Abstract
This study was carried out in the territories of Northern Europe, in the middle taiga subzone of Karelia. The paper presents the results of a study of an experiment on logging to study the impact of controlled logging using supervised logging with controlling cutting (SLCC) and conventional logging (CL) on the properties of soils (horizons O, E and BF) in a spruce forest 15 years after logging. Virgin forest (VF) was used as a control. The volume weight of soils, the contents of carbon, nitrogen and potassium in different soil layers (layers O, E and BF), as well as reserves of C, N and K and their stratification coefficients SRs (SR1 [O:E], SR2 [O:BF] and SR3 [E:BF]) were studied. The results showed a tendency to increase the volume weight of soils of anthropogenically disturbed (CL and SLCC) areas can be measured. The obtained data demonstrated that there was no sharp change in the contents or stocks of the studied elements between the anthropogenically disturbed (CL and SLCC) and undisturbed areas (VF). The largest reserves of carbon, nitrogen and potassium were noted in the upper horizons of the soils of all sites, averaging 35.6, 1.27 and 0.073 t/ha, respectively. In the lower horizons of the studied soils, the values were lower. The values of the stratification coefficients in the studied soils were arranged in decreasing order as SR2 > SR1 > SR3. At the same time, the general trend of unidirectional changes in the SR values for carbon and potassium in soils was noted; the data for nitrogen were somewhat different. The results showed a marked decrease in SOC concentration with an increase in soil depth. Higher rates of cellulose decomposition were observed in anthropogenically disturbed areas (CL—69.0 ± 3.6%; SLCC—57.4 ± 3.5%) compared with virgin forest (VF) (53.7 ± 3.1%), which is consistent with the results of other studies in the taiga zone. The data obtained indicate the importance of a more accurate assessment of the contents and stocks of elements, as well as the need to use tests for soil biological activity.
1. Introduction
Forests are one of the most important parts of terrestrial ecosystems and play an important role in the circulation of chemical elements. According to the FAO, forests account for up to 31% of the land on earth; more than half of the world’s forests are located in only five countries (the Russian Federation, Brazil, Canada, the United States of America and China) and two-thirds (66%) of forests are located in ten countries [1]. Soils are an important part of forests, ensuring that they perform the most important ecological functions. Forest soils are among the main carbon sinks on earth because of their high organic matter content. European forest soils accumulate about 1.5 times more carbon than trees [2]. The nitrogen content in soils is determined by many factors [3]. Factors influencing the formation of carbon, nitrogen and potash pools in soils are important for understanding the ecosystem services performed by forests, their importance in the formation of soil fertility, forest productivity, their role in controlling soil fertility and plant production, as well as in reducing the effects of global climate change [4,5,6].
Currently, due to the increase in the anthropogenic impact on soils, their properties are changing. Deforestation is one of the most powerful anthropogenic impacts on soils. Different machine-based systems (forwarders, tractor skidders and harvesters) can be use in harvesting activities, their engine powers, weights and sizes can be different different. In this regard, logging equipment can directly or indirectly have various effects on the main components of forest ecosystems [6,7]. However, the restoration of forest ecosystems, including soils, will depend on the type of soil, vegetation and climatic conditions. Selective logging is the most delicate management approach that can achieve a differentiation of vertical stand structure. Selective logging (partial forest removal) is used in various natural and climatic conditions, i.e., in tropical forests [7] and boreal forests [8,9,10,11]. This is an alternative method of forest cutting and is a gentler method compared with clear cut. Selective logging is the basis of the practice of sustainable forest management, as it allows us to seize commercially valuable wood, preserve forest resources and enable rapid restoration of forests and the performance of their ecosystem functions [12,13,14,15,16]. In Russia, clear fallings are forbidden in protective forests, which includes forests in protected areas, protective buffer zones along water bodies, shelterbelts by roads and railways, sites of high conservation value, urban forests, etc. In Northwest Russia, the share of protective forests in the forest estate is 38%, so selective logging is important, both economically and environmentally. However, changes in the composition of forest litter, temperature, humidity, etc., occur in anthropogenically disturbed soils [14,17,18]. As we know, the greatest common effects of action of ground-based skidding equipment are forest floor removal, soil compaction and rut deep occurrence [10,18]. The common properties of soils can change under large-scale equipment: heavy vehicles tend to increase