Abstract
The isolation and bottom-up assembly of nano-cellulose by using microorganisms offers unique advantages that fine-tune and meet the main key design criteria of sustainability, rapid renewability, low toxicity and scalability for several industrial applications. As a biomaterial, several properties are required to maintain the quality and functional period of any product. Thus, researchers nowadays are extensively using microorganisms to enhance the yield and properties of plant nanocellulose. A microbial process requires approximately 20%–50% less energy compared to the chemical isolation process that consumes high energy due to the need for intense mechanical processing and harsh chemical treatments. A microbial process can also reduce production costs by around 30%–50% due to the use of renewable feedstocks, fewer chemical additives, and simplified purification steps. A chemical isolation process is typically more expensive due to the extensive use of chemicals, complex processing steps, and higher energy requirements. A microbial process also offers higher yields of nanocellulose with well-defined and uniform dimensions, leading to improved mechanical properties and enhanced performance in various applications, compared with the chemical isolation process, which may result in a wider range of nanocellulose sizes, potentially leading to variations in properties and performance. The present review discusses the role of different microorganisms (bacteria, yeasts and fungi) in the isolation and production of nanocellulose. The types and properties of nanocellulose from different sources are also discussed to show the main differences among them, showing the use of microorganisms and their products to enhance the yield and properties of nanocellulose isolation. Finally, the challenges and propositions regarding the isolation, production and enhancement the quality of nanocellulose are addressed.
1. Introduction
The use of microorganisms in the isolation and production of nanocellulose is a new trend in manufacturing industries. Microorganisms can play a significant role in the isolation of nanocellulose from plant-based sources such as wood, cotton, and various agricultural wastes [1]. Nanocellulose is a nanoscale fibrillated form of cellulose that exhibits excellent mechanical, thermal, and optical properties [2]. Cellulose was isolated for the first time from plants in 1838 by Anselme Payen [3], and since that time, it has continuously been an area of interest and has become the most in-demand biopolymer in most applications. Cellulose, a naturally occurring polysaccharide, is composed of glucose monomer units connected through β (1-4) ringed D-glucose C6H10O5, where each single glucose unit paired with intermolecular hydrogen bonding confirms its linear configuration with the formation of microfibrils [4]. Nanocellulose is generally defined as cellulose particles or fibers with dimensions in the nanometer range [5]. It can be derived from different cellulose sources, such as wood pulp, agricultural residues, or bacterial cultures, using various methods including mechanical treatment, enzymatic hydrolysis, or chemical processes [2,6]. The composition of cellulose remains consistent across all sources, but studies have indicated that there may be slight structural variations between different sources [6,7]. Most scientists have classified nanocellulose into three basic types, depending on its shape: cellulose nanofibers (CNF), cellulose nanocrystals (CNC) and bacterial nanocellulose (BNC) [8]. The demands of this modern age surely cannot be fulfilled by materials based on conventional cellulose production methods. Therefore, in recent years, cellulose has begun to be extracted at nanoscale, to furtherly enhance its unique properties (structural, mechanical, high aspect ratio and low density). Research shows that nanocellulose-based materials have excellent properties, which can be clearly seen from the accelerated number of papers about nanocellulose in the past two decades. The number of scientific publications related to nanocellulose has been increasing steadily over the past two decades (Figure 1), reflecting the growing interest in this field. The acceleration of scientific publications can be influenced by various factors, including research funding, technological advancements, industry demand, and emerging applications. Nanocellulose has attracted attention across multiple disciplines, including materials science, chemistry, biology, engineering, and biomedical research, leading to a multidisciplinary research landscape.
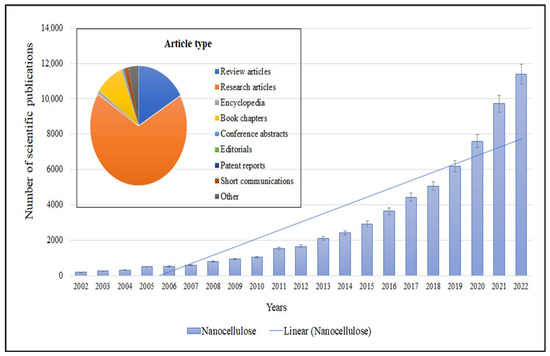
Figure 1.
Accelerated number of publications about nanocellulose in the past two decades.
Microorganisms such as bacteria, fungi, and yeasts have been used in the isolation of nanocellulose from plant sources [9,10]. These microorganisms can contribute to nanocellulose production either by the biosynthesis of nanocellulose (microbial nanocellulose) or by assessing the isolation of plant nanocellulose by breaking down the non-cellulosic components of the plant material, leaving behind a pure cellulose fraction that can be further processed into nanocellulose [11]. Bacteria such as Gluconacetobacter xylinus are well known in nanocellulose production. This bacterium produces cellulose in its extracellular matrix, which can be harvested and further processed into nanocellulose [12], while several fungi such as Trichoderma reesei are used to produce cellulase enzymes that can break down cellulose into its component parts, including nanocellulose [13]. Microbial enzymes are used in a process called enzymatic hydrolysis, which involves treating the plant material with the enzyme solution to break down the cellulose into nanocellulose [14]. The present review discusses the recent advances in the role of different microorganisms and their derived materials in cellulose and nanocellulose production industries. The types and properties of nanocellulose from different sources are also discussed to show the main differences among them, showing the use of microorganisms and their products to enhance the yield and properties of nanocellulose isolation. Finally, the challenges and propositions regarding the isolation, production and enhancement of quality of nanocellulose are addressed.
2. Nanocellulose Functional Material
Nanocellulose refers to cellulose fibers that have been broken down into nanoscale dimensions, typically ranging from a few nanometers to a few hundred nanometers in diameter [15]. Cellulose is the most abundant organic polymer found in nature and is the primary structural component of plant cell walls. The general structure of nanocellulose is derived from the hierarchical structure of cellulose, which is a linear polymer composed of repeating glucose units. In its native form, cellulose consists of long chains of glucose molecules linked together by β-1,4-glycosidic bonds [16,17]. Nanocellulose can be derived from various sources, including wood pulp, agricultural waste, and certain bacteria [18]. Table 1 presents a brief comparison between these three types. Cellulose, in general, possesses unique properties, such as high strength, high aspect ratio, low density, large surface area, and excellent biodegradability [19]. These characteristics make nanocellulose an attractive material for a wide range of applications in various industries.

Table 1.
Types and properties of different nanocellulose types depending on the physical characteristics.
2.1. Structure and Properties of Plant-Based Nanocellulose
Cellulose, hemicellulose, and lignin are the constituents of plant cell walls. Cellulose and hemicelluloses are the primary components, constituting around 34%–75% of the primary cell wall and 50%–80% of the secondary cell wall [31]. Lignin (10%–25% by dry weight) binds the cellulose (30%–45% by dry weight) and hemicellulose (20%–25% by dry weight) confirming the strength and stiffness of the cell wall [32,33,34]. There are different types of nanocellulose, including CNC and CNF. The source of CNF and CNC might be the same, but they differ in the physical properties. CNCs are obtained by hydrolyzing cellulose fibers to remove non-crystalline regions, resulting in highly crystalline and rigid nanoscale rods [35]. CNCs are rod-like structures with a high aspect ratio, meaning they have a long and narrow shape. Typically, CNCs have diameters ranging from a few nanometers to tens of nanometers and lengths ranging from several hundred nanometers to several micrometers [36]. CNCs are highly crystalline, meaning their cellulose chains are tightly packed together in an ordered manner [37]. The surface of CNCs contains hydroxyl (-OH) groups, which can provide sites for chemical modifications and interactions with other materials. CNFs, on the other hand, are produced by disintegrating cellulose fibers through mechanical or enzymatic processes, leading to long and flexible nanofibers [38]. CNFs are long and flexible fibrillar structures. The diameters of CNFs are typically in the range of a few nanometers to tens of nanometers. Their lengths can vary from micrometers to several micrometers, depending on the production method [39]. CNFs also possess a high degree of crystallinity, although they may have a slightly lower crystalline order compared to CNCs. Similar to CNCs, CNFs have abundant hydroxyl groups on their surface, enabling interactions with other substances [40]. Both CNCs and CNFs are derived from cellulose through various methods, such as acid hydrolysis, mechanical fibrillation, or enzymatic treatments. These processes break down the larger cellulose fibers into nanoscale dimensions while retaining the characteristic crystalline structure of cellulose. Nanocellulose fibers can vary depending on factors such as the cellulose source, processing methods, and post-treatment modifications. Additionally, nanocellulose fibers can further assemble into networks or form self-supporting films, offering additional structural complexity and functionality. Figure 2 illustrates the structure of plant nanocellulose from the origin to the cellulose molecules.

Figure 2.
Schematic drawing of plant-based nanocellulose and its role in the formation of plant cell walls (from cellulose fiber to cellulose molecule).
In recent years, there has been significant interest in the isolation of nanocellulose, driven by its versatility in a broad range of applications spanning both medical and non-medical fields. Numerous techniques have been used to produce and to enhance the production of nanocellulose, resulting in material with slightly different properties. Plant cellulose is found in conjunction with other components, primarily hemicellulose and lignin. Therefore, when isolating cellulose, it becomes necessary to eliminate or remove these compounds [41]. Lignin forms ester linkages and hydrogen bonds with hemicellulose and cellulose, and it plays a crucial role in maintaining the structural integrity of cellulosic fibers along with cellulose. As a result, except for cotton, natural or vegetable fibers are commonly referred to as lignocellulosic fibers due to the presence of lignin in their cell walls [42]. The intricate bonding of lignin with hemicellulose creates a matrix that surrounds cellulose molecules, making them highly resilient and resistant to network disruption. The sugar monomers forming long chains are interconnected, leading to robust intermolecular forces between them. Additionally, the cellulose molecule’s high linearity contributes to the tightly packed arrangement of cellulosic fibers [43]. The polymer-like structure and linkages of the polysaccharide contribute to the strong intermolecular forces between the chains of the fiber. Furthermore, the cellulose molecule’s pronounced linearity accounts for the crystalline characteristics observed in cellulosic fibers [43].
2.2. Structure and Properties of Microbial-Based Nanocellulose
Microbial nanocellulose is the type of nanocellulose produced by microorganisms, which exhibit high crystallinity compared with plant nanocellulose [44]. Microbial nanocellulose as a raw material possesses some unique and advanced mechanical properties, unique structural properties with high crystallinity, high water holding capacity, high purity (as compared to cellulose produced by plants) in addition to its excellent biodegradability [44]. Bacterial cellulose is only created as a nanomaterial naturally by various types of bacteria as an exopolysaccharide. Although it has the same molecular formula as plant celluloses, bacterial cellulose is free from lignin, pectin and hemicelluloses, which make it present in high purity, and it can be isolated by using less energy [45]. Microbial nanocellulose production cost may limit the extensive production of bacterial nanocellulose but its unique properties attract more efforts in order to enhance the development of new approaches to develop its production. Bacterial nanocellulose is the most famous type of microbial nanocellulose, which is produced extracellularly by several bacterial genera including Komagataeibacter, Agrobacterium, Aerobacter, Achromobacter, Alcaligenes, Azotobacter, Pseudomonas, Dickeya, Rhodobacter, Rhizobium, Sarcina, Salmonella and even Escherichia [46,47,48]. Acetobacter xylinus, which is a gram-negative strain of acetic-acid-producing bacteria, is the most efficient and researched producer of bacterial nanocellulose and has been reported as the most promising in terms of nanocellulose yield [49]. Most of these bacteria are aerobic and synthesize nanocellulose extracellularly as nano-fibrils with an average diameter ranging from 10 to 100 nm that are able to self-assemble into a 3D layered pellicle with a large water content of about 98% [44].
