The Early- to Latewood Transition Phenology Is Asynchronous between the Different Parts of Abies forrestii var. smithii in Jiaozi Mountain, Yunnan, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Microscopic Observations of the Developing Xylem
2.3. Criteria for Judging Earlywood, Transition Wood, and Latewood
2.4. Measurement of Wood Anatomical and Structural Characteristics
2.5. Gompertz Curve Fitting for Dynamic Changes in Xylem Cell Number
2.6. Relationship between the Earlywood to Latewood Transition and Environmental Factors
3. Results
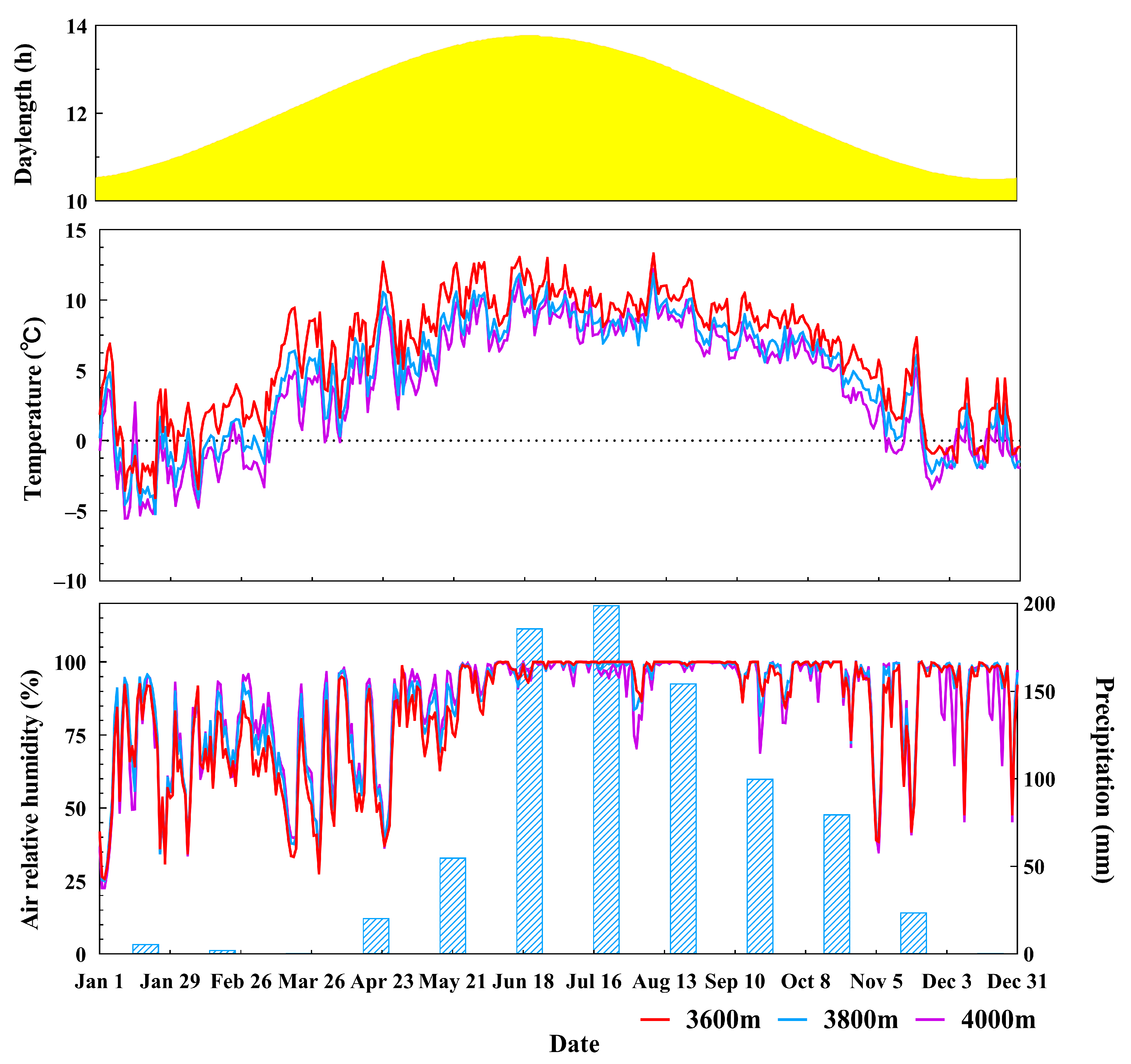
3.1. Seasonal Cycles of the Environmental Factors
3.2. The Earlywood to Latewood Transition Timing
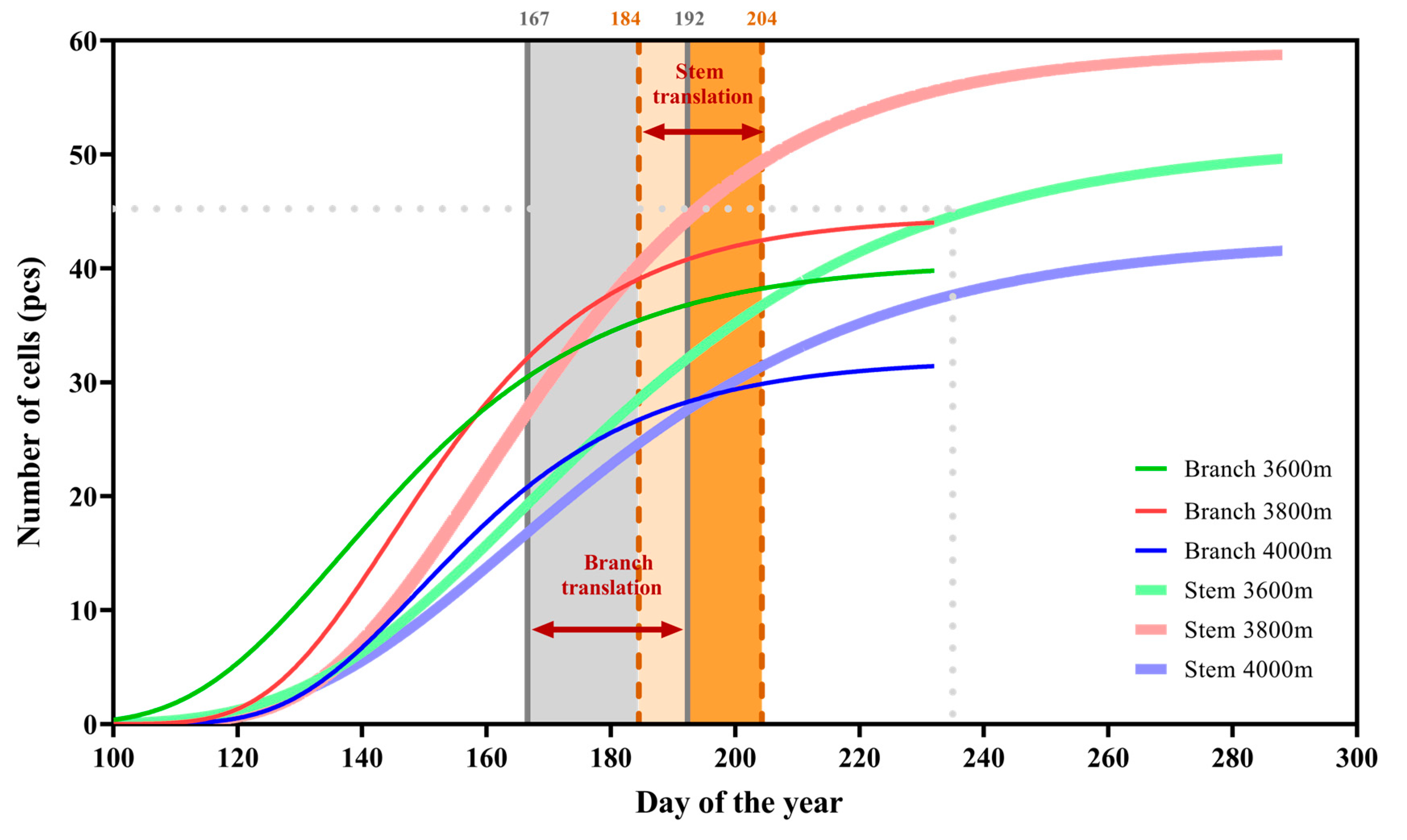
3.3. Kinetics of Tracheid Development and Tree Ring Structure
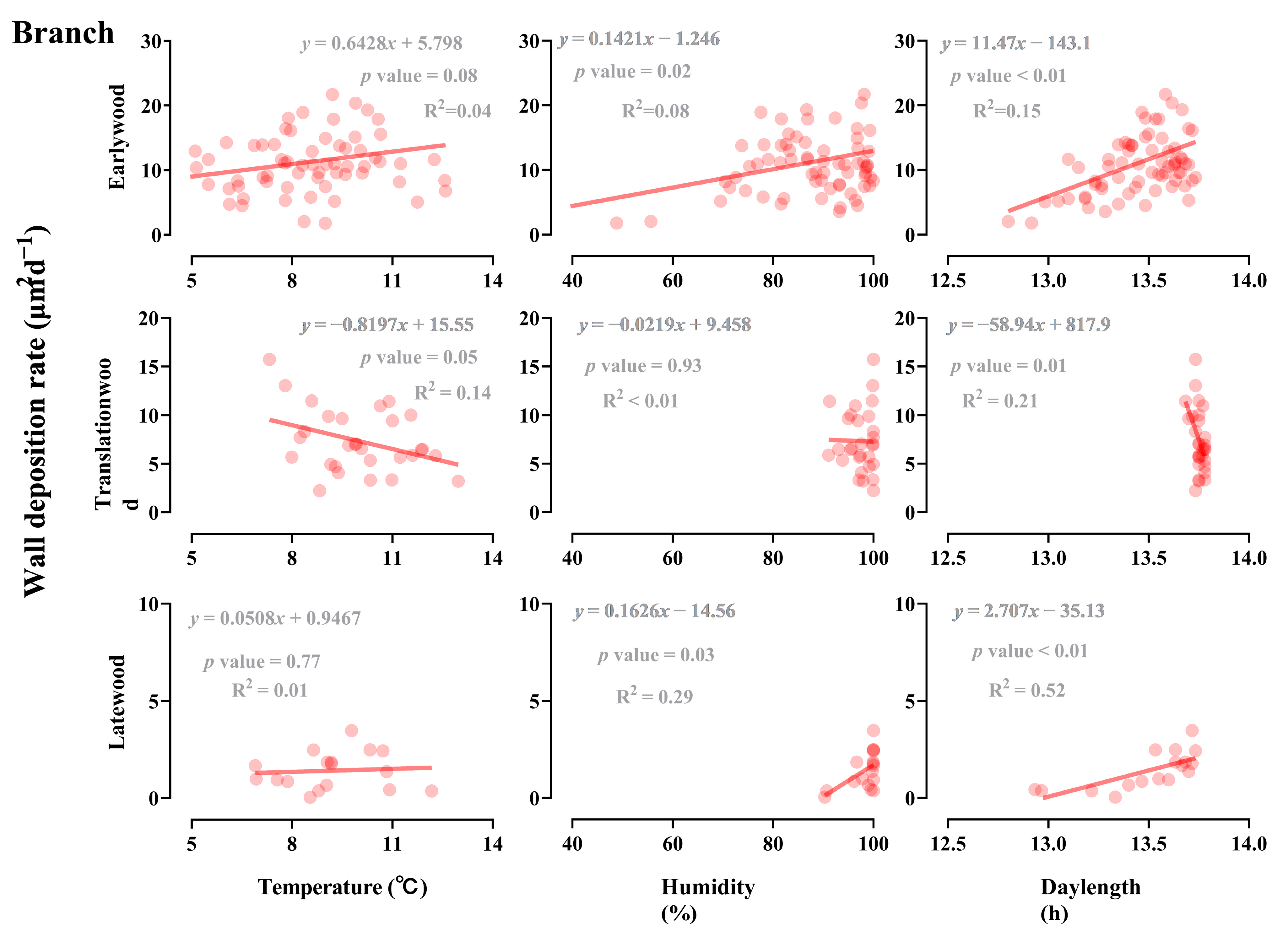
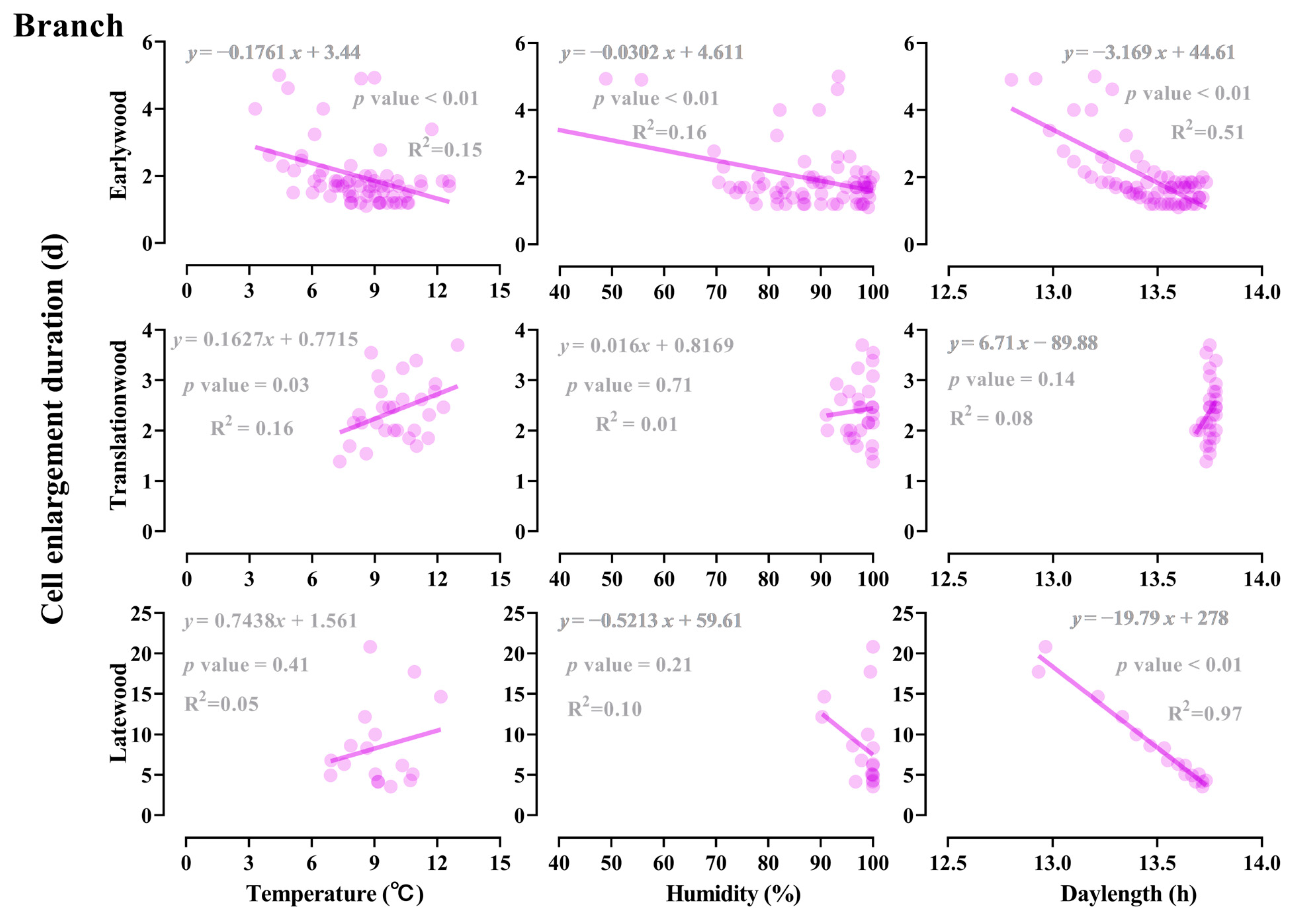
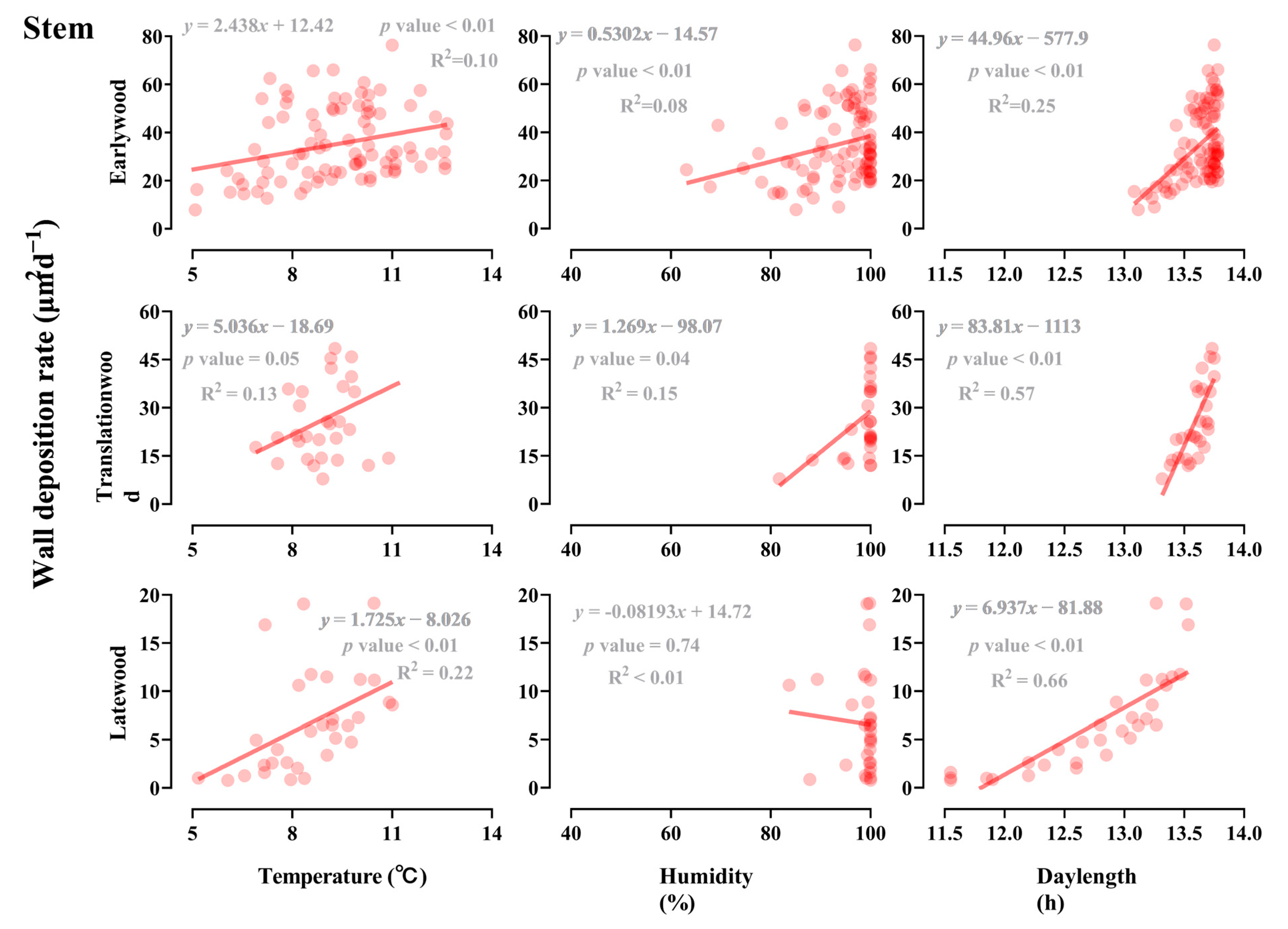
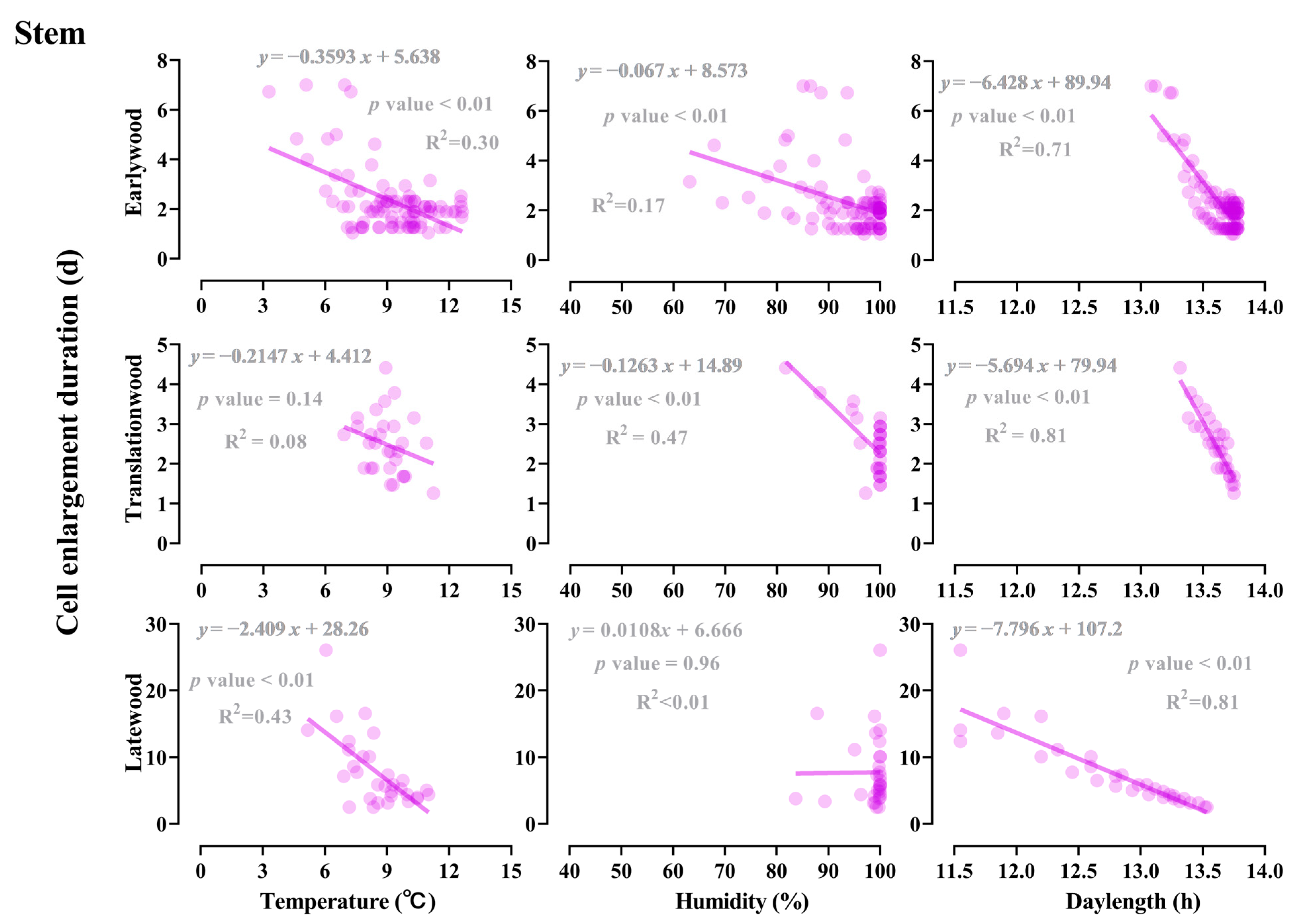
3.4. Effect of Light, Temperature, and Humidity on the Earlywood to Latewood Transition
3.5. Influence of Developmental and Climate Factors on the Earlywood to Latewood Transition
4. Discussion
4.1. The Earlywood to Latewood Transition Time Is the Same along the Altitude Gradient
4.2. Photoperiod Is Closely Related to the Cell Development Process
4.3. The Earlywood to Latewood Transition Occurs Earlier in Branches Than in Stems
4.4. Factors Regulating the Earlywood to Latewood Transition in Different Parts of Trees
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuny, H.E.; Rathgeber, C.B.K.; Frank, D.; Fonti, P.; Fournier, M. Kinetics of tracheid development explain conifer tree-ring structure. New Phytol. 2014, 203, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, J. Microscopic wood anatomy. Structural variability of stems and twigs in recent and subfossil woods from central Europe. J. Archaeol. Sci. 1979, 6, 394–396. [Google Scholar] [CrossRef]
- Schoch, W.; Heller, I.; Schweingruber, F.H.; Kienast, F. Wood Anatomy of Central European Species; Swiss Federal Institute for Forest: Birmensdorf, Switzerland, 2004. [Google Scholar]
- Samusevich, A.; Lexa, M.; Vejpustková, M.; Altman, J.; Zeidler, A. Comparison of methods for the demarcation between earlywood and latewood in tree rings of Norway spruce. Dendrochronologia 2020, 60, 125686. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, S.; Arzac, A.; Cooper, D.J.; Jin, Y.; Yuan, D.; Zhu, Y.; Zhang, X.; Li, Z.; Zhang, Y.; et al. Different response of earlywood vessel features of Fraxinus mandshurica to rapid warming in warm-dry and cold-wet areas. Agric. For. Meteorol. 2021, 307, 108523. [Google Scholar] [CrossRef]