soil compaction more than lighter machines [12]. Deformation of root systems (Picea abies [L.] Karst.) has also been noted under the influence of soil compaction [13]. Changes in ecosystem processes lead to changes in the type of vegetation, carbon recycling in the plant–soil system, microbial transformation of organic matter and patterns of biomass distribution [14,19,20]. There may also be spatial–temporal changes in biomass, tree composition and diversity in the forest [12,20,21]. A change in soil organic matter may indirectly affect global climate warming [22]. Changes in the reserves of elements of the mineral nutrition of plants can lead to a decrease in the adaptive potential of the phytocenosis and deterioration of the forest-growing properties of soils [23,24]. Differentiation of the soil profile into separate horizons determines the specifics of their properties (for example, the stocks of carbon, nitrogen and potassium), and this is a common phenomenon in many natural ecosystems [25]. In this regard, it is possible to use a very informative indicator (soil stratification coefficient) that may change against the background of anthropogenic impact. The stratification coefficient (SR) is defined as the ratio of soil properties in the surface soil layer divided by the properties in deeper soil layers [26,27]. Knowledge about the content of biophile elements in the upper horizons of soils is necessary to establish the quality of soils that can influence ecosystem processes [28]. At the same time, if the indicators in the upper soil horizons are more variable, then in deeper soil layers they are relatively stable and are usually used as a basis for assessing and comparing changes in soils with different levels of anthropogenic impact [17]. Consequently, the content of elements could be associated with changes in soil formation processes; therefore, this coefficient can be used as an indicator of the soil condition and changes in soil quality in various natural ecosystems and under various management methods [29]. Over the past 20 years, deforestation has been very active in Northern Europe, which leads to a disruption of biogeocenosis [30]. The latter becomes a “trigger” for the disruption of soil properties, the circulation of carbon, nitrogen and potassium and changes in the natural rhythms of the intake mineralization of matter [24,31]. We know that nitrogen, carbon and potassium are elements or nutrients that are essential for coniferous plant growth and reproduction. They determine the development of plants, participate in the most important physiological processes and are associated with the fertility of soils. These biophile elements determine the productive capacity of forest ecosystems and their resistance to unfavorable environmental factors [32]. Despite the fact that there are a lot of works on the impact of logging on soil properties, the changes in soil properties in response to various technological elements of the logging process in Karelia have not been considered fully [33]. In this regard, the aim of the work was to study changes in the contents and stocks of carbon, nitrogen and potassium and the cellulolytic activity of soils after the use of logging equipment.
Despite the fact that, at present, the issue of the effects of technology on soils is considered for different aspects, data on changes in soil properties against the background of logging equipment, types of logging, initial soil properties and environmental factors are contradictory. This is due to the various natural and climatic conditions in which the research was conducted [8,11,19,34] and, consequently, with which soils. Therefore, we assume that the obtained data on both physicochemical parameters and biological parameters of soils will emphasize the importance of anthropogenic impact on the ability of soils to accumulate plant nutrition elements, including carbon.
2. Materials and Methods
2.1. Study Area
The research area was located on the territory of Eastern Fennoscandia, in the middle taiga subzone of Karelia (61°45′ north latitude, 33°47′ east longitude) (Figure 1). The spruce forest area occupies up to 300 hectares in the south of the Kivach Nature Reserve (Figure 1). This area is relatively flat, and its height is about 80 m above sea level. According to the climate classification of the studied area, it belongs to the group Df (boreal climate) [35]. The average annual precipitation is 550 mm per year; the average soil temperature in July is 16.6–17.9 °C. The date of transition of the average daily temperature through 10 °C is the period from 21 May to 27 May. A spruce forest grows on this territory. The soil in the study area is an illuvial-ferruginous podzol (Podzols). These soils are widespread on the territory of Fennoscandia [36] and are described in the World Reference Base for Soil Resources [37]. Spruce communities grow on this site. The ground cover is dominated by blueberries, cranberries and blueberries and green mosses such as Dicranum sp. and Pleurocium sp. have become widespread; Sphagnum sp. grow in conditions of stagnant moisture. In general, stands, soils and plants of the ground cover reflect the features of the edapho-phytocenotic complex of the taiga zone of Northern Europe [33].