Microbial cellulose from bacteria can only be produced at nanoscale. The cellulose produced is highly pure, environmentally friendly and also requires less energy than plant cellulose for purification. In the fermenting process, the micro-organisms attach to cellulose fibers or simply move in the media freely and produce highly swollen gel-like structures [50]. Bacterial cellulose is secreted as a thin ribbon-shaped fibril (leather-like white pellicle), 100 nm wide and 2–3 nm long nanofibrils; bacterial cellulose produced through biosynthesis of cellulose is mainly built up of several bundles of microfibrils extracellularly [51]. The biosynthesis of bacterial nanocellulose starts with the creation of a β-1,4-glucan chain, which is done by polymerization of glucose units and then followed by the production and crystallization of the synthesized cellulose chain. Bacterial nanocellulose has been reported to form inside the bacterial cell, between the cytoplasm and the outer membranes of the bacterial cells [52]. After the biosynthesis of nanocellulose inside the cells, it is then spun from the cell by cellulose-exporting components in the cell membrane to form protofibrils only 2 to 4 nm in diameter. It was found that these protofibrils assemble a ribbon-shaped micro-fibril of approximately 80 nm [53]. In the purification process, while removing the culture media and waste, the microorganisms die. In order to produce good-quality cellulose, either microbial cellulose is washed repeatedly in a hot solution of sodium hydroxide and water until it reaches a neutral pH or through other methods, such as gamma radiation [54]. Although identical in chemical structure to plant-based cellulose, microbial nanocellulose is distinctly characterized by its readily extractable nanofiber network, degradability, excellent tensile strength due to high degrees of polymerization and crystallinity (80%–90%), and the possibility to control these and other physical properties including porosity during biosynthesis [55]. The bacterial cellulose is relatively pure and in crystalline form and no chemical treatments are required to isolate it as obtained from plants. The best advantage of bacterial cellulose is that it can be produced in a variety of shapes and textures, such as particles, whiskers, filaments, films, membranes, etc., and to culture bacteria in situ it can also utilize fruit syrup to grow and reproduce [56].
2.3. Structure and Properties of Other Sources of Nanocellulose
Macroalgae or seaweed are multicellular marine organisms that contain huge amounts of different polysaccharides, such as agar, alginates, fucoidan, carrageenan, agarose and cellulose [11]. The production of macroalgae-based nanocellulose has increased in the past few years with the increased production of macroalgae due to the growing market demands [57,58]. It has been reported that cellulose is present in most red and brown algae, such as Rhodophyta phylum and the Phaeophyceae class, respectively [59]. Some red algae, such as Gelidium amansii, are rich in carbohydrates (basically cellulose and agar), which form around 75% [60]. Albuquerque et al. [61] stated that the amount of carbohydrate present in Gracilaria birdie (different red algae) is around 73% and 8% protein. Meanwhile, the brown algae Sargassum muticum, Saccorhiza polyschides, and Sargassum filipendula were found to have carbohydrate content in the vicinity of 45%–52% [62,63]. Green algae also contain nanocellulose. Ulva lactuca was found to contain approximately 54.3% of carbohydrate, which is high for green algae applicable for nanocellulose isolation [59]. It has been also reported that some insects, such as silkworms and beetles, produce natural fibers that contain cellulose [64,65] and these fibers can be processed and treated to obtain nanocellulose. For example, silk fibers produced by silkworms contain fibroin, a protein that can be selectively removed to obtain cellulose-based nanofibers [66]. Insect-based nanocellulose has potential applications in biomedical materials and high-performance textiles. These animal-derived materials contain collagen, which can be chemically treated to isolate and convert cellulose into nanocellulose [67]. It is worth noting that the production and use of animal-based nanocellulose is still in the early stages of research and development. The majority of commercial nanocellulose products are derived from plant-based sources due to their abundance and cost-effectiveness. However, as the field progresses, animal-based nanocellulose may find niche applications in specialized industries.
2.4. Applications of Nanocellulose
Nanocellulose, with its unique properties and versatile nature, holds great potential for various applications across different industries. Nanocellulose finds valuable applications in the biomedical field, including tissue engineering, wound healing, drug delivery systems, and scaffolds for regenerative medicine. Its attractiveness for these applications stems from its biocompatibility, biodegradability, and capacity to mimic the properties of the extracellular matrix [68,69]. Nanocellulose has been also used in wound healing applications due to its excellent moisture-retention capabilities, mechanical strength, and antimicrobial properties [70]. It can promote wound healing by providing a moist environment, preventing infection, and facilitating the migration and proliferation of skin cells [71]. Other researchers have used nanocellulose as a carrier for controlled drug delivery [72]. Its high surface area and ability to encapsulate and release therapeutic agents in a controlled manner make it an ideal material for targeted and sustained drug delivery systems. Nanocellulose can also protect sensitive drugs from degradation and improve their stability [73]. Nanocellulose-based films or membranes can be used as a platform for biosensors [74]. Their high surface area, biocompatibility, and ability to immobilize biomolecules make them suitable for detecting and sensing various biological analytes, such as glucose, proteins, and DNA [75]. These are just a few examples of the wide range of biomedical applications of nanocellulose. Nanocellulose can enhance the properties of packaging materials, making them more sustainable, lightweight, and mechanically strong [76]. It can improve barrier properties against gases and liquids, providing better preservation and protection for food and other sensitive products. Nanocellulose has been incorporated into packaging films to improve their barrier properties against gases, such as oxygen and moisture [77]. Nanocellulose films act as an effective barrier, reducing the permeability of gases and prolonging the shelf life of packaged products [78]. This can help preserve the freshness and quality of food and other sensitive products [79]. Nanocellulose-based coatings can be applied to packaging materials to enhance their performance [80]. Nanocellulose-based packaging materials have a lower carbon footprint and contribute to reducing plastic waste and pollution. Nanocellulose-based packaging can be integrated with sensors or indicators to provide real-time information about the quality or condition of the packaged products [81]. Nanocellulose can be employed in environmental applications, including adsorbents for pollutant removal, oil spill cleanup materials, and sustainable alternatives to single-use plastics. Nanocellulose-based membranes and filters can effectively remove contaminants from water, such as heavy metals [82], dyes [83], and organic pollutants [84]. Nanocellulose-based sorbents offer an eco-friendly alternative to traditional sorbents, aiding in the remediation of oil-contaminated environments [85]. Air filtration has been also benefited from nanocellulose-based materials. Nanocellulose-based air filters can capture and remove particulate matter, allergens, and pollutants from the air [86]. These filters have high filtration efficiency, low pressure drop, and can be produced from sustainable sources, providing cleaner indoor and outdoor air quality [87]. These environmental applications of nanocellulose highlight its potential for mitigating environmental challenges, promoting sustainability, and contributing to a cleaner and healthier planet. Nanocellulose can be also used in several other applications, such as electronic devices, including flexible displays, sensors, and energy storage systems [88]. Its high surface area and electrical conductivity make it a promising material for these applications [89]. The film-forming properties of nanocellulose can help reduce the appearance of fine lines and wrinkles, resulting in a more youthful complexion. It is important to note that the safety and regulatory aspects of nanocellulose in cosmetic products are still being evaluated, and compliance with relevant regulations and guidelines is crucial.
3. The Role of Microorganisms in Nanocellulose Isolation
The isolation of nanocellulose from plant sources can be achieved through various methods, including mechanical, chemical, and biological processes [90]. While mechanical and chemical methods are commonly used, the use of microorganisms presents an alternative and potentially more environmentally friendly approach [91]. Microorganisms such as bacteria and fungi have the ability to degrade cellulose due to the presence of cellulase enzymes. These enzymes can break down cellulose into its individual sugar units, which can then be further processed to obtain nanocellulose [92].
3.1. The Role of Bacteria
Different types of enzymes or combinations of enzymes are produced by various bacteria, which are employed in the sequential or synergistic breakdown of cellulose and its conversion into monosaccharides [93]. Enzymes produced by bacteria such as Bacillus subtillis, Pseudomonas fluorescens, E. coli, and Serratia marcescens are commonly utilized for this purpose [94]. It is important to note that the specific bacteria, cultivation conditions, enzyme production, and isolation methods can vary depending on the desired properties and applications of the nanocellulose. Additionally, optimizing the efficiency and scalability of nanocellulose production using bacteria is an ongoing area of research. As reported in several studies, Caldicellulosiruptor bescii is one of the thermophilic bacteria able to produce multifunction enzymes, which can be utilized for the production of nanocellulose from lignocellulosic biomass [95,96]. It has some advantages, such as reduction of cost of bioconversion, decreased contamination probability; the mixing rate can be improved and the kinetics also enhanced [97]. In one study, Ghosh et al. [98] employed Pseudomonas fluorescens, a bacteria known for its ability to produce cellulolytic enzymes. These enzymes facilitated the degradation of hemicellulose within lignin biomass, resulting in the cleavage of carbohydrates into monosaccharides. The specific conditions and parameters for Pseudomonas fluorescens-based nanocellulose isolation may vary based on several factors, such as the specific strain of bacteria, the cellulase production characteristics, and the properties desired for the final nanocellulose product. Mortabit et al. [99] used three different species of Bacillus including B. subtilis, Bacillus licheniformis and Bacillus spp. for the isolation of carboxymethyl cellulase. The production was characterized according to pH, incubation period, temperature and source of carbon. The enzymatic activity reduced at 60 °C to 50% of what it was at 37 °C and the optimum pH for B. subtilis and Bacillus licheniformis was found to be 7.0, while for Bacillus sp. it was only was 6.0. Again, measured pH was 8.0, when enzymatic activity was 73%, 75% and 66% for Bacillus licheniformis, B. subtilis and Bacillus sp., respectively, at pH 8.0, compared to that of pH at 7.0. It has been reported that the effect of pH on Bacillus enzyme activity can vary depending on the specific enzyme being considered. However, Bacillus enzymes, including cellulases, amylases, proteases, and lipases, generally display activity within a broad pH range [100,101]. In a recent study, Sadalage et al. [102] successfully demonstrated a methodological approach that utilized a cellulolytic bacterial consortium to valorize different lignocellulose biomasses resulting in the production of microcrystalline cellulose and bacterial nanocellulose. Through various spectroscopy and imaging techniques (Figure 3), the authors observed that the microcrystalline cellulose obtained from the valorization process exhibited microcrystalline characteristics, irregular shapes, higher carbon content, and increased crystallinity compared to the original lignocellulose biomasses. They used a novel strain of Bacillus cabrialesii capable of producing BNC, which was cultivated in Hestrin–Schramm medium supplemented with reducing sugars released during the valorization process. This led to the production of nano-sized BNC fibrils that formed ultrafine, aggregated, crystallized networks. This study proposes a straightforward and environmentally friendly methodological approach that can be employed for managing lignocellulose biomasses waste disposal while simultaneously valorizing it to produce various value-added products. This approach holds promise for the sustainable utilization of lignocellulose biomasses waste and the generation of valuable materials.

Figure 3.
Illustration of the isolation approach of microcrystalline cellulose and bacterial nanocellulose using biological treatment and Bacillus cabrialesii for lignocellulosic biomass wastes. Adapted with permission from [102].