- Eckes-Shephard, A.H.; Ljungqvist, F.C.; Drew, D.M.; Rathgeber, C.B.K.; Friend, A.D. Wood Formation Modeling—A Research Review and Future Perspectives. Front. Plant Sci. 2022, 13, 837648. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D.; Dinwoodie, J.M. Studies on the physiology of xylem development. Int. Wood Prod. J. 1960, 3–11. [Google Scholar]
- Larson, P.R. The indirect effect of drought on tracheid diameter in red pine. For. Sci. 1963, 9, 52–62. [Google Scholar]
- Gordon, J.C.; Larson, P.R. Seasonal Course of Photosynthesis, Respiration, and Distribution of 14C in Young Pinus resinosa Trees as Related to Wood Formation. Plant Physiol. 1968, 43, 1617–1624. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.K.; Cuny, H.E.; Rathgeber, C.B.K. Xylogenesis: Coniferous Trees of Temperate Forests Are Listening to the Climate Tale during the Growing Season but Only Remember the Last Words! Plant Physiol. 2016, 171, 306–317. [Google Scholar] [CrossRef]
- Denne, M.P.; Dodd, R.S. The Environmental Control of Xylem Differentiation. In Xylem Cell Development; Castle House Publications Ltd.: Tunbridge Wells, UK, 1981; pp. 236–255. [Google Scholar]
- Vaganov, E.A.; Anchukaitis, K.J.; Evans, M.N. How well understood are the processes that create dendroclimatic records? A mechanistic model of the climatic control on conifer tree-ring growth dynamics. In Dendroclimatology: Progress and Prospects; Hughes, M.K., Swetnam, T.W., Diaz, H.F., Eds.; Developments in Paleoenvironmental Research; Springer: Dordrecht, The Netherlands, 2011; pp. 37–75. ISBN 978-1-4020-5725-0. [Google Scholar]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood Formation in Trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef]
- Cuny, H.E.; Fonti, P.; Rathgeber, C.B.K.; Von Arx, G.; Peters, R.L.; Frank, D.C. Couplings in cell differentiation kinetics mitigate air temperature influence on conifer wood anatomy. Plant Cell Environ. 2019, 42, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Jasińska, A.K.; Alber, M.; Tullus, A.; Rahi, M.; Sellin, A. Impact of elevated atmospheric humidity on anatomical and hydraulic traits of xylem in hybrid aspen. Funct. Plant Biol. 2015, 42, 565–578. [Google Scholar] [CrossRef]