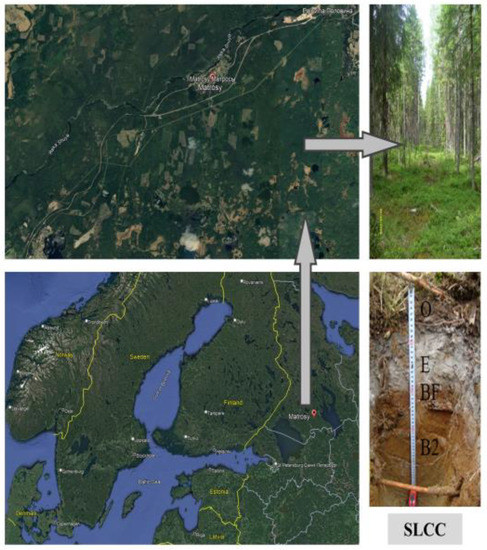
Figure 1.
Geographical location of 3 study sites: supervised logging with controlling cutting (SLCC), conventional logging (CL) and under virgin forest (control) (VF), where the experiment was performed in Karelia, as well as the studied soils and their genetic layers (O, E and BF).
In 1999, a stand of trees (blueberry spruce) was naturally formed at this place. The total volume of felled timber in the harvested area was 106 m3ha−1 in 1999.
2.2. Experimental Project
The plots were laid according to the project, which allowed us to establish the specifics of the processes occurring at various levels of organization of anthropogenically disturbed forest ecosystems [11]. The creation of the site was started in March–June 1999. A high-intensity logging was carried out at the site under study. This technology formed the basis of logging operations—controlled logging using manual felling, cutting branches and skidding wood by tractors over the top. Tractor skidding (TDT-55) was taken as a basis for removing trees from plots. With this forest management technology, the trees were extracted after the portages (CL) were laid. To reduce the negative impact on nearby trees, the felling of trees to the skidding trail was carried out at an angle of 45° to the slip road distance. Fallen trees on the site (CL) were collected and removed from the site. Thus, this biogeocenosis experienced a double anthropogenic impact (in 1929 and in 1999). The changes in the soil properties of which was studied (Figure 2).
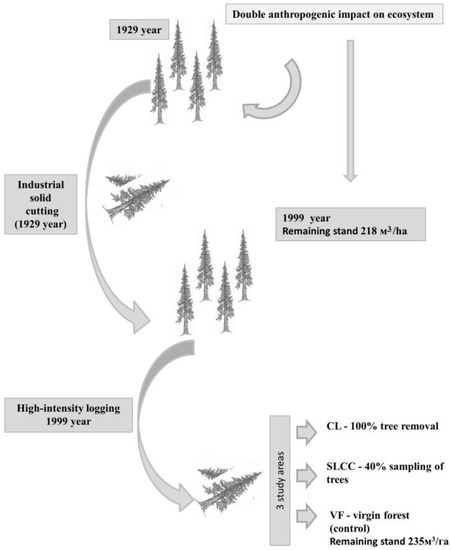
Figure 2.
The scheme of the double anthropogenic impact (continuous logging: controlling cutting areas, conventional logging areas and under virgin forest) on the studied ecosystem and the formation of the studied sites.
As a result of cutting down the stand, three sites were created. The sites proposed for consideration were designated by us as the following.
I—Conventional logging (CL). Total (100%) removal of trees was carried out on this site. The site experienced the greatest anthropogenic impact. The width of the plot was 4 m and the length was 200 m (800 m2).
II—Supervised logging with controlling cutting (SLCC). At this site, 40% removal of the stand was carried out. The site experienced an average anthropogenic impact. After logging in 1999, the remaining stand was 112 m3ha−1. By the time of the survey (2013), the remaining stand was 159 m3ha−1.
III—Control area, virgin forest (VF). The stand was 218 m3ha−1.
The total volume of felled wood in the harvested area was 106 m3 ha−1 in 1999.
According to the handbook on soil resources, soils were classified as illuvial-ferruginous podzols (Podzols), an important diagnostic criterion of which is the alpha-humus process [37]. As noted above, the soils of alpha-humus genesis are widely distributed on the territory of Eastern Fennoscandia and in this area [36,38]. The soil profile includes the following horizons: O-E-BF-B2-BC-C (Figure 1, Table 1). Where the E layer is a diagnostic, bleached soil material—washed out of iron, aluminum and humus by the podzolization process. In this paper, the analysis of the contents and stocks of chemical elements and the cellulolytic ability of soils under anthropogenic influence is carried out.

Table 1.
The most important properties of soils affected by logging: controlling cutting areas (SLCC), conventional logging areas (CL) and under virgin forest (control) (VF).
