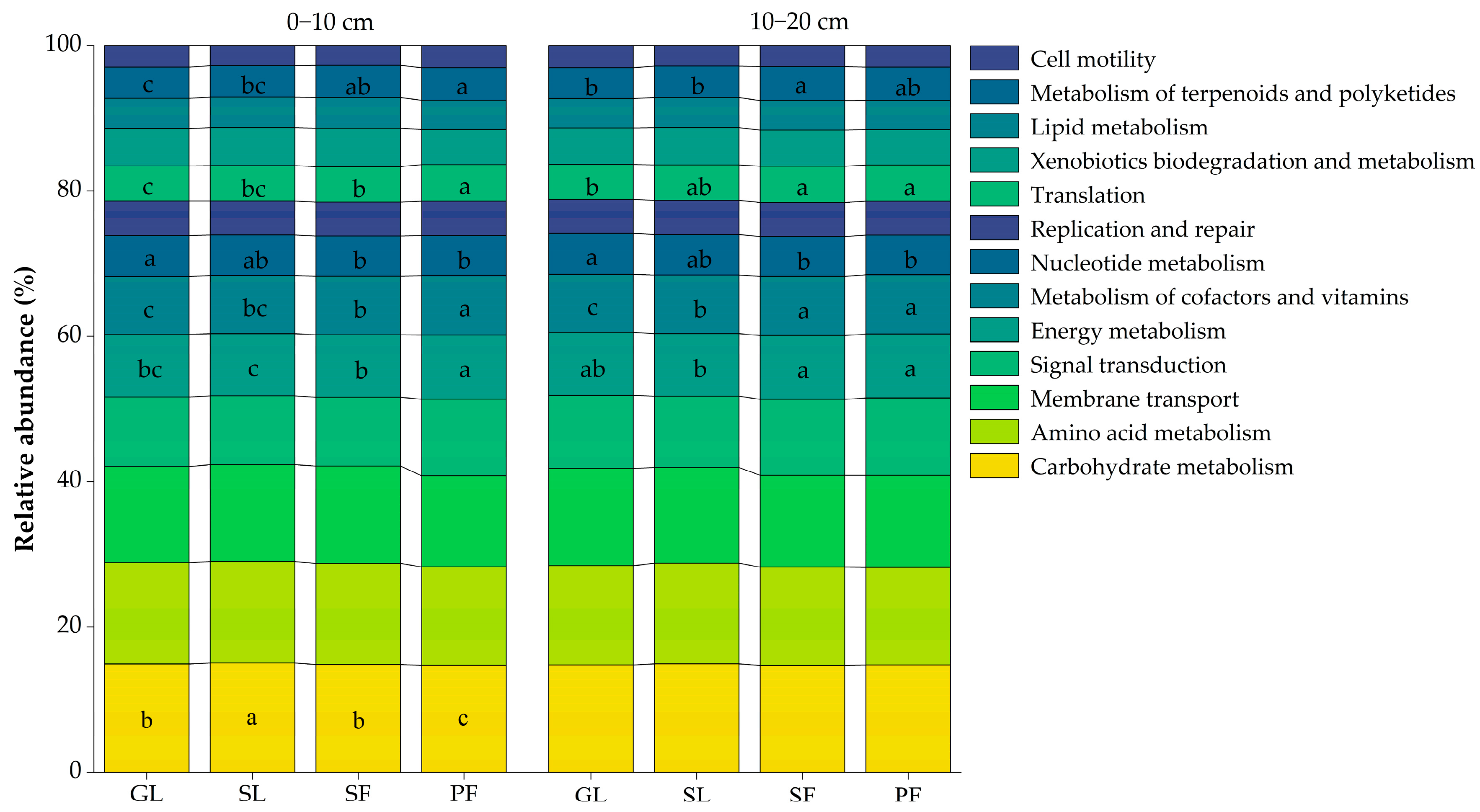
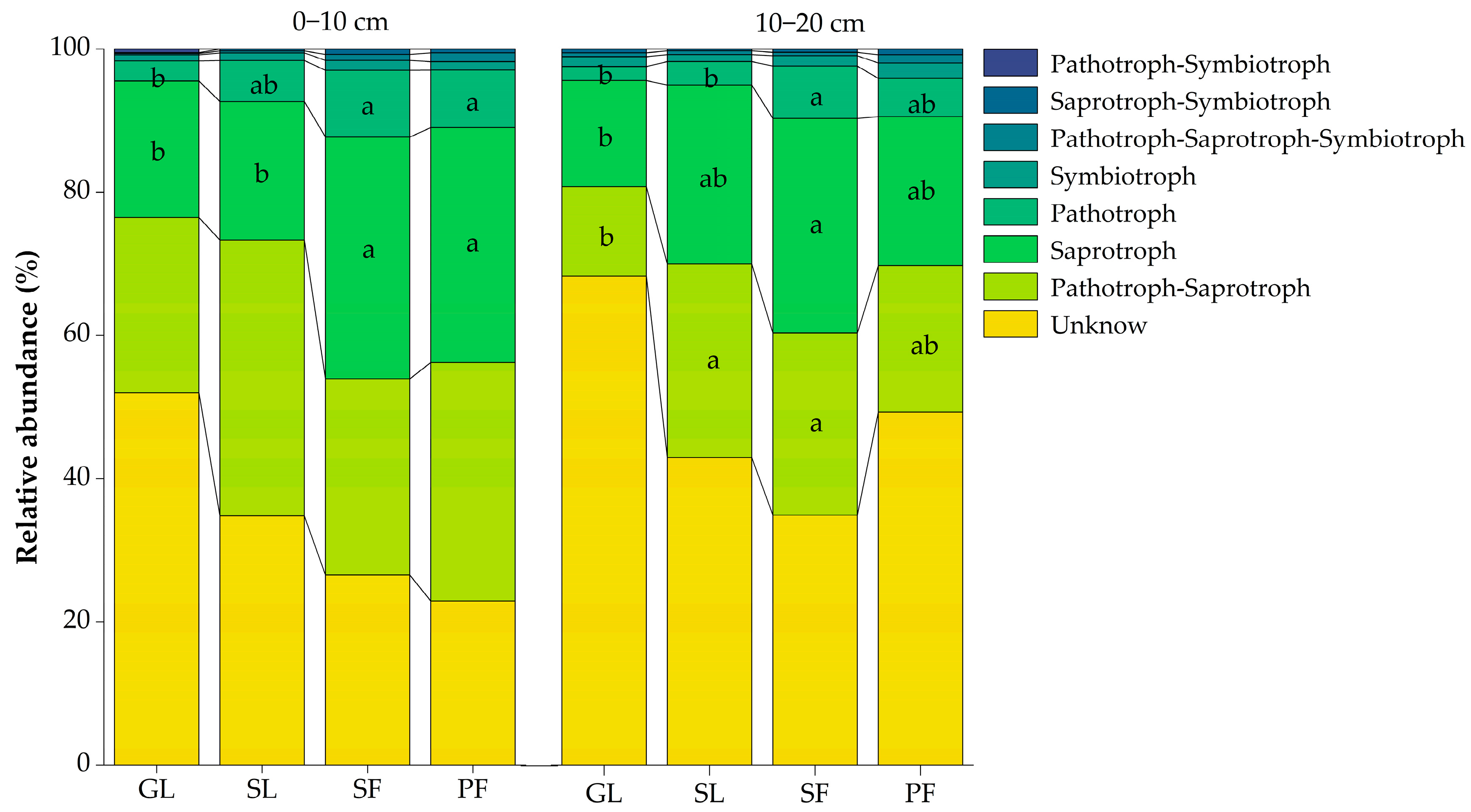
3.3. Soil Microbial Community Function in Different Vegetation Successional Stages
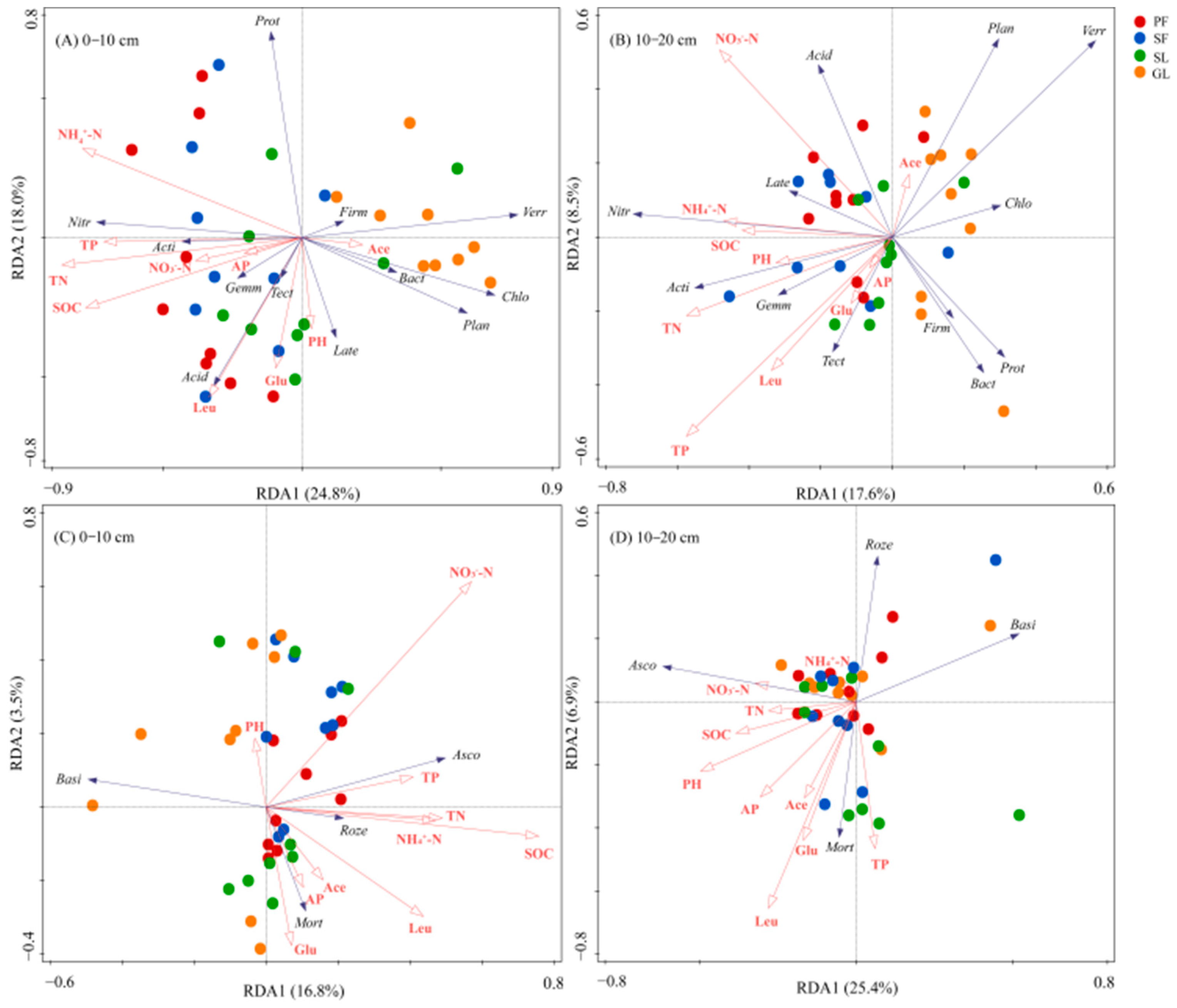
3.4. The Correlation between Soil Microbial Communities and Soil Properties
4. Discussion
4.1. Changes in Soil Microbial Community Diversity during Vegetation Succession
4.2. Changes in Soil Microbial Community Composition during Vegetation Succession
4.3. Changes in Ecological Function of Soil Microbial Communities during Vegetation Succession
4.4. Factors Affecting Soil Microbial Communities during Vegetation Succession
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Liu, X.M.; Yin, Z.Y.; Chen, H.; Cai, X.L.; Xie, Y.H.; Wang, S.J.; Lian, B. Changes in soil microbial communities from exposed rocks to arboreal rhizosphere during vegetation succession in a karst mountainous ecosystem. J. Plant Interact. 2021, 16, 550–563. [Google Scholar] [CrossRef]
- Jiang, S.; Xing, Y.J.; Liu, G.C.; Hu, C.Y.; Wang, X.C.; Yan, G.Y.; Wang, Q.G. Changes in soil bacterial and fungal community composition and functional groups during the succession of boreal forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Li, L.Q.; Wang, D.; Liu, X.Y.; Zhang, B.; Liu, Y.Z.; Xie, T.; Du, Y.X.; Pan, G.X. Soil organic carbon fractions and microbial community and functions under changes in vegetation: A case of vegetation succession in karst forest. Environ. Earth Sci. 2014, 71, 3727–3735. [Google Scholar] [CrossRef]
- Yu, Y.; Zheng, L.; Zhou, Y.J.; Sang, W.G.; Zhao, J.N.; Liu, L.; Li, C.; Xiao, C.W. Changes in soil microbial community structure and function following degradation in a temperate grassland. J. Plant Ecol. 2021, 14, 384–397. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, K.; Krause, S.M.B.; Li, S.P.; Wang, X.; Zhang, Z.C.; Shen, M.W.; Yang, Q.S.; Lian, J.Y.; Wang, X.H.; et al. Changes in assembly processes of soil microbial communities during secondary succession in two subtropical forests. Soil Biol. Biochem. 2021, 154, 108144. [Google Scholar] [CrossRef]
- Ren, C.J.; Liu, W.C.; Zhao, F.Z.; Zhong, Z.K.; Deng, J.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Soil bacterial and fungal diversity and compositions respond differently to forest development. Catena 2019, 181, 104071. [Google Scholar] [CrossRef]
