Pine Bark as a Potential Source of Condensed Tannin: Analysis through Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), and Energy Dispersive X-ray (EDX)
Abstract
1. Introduction
2. Materials and Methods
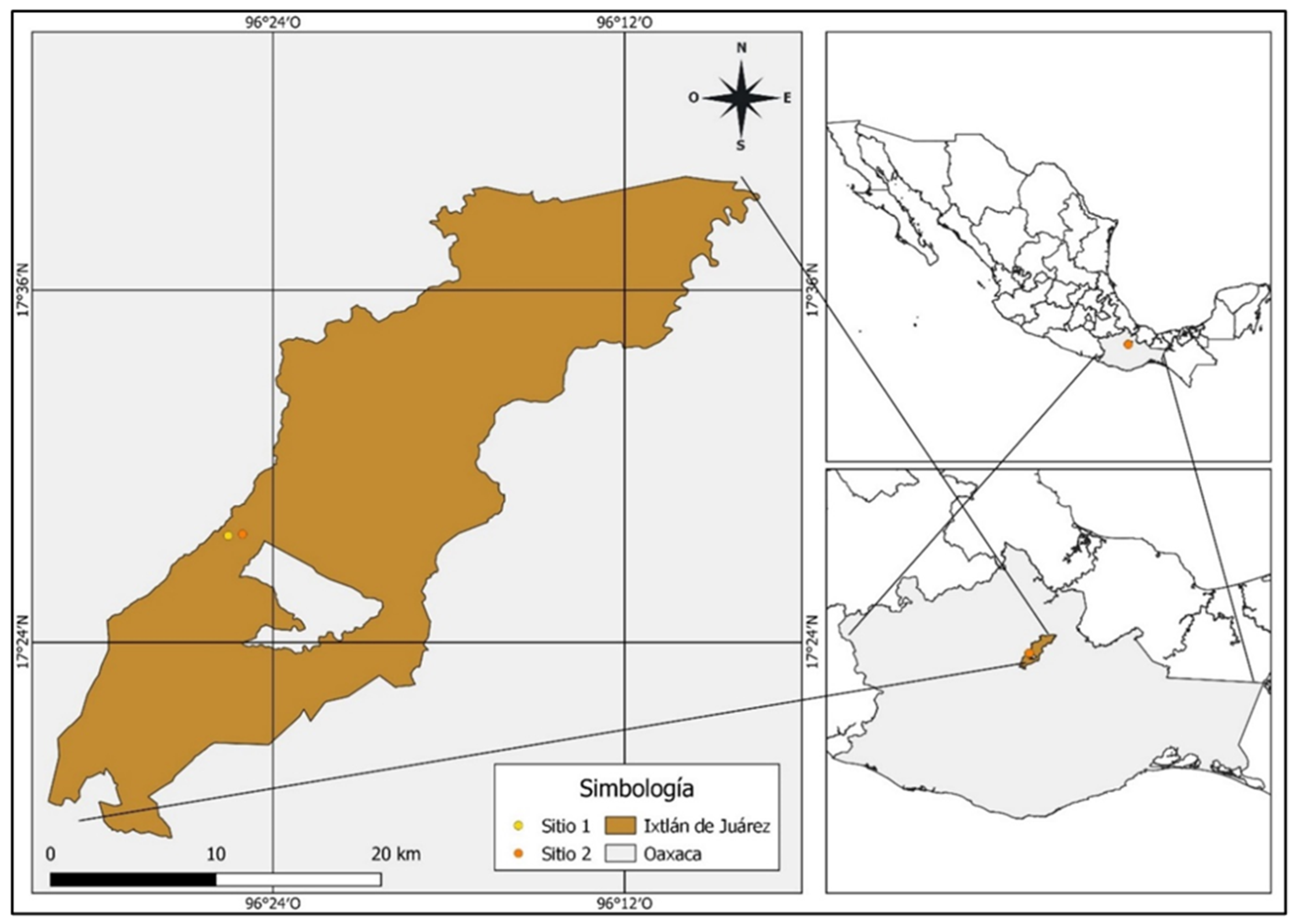
2.1. Study Area
2.2. Tree Selection and Sample Preparation
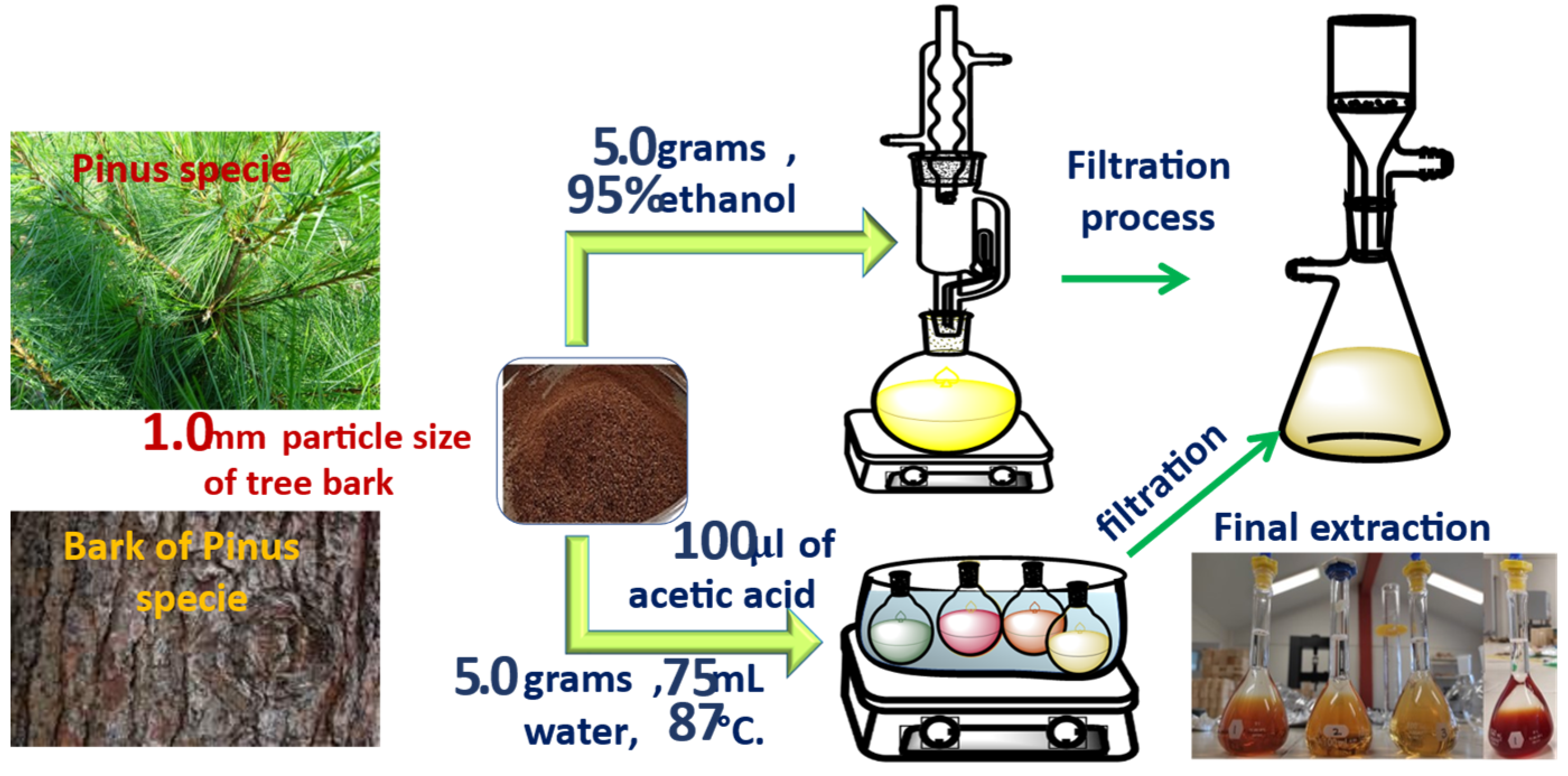
2.3. Extraction of Tannins with Ethanol, Stiasny Number, and Condensed Tannins
2.4. Extraction of Tannins with Distilled Water, Stiasny Number, and Condensed Tannins