It is important to note that the specific bacteria, cultivation conditions, pretreatment methods, and extraction techniques can vary depending on the desired properties and applications of the nanocellulose. Optimization of the bacterial treatment process for nanocellulose isolation is still an ongoing area of research to improve efficiency and scalability. Citrus waste including peels and pulps from citrus fruits has been also utilized in the production of nanocellulose using different types of bacteria. Mariño et al. [103] utilized mixed hydrolytic enzymes produced by Xanthomonas axonopodis pv to isolate nanocellulose from citrus waste. The authors reported significant enhancement in the production yield of nanocellulose by using bacterial treatment and a 13% higher crystallinity index compared with nanocellulose isolated by conventional mechanical treatments. In a different study, the authors compared the production of cellulose nanocrystals and fermentable sugars from two bacteria, namely Caldicellulosiruptor bescci CelA and the classical Hypocrea jecorina (formerly Trichoderma reesei) Cel7A [104]. The mode of action of both the organisms was different; Caldicellulosiruptor bescci CelA hydrolysis occurred locally, while Trichoderma reesei Cel7A hydrolysis occurred progressively. The results obtained were unexpected, as CNCs produced from the bacteria (Caldicellulosiruptor bescci CelA) had higher glucose yield and slightly more suitable characteristics than the nanocellulose produced by the fungi (Trichoderma reesei Cel7A). The choice of bacterial species, cultivation conditions, pretreatment methods, and downstream processing techniques will influence the efficiency and characteristics of the nanocellulose produced. Ongoing research and optimization efforts are focused on maximizing the potential of bacteria in nanocellulose isolation and expanding its applications in various industries, including biomedical, food packaging, and nanocomposite materials.
3.2. The Role of Fungi
Fungi are the microorganisms which produce the cellulase enzymes used for the production of the nanocellulose. Several fungi such as Aspergillus niger, Trichoderma, and other cellulolytic fungi have been explored for their ability to produce cellulase enzymes and contribute to the isolation of nanocellulose [105]. These microorganisms possess cellulolytic enzymes, including endoglucanases, exoglucanases, and cellobiohydrolases, which can efficiently break down cellulose into smaller fragments suitable for nanocellulose production [106]. Most studied fungi belong to genus Aspergillus sp. and Trichoderma sp. for enzymatic treatment of cellulosic biomass for production of nanocellulose. Cellulose nanocrystals were isolated through controlled enzymatic hydrolysis by using fungi Trichoderma reseei from MCC (prepared by acid hydrolysis using hydrochloric acid) from cotton and it was observed that the fungus itself consumed MCC for its growth, as cellulose is the carbon source for microorganisms [107]. Moreover, Janardhnan and Sain [108] isolated CNFs enzymatically with OS1, a fungus (isolated from infected trees with Dutchelm disease) from bleached kraft pulp and observed a notable shift towards lower fiber diameters, which finally improved the properties, also confirmed in two other studies [109,110]. In a recent study, Squinca et al. [111], the authors evaluated the possibility of utilizing fungal enzymes generated on-site for the production of nanocellulose, using eucalyptus cellulose pulp as a representative raw material (Figure 4). The authors used endoglucanase activity (17.09 IU/mg protein), which was produced by Aspergillus niger and, subsequent to hydrolysis, the resulting materials were subjected to sonication. It is well known that endoglucanase is an enzyme that specifically targets the internal bonds within cellulose molecules and cleaves them, resulting in the degradation of cellulose into smaller fragments [112]. The authors revealed that longer ball-milling pretreatment and reaction durations favored the extraction of CNCs. The optimal yield of CNCs (24.6%) was achieved by subjecting the ball-milled cellulose pulp to enzymatic hydrolysis for 96 h, followed by sonication [111]. The same authors reported that their isolated CNCs exhibited an approximate length of 294.0 nm and a diameter of 24.0 nm. These findings not only demonstrated the successful extraction of nanocelluloses using on-site generated enzymes but also highlighted the potential of the sustainable integrated process described in this study to contribute to the advancement of the emerging biobased economy.

Figure 4.
Illustration of using endoglucanase produced by Aspergillus niger in nanocellulose isolation from eucalyptus cellulose pulp. Adapted with permission from ACS [111].
To modify the surface of the produced nanocellulose, enzymatic treatment with xylanase can be employed to eliminate lignin and hemicellulose residues [113]. Xylanase, which is primarily produced by fungi, particularly from the genera Aspergillus and Trichoderma, is used for this purpose [114]. Various types of fungi can be found and utilized for enzymatic treatment. In one report, nanocellulose was isolated from flax straw (biomass) enzymatically through lignolytic enzymes produced in the lab by fungal strains of Aspergillus niger by solid fermentation and resulted in reduction of fibers; the authors concluded that the CNFs produced are of low cost and ecofriendly and can potentially be used for textile fabrication [115]. The kinetics and thermodynamic properties of cellobiohydrolase (CBH) cellulase from the fungus Trichoderma harzianum were examined and showed maximum activity at pH 5 at 60 °C, confirming that it has great potential for industrial applications [116]. In a pioneering study, nanocellulose was successfully obtained from agricultural waste through enzymatic treatment using the fungus Humicola Fuscoatra Egyptia X4 [117]. The findings demonstrated that the produced nanocellulose exhibited excellent quality, characterized by high crystallinity, a large surface area, and remarkable stability.
The acyl modification of hydroxyethylcellulose has been documented to occur in the presence of β-galactosidase derived from the fungus Aspergillus oryzae. Moreover, the introduction of acylating agents such as vinyl acrylate and vinyl propionate through transesterification has the potential to enhance the hydrophobicity of nanocellulose [118]. In one research study, it was confirmed that CNCs and CNFs can be efficiently isolated from citrus-processing waste from oranges by adding it to a enzymatic cocktail of cellulast, pulpzyme HA and b-galactosidase from Aspergillus oryzae, and the efficiency depends on the amount of individual units of enzymes in the cocktail [119]. In another report, it was explained that xylanase isolated from a T. reesei addition in an enzymatic cocktail can enhance the enzymatic activity and thus enhance the property of CNCs [120]. To isolate CNCs at higher yields, reaction time, temperature and enzyme activities should be considered for each substrate; otherwise, hydrolysis of residues of lignin/hemicellulose with EG can become difficult. This fact was experienced during the isolation of CNCs from sugarcane bagasse, bleached cellulose, unbleached kraft pulp and holocellulose, enzymatically by using a combination of EG from Pyrococcus horikoshii and ß-glucosidase from Pyrococcus furiosus [121]. It is important to note that the specific fungal species, cultivation conditions, enzyme production, and isolation methods can vary based on the desired properties and applications of the nanocellulose. Research in this field is ongoing to optimize fungal strains, enzyme production, and isolation techniques to improve the efficiency and scalability of nanocellulose production using fungi.
Yeasts also play a significant role in nanocellulose isolation, particularly in the production of yeast-derived nanocellulose. Yeast cellulases are the area of interest now-a-days as they are stable at high temperatures up to 700 °C and are capable of producing thermostable cellulase and can be used at a broader range of pH [122]. Yeasts, under suitable conditions, can convert glucose or other carbon sources into cellulose through the action of cellulose synthase enzymes [123]. This results in the formation of nanocellulose fibrils, which can be further processed into nanocellulose materials. Yeasts are cultivated in nutrient-rich media containing carbon sources, nitrogen sources, and other essential nutrients. The cultivation conditions, such as temperature, pH, and oxygen availability, are optimized to promote cellulose synthesis and nanocellulose production. Yeasts also produce other enzymes such as carboxy methyl cellulases enzyme, filter paperase (FPase), and β-glucosidases [124]. These enzymes have been isolated from wood chips, decaying wood grown in CMC for 51 yeast strains of the genera Kluyveromyces, Candida, Pichia and Filobasidium [125]. Nanocellulose isolation from citrus processing waste from oranges using fermentation in three different strains of Saccharomyces cerevisiae and two Candida sp., where citrus processing waste is enzymatically converted into fermentable sugars and finally nanocellulose, was reported for the first time [119]. After the yeast culture has produced nanocellulose, the material needs to be separated and purified. The process typically involves physical and chemical treatments to remove yeast cells, impurities, and residual media components. Techniques such as washing, filtration, and centrifugation are commonly used for purification. Božič et al. [126] reported that biocompatibility of CNFs can be improved by using commercial hexokinase (Saccharomyces cerevisiae), which phosphorylate the surface of CNFs and improve the degree of substitution. In a recent study, Atatoprak et al. [127] fractionated white and red grape stalk biomass to maximize its economic value by generating fermentable sugars and other valuable products. The authors initially obtained high yields of extractives and lignin, resulting in a biomass rich in cellulose and hemicellulose, which was then subjected to both acid and enzymatic hydrolysis processes to produce fermentable sugars (Figure 5). The authors then utilized the obtained biosugars in fermentation processes employing two yeasts, Pichia stipitis and Saccharomyces cerevisiae. Owing to the presence of higher quantities of xylose, P. stipitis demonstrated higher ethanol yields compared to S. cerevisiae, which has a preference for glucose. In the same study, cellulose nanocrystals were produced from the residual biomass that did not contain monosaccharides. This integrated valorization approach of grape stalks, followed by the application of one of the valorized streams, represents a significant advancement in the field. The fractionation of grape stalks biomass resulted in the production of fermentable sugars and other valuable products, including cellulose nanocrystals. The utilization of these streams demonstrates a novel and comprehensive valorization approach for grape stalks, highlighting their potential for sustainable and economically viable applications.
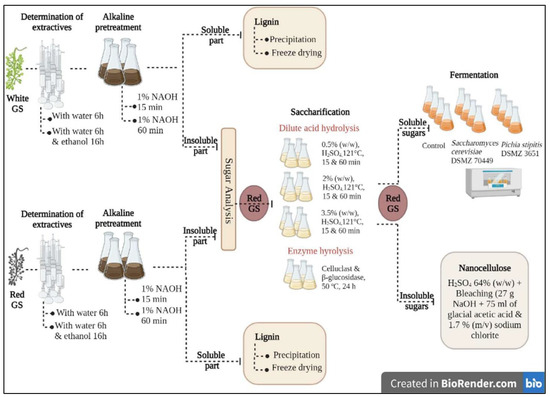
Figure 5.
Schematic drawing of nanocellulose isolation using red grape stalk valorization and yeast fermentation. Created with BioRender.com and adapted with permission from Atatoprak et al. [127].
4. Mechanism of Microbial Process in Nanocellulose Isolation
The microbial process for nanocellulose isolation from plants involves the use of microorganisms that possess cellulolytic enzymes capable of breaking down cellulose into its individual components [128]. Microbes with cellulolytic capabilities are selected based on their ability to produce a variety of cellulases, which are enzymes that specifically break down cellulose. Common examples include bacteria like Bacillus subtillis, Pseudomonas fluorescens, and Serratia marcescens, as well as fungi from the genera Aspergillus and Trichoderma [94]. The plant biomass undergoes a pretreatment step to remove lignin and hemicellulose, which can hinder cellulose accessibility. Various methods, such as chemical or enzymatic pretreatment, can be employed to facilitate the breakdown of these components [129,130]. The pretreated biomass is then subjected to microorganisms or their enzymes for enzymatic hydrolysis, where the cellulolytic enzymes produced by the selected microorganisms are introduced [131]. In a recent work, Zielińska et al. [132] developed a novel microbial process to isolate nanocellulose with strictly and well defined dispersion and structural parameters (Figure 6a). The authors utilized microscopic fungi Trichoderma reesei and Aspergillus sp. for the isolation process and controlled enzymatic hydrolysis and they reported that their findings have demonstrated that the effectiveness of converting cellulose material through biological means relies on the specific enzymes employed. Utilizing a mixture of cellulases derived from a Trichoderma fungus has proven to be a successful approach for obtaining nanoscale cellulose with minimal variation in particle size. Microbial enzymes act on the cellulose chains, breaking them down into smaller fragments and ultimately into monosaccharides, such as glucose (Figure 6b). Following enzymatic hydrolysis, the resulting cellulose hydrolysate, containing monosaccharides and other byproducts, is typically separated from the enzyme mixture [128]. It has been reported that the effectiveness of the microbial enzyme treatment procedure is impacted by the cell wall structure of cellulose pulp fibers, which includes factors like pore size, pore distribution, and surface characteristics [133]. The application of mechanical treatments to the fibers can enhance the specific surface area and porosity, thereby improving the accessibility of cellulose fibers to enzymes [128]. Consequently, this leads to an enhancement in enzymatic treatment efficiency. By employing mechanical refining as a pre-treatment step, the effectiveness of enzymatic treatment is enhanced, resulting in a significant increase in the reactivity of dissolving pulp [134,135]. This configuration facilitates better accessibility of cellulose fibers to enzyme molecules, leading to improved process efficiency in terms of enzyme utilization and energy consumption. Additional purification steps, such as washing and drying, may be employed to obtain the desired nanocellulose product. During the microbial process, the microorganisms not only produce cellulolytic enzymes but also aid in the breakdown of cellulose through synergistic or sequential action [136]. The enzymes work cooperatively, cleaving the glycosidic bonds of cellulose chains and converting them into soluble monosaccharides. This enzymatic action, combined with the microorganisms’ ability to grow and metabolize cellulose, contributes to the efficient conversion of plant cellulose into nanocellulose [137]. Overall, the microbial process for nanocellulose isolation from plants offers a promising and sustainable alternative to traditional methods. By harnessing the enzymatic capabilities of microorganisms, it allows for the production of high-quality nanocellulose with reduced energy consumption and potential scalability for industrial applications. Ongoing research in this area continues to explore and optimize the microbial process for improved nanocellulose production.