- Zhang, K.R.; Cheng, X.L.; Shu, X.; Liu, Y.; Zhang, Q.F. Linking soil bacterial and fungal communities to vegetation succession following agricultural abandonment. Plant Soil 2018, 431, 19–36. [Google Scholar] [CrossRef]
- Yin, R.; Deng, H.; Wang, H.L.; Zhang, B. Vegetation type affects soil enzyme activities and microbial functional diversity following re-vegetation of a severely eroded red soil in sub-tropical China. Catena 2014, 115, 96–103. [Google Scholar] [CrossRef]
- Baldrian, P. Microbial activity and the dynamics of ecosystem processes in forest soils. Curr. Opin. Microbiol. 2017, 37, 128–134. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Jiang, L.F.; Luo, Y.Q. Trends in soil microbial communities during secondary succession. Soil Biol. Biochem. 2017, 115, 92–99. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Wang, Q.C.; Zhang, Y.; Zarif, N.; Ma, S.J.; Xu, L.Q. Variation in soil bacterial and fungal community composition at different successional stages of a broad-leaved Korean pine forest in the Lesser Hinggan Mountains. Forests 2022, 13, 625. [Google Scholar] [CrossRef]
- Peng, J.W.; Liu, H.; Hu, Y.; Sun, Y.; Liu, Q.; Li, J.G.; Dong, Y.H. Shift in soil bacterial communities from k- to r-strategists facilitates adaptation to grassland degradation. Land Degrad. Dev. 2022, 33, 2076–2091. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.R.; Huang, Y.T.; Fu, S.L.; Wang, J.X.; Ming, A.G.; Li, X.Z.; Yao, M.J.; Li, H. Tree species mixture inhibits soil organic carbon mineralization accompanied by decreased r-selected bacteria. Plant Soil 2018, 431, 203–216. [Google Scholar] [CrossRef]
- Cai, Z.Q.; Zhang, Y.H.; Yang, C.; Wang, S. Land-use type strongly shapes community composition, but not always diversity of soil microbes in tropical China. Catena 2018, 165, 369–380. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Luo, Y.Q. Effects of forest degradation on microbial communities and soil carbon cycling: A global meta-analysis. Global. Ecol. Biogeogr. 2018, 27, 110–124. [Google Scholar] [CrossRef]