- Fonti, P.; Solomonoff, N.; García-González, I. Earlywood vessels of Castanea sativa record temperature before their formation. New Phytol. 2007, 173, 562–570. [Google Scholar] [CrossRef]
- Dolezal, J.; Kopecky, M.; Dvorsky, M.; Macek, M.; Rehakova, K.; Capkova, K.; Borovec, J.; Schweingruber, F.; Liancourt, P.; Altman, J. Sink limitation of plant growth determines tree line in the arid Himalayas. Funct. Ecol. 2019, 33, 553–565. [Google Scholar] [CrossRef]
- Jochner, M.; Bugmann, H.; Nötzli, M.; Bigler, C. Tree growth responses to changing temperatures across space and time: A fine-scale analysis at the treeline in the Swiss Alps. Trees 2018, 32, 645–660. [Google Scholar] [CrossRef]
- Li, X.; Liang, E.; Gričar, J.; Rossi, S.; Čufar, K.; Ellison, A.M. Critical minimum temperature limits xylogenesis and maintains treelines on the southeastern Tibetan Plateau. Sci. Bull. 2017, 62, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Simard, S.; Deslauriers, A.; Morin, H. Wood formation in Abies balsamea seedlings subjected to artificial defoliation. Tree Physiol. 2009, 29, 551–558. [Google Scholar] [CrossRef]
- De Micco, V.; Aronne, G. Morpho-anatomical traits for plant adaptation to drought. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 37–61. ISBN 978-3-642-32653-0. [Google Scholar]
- Du, X.; Du, F.; Zeng, H.; Chen, Y.; Yao, Y.; Zhang, H.; Li, Z. A study on the community of Abies George var. smithii in Jiaozi Mountain Nature Reserve. J. Yunnan Univ. (Nat. Sci. Ed.) 2010, 32, 358–364. [Google Scholar]
- Li, Z.; Liu, K.; Chen, Y.; Yao, Y.; Du, X.; Zhang, H. Studies on the vegetation form and distribution characteristics in Jiaozishan Nature Reserve. Shandong For. Sci. Technol. 2010, 40, 32–35. [Google Scholar]
- Rossi, S.; Anfodillo, T.; Menardi, R. Trephor: A New Tool for Sampling Microcores from tree stems. IAWA J. 2006, 27, 89–97. [Google Scholar] [CrossRef]
- Denne, M.P. Definition of Latewood According to Mork (1928). IAWA J. 1989, 10, 59–62. [Google Scholar] [CrossRef]
- Park, Y.-I.; Spiecker, H. Variations in the tree-ring structure of Norway spruce (Picea abies) under contrasting climates. Dendrochronologia 2005, 23, 93–104. [Google Scholar] [CrossRef]
- Deslauriers, A.; Morin, H.; Begin, Y. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada). Can. J. For. Res. 2003, 33, 190–200. [Google Scholar] [CrossRef]