Figure 6.
Mechanism of microbial process in nanocellulose isolation. (a) Schematic drawing for enzymatic hydrolysis of cellulose. (b) The effect of incorporating mechanical treatment with enzymatic treatment in nanocellulose production. Adapted with permission from Zielińska et al. [132] (a) and Tong et al. [128] (b).
5. Advantages of Microbial Process Compared to Traditional Isolation Processes
The microbial process for nanocellulose isolation from plant biomass offers several advantages over traditional methods, including lower energy consumption, milder reaction conditions, and potentially higher yields of nanocellulose [32,138]. Utilizing microorganisms for nanocellulose isolation aligns with the principles of sustainability and renewable resources. Microorganisms produce a range of cellulolytic enzymes that are highly specific to cellulose, allowing for selective breakdown and isolation of nanocellulose from plant biomass. This specificity results in higher purity and quality of the obtained nanocellulose. The microbial process offers advantages such as energy efficiency, environmental friendliness, higher yields, versatility, sustainability, and the potential for functionalization. These benefits make the microbial process an attractive approach for the isolation of nanocellulose from plant biomass.
5.1. Lower Energy Consumption
Lower energy consumption is one of the key advantages of enzymatic treatment, making it an attractive option for the commercial production of nanocellulose. The conventional methods of nanocellulose production often involve high-energy processes such as mechanical refining or chemical treatments, which can be energy-intensive and costly [139,140]. The homogenization process as an example has posed a significant challenge primarily due to its high energy requirements, ranging from 25,000 to 70,000 kWh per tonne [128,141]. Most of other conventional mechanical processes are also energy-intensive and linked with the main drawback of larger fragments of cellulose [142]. Microbial processes generally require lower energy inputs compared to chemical or mechanical methods for nanocellulose isolation. The enzymatic breakdown of cellulose by microorganisms can proceed under milder reaction conditions, reducing energy consumption. Enzymatic treatment offers a more sustainable and efficient alternative by utilizing enzymes to selectively degrade cellulose fibers [143]. Compared to mechanical or chemical methods, enzymatic treatment requires milder process conditions, such as lower temperatures and less severe chemical agents, resulting in reduced energy consumption.
The energy consumption in the production of MFC from bleached hardwood pulp was examined in a study by Spence et al. [144]. The findings showed that the total energy consumption varied depending on the processing methods employed. Specifically, for homogenization with a homogenizer and pretreatment with a Valley beater, the energy consumption was 22,000 kWh per tonne. In contrast, homogenization with a microfluidizer and pretreatment with a Valley beater resulted in a lower energy consumption of 3558 kWh per tonne. Micro-grinding without pretreatment had even lower energy requirements, measuring 1550 kWh per tonne. Enzymes, such as cellulases, can specifically target and break down the amorphous regions of cellulose, facilitating the isolation of nanocellulose with high purity and yield. This enzymatic approach minimizes the need for extensive mechanical processing, which often requires significant energy input. In a study conducted by Mohlin et al. [145], bleached softwood pulp was treated with one unit of microbial cellulases per gram prior to refining using an industrial disc refiner. The authors observed a significant reduction in refining energy, ranging from 45 to 65 kWh per tonne or approximately 40% to 70%. Similarly, Bajpai et al. [146] reported a laboratory study where pre-treatment with an enzyme product containing both hemicellulase and cellulase resulted in a decrease in refining energy by 18% to 45%. In pilot trials, the energy savings were measured at 25 to 54 kWh per metric tonne. These findings were further validated in mill trials, which showed refining-energy savings of about 70 kWh per metric tonne for softwood pulp and 30 kWh per metric tonne for hardwood pulp. In a study by Lecourt et al. [147], pretreatment of softwood bleached kraft pulp with a commercial cellulase prior to disc refining led to approximately 20% electric energy savings, equivalent to 50 kWh per tonne, while maintaining a specific freeness or tensile breaking length. These studies demonstrate the effectiveness of enzyme pre-treatments in reducing the energy consumption during the refining process for various types of pulps. The use of a microbial process resulted in significant energy savings.
5.2. Higher Production Yields of Nanocellulose
Microbial processes have the potential to achieve higher yields of nanocellulose compared to traditional methods. The synergistic action of multiple cellulolytic enzymes produced by microorganisms enables efficient cellulose degradation, leading to increased nanocellulose production. Numerous recent studies have explored the enzymatic approach for producing nanocellulose using microbial processes and cellulase enzymes. In a recent study, Beltramino et al. [129] conducted research on cotton linter and achieved an approximately 82% yield of cellulose nanocrystals by treating it with a cellulase enzyme derived from the Cerrena sp. Fungus. This was accomplished by optimizing the conditions, which involved a 2-h hydrolysis using an enzyme dosage of 20 U g−1 odp, followed by sulfuric acid hydrolysis. The nanocellulose yield obtained with the enzyme treatment was 21 percentage points higher compared to the yield obtained with the conventional chemical approach. Furthermore, the microbial pretreatment led to a reduction in surface charge while increasing the crystallinity of the resulting nanocellulose. Traditional isolation techniques for nanocellulose typically involve extensive mechanical processing, such as high-pressure homogenization or ultrasonication, to break down cellulose fibers into smaller dimensions. However, these methods often result in lower yields and require harsh chemicals or energy-intensive processes [148]. In contrast, microbial enzyme-based methods utilize specific enzymes, typically cellulases, that are highly effective in breaking down cellulose fibers, leading to a higher yield of nanocellulose. The enzymes act specifically on cellulose, selectively degrading it into nanoscale particles, thereby maximizing the yield. In a research study conducted by Liu et al. [149], a mono-component endoglucanase (with a cellulolytic activity of 2036 U/mL) was employed for enzymatic pretreatment of bleached bagasse and softwood kraft pulp before grinding them to produce cellulose nanofibrils. The authors reported that the enzymatic treatment (carried out at a temperature of 50 °C, pH 7, and 200 rpm for 12 h) significantly improved the nanocellulose yield. The highest yield of 70.56% was achieved in enzyme-treated bleached bagasse after 2 h of grinding. Furthermore, the enzymatic pretreatment resulted in a reduction of specific net energy consumption during defibrillation by 59.71% for bleached bagasse and 42.98% for softwood kraft pulp [149]. In another investigation by Squinca et al. [111], eucalyptus cellulose kraft pulp was pretreated using ball milling for 90 min, followed by enzyme hydrolysis using a cellulolytic enzymatic complex. This complex, produced on-site using Aspergillus niger, exhibited high endoglucanase specific activity (17.09 IU/mg protein). Sonication for 5 min was also performed. The maximum yield of cellulose nanocrystals obtained was 24.6%, which is higher than from a non-enzymatic-based approach (22.3%). These nanocelluloses had a diameter of 24.0 nm, a length of 294 nm, and a crystallinity index of 78.3%. These findings indicate the successful isolation of nanocellulose from biomass using on-site produced enzymes. Overall, microbial enzyme-based methods have demonstrated their superiority over traditional isolation techniques in terms of higher yields of nanocellulose. Their ability to efficiently degrade cellulose fibers into nanoscale particles under milder conditions, while providing greater control and sustainability, makes them a promising avenue for the production of nanocellulose in various applications.
5.3. Milder Reaction Conditions and Ease of Scale-Up
Microbial processes are often more environmentally friendly compared to chemical processes. They eliminate the need for harsh chemicals and solvents, thereby reducing the generation of hazardous waste and minimizing environmental impact. The production of microbial enzymes can contribute to the overall cost of the process. Factors such as the choice of microbial strain, fermentation conditions, downstream processing, and scale of production can impact the cost of enzyme production. Optimization of enzyme production processes can help reduce costs [104]. The scale of nanocellulose production can impact the cost per unit. Scaling up microbial processes can lead to economies of scale, resulting in reduced costs. However, initial investments in infrastructure and equipment may be required for large-scale production, which should be considered when assessing the overall costs [150,151]. Another technique that can be used to significantly reduce the cost of nanocellulose production using a microbial process is enzyme-immobilization on a support matrix. This method offers a cost-effective approach for nanocellulose production, as the enzymes can be reused multiple times. Various immobilization methods can be employed, such as physical adsorption, covalent binding, entrapment, and cross-linking. The choice of method depends on the enzyme, nanocellulose matrix, and desired characteristics of the immobilized enzyme [152]. The immobilization conditions, such as enzyme concentration, pH, temperature, and reaction time, must be optimized to achieve maximum enzyme loading and immobilization efficiency. These conditions may vary depending on the specific enzyme and immobilization method employed [153]. Developing scalable methods for the separation and purification of nanocellulose from the reaction mixture should be done. Techniques such as filtration, centrifugation, or sedimentation can be employed, followed by washing, drying, and size reduction to obtain the desired nanocellulose product.
5.4. Controlled and Enhanced Properties of Nanocellulose
Microbial processes can be tailored to introduce desired surface modifications or even functional groups onto the nanocellulose surface during the isolation process. This allows for the development of nanocellulose materials with enhanced properties and tailored functionalities for specific applications. Chen et al. [154] recently prepared ribbon-like cellulose nanocrystals by subjecting cotton pulp fibers to enzyme hydrolysis using cellulase derived from Aspergillus niger. The hydrolysis process was carried out at 50 °C for 5 to 11 h, employing cellulase with an enzyme activity of 1.10 × 104 μ/mL. When a lower concentration of cellulase was utilized, the cellulose chains were truncated by the endoglucanase enzyme specifically at the amorphous zone, leading to the disintegration of the chains and the formation of ribbon-like CNCs measuring approximately 45 nm in diameter. Conversely, at an enzyme concentration of 100 μ/mL, granular CNCs were observed in the enzymolysis product. However, upon further increasing the concentration to 300 μ/mL, entirely granular CNCs were obtained [154]. This indicates that the higher concentration of endoglucanase resulted in the truncation of cellulose chains at both the crystalline and amorphous regions. By adjusting the enzymatic reaction conditions such as enzyme concentration, reaction time, temperature, and pH, researchers can influence the degree of cellulose fiber degradation and fragmentation, resulting in nanocellulose particles with desired dimensions. Zhang et al. [155] conducted a study where they employed xylanase with an enzyme activity of 2980 IU/g. Different enzyme concentrations were utilized for enzyme hydrolysis of unbleached bagasse pulp. The hydrolysis process was conducted at a temperature of 50 °C for 2 h. Subsequently, superfine grinding and microfluidization techniques were applied to prepare cellulose nanofibrils. The authors indicated that as the enzyme concentration increased, there was a decrease in the particle diameter of the CNFs. Additionally, the thermal stability of the CNFs decreased with the increase in enzyme concentration. Conversely, the carboxyl group content and zeta potential of the CNFs exhibited an increase as the enzyme concentration increased [155]. Microbial enzymes can also influence the surface morphology of nanocellulose. By selectively breaking down cellulose fibers, the enzymatic treatment can create a rough or smooth surface texture, affecting the surface area, porosity, and interactions with other materials. Overall, microbial processes for nanocellulose isolation offer precise control over various properties of nanocellulose. These controlled properties enable researchers to tailor nanocellulose for specific applications, ranging from nanocomposites, biomedical materials, packaging, to functional coatings and sensors.