2.5. Characterization of Tannins by Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Analyses by Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX) Spectrometry
2.7. Statistical Analysis
3. Results and Discussion
3.1. Tannin Extraction with Ethanol, Stiasny Number, and Condensed Tannins
3.2. Extraction of Tannins with Water, Stiasny Number, and Condensed Tannins
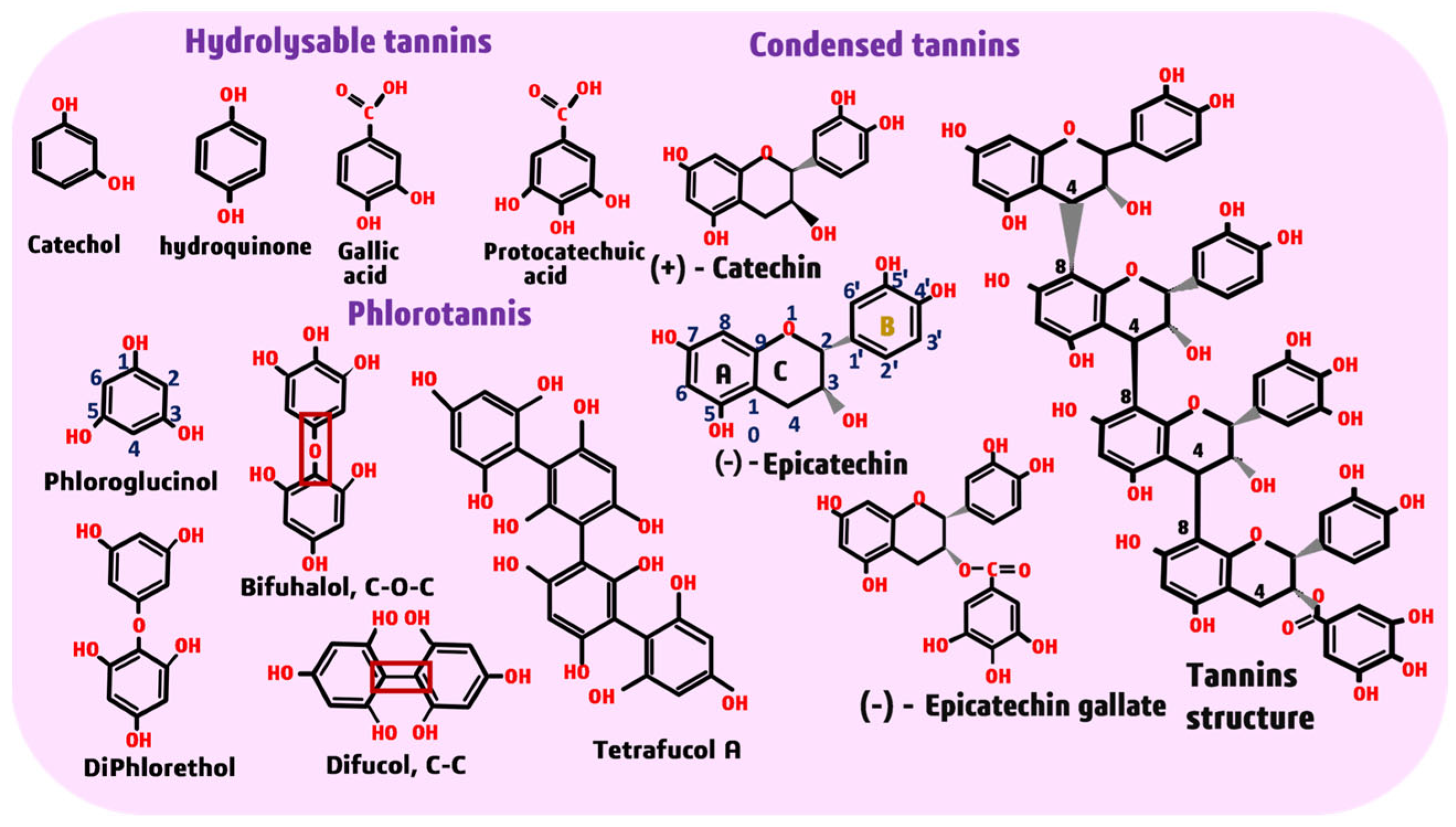
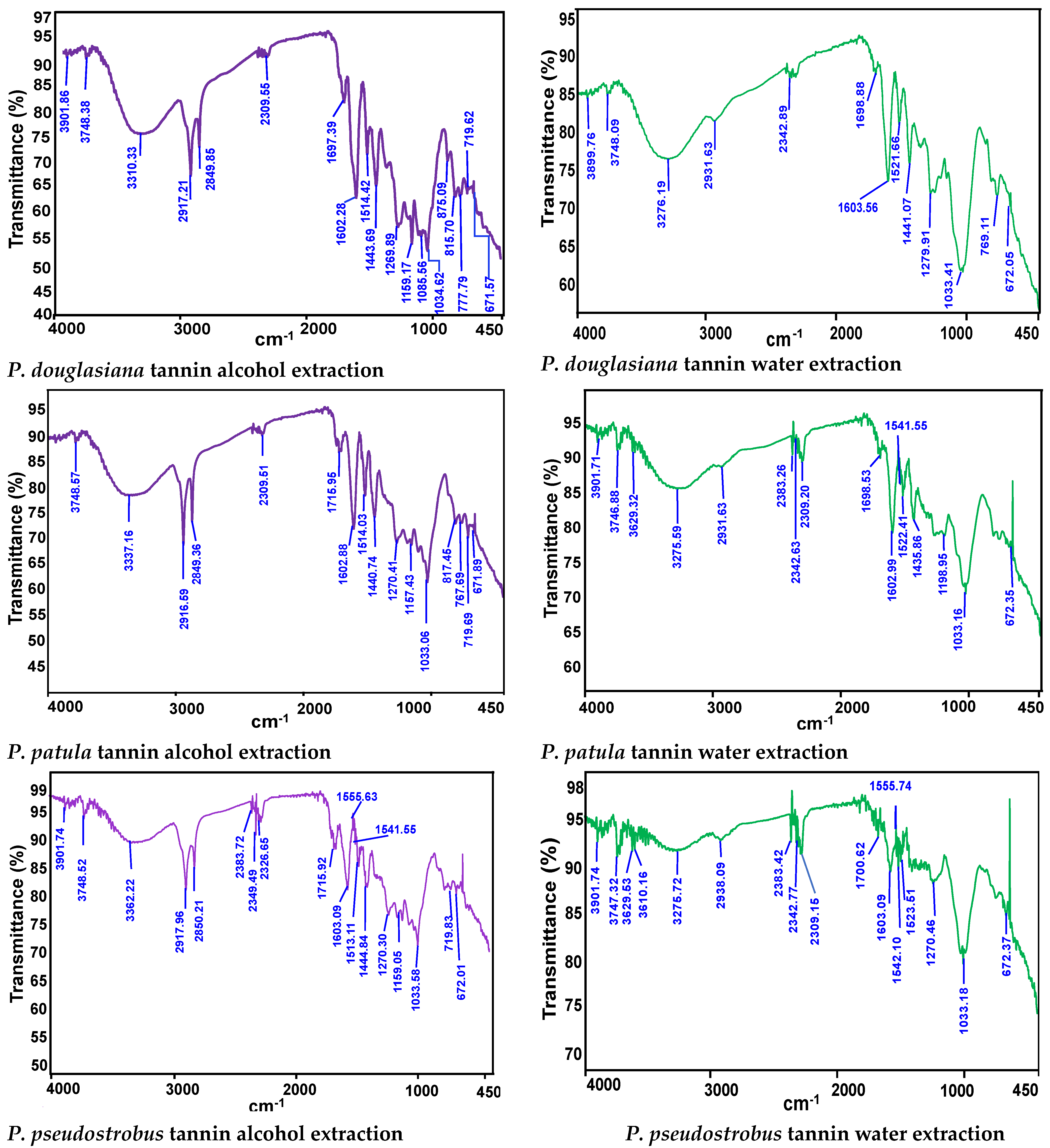
3.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.4. Scanning Electron Microscopy (SEM) Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CONAFOR (Comisión Nacional Forestal). Estudio Regional Forestal Para el Fortalecimiento de Las Unidades de Manejo Forestal en la Sierra Norte de Oaxaca. Fondo CONAFOR-CONACYT, Proyecto Número 41808. 2006. Available online: http://www.conafor.gob.mx:8080/documentos/docs/9/1091ERF_UMAFOR2001.pdf (accessed on 12 December 2021).