- Gričar, J.; Čufar, K.; Eler, K.; Gryc, V.; Vavrčík, H.; de Luis, M.; Prislan, P. Transition Dates from Earlywood to Latewood and Early Phloem to Late Phloem in Norway Spruce. Forests 2021, 12, 331. [Google Scholar] [CrossRef]
- Miller, T.W.; Stangler, D.F.; Larysch, E.; Seifert, T.; Spiecker, H.; Kahle, H.-P. Plasticity of seasonal xylem and phloem production of Norway spruce along an elevational gradient. Trees 2020, 34, 1281–1297. [Google Scholar] [CrossRef]
- Moser, L.; Fonti, P.; Büntgen, U.; Esper, J.; Luterbacher, J.; Franzen, J.; Frank, D. Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol. 2010, 30, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Prislan, P.; Gričar, J.; de Luis, M.; Smith, K.T.; Čufar, K. Phenological variation in xylem and phloem formation in Fagus sylvatica from two contrasting sites. Agric. For. Meteorol. 2013, 180, 142–151. [Google Scholar] [CrossRef]
- Gričar, J.; Prislan, P.; Gryc, V.; Vavrčík, H.; De Luis, M.; Čufar, K. Plastic and locally adapted phenology in cambial seasonality and production of xylem and phloem cells in Picea abies from temperate environments. Tree Physiol. 2014, 34, 869–881. [Google Scholar] [CrossRef]
- DesLauriers, A.; Rossi, S.; Anfodillo, T.; Saracino, A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol. 2008, 28, 863–871. [Google Scholar] [CrossRef]
- Ziaco, E. A phenology-based approach to the analysis of conifers intra-annual xylem anatomy in water-limited environments. Dendrochronologia 2020, 59, 125662. [Google Scholar] [CrossRef]
- Körner, C.; Basler, D. Phenology Under Global Warming. Science 2010, 327, 1461–1462. [Google Scholar] [CrossRef] [PubMed]
- Renninger, H.J.; Gartner, B.L.; Grotta, A.T. No Correlation between Latewood Formation and Leader Growth in Douglas-Fir Saplings. IAWA J. 2006, 27, 183–191. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Morin, H.; Saracino, A.; Motta, R.; Borghetti, M. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 2006, 170, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Jin, X.; Tian, Y.; Huang, Z.; Wang, X.; Zhang, Y.; Sun, Y.; Shao, C. Molecular regulation of bud dormancy in perennial plants. Plant Growth Regul. 2023. [Google Scholar] [CrossRef]