6. Conclusions
Due to developing technology, ongoing research as well as demand, there always will be some challenges in the production of nanocellulose at low cost for commercial purposes. Several factors are involved in this category, such as high operating and investment costs, fermentation systems, requirement of low yield [156,157]. This clearly shows that nanocellulose is still in developing stages and needs to be developed more. In order to widen the scope for applications, the whole process of production needs to be optimized, including fermentation processes, culture media, genetic make-up of organisms and post-production processes [158]. Also, there were several research proposals proposing the replacement of the Hestrin and Schramm (HS) conventional medium as it was too expensive, and by introducing an economical fermentation system, production costs could be reduced. As culture media plays a crucial role in total production cost of microbial cellulose, it should also be optimized, made economical to enhance yields and to increase the range of applications [159,160]. It should be very clear that whatever development is done, ultimately it is going to affect the total cost of production. To increase the production of microbial cellulose, carbon sources also play a vital role. Several sources are available, such as fructose, glucose, mannitol, sucrose, glycerol, glucose and arabitol. Being the best among these, fructose and mannitol are considered good sources of carbon. However, due to their high costs and low yield, production is limited and so is the application range of microbial cellulose. Therefore, it makes complete sense to find an alternate solution or a way to develop a carbon source for producing low-cost microbial cellulose with high yields [161]. In the surface culture method, generally an organism grows on a liquid surface without agitation and after a fixed period of incubation, a culture filtrate is removed from the cell mass and further processed to produce a desirable product, like beer and alcohol. Biomass can also be re-used sometimes, but this fermentation has its own disadvantages such as a big space requirement, and time consumption was also too long.
Microbial-producing industries generally have used a submerged process for fermentation. In the case of agitated fermenters, shear stress during synthesis can damage cellulose [162,163]. To overcome this issue, rotating disc reactors are new methods to be used for microbial cellulose production by Acetobacter xylinium, where agitated and stationary cultures are used in combination in a horizontal fermenter and culture media is supplied under optimal conditions. Also, it is a well-known fact that a major issue is not just the fermentation process but also the biosynthesis process as whole and so it needs to be optimized. Several researchers have given different factors which need to be improved, such as fermenter design, fermentation period, carbon sources, their concentrations and surface-to-volume ratios [164,165]. A high-quality microbial cellulose can be produced by combining the improved biosynthesis technology and genetically enhanced bacterial strain like the Acetobacter strain, which can be produced at low-cost and contribute to the mass production of microbial cellulose. Already various propositions are given to modify microbial nanocellulose, to reduce production costs, to control various physical properties, to develop design of bioreactors, to modify genetics of microbial cellulose or to widen the range of applications by uncovering other sources [166,167]. Further enhancements are required to produce economic microbial cellulose without hindering the quality of cellulose. Biosynthesis and genetics of microbial cellulose need to be more focused and requires research. To increase the mass production, cultivation of bacterial strains, specifically Acetobacter strains, needs more research. Still, there is a need for interdisciplinary research and genetic investigations for its wide commercialization and large-scale production.
Author Contributions
Conceptualization, E.B.Y. and S.S.E.; methodology, R.D.B.; software, M.M.I.M.; validation, N.B.K. and P.W.H.; formal analysis, E.B.Y.; investigation, S.S.E.; resources, H.P.S.A.K.; data curation, E.B.Y.; writing—original draft preparation, S.S.E.; writing—review and editing, E.B.Y.; visualization, M.M.I.M.; supervision, E.B.Y.; project administration, H.P.S.A.K.; funding acquisition, M.M.I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The authors confirm that the data supporting the findings of this 544 study are available within the article.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through largegroup Research Project under grant number RGP2/388/44.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Osama, S.; Hussain, A.A.; Roushdy, M.M.; Shehabeldine, A.M.; Hasanin, M.S. Preliminary study for isolation, characterizations of the cellulolytic microorganisms: Green convert of microcrystalline cellulose to nanofibers. Egypt. J. Chem. 2022, 65, 1265–1273. [Google Scholar] [CrossRef]
- Abdul Khalil, H.; Adnan, A.; Yahya, E.B.; Olaiya, N.; Safrida, S.; Hossain, M.S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.; Oyekanmi, A. A review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef]
- Magalhães, M.I.; Almeida, A.P. Nature-Inspired Cellulose-Based Active Materials: From 2D to 4D. Appl. Biosci. 2023, 2, 94–114. [Google Scholar] [CrossRef]
- Nasution, H.; Yahya, E.B.; Abdul Khalil, H.; Shaah, M.A.; Suriani, A.; Mohamed, A.; Alfatah, T.; Abdullah, C. Extraction and Isolation of Cellulose Nanofibers from Carpet Wastes Using Supercritical Carbon Dioxide Approach. Polymers 2022, 14, 326. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, F.; Tao, H.; Gao, W.; Guo, L.; Cui, B.; Yuan, C.; Liu, P.; Lu, L.; Wu, Z. Effects of different sources of cellulose on mechanical and barrier properties of thermoplastic sweet potato starch films. Ind. Crop. Prod. 2023, 194, 116358. [Google Scholar] [CrossRef]
- Kadier, A.; Ilyas, R.; Huzaifah, M.; Harihastuti, N.; Sapuan, S.; Harussani, M.; Azlin, M.; Yuliasni, R.; Ibrahim, R.; Atikah, M. Use of industrial wastes as sustainable nutrient sources for bacterial cellulose (BC) production: Mechanism, advances, and future perspectives. Polymers 2021, 13, 3365. [Google Scholar] [CrossRef] [PubMed]
- Nicu, R.; Ciolacu, F.; Ciolacu, D.E. Advanced functional materials based on nanocellulose for pharmaceutical/medical applications. Pharmaceutics 2021, 13, 1125. [Google Scholar] [CrossRef]
- Baltaci, M.O. Enhancement of cellulase production by co-culture of Streptomyces ambofaciens OZ2 and Cytobacillus oceanisediminis OZ5 isolated from rumen samples. Biocatal. Biotransformation 2022, 40, 144–152. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Shim, J.; Chang, S.; Chung, W. Coconut mesocarp-based lignocellulosic waste as a substrate for cellulase production from high promising multienzyme-producing Bacillus amyloliquefaciens FW2 without pretreatments. Microorganisms 2022, 10, 327. [Google Scholar] [CrossRef]
- Zaki, M.; Abdul Khalil, H.P.S.; Sabaruddin, F.; Bairwan, R.; Oyekanmi, A.A.; Alfatah, T.; Danish, M.; Mistar, E.; Abdullah, C. Microbial treatment for nanocellulose extraction from marine algae and its applications as sustainable functional material. Bioresour. Technol. Rep. 2021, 16, 100811. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.H.; Kim, S.; Son, H.; Chun, Y.; Park, C.; Yoo, H.Y. Improved production of bacterial cellulose using Gluconacetobacter sp. LYP25, a strain developed in UVC mutagenesis with limited viability conditions. Int. J. Biol. Macromol. 2023, 232, 123230. [Google Scholar] [CrossRef]
- Adsul, M.G.; Dixit, P.; Saini, J.K.; Gupta, R.P.; Ramakumar, S.S.V.; Mathur, A.S. Morphologically favorable mutant of Trichoderma reesei for low viscosity cellulase production. Biotechnol. Bioeng. 2022, 119, 2167–2181. [Google Scholar] [CrossRef] [PubMed]
- Tsudome, M.; Tachioka, M.; Miyazaki, M.; Uchimura, K.; Tsuda, M.; Takaki, Y.; Deguchi, S. An ultrasensitive nanofiber-based assay for enzymatic hydrolysis and deep-sea microbial degradation of cellulose. Iscience 2022, 25, 104732. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.A.; Jummaat, F.; Yahya, E.B.; Olaiya, N.; Adnan, A.; Abdat, M.; NAM, N.; Halim, A.S.; Kumar, U.S.U.; Bairwan, R. A review on micro-to nanocellulose biopolymer scaffold forming for tissue engineering applications. Polymers 2020, 12, 2043. [Google Scholar] [CrossRef]
- Durand, H.; Smyth, M.; Bras, J. Nanocellulose: A new biopolymer for biomedical application. In Biopolymers for Biomedical and Biotechnological Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 129–179. [Google Scholar]
- Rizal, S.; Alfatah, T.; Abdul Khalil, H.; Yahya, E.B.; Abdullah, C.; Mistar, E.M.; Ikramullah, I.; Kurniawan, R.; Bairwan, R. Enhanced Functional Properties of Bioplastic Films Using Lignin Nanoparticles from Oil Palm-Processing Residue. Polymers 2022, 14, 5126. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.; Sapuan, S.; Norrrahim, M.N.F.; Yasim-Anuar, T.A.T.; Kadier, A.; Kalil, M.S.; Atikah, M.; Ibrahim, R.; Asrofi, M.; Abral, H. Nanocellulose/starch biopolymer nanocomposites: Processing, manufacturing, and applications. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 65–88. [Google Scholar]
- Xu, Y.; Xu, Y.; Chen, H.; Gao, M.; Yue, X.; Ni, Y. Redispersion of dried plant nanocellulose: A review. Carbohydr. Polym. 2022, 294, 119830. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Mishra, R.; Sabu, A.; Tiwari, S. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Moon, R.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathala, B.; Capron, I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 2013, 9, 952–959. [Google Scholar] [CrossRef]
- Bettaieb, F.; Khiari, R.; Dufresne, A.; Mhenni, M.F.; Belgacem, M.N. Mechanical and thermal properties of Posidonia oceanica cellulose nanocrystal reinforced polymer. Carbohydr. Polym. 2015, 123, 99–104. [Google Scholar] [CrossRef]
- Choi, S.M.; Shin, E.J. The nanofication and functionalization of bacterial cellulose and its applications. Nanomaterials 2020, 10, 406. [Google Scholar] [CrossRef]