- Wang, Z.H.; Bai, Y.; Hou, J.F.; Li, F.; Li, X.Q.; Cao, R.; Deng, Y.Y.; Wang, H.B.; Jiang, Y.R.; Yang, W.Q. The changes in soil microbial communities across a subalpine forest successional series. Forests 2022, 13, 289. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Bai, L.; Wang, J.Y.; Deng, J.; Ren, C.J.; Han, X.H.; Yang, G.H.; Wang, J. Change in soil bacterial community during secondary succession depend on plant and soil characteristics. Catena 2019, 173, 246–252. [Google Scholar] [CrossRef]
- Yang, N.; Ji, L.; Salahuddin; Yang, Y.C.; Yang, L.X. The influence of tree species on soil properties and microbial communities following afforestation of abandoned land in northeast China. Eur. J. Soil Biol. 2018, 85, 73–78. [Google Scholar] [CrossRef]
- Zhao, C.; Long, J.; Liao, H.K.; Zheng, C.L.; Li, J.; Liu, L.F.; Zhang, M.J. Dynamics of soil microbial communities following vegetation succession in a karst mountain ecosystem, Southwest China. Sci. Rep. 2019, 9, 2160. [Google Scholar] [CrossRef]
- Wakelin, S.A.; Gerard, E.; van Koten, C.; Banabas, M.; O’ Callaghan, M.; Nelson, P.N. Soil physicochemical properties impact more strongly on bacteria and fungi than conversion of grassland to oil palm. Pedobiologia 2016, 59, 83–91. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Banning, N.C.; Gleeson, D.B.; Grigg, A.H.; Grant, C.D.; Andersen, G.L.; Brodie, E.L.; Murphy, D.V. Soil microbial community successional patterns during forest ecosystem restoration. Appl. Environ. Microb. 2011, 77, 6158–6164. [Google Scholar] [CrossRef]
- Peng, S.M.; Liu, W.; Xu, G.; Pei, X.J.; Millerick, K.; Duan, B.L. A meta-analysis of soil microbial and physicochemical properties following native forest conversion. Catena 2021, 204, 105447. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Ren, C.J.; Han, X.H.; Yang, G.H.; Wang, J.; Doughty, R. Changes of soil microbial and enzyme activities are linked to soil C, N and P stoichiometry in afforested ecosystems. Forest. Ecol. Manag. 2018, 427, 289–295. [Google Scholar] [CrossRef]
- Maron, P.A.; Sarr, A.; Kaisermann, A.; Lévêque, J.; Mathieu, O.; Guigue, J.; Karimi, B.; Bernard, L.; Dequiedt, S.; Terrat, S.; et al. High microbial diversity promotes soil ecosystem functioning. Appl. Environ. Microb. 2018, 84, e02738-17. [Google Scholar] [CrossRef]