- Huang, J.-G.; Ma, Q.; Rossi, S.; Biondi, F.; Deslauriers, A.; Fonti, P.; Liang, E.; Mäkinen, H.; Oberhuber, W.; Rathgeber, C.B.K.; et al. Photoperiod and temperature as dominant environmental drivers triggering secondary growth resumption in Northern Hemisphere conifers. Proc. Natl. Acad. Sci. USA 2020, 117, 20645–20652. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; de Oliveira, D.M.; Andreotti, G.C.; de Souza, L.A.; dos Santos, W.D.; Bonato, C.M.; Antunes, W.C. Increased Gibberellins and Light Levels Promotes Cell Wall Thickness and Enhance Lignin Deposition in Xylem Fibers. Front. Plant Sci. 2018, 9, 1391. [Google Scholar] [CrossRef]
- Körner, C. Alpine Treelines: Functional Ecology of the Global High Elevation Tree Limits; Springer: Basel, Switzerland, 2012; ISBN 978-3-0348-0396-0. [Google Scholar]
- Davik, J.; Kjersti Bakken, A.; Holte, K.; Blomhoff, R. Effects of genotype and environment on total anti-oxidant capacity and the content of sugars and acids in strawberries (Fragaria × ananassa Duch.). J. Hortic. Sci. Biotechnol. 2006, 81, 1057–1063. [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Hyde, P.T.; Setter, T.L. Long-day photoperiod and cool temperature induce flowering in cassava: Expression of signaling genes. Front. Plant Sci. 2022, 13, 973206. [Google Scholar] [CrossRef]
- Osnato, M.; Cota, I.; Nebhnani, P.; Cereijo, U.; Pelaz, S. Photoperiod Control of Plant Growth: Flowering Time Genes Beyond Flowering. Front. Plant Sci. 2022, 12, 805635. [Google Scholar] [CrossRef]
- Siemann, J.K.; Williams, P.; Malik, T.N.; Jackson, C.R.; Green, N.H.; Emeson, R.B.; Levitt, P.; McMahon, D.G. Photoperiodic effects on monoamine signaling and gene expression throughout development in the serotonin and dopamine systems. Sci. Rep. 2020, 10, 15437. [Google Scholar] [CrossRef] [PubMed]
- Boöhlenius, H.; Huang, T.; Charbonnel-Campaa, L.; Brunner, A.M.; Jansson, S.; Strauss, S.H.; Nilsson, O. CO/FT Regulatory Module Controls Timing of Flowering and Seasonal Growth Cessation in Trees. Science 2006, 312, 1040–1043. [Google Scholar] [CrossRef]
- Olsen, J.E.; Junttila, O.; Nilsen, J.; Eriksson, M.E.; Martinussen, I.; Olsson, O.; Sandberg, G.; Moritz, T. Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimatization. Plant J. 1997, 12, 1339–1350. [Google Scholar] [CrossRef]
- Rohde, A.; Bhalerao, R.P. Plant dormancy in the perennial context. Trends Plant Sci. 2007, 12, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Deslauriers, A.; Morin, H. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia 2003, 21, 33–39. [Google Scholar] [CrossRef]