- Islam, M.N.; Rahman, F. Production and modification of nanofibrillated cellulose composites and potential applications. In Green Composites for Automotive Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 115–141. [Google Scholar]
- Vieira, D. Obtenção e Caracterização de Nanocelulose a Partir de Fibras de Chorisia Speciosa St. Hil. Master’s Thesis, Universidade Estadual Paulista, São Paulo, Brazil, 2015. [Google Scholar]
- Das, R.; Lindström, T.; Sharma, P.R.; Chi, K.; Hsiao, B.S. Nanocellulose for sustainable water purification. Chem. Rev. 2022, 122, 8936–9031. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, A.; Dutta, S.D.; Ganguly, K.; Patil, T.V.; Patel, D.K.; Lim, K.-T. A review of properties of nanocellulose, its synthesis, and potential in biomedical applications. Appl. Sci. 2022, 12, 7090. [Google Scholar] [CrossRef]
- Madsen, B.; Gamstedt, E.K. Wood versus plant fibers: Similarities and differences in composite applications. Adv. Mater. Sci. Eng. 2013, 2013, 564346. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging technologies for the production of nanocellulose from lignocellulosic biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef]
- Louis, A.C.F.; Venkatachalam, S. Energy efficient process for valorization of corn cob as a source for nanocrystalline cellulose and hemicellulose production. Int. J. Biol. Macromol. 2020, 163, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Oun, A.A.; Rhim, J.-W. Isolation of oxidized nanocellulose from rice straw using the ammonium persulfate method. Cellulose 2018, 25, 2143–2149. [Google Scholar] [CrossRef]
- Rai, R.; Dhar, P. Biomedical engineering aspects of nanocellulose: A review. Nanotechnology 2022, 33, 362001. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Sherje, A.P. Cellulose nanocrystals: Fundamentals and biomedical applications. Carbohydr. Polym. 2022, 275, 118668. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.L.; Tiong, S.I.X.; Siva, S.P.; Ahamed, F.; Chan, C.-H.; Lee, C.L.; Chew, I.M.L.; Ho, Y.K. Morphological control of cellulose nanocrystals via sulfuric acid hydrolysis based on sustainability considerations: An overview of the governing factors and potential challenges. J. Environ. Chem. Eng. 2022, 10, 108145. [Google Scholar] [CrossRef]
- Zhao, X.; Bhagia, S.; Gomez-Maldonado, D.; Tang, X.; Wasti, S.; Lu, S.; Zhang, S.; Parit, M.; Rencheck, M.L.; Korey, M. Bioinspired design toward nanocellulose-based materials. Mater. Today 2023, 66, 409–430. [Google Scholar] [CrossRef]
- Surendran, G.; Sherje, A.P. Cellulose nanofibers and composites: An insight on basics and biomedical applications. J. Drug Deliv. Sci. Technol. 2022, 75, 103601. [Google Scholar] [CrossRef]
- Meftahi, A.; Momeni Heravi, M.E.; Barhoum, A.; Samyn, P.; Najarzadeh, H.; Alibakhshi, S. Cellulose Nanofibers: Synthesis, Unique Properties, and Emerging Applications. In Handbook of Nanocelluloses: Classification, Properties, Fabrication, and Emerging Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 233–262. [Google Scholar]
- Bourmaud, A.; Mayer-Laigle, C.; Baley, C.; Beaugrand, J. About the frontier between filling and reinforcement by fine flax particles in plant fibre composites. Ind. Crop. Prod. 2019, 141, 111774. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45. [Google Scholar] [CrossRef]
- Hurtado, P.L.; Rouilly, A.; Vandenbossche, V.; Raynaud, C. A review on the properties of cellulose fibre insulation. Build. Environ. 2016, 96, 170–177. [Google Scholar] [CrossRef]
- Schiros, T.N.; Antrobus, R.; Farías, D.; Chiu, Y.-T.; Joseph, C.T.; Esdaille, S.; Sanchirico, G.K.; Miquelon, G.; An, D.; Russell, S.T. Microbial nanocellulose biotextiles for a circular materials economy. Environ. Sci. Adv. 2022, 1, 276–284. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial cellulose: Molecular regulation of biosynthesis, supramolecular assembly, and tailored structural and functional properties. Prog. Mater. Sci. 2022, 129, 100972. [Google Scholar] [CrossRef]
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa-Jr, A.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef]
- Stanisławska, A. Bacterial nanocellulose as a microbiological derived nanomaterial. Adv. Mater. Sci. 2016, 16, 45–57. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Thomas, D.; Madhavan, A.; Sindhu, R.; Binod, P.; Varjani, S.; Awasthi, M.K.; Pandey, A. Bacterial nanocellulose: Engineering, production, and applications. Bioengineered 2021, 12, 11463. [Google Scholar]
- Skočaj, M. Bacterial nanocellulose in papermaking. Cellulose 2019, 26, 6477–6488. [Google Scholar] [CrossRef]
- Cottet, C.; Ramirez-Tapias, Y.A.; Delgado, J.F.; de la Osa, O.; Salvay, A.G.; Peltzer, M.A. Biobased materials from microbial biomass and its derivatives. Materials 2020, 13, 1263. [Google Scholar] [CrossRef] [PubMed]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Mater. Sci. Eng. R Rep. 2021, 145, 100623. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Moniri, M.; Boroumand Moghaddam, A.; Azizi, S.; Abdul Rahim, R.; Bin Ariff, A.; Zuhainis Saad, W.; Navaderi, M.; Mohamad, R. Production and status of bacterial cellulose in biomedical engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef]
- Pacheco, G.; de Mello, C.V.; Chiari-Andréo, B.G.; Isaac, V.L.B.; Ribeiro, S.J.L.; Pecoraro, É.; Trovatti, E. Bacterial cellulose skin masks—Properties and sensory tests. J. Cosmet. Dermatol. 2018, 17, 840–847. [Google Scholar] [CrossRef]
- Lee, K.Y.; Buldum, G.; Mantalaris, A.; Bismarck, A. More than meets the eye in bacterial cellulose: Biosynthesis, bioprocessing, and applications in advanced fiber composites. Macromol. Biosci. 2014, 14, 10–32. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Bar-Shai, N.; Sharabani-Yosef, O.; Zollmann, M.; Lesman, A.; Golberg, A. Seaweed cellulose scaffolds derived from green macroalgae for tissue engineering. Sci. Rep. 2021, 11, 11843. [Google Scholar] [CrossRef]
- Oyekanmi, A.A.; Saharudin, N.; Hazwan, C.M.; HPS, A.K.; Olaiya, N.G.; Abdullah, C.K.; Alfatah, T.; Gopakumar, D.A.; Pasquini, D. Improved hydrophobicity of macroalgae biopolymer film incorporated with kenaf derived CNF using silane coupling agent. Molecules 2021, 26, 2254. [Google Scholar] [CrossRef]
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal cellulose, production and potential use in plastics: Challenges and opportunities. Algal Res. 2021, 56, 102288. [Google Scholar] [CrossRef]
- Jeong, T.S.; Choi, C.H.; Lee, J.Y.; Oh, K.K. Behaviors of glucose decomposition during acid-catalyzed hydrothermal hydrolysis of pretreated Gelidium amansii. Bioresour. Technol. 2012, 116, 435–440. [Google Scholar] [CrossRef]
- Albuquerque, J.C.S.; Araújo, M.L.H.; Rocha, M.V.P.; de Souza, B.W.S.; de Castro, G.M.C.; Cordeiro, E.M.S.; de Sousa Silva, J.; Benevides, N.M.B. Acid hydrolysis conditions for the production of fine chemicals from Gracilaria birdiae alga biomass. Algal Res. 2021, 53, 102139. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef]
- Fraly Erbabley, N.Y.G.; Junianto, J. Chemical characteristics and phytochemicals of the brown alga Sargassum filipendulla from kelanit waters of southeast Maluku. Egypt. J. Aquat. Biol. Fish. 2020, 24, 535–547. [Google Scholar] [CrossRef]
- Ennab, R.M.; Aljabali, A.A.; Charbe, N.B.; Barhoum, A.; Alqudah, A.; Tambuwala, M.M. Nanocelluloses in Wound Healing Applications. In Handbook of Nanocelluloses: Classification, Properties, Fabrication, and Emerging Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–28. [Google Scholar]
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef]
- Calvo, V.; González-Domínguez, J.M.; Benito, A.M.; Maser, W.K. Synthesis and Processing of Nanomaterials Mediated by Living Organisms. Angew. Chem. 2022, 134, e202113286. [Google Scholar] [CrossRef]
- Noremylia, M.; Hassan, M.Z.; Ismail, Z. Recent advancement in isolation, processing, characterization and applications of emerging nanocellulose: A review. Int. J. Biol. Macromol. 2022, 206, 954–976. [Google Scholar] [CrossRef]
- Fang, B.; Wan, Y.-Z.; Tang, T.-T.; Gao, C.; Dai, K.-R. Proliferation and osteoblastic differentiation of human bone marrow stromal cells on hydroxyapatite/bacterial cellulose nanocomposite scaffolds. Tissue Eng. Part A 2009, 15, 1091–1098. [Google Scholar] [CrossRef]
- Yahya, E.B.; Amirul, A.; HPS, A.K.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; AK, A.S.; Adnan, A. Insights into the role of biopolymer aerogel scaffolds in tissue engineering and regenerative medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef]
- Eskilson, O.; Zattarin, E.; Berglund, L.; Oksman, K.; Hanna, K.; Rakar, J.; Sivlér, P.; Skog, M.; Rinklake, I.; Shamasha, R. Nanocellulose composite wound dressings for real-time pH wound monitoring. Mater. Today Bio 2023, 19, 100574. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, N.; Sun, Y.; Shao, J.; Liu, Q.; Zhuang, X.; Twebaze, C.B. Nanocellulose aerogels from banana pseudo-stem as a wound dressing. Ind. Crop. Prod. 2023, 194, 116383. [Google Scholar] [CrossRef]
- Puppala, N.V.; Doddipatla, P.; Mohannath, G. Use of nanocellulose in the intracellular delivery of biological and non-biological drugs: A review. Cellulose 2023, 30, 1335–1354. [Google Scholar] [CrossRef]
- Bellmann, T.; Thamm, J.; Beekmann, U.; Kralisch, D.; Fischer, D. In situ Formation of Polymer Microparticles in Bacterial Nanocellulose Using Alternative and Sustainable Solvents to Incorporate Lipophilic Drugs. Pharmaceutics 2023, 15, 559. [Google Scholar] [CrossRef]
- de Assis, S.C.; Morgado, D.L.; Scheidt, D.T.; de Souza, S.S.; Cavallari, M.R.; Ando Junior, O.H.; Carrilho, E. Review of Bacterial Nanocellulose-Based Electrochemical Biosensors: Functionalization, Challenges, and Future Perspectives. Biosensors 2023, 13, 142. [Google Scholar] [CrossRef]
- Eldhose, M.; Roy, R.; George, C.; Joseph, A. Sensing and Biosensing Applications of Nanocellulose. In Handbook of Biopolymers; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–26. [Google Scholar]
- Vilarinho, F.; Sanches Silva, A.; Vaz, M.F.; Farinha, J.P. Nanocellulose in green food packaging. Crit. Rev. Food Sci. Nutr. 2018, 58, 1526–1537. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kitaoka, T. Ultraselective gas separation by nanoporous metal− organic frameworks embedded in gas-barrier nanocellulose films. Adv. Mater. 2016, 28, 1765–1769. [Google Scholar] [CrossRef]