- Aquino Vásquez, C.; Ruiz Aquino, F.; Fuente Carrasco, M.E. Caracterización del patrimonio natural de la comunidad de Ixtlán de Juárez: Una aproximación desde el espacio territorial. In Conocimiento Indígena Contemporáneo Y Patrimonio Biocultural En La Sierra Juárez De Oaxaca: Aportaciones Empíricas Y Analíticas Hacia La Sustentabilidad; Fuente Carrasco, M.E., Ruiz Aquino, F., y Aquino Vásquez, C., Eds.; Universidad de la Sierra Juárez: Ixtlán de Juárez, México, 2012; Volume 201, pp. 35–59. [Google Scholar]
- Camacho-Montoya, J.A.; Santiago-García, W.; Rodríguez-Ortiz, G.; Antúnez, P.; Santiago-García, E.; Suárez-Mota, M.E. Autoaclareo y manejo de la densidad en rodales coetáneos de Pinus patula Schiede ex Schlechtdl. & Cham. Rev. Mex. Cienc. For. 2018, 9, 188–212. [Google Scholar]
- Serret-Guasch, N.; Giralt-Ortega, G.; Quintero-Ríos, M. Caracterización de aserrín de diferentes maderas. Tecnol. Química 2016, 36, 395–405. [Google Scholar]
- Haslam, E. Plant Polyphenols: Vegetable Tannins Revisited; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Fraga-Corral, M.; Otero, P.; Cassani, L.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Chamorro, F.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility. Foods 2021, 10, 251. [Google Scholar] [CrossRef]
- Ruiz-Aquino, F.; Feria-Reyes, R.; Rutiaga-Quiñones, J.G.; Santiago-García, W.; Suárez-Mota, M.E.; Esquivel-Reyes, H.H. Development and validation of an analytical method for condensed tannin extracts obtained from the bark of four tree species using HPLC. Wood Res. 2021, 66, 171–182. [Google Scholar] [CrossRef]
- Brennan, M.; Fritsch, C.; Cosgun, S.; Dumarcay, S.; Colin, F.; Gérardin, P. Quantitative and qualitative composition of bark polyphenols changes longitudinally with bark maturity in Abies alba Mill. Ann. For. Sci. 2020, 77, 9. [Google Scholar] [CrossRef]
- Krekling, T.; Franceschi, V.R.; Berryman, A.A.; Christiansen, E. The structure and development of polyphenolic parenchyma cells in Norway spruce (Picea abies) bark. Flora 2000, 195, 354–369. [Google Scholar] [CrossRef]
- Swaminathan, S.; Lionetti, V.; Zabotina, O.A. Plant Cell Wall Integrity Perturbations and Priming for Defense. Plants 2022, 11, 3539. [Google Scholar] [CrossRef]
- Abilleira, F.; Varela, P.; Cancela, Á.; Álvarez, X.; Sánchez, Á.; Valero, E. Tannins extraction from Pinus pinaster and Acacia dealbata bark with applications in the industry. Ind. Crops Prod. 2021, 164, 113394. [Google Scholar] [CrossRef]
- Dababi, I.; Gimello, O.; Elaloui, E.; Brosse, N. Water Extraction of Tannins from Aleppo Pine Bark and Sumac Root for the Production of Green Wood Adhesives. Molecules 2020, 25, 5041. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Das, R.K.; Mizan, A.; Zohra, F.T.; Ahmed, S.; Ahmed, K.S.; Hossain, H. Extraction of a novel tanning agent from indigenous plant bark and its application in leather processing. J. Leather Sci. Eng. 2022, 4, 18. [Google Scholar] [CrossRef]
- Bacelo, H.; Santos, S.C.R.; Botelho, C.M.S. Removal of arsenic from water by an iron-loaded resin prepared from Pinus pinaster bark tannins. Euro-Mediterr. J. Environ. Integr. 2020, 5, 3. [Google Scholar] [CrossRef]
- Byrne, C.E.; D’Alessandro, O.; Deyá, C. Tara Tannins as a Green Sustainable Corrosion Inhibitor for Aluminum. J. Mater. Eng Perform 2022, 31, 2918–2933. [Google Scholar] [CrossRef]
- Fokuo, M.K.; Aggrey, W.N.; Rockson, M.A.D.; Sokama-Neuyam, Y.A.; Boakye, P.; Amenuvor, G.; Sarkodie, K.; Pinto, E.; Karimaie, H. Tannin-Based Deflocculants in High Temperature High Pressure Wells: A Comprehensive Review. Adv.Chem. Eng. Sci. 2021, 11, 263–289. [Google Scholar] [CrossRef]
- Arias, A.; González-García, S.; Feijoo, G.; Moreira, M.T. Tannin-based bio-adhesives for the wood panel industry as sustainable alternatives to petrochemical resins. J. Ind. Ecol. 2022, 26, 627–642. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and actual industrial applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Seijas, S.; Seijas, P.; Seijas, N.; Salgado, L.; Esquivel, C.; Gonzalez, J.L. Parameters energy in the industrial production of tannins of Caesalpinia spinosakuntze (Tara). Rev. Cienc. Tecnol. 2022, 18, 189–201. [Google Scholar]
- Petchidurai, G.; Amruthraj, N.J.; John, M.S.; Sahayaraj, K.; Murugesan, N.; Pucciarelli, S. Standardization of quantification of total tannins, condensed tannin and soluble phlorotannins extracted from thirty-two drifted coastal macroalgae using high performance liquid chromatography. Bioresour. Technol. Rep. 2019, 7, 100273. [Google Scholar] [CrossRef]
- Mailoa, M.N.; Mahendradatta, M.; Laga, A.; Djide, N. Tannin extract of guava leaves (Psidium guajava L.) variation with concentration organic solvents. Int. J. Sci. Technol. Res. 2013, 2, 106–110. [Google Scholar]
- Duraisamy, R.; Shuge, T.; Worku, B.; Berekete, A.K.; Ramasamy, K.M. Extraction, screening and spectral characterization of tannins from acacia xanthophloea (Fever Tree) Bark. Res. J. Textile Leather 2020, 1, 1–10. [Google Scholar] [CrossRef]
- Luo, X.; Bai, R.; Zhen, D.; Yang, Z.; Huang, D.; Mao, H.; Fu, C. Response surface optimization of the enzyme-based ultrasound-assisted extraction of acorn tannins and their corrosion inhibition properties. Ind. Crops Prod. 2019, 129, 405–413. [Google Scholar] [CrossRef]
- Poaty, B.; Dumarçay, S.; Gérardin, P.; Perrin, D. Modification of grape seed and wood tannins to lipophilic antioxidant derivatives. Ind. Crops Prod. 2010, 31, 509–515. [Google Scholar] [CrossRef]