- Wagg, C.; Hautier, Y.; Pellkofer, S.; Banerjee, S.; Schmid, B.; van der Heijden, M.G.; Weigel, D.; Mori, A. Diversity and asynchrony in soil microbial communities stabilizes ecosystem functioning. Elife 2021, 10, e62813. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Wagg, C. Soil microbial diversity and agro-ecosystem functioning. Plant Soil 2013, 363, 1–5. [Google Scholar] [CrossRef]
- Yang, L.Y.; Barnard, R.; Kuzyakov, Y.; Tian, J. Bacterial communities drive the resistance of soil multifunctionality to land-use change in karst soils. Eur. J. Soil Biol. 2021, 104, 103313. [Google Scholar] [CrossRef]
- Li, J.; Delgado-Baquerizo, M.; Wang, J.T.; Hu, H.W.; Cai, Z.J.; Zhu, Y.N.; Singh, B.K. Fungal richness contributes to multifunctionality in boreal forest soil. Soil Biol. Biochem. 2019, 136, 107526. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.J.; Li, D.J.; Chen, H.S.; Wang, K.L. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Zeng, F.P.; Peng, W.X.; Song, T.Q.; Wang, K.L.; Wu, H.Y.; Song, X.J.; Zeng, Z.X. Changes in vegetation after 22 years’ natural restoration in the Karst disturbed area in northwestern Guangxi, China. Acta Ecol. Sin. 2007, 27, 5110–5119. [Google Scholar] [CrossRef]
- Ding, F.J.; Yuan, C.J.; Zhou, T.; Cheng, J.; Wu, P.; Ye, Y.Y. Water-use strategies and habitat adaptation of four tree species in karstic climax forest in Maolan. Water 2023, 15, 203. [Google Scholar] [CrossRef]
- Wu, P.; Zhou, H.; Cui, Y.C.; Zhao, W.J.; Hou, Y.J.; Tan, C.J.; Yang, G.N.; Ding, F.J. Stoichiometric characteristics of leaf, litter and soil during vegetation succession in Maolan National Nature Reserve, Guizhou, China. Sustainability 2022, 14, 16517. [Google Scholar] [CrossRef]
- Lu, M.Z.; Du, H.; Song, T.Q.; Peng, W.X.; Su, L.; Zhang, H.; Zeng, Z.X.; Wang, K.L.; Zeng, F.P. Effects of density dependence in an evergreen-deciduous broadleaf karst forest in southwest China. Forest. Ecol. Manag. 2021, 490, 119142. [Google Scholar] [CrossRef]
- Thomas, G.W. Methods of Soil Analysis. Part. 2. Chemical and Microbiological Properties: Exchangeable Cations; Soil Science Society of America: Madison, WI, USA, 1982; pp. 159–166. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis. Part. 3. Chemical Methods; John Wiley&Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Tang, Q.; Ding, F.J.; Zhu, S.X.; Wu, P.; Cui, Y.C.; Zhao, W.J.; Hou, Y.J. Effects of different vegetative succession stages on soil chemical properties and enzyme activities in karst region of Maolan. Ecol. Environ. Sci. 2020, 29, 1943–1952. [Google Scholar]