- Aulin, C.; Karabulut, E.; Tran, A.; Wågberg, L.; Lindström, T. Transparent nanocellulosic multilayer thin films on polylactic acid with tunable gas barrier properties. ACS Appl. Mater. Interfaces 2013, 5, 7352–7359. [Google Scholar] [CrossRef]
- Parvathy, S.; Hema, S.; Sajith, M.; Sulthan, R.; Sreelekshmi, C.; Sambhudevan, S.; Shankar, B. A Review on Barrier Properties of Nanocellulose and Polylactic acid Composites. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2022; p. 012017. [Google Scholar]
- Rizal, S.; Olaiya, F.G.; Saharudin, N.; Abdullah, C.; NG, O.; Mohamad Haafiz, M.; Yahya, E.B.; Sabaruddin, F.; Ikramullah; Abdul Khalil, H.P.S. Isolation of textile waste cellulose nanofibrillated fibre reinforced in polylactic acid-chitin biodegradable composite for green packaging application. Polymers 2021, 13, 325. [Google Scholar] [CrossRef]
- Ginja, G.A.; da Costa, J.P.d.C.; Gounella, R.H.; Izquierdo, J.E.E.; Carmo, J.P.; Fonseca, F.J.; Cavallari, M.R.; Junior, O.H.A.; de Souza, S.S. A Humidity Sensor Based on Bacterial Nanocellulose Membrane (BNC). IEEE Sens. J. 2023, 23, 3485–3492. [Google Scholar] [CrossRef]
- Suman; Kardam, A.; Gera, M.; Jain, V. A novel reusable nanocomposite for complete removal of dyes, heavy metals and microbial load from water based on nanocellulose and silver nano-embedded pebbles. Environ. Technol. 2015, 36, 706–714. [Google Scholar] [CrossRef]
- Tasrin, S.; Mohamed Madhar Fazil, S.; Senthilmurugan, S.; Selvaraju, N. Facile preparation of nanocellulose embedded polypyrrole for dye removal: Unary and binary process optimization and seed toxicity. Int. J. Environ. Sci. Technol. 2021, 18, 365–378. [Google Scholar] [CrossRef]
- Maqbool, Q.; Barucca, G.; Sabbatini, S.; Parlapiano, M.; Ruello, M.L.; Tittarelli, F. Transformation of industrial and organic waste into titanium doped activated carbon–cellulose nanocomposite for rapid removal of organic pollutants. J. Hazard. Mater. 2022, 423, 126958. [Google Scholar] [CrossRef]
- Yin, Z.; Cheng, Y.; Deng, Y.; Li, Z.; Liu, K.; Li, M.; Chen, X.; Xue, M.; Ou, J.; Lei, S. Functional and versatile colorful superhydrophobic nanocellulose-based membrane with high durability, high-efficiency oil/water separation and oil spill cleanup. Surf. Coat. Technol. 2022, 445, 128714. [Google Scholar] [CrossRef]
- Xiong, Z.; Lin, J.; Li, X.; Bian, F.; Wang, J. Hierarchically structured nanocellulose-implanted air filters for high-efficiency particulate matter removal. ACS Appl. Mater. Interfaces 2021, 13, 12408–12416. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, Z.; Guo, L.; Hu, S.; Wang, H.; Meng, L.; Tang, M.; Qi, H. Acetylated tunicate nanocellulose-based high-efficient air filter media with antibacterial property. J. Membr. Sci. 2023, 669, 121307. [Google Scholar] [CrossRef]
- Dias, O.A.T.; Konar, S.; Leão, A.L.; Yang, W.; Tjong, J.; Sain, M. Current state of applications of nanocellulose in flexible energy and electronic devices. Front. Chem. 2020, 8, 420. [Google Scholar] [CrossRef]
- Fan, W.; Wang, J.; Zhang, Z.; Li, Z. Synergistic enhancement of UV-resistance and electrical conductivity of waterborne polyurethane composite with combination of functionalized 2D graphene oxide and 1D nanocellulose. Prog. Org. Coat. 2021, 151, 106017. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, Q.; Liu, J.; Sun, J.; Zhu, Q.; Chen, H. Processing nanocellulose to bulk materials: A review. Cellulose 2019, 26, 7585–7617. [Google Scholar] [CrossRef]
- Ilyas, R.; Asyraf, M.; Aisyah, H.; Sapuan, S.; Norrrahim, M.; Ibrahim, R.; Atikah, M.; Atiqah, A.; Zainudin, E.; Ishak, M. Introduction to nanocellulose production from biological waste. In Industrial Applications of Nanocellulose and Its Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–37. [Google Scholar]
- Squinca, P.; Bilatto, S.; Badino, A.C.; Farinas, C.S. The use of enzymes to isolate cellulose nanomaterials: A systematic map review. Carbohydr. Polym. Technol. Appl. 2022, 3, 100212. [Google Scholar] [CrossRef]
- Oktiarni, D.; Nofyan, E.; Kasmiarti, G. Isolation and identification cellulolytic bacteria from termite gut obtained from Indralaya peatland area. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; p. 012024. [Google Scholar]
- Singh, R.S.; Singh, T.; Pandey, A. Microbial enzymes—An overview. In Advances in Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–40. [Google Scholar]
- Straub, C.T.; Bing, R.G.; Otten, J.K.; Keller, L.M.; Zeldes, B.M.; Adams, M.W.; Kelly, R.M. Metabolically engineered Caldicellulosiruptor bescii as a platform for producing acetone and hydrogen from lignocellulose. Biotechnol. Bioeng. 2020, 117, 3799–3808. [Google Scholar] [CrossRef]
- Straub, C.T.; Bing, R.G.; Wang, J.P.; Chiang, V.L.; Adams, M.W.; Kelly, R.M. Use of the lignocellulose-degrading bacterium Caldicellulosiruptor bescii to assess recalcitrance and conversion of wild-type and transgenic poplar. Biotechnol. Biofuels 2020, 13, 43. [Google Scholar] [CrossRef]
- Zhu, X.; Xin, S.; Ding, H.; Yang, Y.; Chen, Y.; Li, X.; Shi, H.; Tan, Z.; Zhou, J.; Liu, P. Functional characterization of a noncatalytic protein, Athe_0181, from Caldicellulosiruptor bescii in promoting lignocellulose hydrolysis. BioResources 2022, 17, 3067–3081. [Google Scholar] [CrossRef]
- Ghosh, T.; Ngo, T.-D.; Kumar, A.; Ayranci, C.; Tang, T. Cleaning carbohydrate impurities from lignin using Pseudomonas fluorescens. Green Chem. 2019, 21, 1648–1659. [Google Scholar] [CrossRef]
- Mortabit, D.; Zyani, M.; Haggoud, A.; Housaini Iraqui, M.; Houari, A.; Fikri Benbrahim, K.; Koraichi Ibnsouda, S.; Koraichi, S. Carboxymethyl cellulase production by moroccan bacillus isolates. Moroc. J. Biol. 2010, 1, 43–49. [Google Scholar]
- Adorian, T.J.; Jamali, H.; Farsani, H.G.; Darvishi, P.; Hasanpour, S.; Bagheri, T.; Roozbehfar, R. Effects of probiotic bacteria Bacillus on growth performance, digestive enzyme activity, and hematological parameters of Asian sea bass, Lates calcarifer (Bloch). Probiotics Antimicrob. Proteins 2019, 11, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kuebutornye, F.K.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef]
- Sadalage, P.S.; Pawar, K.D. Production of microcrystalline cellulose and bacterial nanocellulose through biological valorization of lignocellulosic biomass wastes. J. Clean. Prod. 2021, 327, 129462. [Google Scholar] [CrossRef]
- Mariño, M.; Lopes da Silva, L.; Durán, N.; Tasic, L. Enhanced materials from nature: Nanocellulose from citrus waste. Molecules 2015, 20, 5908–5923. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, J.M.; Zhang, R.; Mittal, A.; Vander Wall, T.; Bomble, Y.J.; Decker, S.R.; Himmel, M.E.; Ciesielski, P.N. Multifunctional cellulolytic enzymes outperform processive fungal cellulases for coproduction of nanocellulose and biofuels. ACS Nano 2017, 11, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- Septiani, D.; Suryadi, H.; Mun'im, A.; Mangunwardoyo, W. Production of cellulase from Aspergillus niger and Trichoderma reesei mixed culture in carboxymethylcellulose medium as sole carbon. Biodiversitas J. Biol. Divers. 2019, 20. [Google Scholar] [CrossRef]
- Wu, D.; Qu, F.; Li, D.; Zhao, Y.; Li, X.; Niu, S.; Zhao, M.; Qi, H.; Wei, Z.; Song, C. Effect of Fenton pretreatment and bacterial inoculation on cellulose-degrading genes and fungal communities during rice straw composting. Sci. Total Environ. 2022, 806, 151376. [Google Scholar] [CrossRef]
- Fritz, C.; Jeuck, B.; Salas, C.; Gonzalez, R.; Jameel, H.; Rojas, O.J. Nanocellulose and proteins: Exploiting their interactions for production, immobilization, and synthesis of biocompatible materials. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Springer: Berlin/Heidelberg, Germany, 2016; pp. 207–224. [Google Scholar]
- Janardhnan, S.; Sain, M.M. Isolation of cellulose microfibrils–an enzymatic approach. Bioresources 2006, 1, 176–188. [Google Scholar] [CrossRef]
- López-Rubio, A.; Lagaron, J.; Ankerfors, M.; Lindström, T.; Nordqvist, D.; Mattozzi, A.; Hedenqvist, M.S. Enhanced film forming and film properties of amylopectin using micro-fibrillated cellulose. Carbohydr. Polym. 2007, 68, 718–727. [Google Scholar] [CrossRef]
- Svagan, A.J.; Azizi Samir, M.A.; Berglund, L.A. Biomimetic polysaccharide nanocomposites of high cellulose content and high toughness. Biomacromolecules 2007, 8, 2556–2563. [Google Scholar] [CrossRef]
- Squinca, P.; Bilatto, S.; Badino, A.C.; Farinas, C.S. Nanocellulose production in future biorefineries: An integrated approach using tailor-made enzymes. ACS Sustain. Chem. Eng. 2020, 8, 2277–2286. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Wang, L.; Song, X.; Qin, C.; Wang, S. Tuning of size and properties of cellulose nanofibers isolated from sugarcane bagasse by endoglucanase-assisted mechanical grinding. Ind. Crop. Prod. 2020, 146, 112201. [Google Scholar] [CrossRef]
- Colonia, B.S.O.; Woiciechowski, A.L.; Malanski, R.; Letti, L.A.J.; Soccol, C.R. Pulp improvement of oil palm empty fruit bunches associated to solid-state biopulping and biobleaching with xylanase and lignin peroxidase cocktail produced by Aspergillus sp. LPB-5. Bioresour. Technol. 2019, 285, 121361. [Google Scholar] [CrossRef] [PubMed]
- Anand, G.; Leibman-Markus, M.; Elkabetz, D.; Bar, M. Method for the production and purification of plant immuno-active xylanase from Trichoderma. Int. J. Mol. Sci. 2021, 22, 4214. [Google Scholar] [CrossRef] [PubMed]
- Legodi, L.M.; La Grange, D.C.; van Rensburg, E.L.J. Production of the Cellulase Enzyme System by Locally Isolated Trichoderma and Aspergillus Species Cultivated on Banana Pseudostem during Solid-State Fermentation. Fermentation 2023, 9, 412. [Google Scholar] [CrossRef]
- Butt, K.Y.; Saleemi, K.B.; Gilani, S.R.; Ghori, M.I. Kinetics of cellobiohydrolase from a phytopathogenic fungus Trichoderma harzianum. Pak. J. Life Soc. Sci. 2018, 16, 72–76. [Google Scholar]