- Guo, L.; Qiang, T.; Ma, Y.; Wang, K.; Du, K. Optimisation of tannin extraction from Coriaria nepalensis bark as a renewable resource for use in tanning. Ind. Crops Prod. 2020, 149, 112360. [Google Scholar] [CrossRef]
- Antwi-Boasiako, C.; Animapauh, S.O. Tannin extraction from the barks of three tropical hardwoods for the production of adhesives. J. Appl. Sci. Res. 2012, 8, 2959–2965. [Google Scholar]
- Rhazi, N.; Hannache, H.; Oumam, M.; Sesbou, A.; Charrier, B.; Pizzi, A.; Charrier-El Bouhtoury, F. Green extraction process of tannins obtained from Moroccan Acacia mollissima barks by microwave: Modeling and optimization of the process using the response surface methodology RSM. Arab. J. Chem. 2019, 12, 2668–2684. [Google Scholar] [CrossRef]
- de Hoyos-Martinez, P.L.; Merle, J.; Labidi, J.; Charrier–El Bouhtoury, F. Tannins extraction: A key point for their valorization and cleaner production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef]
- Vásquez-Cortez, V.F.; Clark-Tapia, R.; Manzano-Méndez, F.; González-Adame, G.; Aguirre-Hidalgo, V. Estructura, composición y diversidad arbórea y arbustiva en tres condiciones de manejo forestal de Ixtlán de Juárez, Oaxaca. Madera Y Bosques 2018, 24, 2431649. [Google Scholar] [CrossRef]
- Valencia-Avalos, S.; Nixon, K. Encinos. In Biodiversidad de Oaxaca; García-Mendoza, A.J., Ordóñez, M.J., Briones-Salas, M., Eds.; Instituto de Biología-UNAM, Fondo Oaxaqueño Para la Conservación de la Naturaleza, World Wildlife Fund. D.F.: Oaxaca, México, 2004; pp. 219–225. [Google Scholar]
- Del Castillo, R.F.; Pérez de la Rosa, J.A.; Vargas, A.; Rivera, R. Coníferas. In Biodiversidad de Oaxaca; García-Mendoza, A.J., Ordóñez, M.J., Briones-Salas, M., Eds.; Instituto de Biología-UNAM, Fondo Oaxaqueño para la Conservación de la Naturaleza, World Wildlife Fund. D.F.: Oaxaca, México, 2004; pp. 141–158. [Google Scholar]
- INEGI. Prontuario de Información Geográfica Municipal de los Estados Unidos Mexicanos Ixtlán de Juárez, Oaxaca. Clave Geoestadística 20042. 9p. 2008. Available online: https://1library.co/document/zl9lkdlz-prontuario-informacion-geografica-municipal-mexicanos-ixtlan-juarez-geoestadistica.html (accessed on 15 December 2021).
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen (Para Adaptarlo a las Condiciones de la República Mexicana); Instituto de Geografía UNAM: Mexico City, México, 1987. [Google Scholar]
- Rosales Castro, M.; González Laredo, R.F. Comparación del contenido de compuestos fenólicos en la corteza de ocho especies de pino. Madera Bosques 2003, 9, 41–49. [Google Scholar] [CrossRef]
- Pedraza-Bucio, F.E.; Rutiaga-Quiñones, J.G. Extracto tánico de la madera de palo de Brasil. Rev. Concienc. Tecnológica 2011, 42, 36–41. [Google Scholar]
- Ruiz-Aquino, F.; Feria-Reyes, R.; Rutiaga-Quiñones, J.G.; Robledo-Taboada, L.H.; Gabriel-Parra, R. Characterization of tannin extracts derived from the bark of four tree species by HPLC and FTIR. For. Sci. Technol. 2023, 9, 38–46. [Google Scholar] [CrossRef]
- SAS (Statistical Analysis System). SAS/ETS* 9.0 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Sarria-Villa, R.A.; Gallo, J.A.; Benítez, R. Extracción de compuestos fenólicos y contenido de catequina en cortezas de tres especies forestales del Cauca-Colombia. Entre Cienc. Ing. 2021, 15, 19–27. [Google Scholar] [CrossRef]
- Chemetova, C.; Ribeiro, H.; Fabião, A.; Gominho, J. Towards sustainable valorisation of Acacia melanoxylon biomass: Characterization of mature and juvenile plant tissues. Environ. Res. 2020, 191, 110090. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.S.; Ryazanova, T.V. Bark of Siberian conifers: Composition, use, and processing to extract tannin. Forests 2021, 12, 1043. [Google Scholar] [CrossRef]
- Bello, A.; Bergmann, U.; Vepsäläinen, J.; Leiviskä, T. Effects of tree harvesting time and tannin cold/hot-water extraction procedures on the performance of spruce tannin biocoagulant for water treatment. Chem. Eng. J. 2022, 449, 137809. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.; Teixeira, J.A. Green and sustainable valorization of bioactive phenolic compounds from pinus by-products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef] [PubMed]
- Frutos, P.; Hervas, G.; Giráldez, F.J.; Mantecón, A.R. Tannins and ruminant nutrition. Span. J. Agric. Res. 2004, 2, 191–202. [Google Scholar] [CrossRef]
- Quito-Torres, J.; Jumbo-Benítez, N.; Fernández-Guarnizo, P. The chemical composition of the wood of Schizolobium parahyba and its relationship with the chemical properties of the soil, in the Experimental Station “El Padmi”, province of Zamora Chinchipe. Bosques Latid. Cero. 2019, 9, 47–60. [Google Scholar]
- Valverde-Rodríguez, J.X.; del Carmen Jumbo–Benítez, N.; Fernández–Guarnizo, P.V.; Rogel, J.B.G.; Iñiguez–Ordoñez, D.P.; Pucha–Cofrep, D.A. Chemical composition of the wood of Juglans neotropica Diels., and its relationship with the chemical properties of the soil in Valladolid parish, Zamora Chinchipe province, Ecuador. Investig. Agrar. 2020, 2, 68–82. [Google Scholar]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content, soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Rosales Castro, M.; Galindo, A.; González Laredo, R.F. Taninos condensados en la corteza de Pinus chihuahuana y Pinus durangensis. Inf. Tecnológica 2002, 13, 39–42. Available online: https://www.researchgate.net/publication/258994854_Taninos_condensados_en_la_corteza_de_Pinus_chihuahuana_y_pinus_durangensis (accessed on 22 April 2022).