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Nagata, Y.; Fujiyama, A.; Tsuda, M.; Kurokawa, K. Design and Experimental Application of a Novel Non-Degenerate Universal Primer Set that Amplifies Prokaryotic 16S rRNA Genes with a Low Possibility to Amplify Eukaryotic rRNA Genes. DNA Res. 2014, 21, 217–227. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef]
- Aßhauer, K.P.; Wemheuer, B.; Daniel, R.; Meinicke, P. Tax4Fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 2015, 31, 2882–2884. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.W.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Thompson, H.W.; Mera, R.; Prasad, C. The Analysis of Variance (ANOVA). Nutr. Neurosci. 1999, 2, 43–55. [Google Scholar] [CrossRef]
- Somerfield, P.J.; Clarke, K.R.; Gorley, R.N. Analysis of similarities (ANOSIM) for 3-way designs. Austral Ecol. 2021, 46, 927–941. [Google Scholar] [CrossRef]
- Liu, J.; Jia, X.Y.; Yan, W.M.; Zhong, Y.Q.; Shangguan, Z.P. Changes in soil microbial community structure during long-term secondary succession. Land Degrad. Dev. 2020, 31, 1151–1166. [Google Scholar] [CrossRef]
- Qiang, W.; He, L.L.; Zhang, Y.; Liu, B.; Liu, Y.; Liu, Q.H.; Pang, X.Y. Aboveground vegetation and soil physicochemical properties jointly drive the shift of soil microbial community during subalpine secondary succession in southwest China. Catena 2021, 202, 105251. [Google Scholar] [CrossRef]
- Tian, Q.X.; Jiang, Y.; Tang, Y.N.; Wu, Y.; Tang, Z.Y.; Liu, F. Soil pH and organic carbon properties drive soil bacterial communities in surface and deep layers along an elevational gradient. Front. Microbiol. 2021, 12, 646124. [Google Scholar] [CrossRef]
- Song, S.Z.; Xiong, K.N.; Chi, Y.K.; He, C.; Fang, J.Z.; He, S.Y. Effect of cultivated pastures on soil bacterial communities in the karst rocky desertification area. Front. Microbiol. 2022, 13, 922989. [Google Scholar] [CrossRef]
- Burton, J.I.; Zenner, E.K.; Frelich, L.E.; Cornett, M.W. Patterns of plant community structure within and among primary and second-growth northern hardwood forest stands. Forest. Ecol. Manag. 2009, 258, 2556–2568. [Google Scholar] [CrossRef]