- Hasanin, M.S.; Mostafa, A.M.; Mwafy, E.A.; Darwesh, O.M. Eco-friendly cellulose nano fibers via first reported Egyptian Humicola fuscoatra Egyptia X4: Isolation and characterization. Environ. Nanotechnol. Monit. Manag. 2018, 10, 409–418. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, J.; Durán, N.; Tasic, L. Nanocellulose and bioethanol production from orange waste using isolated microorganisms. J. Braz. Chem. Soc. 2013, 24, 1537–1543. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Deng, X.-Y.; Shen, W.-H.; Jia, M.-Y. Preparation and characterization of the spherical nanosized cellulose by the enzymatic hydrolysis of pulp fibers. Carbohydr. Polym. 2018, 181, 879–884. [Google Scholar] [CrossRef]
- Teixeira, R.S.S.; da Silva, A.S.A.; Jang, J.-H.; Kim, H.-W.; Ishikawa, K.; Endo, T.; Lee, S.-H.; Bon, E.P. Combining biomass wet disk milling and endoglucanase/β-glucosidase hydrolysis for the production of cellulose nanocrystals. Carbohydr. Polym. 2015, 128, 75–81. [Google Scholar] [CrossRef]
- Black, K.; Walker, G. Yeast Fermentation for Production of Neutral Distilled Spirits. Appl. Sci. 2023, 13, 4927. [Google Scholar] [CrossRef]
- Chung, S.Y.; Seki, H.; Fujisawa, Y.; Shimoda, Y.; Hiraga, S.; Nomura, Y.; Saito, K.; Ishimoto, M.; Muranaka, T. A cellulose synthase-derived enzyme catalyses 3-O-glucuronosylation in saponin biosynthesis. Nat. Commun. 2020, 11, 5664. [Google Scholar] [CrossRef]
- Giese, E.C.; Cadete, R.M.; Pierozzi, M.; Philippini, R.R.; Martiniano, S.E.; Pagnocca, F.C.; Rosa, C.A.; Da Silva, S.S. Cellulases production by new yeast isolates from Brazilian biodiversity. Curr. Opin. Biotechnol. 2011, 22, S147–S148. [Google Scholar] [CrossRef]
- Jimenez, M.; Gonzalez, A.; Martinez, M.; Martinez, A.; Dale, B. Screening of yeasts isolated from decayed wood for lignocellulose-degrading enzyme activities. Mycol. Res. 1991, 95, 1299–1302. [Google Scholar] [CrossRef]
- Božič, M.; Liu, P.; Mathew, A.P.; Kokol, V. Enzymatic phosphorylation of cellulose nanofibers to new highly-ions adsorbing, flame-retardant and hydroxyapatite-growth induced natural nanoparticles. Cellulose 2014, 21, 2713–2726. [Google Scholar] [CrossRef]
- Atatoprak, T.; Amorim, M.M.; Ribeiro, T.; Pintado, M.; Madureira, A.R. Grape stalk valorization for fermentation purposes. Food Chem. Mol. Sci. 2022, 4, 100067. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; He, Z.; Zheng, L.; Pande, H.; Ni, Y. Enzymatic treatment processes for the production of cellulose nanomaterials: A review. Carbohydr. Polym. 2022, 299, 120199. [Google Scholar] [CrossRef]
- Beltramino, F.; Roncero, M.B.; Vidal, T.; Valls, C. A novel enzymatic approach to nanocrystalline cellulose preparation. Carbohydr. Polym. 2018, 189, 39–47. [Google Scholar] [CrossRef]
- Banvillet, G.; Depres, G.; Belgacem, N.; Bras, J. Alkaline treatment combined with enzymatic hydrolysis for efficient cellulose nanofibrils production. Carbohydr. Polym. 2021, 255, 117383. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Rodrigues, M.I.; Menegalli, F.C. Cellulose nanofibers produced from banana peel by enzymatic treatment: Study of process conditions. Ind. Crop. Prod. 2017, 95, 664–674. [Google Scholar] [CrossRef]
- Zielińska, D.; Szentner, K.; Waśkiewicz, A.; Borysiak, S. Production of nanocellulose by enzymatic treatment for application in polymer composites. Materials 2021, 14, 2124. [Google Scholar] [CrossRef]
- Li, H.; Legere, S.; He, Z.; Zhang, H.; Li, J.; Yang, B.; Zhang, S.; Zhang, L.; Zheng, L.; Ni, Y. Methods to increase the reactivity of dissolving pulp in the viscose rayon production process: A review. Cellulose 2018, 25, 3733–3753. [Google Scholar] [CrossRef]
- Duan, C.; Verma, S.K.; Li, J.; Ma, X.; Ni, Y. Combination of mechanical, alkaline and enzymatic treatments to upgrade paper-grade pulp to dissolving pulp with high reactivity. Bioresour. Technol. 2016, 200, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Chen, L.; Huang, L.; Tian, C.; Zheng, L.; Ni, Y. A process for enhancing the accessibility and reactivity of hardwood kraft-based dissolving pulp for viscose rayon production by cellulase treatment. Bioresour. Technol. 2014, 154, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Houfani, A.A.; Anders, N.; Spiess, A.C.; Baldrian, P.; Benallaoua, S. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars–a review. Biomass Bioenergy 2020, 134, 105481. [Google Scholar] [CrossRef]
- de Aguiar, J.; Bondancia, T.J.; Claro, P.I.C.; Mattoso, L.H.C.; Farinas, C.S.; Marconcini, J.M. Enzymatic deconstruction of sugarcane bagasse and straw to obtain cellulose nanomaterials. ACS Sustain. Chem. Eng. 2020, 8, 2287–2299. [Google Scholar] [CrossRef]
- Afrin, S.; Karim, Z. Isolation and surface modification of nanocellulose: Necessity of enzymes over chemicals. ChemBioEng Rev. 2017, 4, 289–303. [Google Scholar] [CrossRef]
- Pires, J.R.; Souza, V.G.; Fernando, A.L. Valorization of energy crops as a source for nanocellulose production–current knowledge and future prospects. Ind. Crop. Prod. 2019, 140, 111642. [Google Scholar] [CrossRef]
- Ai, Y.; Zhang, L.; Cui, M.; Huang, R.; Qi, W.; He, Z.; Klemeš, J.J.; Su, R. Toward cleaner production of nanocellulose: A review and evaluation. Green Chem. 2022, 24, 6406–6434. [Google Scholar] [CrossRef]
- Eriksen, Ø.; Syverud, K.; Gregersen, Ø. The use of microfibrillated cellulose produced from kraft pulp as strength enhancer in TMP paper. Nord. Pulp Pap. Res. J. 2008, 23, 299–304. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Anderson, S.R.; Esposito, D.; Gillette, W.; Zhu, J.; Baxa, U.; Mcneil, S.E. Enzymatic preparation of nanocrystalline and microcrystalline cellulose. Tappi J. 2014, 13, 35–42. [Google Scholar] [CrossRef]
- Spence, K.L.; Venditti, R.A.; Rojas, O.J.; Habibi, Y.; Pawlak, J.J. A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose 2011, 18, 1097–1111. [Google Scholar] [CrossRef]
- Mohlin, U.; Pettersson, B. Improved papermaking by cellulase treatment before refining. ICBPPI 2001, 21, 291–299. [Google Scholar]
- Bajpai, P.; Mishra, S.P.; Mishra, O.P.; Kumar, S.; Bajpai, A. Use of enzymes for reduction in refining energy-laboratory studies. Tappi J. 2006, 5, 25. [Google Scholar]
- Lecourt, M.; Meyer, V.; Sigoillot, J.-C.; Petit-Conil, M. Energy reduction of refining by cellulases. Holzforschung 2010, 64, 441–446. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Y.; Jiang, F. Sustainable isolation of nanocellulose from cellulose and lignocellulosic feedstocks: Recent progress and perspectives. Carbohydr. Polym. 2021, 267, 118188. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, Y.; Qin, C.; Yang, S.; Song, X.; Wang, S.; Li, K. Enzyme-assisted mechanical grinding for cellulose nanofibers from bagasse: Energy consumption and nanofiber characteristics. Cellulose 2018, 25, 7065–7078. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Predicting the environmental impact of a future nanocellulose production at industrial scale: Application of the life cycle assessment scale-up framework. J. Clean. Prod. 2018, 174, 283–295. [Google Scholar] [CrossRef]
- Li, Q.; McGinnis, S.; Wong, A.; Renneckar, S. Nanocellulose life cycle assessment. ACS Sustain. Chem. Eng. 2013, 1, 919–928. [Google Scholar] [CrossRef]
- Yassin, M.A.; Gad, A.A.M.; Ghanem, A.F.; Rehim, M.H.A. Green synthesis of cellulose nanofibers using immobilized cellulase. Carbohydr. Polym. 2019, 205, 255–260. [Google Scholar] [CrossRef]
- Ashkan, Z.; Hemmati, R.; Homaei, A.; Dinari, A.; Jamlidoost, M.; Tashakor, A. Immobilization of enzymes on nanoinorganic support materials: An update. Int. J. Biol. Macromol. 2021, 168, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Q.; Pang, G.-X.; Shen, W.-H.; Tong, X.; Jia, M.-Y. Preparation and characterization of the ribbon-like cellulose nanocrystals by the cellulase enzymolysis of cotton pulp fibers. Carbohydr. Polym. 2019, 207, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Yan, D.; Zhang, C.; Nie, S. Enzyme-assisted mechanical production of cellulose nanofibrils: Thermal stability. Cellulose 2018, 25, 5049–5061. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Pohlmann, B.C.; Calado, V.; Bojorge, N.; Pereira Jr, N. Production of nanocellulose by enzymatic hydrolysis: Trends and challenges. Eng. Life Sci. 2019, 19, 279–291. [Google Scholar] [CrossRef]
- Samyn, P.; Meftahi, A.; Geravand, S.A.; Heravi, M.E.M.; Najarzadeh, H.; Sabery, M.S.K.; Barhoum, A. Opportunities for bacterial nanocellulose in biomedical applications: Review on biosynthesis, modification and challenges. Int. J. Biol. Macromol. 2023, 231, 123316. [Google Scholar] [CrossRef]
- da Gama, F.M.P.; Dourado, F. Bacterial NanoCellulose: What future? BioImpacts BI 2018, 8, 1. [Google Scholar]
- Padmanaban, S.; Balaji, N.; Muthukumaran, C.; Tamilarasan, K. Statistical optimization of process parameters for exopolysaccharide production by Aureobasidium pullulans using sweet potato based medium. 3 Biotech 2015, 5, 1067–1073. [Google Scholar] [CrossRef]
- Cakar, F.; Özer, I.; Aytekin, A.Ö.; Şahin, F. Improvement production of bacterial cellulose by semi-continuous process in molasses medium. Carbohydr. Polym. 2014, 106, 7–13. [Google Scholar] [CrossRef]
- Kamal, I. Novel Approaches in Production Pathway of Microbial, Microcrystalline and Nano-Cellulose. 2022. Available online: https://www.researchgate.net/publication/271829336_Novel_approaches_In_Production_Pathway_Of_Microbial_Microcrystalline_and_Nano-Cellulose (accessed on 25 May 2022).
- Zhang, H.; Chen, C.; Yang, J.; Sun, B.; Lin, J.; Sun, D. Effect of Culture Conditions on Cellulose Production by a Komagataeibacter xylinus Strain. Macromol. Biosci. 2022, 22, 2100476. [Google Scholar] [CrossRef] [PubMed]
- Tapias, Y.A.R.; Di Monte, M.V.; Peltzer, M.A.; Salvay, A.G. Kombucha fermentation in yerba mate: Cellulose production, films formulation and its characterisation. Carbohydr. Polym. Technol. Appl. 2023, 5, 100310. [Google Scholar] [CrossRef]
- Blanco Parte, F.G.; Santoso, S.P.; Chou, C.-C.; Verma, V.; Wang, H.-T.; Ismadji, S.; Cheng, K.-C. Current progress on the production, modification, and applications of bacterial cellulose. Crit. Rev. Biotechnol. 2020, 40, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Mazhar, S.; Abidi, S.H.; Syed, Q.; Abbas, N.; Saleem, Y.; Nadeem, A.A.; Maryam, M.; Essa, R.; Ashfaq, S. Biobutanol production from sustainable biomass process of anaerobic ABE fermentation for industrial applications. Arch. Microbiol. 2022, 204, 672. [Google Scholar] [CrossRef]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trček, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).