- Aguilar López, J.; Jaén Jiménez, J.C.; Vargas Abarca, A.S.; Jiménez Bonilla, P.; Vega Guzmán, L.; Herrera Núñez, J.; Borbón Alpízar, H.; Soto Fallas, R.M. Extracción y evaluación de taninos condensados a partir de la corteza de once especies maderables de Costa Rica. Tecnol. Marcha 2012, 25, 15–22. [Google Scholar] [CrossRef]
- Nurhanan, A.R.; Wan Rosli, W.I. Evaluation of polyphenol content and antioxidant activities of some selected organic and aqueous extracts of cornsilk (Zea Mays Hairs). J. Med. Biol. Eng. 2012, 1, 48–51. [Google Scholar] [CrossRef]
- Gonçalves, F.; Chávez, I.L.; Fassarella, M.; Brito, A.; Soares, E.; López, Y.; Oliveira, R.E. Extracción de taninos de la corteza de Pinus spp tratada térmicamente—Aplicación como adhesivo. Madera Bosques 2021, 27, 1–9. [Google Scholar] [CrossRef]
- Soto, R.; Freer, J.; Reyes, N.; Baeza, J. Extraction of polyflavonoids from Pinus radiata D. Don bark: Evaluation of effects of solvent composition and of the height on tree bark. Bol. Soc. Chil. Quím. 2001, 46, 41–49. [Google Scholar] [CrossRef]
- Inoue, S.; Asaga, M.; Ogi, T.; Yazaki, Y. Extraction of polyflavonoids from radiata pine bark using hot compressed water al temperatures higher than 100 °C. Holzforschung 1998, 52, 139–145. [Google Scholar] [CrossRef]
- Thummajitsakul, S.; Samaikam, S.; Tacha, S.; Silprasit, K. Study on FTIR spectroscopy, total phenolic content, antioxidant activity and anti-amylase activity of extracts and different tea forms of Garcinia schomburgkiana leaves. LWT 2020, 134, 110005. [Google Scholar] [CrossRef]
- Tondi, G.; Petutschnigg, A. Middle infrared (ATR FT-MIR) characterization of industrial tannin extracts. Ind. Crops Prod. 2015, 65, 422–428. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of Fourier Transform Infrared (FTIR) Spectroscopy in the Characterization of Tannins. Appl. Spectrosc. Rev. 2015, 50, 15. [Google Scholar] [CrossRef]
- Grossman, R.B. Free-Radical Reactions. In The Art of Writing Reasonable Organic Reaction Mechanisms; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Finch, C.A. Fundamentals of leather manufacturing. Polym. Int. 1995, 37, 149. [Google Scholar] [CrossRef]