- Wang, G.Z.; Liu, Y.G.; Cui, M.; Zhou, Z.Y.; Zhang, Q.; Li, Y.J.; Ha, W.X.; Pang, D.B.; Luo, J.F.; Zhou, J.X. Effects of secondary succession on soil fungal and bacterial compositions and diversities in a karst area. Plant Soil 2022, 475, 91–102. [Google Scholar] [CrossRef]
- Wang, K.B.; Zhang, Y.W.; Tang, Z.S.; Shangguan, Z.P.; Chang, F.; Jia, F.A.; Chen, Y.P.; He, X.H.; Shi, W.Y.; Deng, L. Effects of grassland afforestation on structure and function of soil bacterial and fungal communities. Sci. Total Environ. 2019, 676, 396–406. [Google Scholar] [CrossRef]
- Spain, A.M.; Krumholz, L.R.; Elshahed, M.S. Abundance, composition, diversity and novelty of soil Proteobacteria. ISME J. 2009, 3, 992–1000. [Google Scholar] [CrossRef]
- Viitamäki, S.; Pessi, I.S.; Virkkala, A.; Niittynen, P.; Kemppinen, J.; Eronen-Rasimus, E.; Luoto, M.; Hultman, J. The activity and functions of soil microbial communities in the Finnish sub-Arctic vary across vegetation types. Fems Microbiol. Ecol. 2022, 98, c79. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, X.R.; Feng, J.; Zhu, S.F.; Shui, W. Metagenomic analysis reveals the different characteristics of microbial communities inside and outside the karst tiankeng. BMC Microbiol. 2022, 22, 115. [Google Scholar] [CrossRef]
- Xin, Y.; Ji, L.H.; Wang, Z.H.; Li, K.; Xu, X.Y.; Guo, D.F. Functional diversity and CO2 emission characteristics of soil bacteria during the succession of halophyte vegetation in the Yellow River Delta. Int. J. Environ. Res. Public Health 2022, 19, 12919. [Google Scholar] [CrossRef]
- Liu, M.L.; Li, X.R.; Zhu, R.Q.; Chen, N.; Ding, L.; Chen, C.Y. Vegetation richness, species identity and soil nutrients drive the shifts in soil bacterial communities during restoration process. Environ. Microbiol. Rep. 2021, 13, 411–424. [Google Scholar] [CrossRef]
- Zhao, W.; Yin, Y.L.; Li, S.X.; Dong, Y.L.; Su, S.F. Changes in soil fungal community composition and functional groups during the succession of Alpine grassland. Plant Soil 2023, 484, 201–216. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Peng, X.H.; Tamura, K.J.; Asano, M.; Takano, A.; Kawagoe, M.; Kamijo, T. Changes in soil physical and chemical properties during vegetation succession on Miyake-jima Island. Forests 2021, 12, 1435. [Google Scholar] [CrossRef]