- Zheng, Z.; Fang, K.; Chen, Y.; Dong, Z.; Zhou, F.; Li, Y. Is the Pinus massoniana Lamb. Tree-Ring Latewood Formation Influenced by the Diurnal Temperature Range in Humid Subtropical China? Forests 2022, 13, 1439. [Google Scholar] [CrossRef]
- Fritts, H. Tree Rings and Climate; Elsevier: Amsterdam, The Netherlands, 1976; ISBN 978-0-323-14528-2. [Google Scholar]
- Traversari, S.; Giovannelli, A.; Emiliani, G. Wood Formation under Changing Environment: Omics Approaches to Elucidate the Mechanisms Driving the Early-to-Latewood Transition in Conifers. Forests 2022, 13, 608. [Google Scholar] [CrossRef]
- Jang, G.; Yoon, Y.; Choi, Y.D. Crosstalk with Jasmonic Acid Integrates Multiple Responses in Plant Development. Int. J. Mol. Sci. 2020, 21, 305. [Google Scholar] [CrossRef]
- Lindsey, K. Peptides: New signalling molecules in plants. Trends Plant Sci. 2002, 7, 78–83. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Čufar, K.; Cuny, H.E.; Deslauriers, A.; Fonti, P.; Frank, D.; Gričar, J.; Gruber, A.; King, G.M.; et al. A meta-analysis of cambium phenology and growth: Linear and non-linear patterns in conifers of the northern hemisphere. Ann. Bot. 2013, 112, 1911–1920. [Google Scholar] [CrossRef]
- Bouriaud, O.; Leban, J.-M.; Bert, D.; Deleuze, C. Intra-annual variations in climate influence growth and wood density of Norway spruce. Tree Physiol. 2005, 25, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Cartenì, F.; Deslauriers, A.; Rossi, S.; Morin, H.; De Micco, V.; Mazzoleni, S.; Giannino, F. The Physiological Mechanisms Behind the Earlywood-To-Latewood Transition: A Process-Based Modeling Approach. Front. Plant Sci. 2018, 9, 1053. [Google Scholar] [CrossRef] [PubMed]
- Cregg, B.M.; Dougherty, P.M.; Hennessey, T.C. Growth and wood quality of young loblolly pine trees in relation to stand density and climatic factors. Can. J. For. Res. 1988, 18, 851–858. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Bréda, N.; Ulrich, E.; Granier, A. Climate-tree-growth relationships of European beech (Fagus sylvatica L.) in the French Permanent Plot Network (RENECOFOR). Trees 2005, 19, 385–401. [Google Scholar] [CrossRef]
- Deslauriers, A.; Rossi, S.; Anfodillo, T. Dendrometer and intra-annual tree growth: What kind of information can be inferred? Dendrochronologia 2007, 25, 113–124. [Google Scholar] [CrossRef]
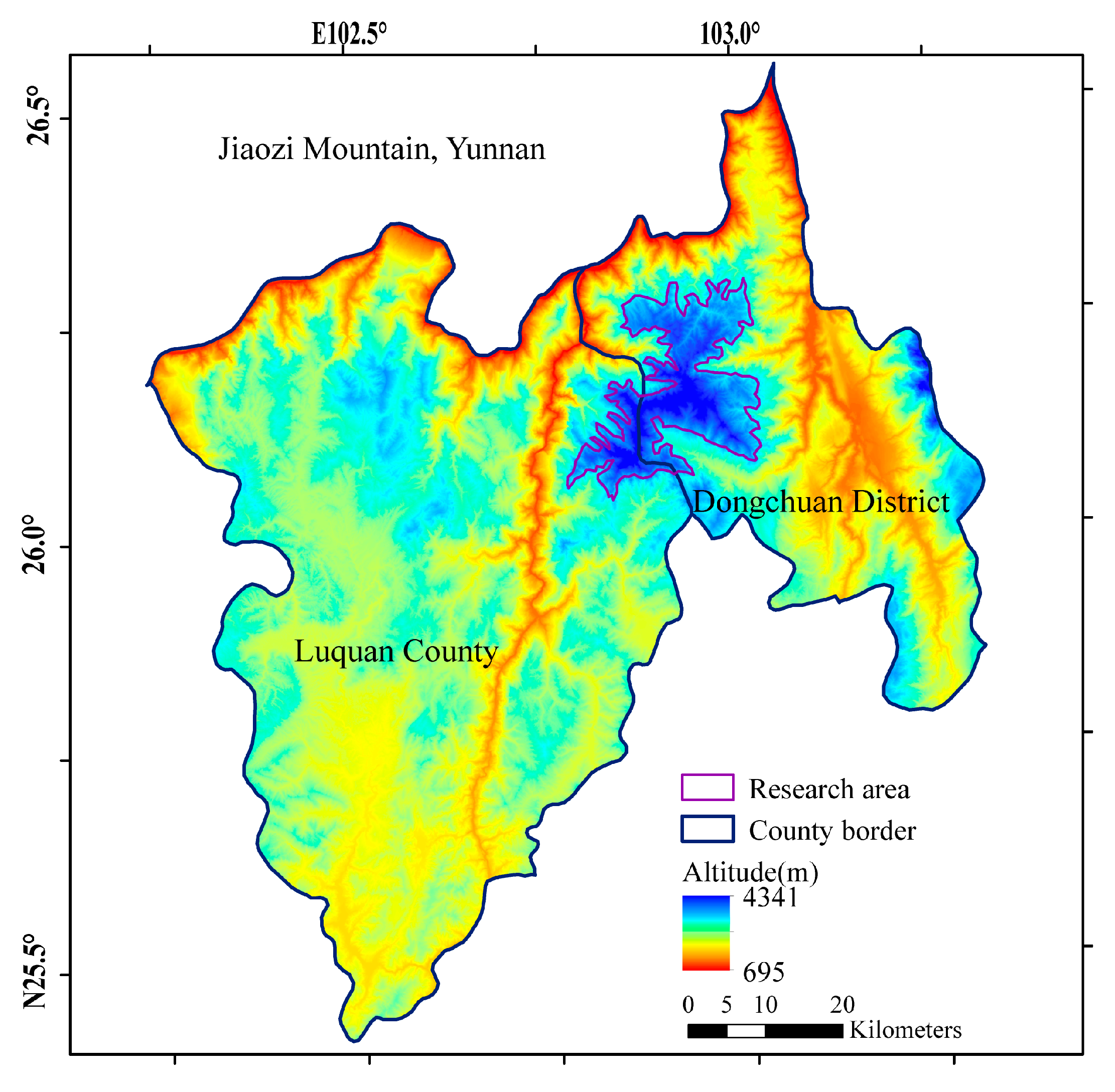