- Zhou, W.; Apkarian, R.; Wang, Z.; Joy, D. Fundamentals of scanning electron microscopy. In Scanning Microscopy for Nanotechnology; Zhou, W., Wang, Z., Eds.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Gupta, N.; Ram, H.; Cakmak, I. Micronutrients: Soil to Seed. In Biofortification of Staple Crops; Kumar, S., Dikshit, H.K., Mishra, G.P., Singh, A., Eds.; Springer: Singapore, 2022. [Google Scholar]
- Mohammadzadeh, H.; Mirzaei, J.; Farashiyani, M.E.; Soheili, F.; Woodward, S.; Abdul-Hamid, H.; Naji, H.R. Variation in the nutrient contents of leaves, bark, and wood of Persian oak trees (Quercus brantii) affected by decline. BioResources 2021, 16, 4704–4715. [Google Scholar] [CrossRef]
- Inkson, B.J. Materials Characterization Using Nondestructive Evaluation (NDE) Methods. In Chapter: 2 Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) for Materials Characterization; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Deladino, L.; Anbinder, P.S.; Navarro, A.S.; Martino, M.N. Encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydr. Polym. 2008, 71, 126–134. [Google Scholar] [CrossRef]
- Vasile-Dan, H. Characterization of Nanoparticles. In Chapter 4.4 Energy-Dispersive X-ray Spectroscopy (EDS); Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]


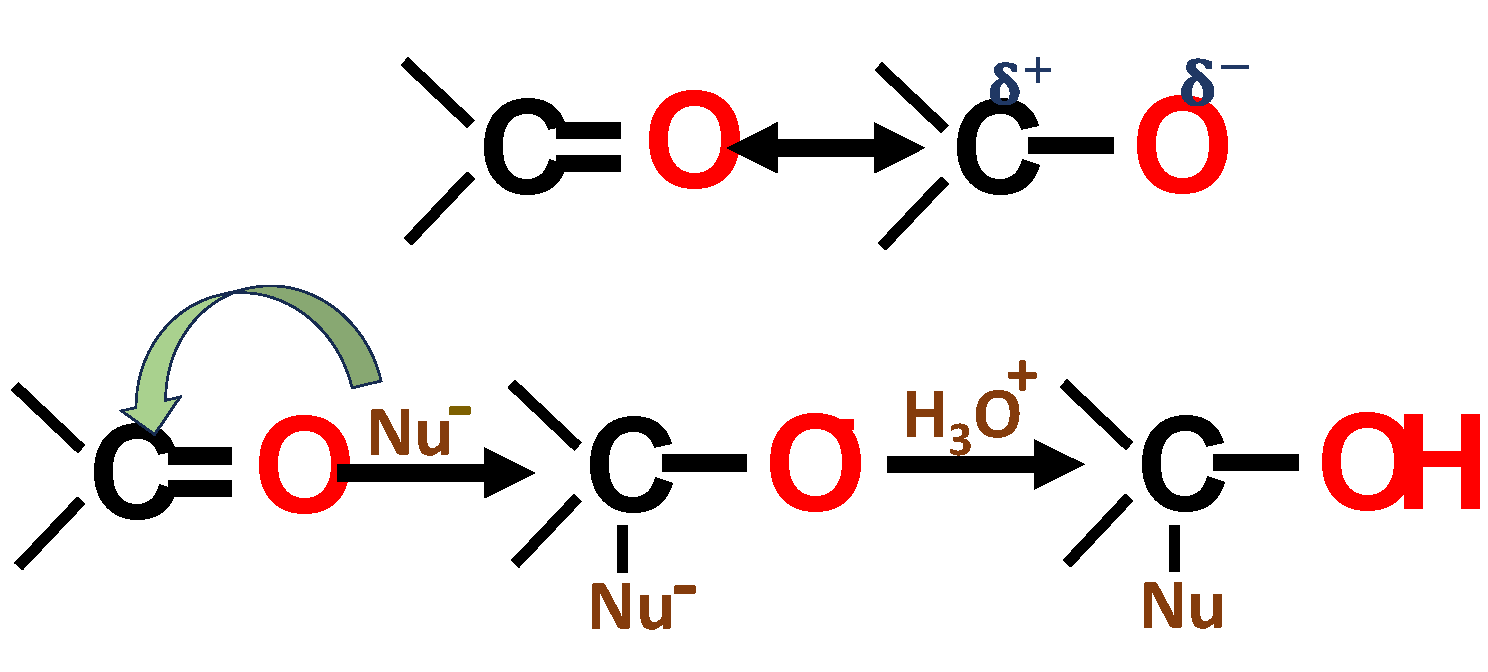
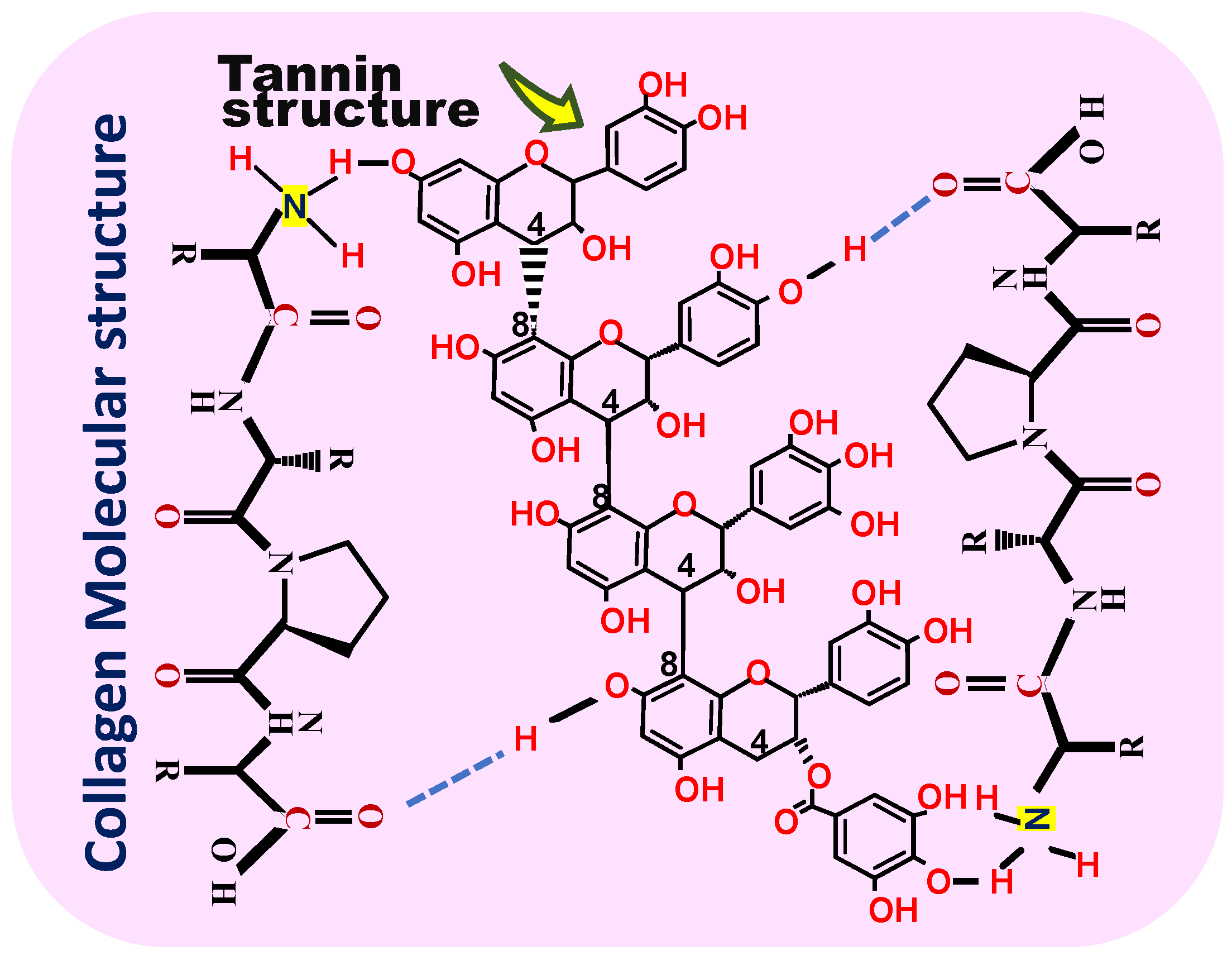

| Specie | Age (Years) | DBH (cm) | H (m) | Geographic Location |
|---|---|---|---|---|
| Pinus ayacahuite | 41 | 33.5 | 17.5 | 17°27′38.1″ N 96°25′31.9″ O |
| Pinus douglasiana | 45 | 35.7 | 18.45 | 17°27′38.1″ N 96°25′31.9″ O |
| Pinus patula | 44 | 36 | 21.6 | 17°27′38.1″ N 96°25′31.9″ O |
| Pinus pseudostrobus | 55 | 48.5 | 18.35 | 17°27′41.3″ N 96°25′02.6″ O |
| Pinus rudis | 52 | 36 | 21.45 | 17°24′15.3″ N 96°29′29.9″ O |
| Species | Total Extract (%) | Stiasny Number (%) | Condensed Tannins (%) |
|---|---|---|---|
| P. ayacahuite | 8.44 (0.128) b | 55.74 (0.529) b | 4.71 (0.108) ab |
| P. douglasiana | 14.53 (0.176) a | 31.29 (2.001) d | 4.55 (0.346) b |
| P. patula | 7.54 (0.339) c | 68.14 (0.959) a | 5.14 (0.271) a |
| P. pseudostrobus | 4.92 (0.109) d | 47.12 (0.268) c | 2.32 (0.042) c |
| P. rudis | 7.58 (0.238) c | 8.61 (0.682) e | 0.65 (0.065) d |