- Xu, M.P.; Wang, J.Y.; Zhu, Y.F.; Han, X.H.; Ren, C.J.; Yang, G.H. Plant biomass and soil nutrients mainly explain the variation of soil microbial communities during secondary succession on the Loess Plateau. Microb. Ecol. 2022, 83, 114–126. [Google Scholar] [CrossRef]
- Fang, J.; Deng, Y.C.; Che, R.X.; Han, C.; Zhong, W.H. Bacterial community composition in soils covered by different vegetation types in the Yancheng tidal marsh. Environ. Sci. Pollut. R. 2020, 27, 21517–21532. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Zhao, X.Q.; Shi, Y.; Liang, Y.T.; Shen, R.F. Changes in soil bacterial communities with increasing distance from maize roots affected by ammonium and nitrate additions. Geoderma 2021, 398, 115102. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, Q.H.; Ding, P.W.; Li, K.F.; Yi, X.S.; He, J.; Peng, X.D.; Yan, Y.J.; Zhao, M.; Yang, Y.C. Rapid response of runoff carrying nitrogen loss to extreme rainfall in gentle slope farmland in the karst area of SW China. Water 2022, 14, 3341. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Si, G.C.; Wang, J.; Luo, T.X.; Zhang, G.X. Bacterial community in alpine grasslands along an altitudinal gradient on the Tibetan Plateau. FEMS Microbiol. Ecol. 2014, 87, 121–132. [Google Scholar] [CrossRef]
- Liu, M.S.; Zhang, W.H.; Wang, X.G.; Wang, F.H.; Dong, W.X.; Hu, C.S.; Liu, B.B.; Sun, R.B. Nitrogen leaching greatly impacts bacterial community and denitrifiers abundance in subsoil under long-term fertilization. Agric. Ecosyst. Environ. 2020, 294, 106885. [Google Scholar] [CrossRef]
| Soil Properties | Depth (cm) | Successional Stages | |||
|---|---|---|---|---|---|
| Grassland | Shrubland | Secondary Forest | Primary Forest | ||
| pH | 0–10 | 7.11 ± 0.08 a | 7.07 ± 0.21 a | 6.95 ± 0.25 a | 6.84 ± 0.19 a |
| 10–20 | 7.13 ± 0.07 a | 7.06 ± 0.23 a | 7.04 ± 0.09 a | 7.02 ± 0.14 a | |
| SOC (g·kg−1) | 0–10 | 33.37 ± 2.37 b | 60.4 ± 7.71 a | 58.82 ± 8.26 a | 77.19 ± 5.98 a |
| 10–20 | 27.98 ± 3.84 b | 44.38 ± 9.82 ab | 33.63 ± 5.36 b | 55.16 ± 6.17 a | |
| TN (g·kg−1) | 0–10 | 3.41 ± 0.89 c | 6.35 ± 0.77 b | 6.60 ± 0.68 b | 8.82 ± 0.54 a |
| 10–20 | 2.68 ± 0.59 b | 5.05 ± 1.13 a | 4.96 ± 0.58 a | 6.01 ± 0.35 a | |
| TP (g·kg−1) | 0–10 | 0.49 ± 0.10 b | 1.12 ± 0.15 a | 0.84 ± 0.08 a | 0.95 ± 0.05 a |