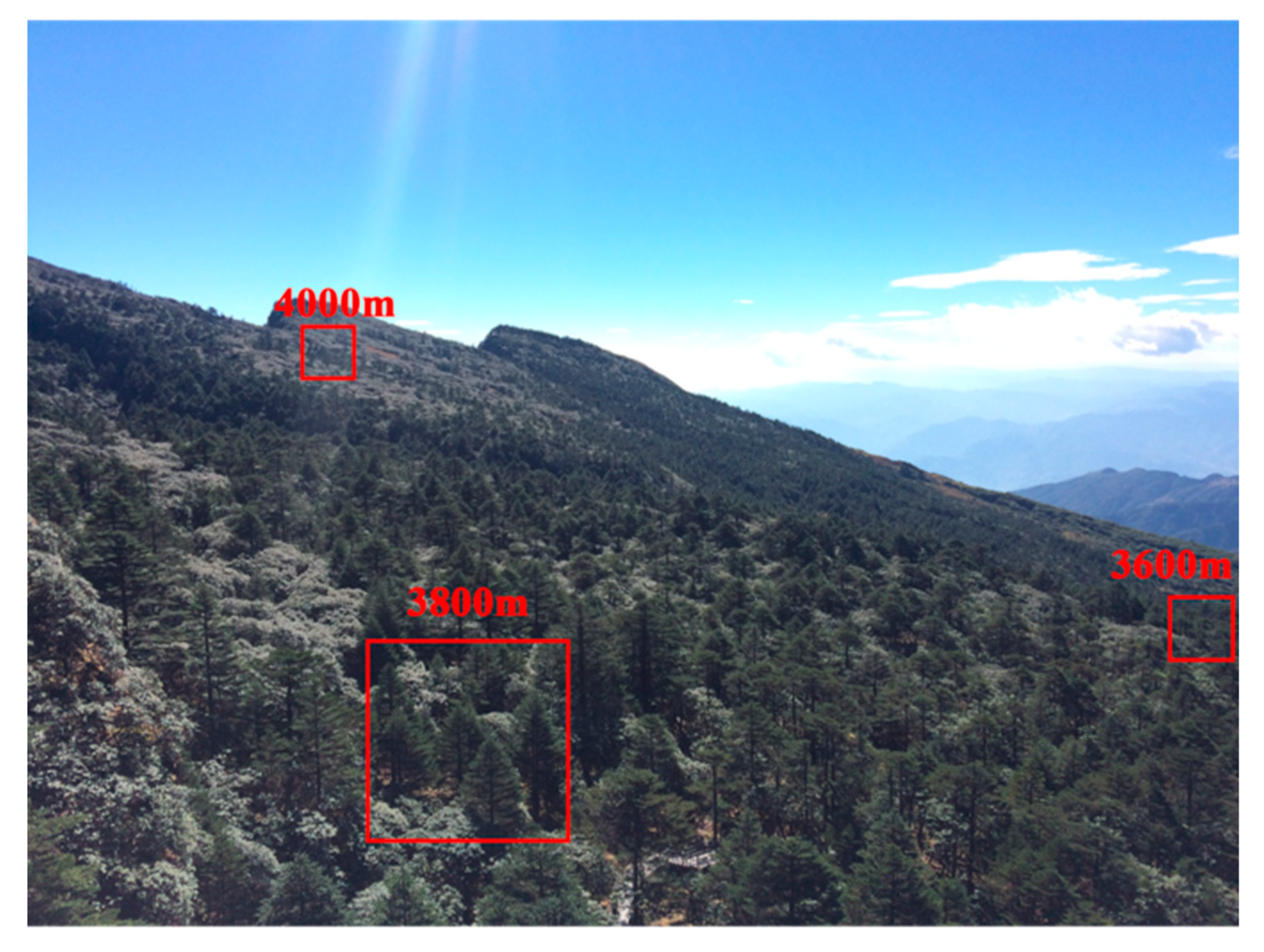

| Elevation (m) | Number | Basal Diameter (cm) | Diameter at Breast Height (cm) | Height (cm) | Age (years) |
|---|---|---|---|---|---|
| 3600 | 1 | 32 | 25 | 1250 | 58 |
| 2 | 21 | 16.5 | 1100 | 27 | |
| 3 | 23 | 18 | 1200 | 45 | |
| 4 | 20 | 15.5 | 1150 | 51 | |
| 5 | 22 | 17 | 1160 | 40 | |
| 3800 | 6 | 45 | 35 | 1200 | 32 |
| 7 | 32 | 25 | 1050 | 24 | |
| 8 | 16 | 12 | 1180 | 20 | |
| 9 | 34 | 27 | 1300 | 24 | |
| 10 | 22 | 17 | 1020 | 18 | |
| 4000 | 11 | 20 | 17 | 510 | 27 |
| 12 | 57 | 44 | 680 | 55 | |
| 13 | 19 | 15 | 500 | 27 | |
| 14 | 19 | 14.5 | 390 | 22 | |
| 15 | 21 | 16 | 520 | 20 | |
| Mean ± SE | 25.87 ± 2.93 | 20.97 ± 2.27 | 947.33 ± 83.93 | 32.66 ± 3.50 |
| Elevation (m) | Air Temperature (°C) | Humidity (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Average | Maximum | Minimum | Direct Reduction Rate (°C m−1) | Average | Maximum | Minimum | Direct Reduction Rate (°C m−1) | |
| 3600 | 6.52 | 13.28 | −4.06 | 83.59 | 100 | 25.74 | ||
| 3800 | 4.91 | 11.84 | −5.21 | 0.0060 ± 0.0021 | 85.76 | 100 | 25.00 | 0 |
| 4000 | 4.14 | 12.18 | −5.54 | 85.24 | 100 | 22.63 | ||
| Elevation (m) | T-Onset | T-Duration | T-Ending | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Branches | Stem | p | F | n | Branches | Stem | p | F | n | Branches | Stem | p | F | n | |
| 3600 m | 167 ± 9 | 190 ± 9 | 0.004 | 16.235 | 10 | 30 ± 10 | 20 ± 2 | 0.065 | 4.567 | 10 | 197 ± 11 | 211 ± 9 | 0.078 | 4.088 | 10 |
| 3800 m | 163 ± 4 | 184 ± 11 | 0.005 | 14.301 | 10 | 27 ± 4 | 21 ± 3 | 0.037 | 6.228 | 10 | 190 ± 8 | 205 ± 13 | 0.037 | 6.228 | 10 |
| 4000 m | 169 ± 15 | 179 ± 9 | 0.201 | 1.941 | 10 | 22 ± 9 | 18 ± 5 | 0.453 | 0.621 | 10 | 191 ± 20 | 198 ± 14 | 0.567 | 0.356 | 10 |
| p | 0.689 | 0.236 | 0.307 | 0.566 | 0.705 | 0.292 | |||||||||
| F | 0.384 | 1.635 | 1.306 | 0.597 | 0.360 | 1.367 | |||||||||
| n | 15 | 15 | 15 | 15 | 15 | 15 | |||||||||
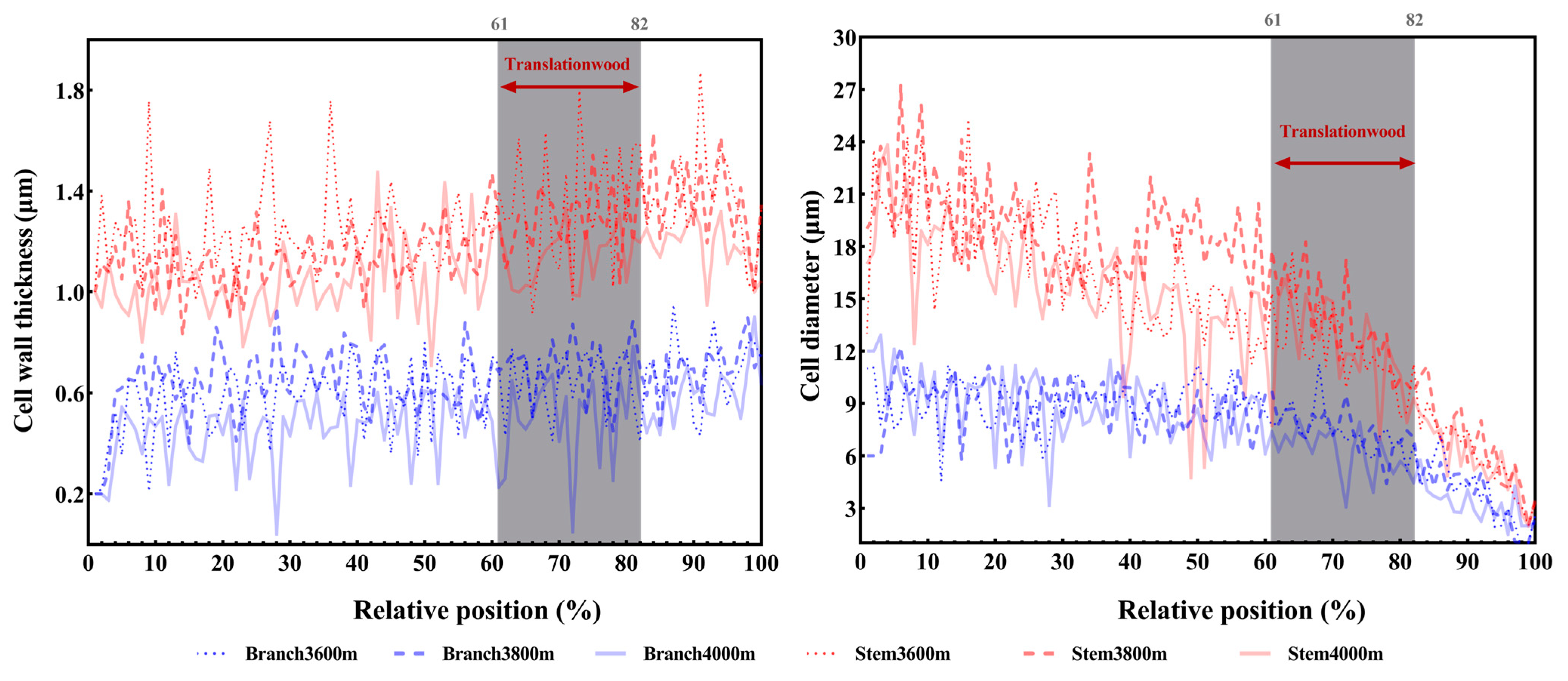
| Elevation (m) | Cell Wall Thickness (μm) | Cell Diameter (μm) | Cell Enlargement Duration (d) | Wall Deposition Rate (μm2d−1) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Branches | Stem | p | F | n | Branches | Stem | p | F | n | Branches | Stem | p | F | n | Branches | Stem | p | F | n | |
| 3600 | 0.61 ± 0.13 | 1.30 ± 0.18 | 0.001 | 49.65 | 10 | 7.91 ± 1.30 | 14.13 ± 3.20 | 0.004 | 16.17 | 10 | 3.28 ± 3.15 | 3.56 ± 2.91 | 0.677 | 0.175 | 10 | 8.668 ± 1.68 | 23.23 ± 3.56 | 0.001 | 23.215 | 10 |