| Species | Total Extract (%) | Stiasny Number (%) | Condensed Tannins (%) |
|---|---|---|---|
| P. ayacahuite | 2.99 (0.022) b | 44.92 (0.539) b | 1.34 (0.026) b |
| P. douglasiana | 3.19 (0.338) a | 44.29 (1.387) b | 1.41 (0.031) ab |
| P. patula | 2.26 (0.046) c | 64.60 (3.306) a | 1.46 (0.049) a |
| P. pseudostrobus | 1.16 (0.030) e | 33.78 (1.247) c | 0.35 (0.026) b |
| P. rudis | 1.70 (0.057) d | 8.18 (0.667) d | 0.14 (0.006) d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feria-Reyes, R.; Ramírez-Cruz, S.O.; Ruiz-Aquino, F.; Robledo-Taboada, L.H.; Sánchez-Medina, M.A.; Mijangos-Ricárdez, O.F.; Gabriel-Parra, R.; Suárez-Mota, M.E.; Puc-Kauil, R.; Porcallo-Vargas, J. Pine Bark as a Potential Source of Condensed Tannin: Analysis through Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), and Energy Dispersive X-ray (EDX). Forests 2023, 14, 1433. https://doi.org/10.3390/f14071433
Feria-Reyes R, Ramírez-Cruz SO, Ruiz-Aquino F, Robledo-Taboada LH, Sánchez-Medina MA, Mijangos-Ricárdez OF, Gabriel-Parra R, Suárez-Mota ME, Puc-Kauil R, Porcallo-Vargas J. Pine Bark as a Potential Source of Condensed Tannin: Analysis through Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), and Energy Dispersive X-ray (EDX). Forests. 2023; 14(7):1433. https://doi.org/10.3390/f14071433
Chicago/Turabian StyleFeria-Reyes, Rossy, Sergio Obed Ramírez-Cruz, Faustino Ruiz-Aquino, Luis Humberto Robledo-Taboada, Marco Antonio Sánchez-Medina, Oscar Francisco Mijangos-Ricárdez, Rosalío Gabriel-Parra, Mario Ernesto Suárez-Mota, Ramiro Puc-Kauil, and Jhazeel Porcallo-Vargas. 2023. "Pine Bark as a Potential Source of Condensed Tannin: Analysis through Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), and Energy Dispersive X-ray (EDX)" Forests 14, no. 7: 1433. https://doi.org/10.3390/f14071433
APA StyleFeria-Reyes, R., Ramírez-Cruz, S. O., Ruiz-Aquino, F., Robledo-Taboada, L. H., Sánchez-Medina, M. A., Mijangos-Ricárdez, O. F., Gabriel-Parra, R., Suárez-Mota, M. E., Puc-Kauil, R., & Porcallo-Vargas, J. (2023). Pine Bark as a Potential Source of Condensed Tannin: Analysis through Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), and Energy Dispersive X-ray (EDX). Forests, 14(7), 1433. https://doi.org/10.3390/f14071433