| 10–20 | 0.42 ± 0.09 b | 0.96 ± 0.17 a | 0.84 ± 0.08 a | 0.73 ± 0.10 ab | |
| AP (mg·kg−1) | 0–10 | 7.08 ± 1.03 a | 6.79 ± 1.22 a | 5.53 ± 0.69 a | 7.05 ± 0.72 a |
| 10–20 | 4.12 ± 0.77 a | 5.72 ± 1.27 a | 3.36 ± 0.62 a | 3.77 ± 0.49 a | |
| NH4+-N (mg·kg−1) | 0–10 | 13.69 ± 2.05 c | 22.03 ± 4.77 bc | 25.60 ± 3.34 b | 36.52 ± 4.17 a |
| 10–20 | 8.98 ± 1.26 c | 15.12 ± 3.66 bc | 18.50 ± 1.99 b | 26.60 ± 2.33 a | |
| NO3−-N (mg·kg−1) | 0–10 | 0.23 ± 0.11 b | 0.49 ± 0.11 ab | 1.35 ± 0.22 a | 1.37 ± 0.66 a |
| 10–20 | 0.11 ± 0.06 b | 0.55 ± 0.33 ab | 1.48 ± 0.43 ab | 2.13 ± 1.00 a | |
| Leu (μmol·h−1·g−1) | 0–10 | 262.88 ± 54.62 b | 555.51 ± 98.54 a | 418.59 ± 46.35 ab | 305.93 ± 60.21 b |
| 10–20 | 146.17 ± 36.44 b | 644.16 ± 119.56 a | 354.75 ± 90.11 b | 173.13 ± 38.00 b | |
| Ace (μmol·h−1·g−1) | 0–10 | 18.21 ± 3.86 b | 74.07 ± 17.46 a | 35.16 ± 6.83 b | 26.25 ± 2.98 b |
| 10–20 | 19.90 ± 4.81 b | 64.59 ± 8.90 a | 25.25 ± 5.15 b | 24.63 ± 4.81 b | |
| Glu (μmol·h−1·g−1) | 0–10 | 45.27 ± 12.43 ab | 97.88 ± 28.54 a | 66.32 ± 16.01 ab | 34.72 ± 9.89 b |
| 10–20 | 31.83 ± 5.54 b | 114.91 ± 32.74 a | 34.69 ± 7.45 b | 27.97 ± 6.35 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Tang, Q.; Yuan, C.; Zhu, S.; Ye, Y.; Wu, P.; Cui, Y.; Ding, F. Changes in Community Structure and Functional Characteristics of Soil Bacteria and Fungi along Karst Vegetation Succession. Forests 2023, 14, 1562. https://doi.org/10.3390/f14081562
Li Y, Tang Q, Yuan C, Zhu S, Ye Y, Wu P, Cui Y, Ding F. Changes in Community Structure and Functional Characteristics of Soil Bacteria and Fungi along Karst Vegetation Succession. Forests. 2023; 14(8):1562. https://doi.org/10.3390/f14081562
Chicago/Turabian StyleLi, Yuanyong, Qian Tang, Congjun Yuan, Sixi Zhu, Yuyan Ye, Peng Wu, Yingchun Cui, and Fangjun Ding. 2023. "Changes in Community Structure and Functional Characteristics of Soil Bacteria and Fungi along Karst Vegetation Succession" Forests 14, no. 8: 1562. https://doi.org/10.3390/f14081562
APA StyleLi, Y., Tang, Q., Yuan, C., Zhu, S., Ye, Y., Wu, P., Cui, Y., & Ding, F. (2023). Changes in Community Structure and Functional Characteristics of Soil Bacteria and Fungi along Karst Vegetation Succession. Forests, 14(8), 1562. https://doi.org/10.3390/f14081562