| 3800 | 0.61 ± 0.04 | 1.21 ± 0.14 | 0.001 | 82.12 | 10 | 7.41 ± 1.57 | 15.82 ± 3.39 | 0.001 | 25.31 | 10 | 2.64 ± 3.51 | 3.35 ± 4.72 | 0.734 | 0.116 | 10 | 11.21 ± 1.83 | 35.50 ± 2.00 | 0.031 | 4.766 | 10 |
| 4000 | 0.44 ± 0.04 | 1.09 ± 0.31 | 0.002 | 21.65 | 10 | 7.22 ± 1.78 | 11.99 ± 2.53 | 0.009 | 11.87 | 10 | 3.54 ± 3.10 | 4.11 ± 3.47 | 0.212 | 1.584 | 10 | 5.304 ± 1.20 | 17.76 ± 3.37 | 0.001 | 40.954 | 10 |
| p | 0.008 | 0.349 | 0.775 | 0.183 | 0.554 | 0.166 | 0.001 | 0.001 | ||||||||||||
| F | 7.373 | 1.151 | 0.260 | 1.960 | 0.594 | 1.820 | 42.288 | 21.123 | ||||||||||||
| n | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | ||||||||||||
| Organ | Daylength | Temperature | Humidity | |||
|---|---|---|---|---|---|---|
| Odd Ratio (95%CI) | p Value | Odd Ratio (95%CI) | p Value | Odd Ratio (95%CI) | p Value | |
| Branch | 12.44 | <0.01 | 0.60 | <0.01 | 0.87 | <0.01 |
| Stem | 79.49 | <0.01 | 0.80 | <0.01 | 0.90 | <0.01 |
| Stage | Cell Enlargement Duration of the Branches (d) | Cell Wall Deposition Rate of the Branches (μm2d−1) | ||
|---|---|---|---|---|
| Odd Ratio (95%CI) | p Value | Odd Ratio (95%CI) | p Value | |
| Stem transition | 0.59 | <0.01 | 1.03 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhu, M.; Lin, L.; Yang, Z.; Yao, F. The Early- to Latewood Transition Phenology Is Asynchronous between the Different Parts of Abies forrestii var. smithii in Jiaozi Mountain, Yunnan, China. Forests 2023, 14, 1456. https://doi.org/10.3390/f14071456
Wang W, Zhu M, Lin L, Yang Z, Yao F. The Early- to Latewood Transition Phenology Is Asynchronous between the Different Parts of Abies forrestii var. smithii in Jiaozi Mountain, Yunnan, China. Forests. 2023; 14(7):1456. https://doi.org/10.3390/f14071456
Chicago/Turabian StyleWang, Wenli, Mingyang Zhu, Lin Lin, Ziyu Yang, and Fenjie Yao. 2023. "The Early- to Latewood Transition Phenology Is Asynchronous between the Different Parts of Abies forrestii var. smithii in Jiaozi Mountain, Yunnan, China" Forests 14, no. 7: 1456. https://doi.org/10.3390/f14071456
APA StyleWang, W., Zhu, M., Lin, L., Yang, Z., & Yao, F. (2023). The Early- to Latewood Transition Phenology Is Asynchronous between the Different Parts of Abies forrestii var. smithii in Jiaozi Mountain, Yunnan, China. Forests, 14(7), 1456. https://doi.org/10.3390/f14071456