2.3. Assessment of the Contents of Carbon, Nitrogen and Potassium in the Soil
The selection of soils for analysis was carried out on anthropogenically disturbed (CL and SLCC) and control (VF) sites. Sampling, determination of soil properties and cellulose decomposition were carried out according to [39]. A total of 9 soils pit were made at each site. Samples were taken from three genetic horizons of soils: forest litter (O), podzolic (E) and alpha-humus (BF). The total number of soil samples at the 9 points was 3 for each horizon. The soil depth interval of the horizons was determined at each point. To determine the volume weight of the soils, samples from the upper organogenic horizons (O) were taken using a frame (size 20 × 20 cm) and the mineral horizons of soils (E, BF) were taken using a cylinder (volume 54.59 cm3). These samples were placed in plastic bags. Samples were also taken from these soil horizons for chemical analysis. Then, all the samples were taken to the laboratory for further analysis.
In the laboratory, analyses were carried out to determine the volume weight of the soils, the acidity (pH H2O) and the contents and stocks of C, N and K. The bulk density of the soil (BD) was expressed as the ratio of dry mass to its volume [40]. To measure the pH of the soil we used the method from [40]; the pH of the soil was determined using a pH meter (Hanna, Vöhringen, Germany) after the solution was shaken with a shaker for 30 min. This pH meter was calibrated using pH buffer solutions of 4.0, 7.0 and 9.0 before analysis. The percentage of carbon and nitrogen was determined on a CNH analyzer (2400 Series II CHNS/O Elemental Analyzer, Perkin Elmer, Waltham, MA, USA). The potassium content was determined on a flame atomic spectrophotometer (Shimadzu AA 7000, Yokohama, Japan). The research was carried out using the equipment of the Core Facility of the Karelian Research Centre of the Russian Academy of Sciences.
2.4. Assessment of Carbon, Nitrogen and Potassium Reserves in the Organic Layer of Soils
To determine the reserves of elements, organogenic layer (O) soil samples were selected at nine random points on the plots using a sampling frame of 20 cm × 20 cm (400 cm2). At the same time, the soil depth interval (SDI) of the organogenic soil horizon was measured simultaneously with a ruler. The selected samples were placed into plastic bags and delivered to the laboratory. The drying of soil samples in the laboratory was carried out first in air and then in a drying cabinet to a constant weight.
The stocks of carbon, nitrogen and potassium in the soil were calculated using information on bulk density (BD), soil depth interval (SDI) and concentrations of C, N and K. The calculation used the equation is given below (for example, carbon) [41]:
Csoil (mg ha−1) = BD (g cm−3) × SDI (cm) × C (%)
Calculations for nitrogen and potassium were made according to the same equation.
The coefficient of stratifications (SRs) for the contents of C, N and K were determined as the content of an element in the upper layer of the soil divided by its value at a lower depth [41]. SR concentrations of C, N and K in horizon O, relative to those in horizons E and BF (SR1 [O:E], SR2 [O:BF], SR3 [E:BF], respectively) were calculated in this study.
2.5. Measurement of the Cellulolytic Capacity of Soils
The cellulolytic capacity of soils was measured according to the methodology recommended by [39]. Cellulose was laid in the upper organogenic (O) and mineral (E, BF) horizons at 9 points. The exposure period of the cellulose web was carried out during the vegetation period of plants from 15 May to 28 October (the exposure time was 5 months).
2.6. Statistical Analysis
To analyze the data, we used MS Excel and Statistica 10.0. To check the distributions of BD (g cm−3), C, N and K stocks and CRs’ dates, Spearman’s t-test was used. Comparisons of p values were used to identify significant differences between the same measurements in the study areas (CL, SLCC and VF), as well as to identify differences between pairs of measurement locations within a given forest stand for the BD (g/cm3), C, N and K stocks and SR variables. To study the statistical differences between the obtained soil data from logging sites and the control, one-way analysis of variance (ANOVA) was applied, followed by testing using Student’s criterion with a significant value of less than 0.05.
3. Results
3.1. Physical and Chemical Properties of Soils
Table 2 shows the volume weight of soils in the control area in the apiary and on the drag. The volume weight of the upper horizon of all soils varies between 0.103 and 0.147 g/cm3. The volume weight of the mineral horizons is higher; the horizon E varies within 1.27–1.50 g/cm3 and the horizon BF varies within 1.43–1.54 g/cm3.

Table 2.
Bulk density of soils after continuous logging in controlling cutting areas (SLCC), conventional logging areas (CL) and under virgin forest (control) (VF).
Table 2 also shows trends in changes in the volume weight of soils depending on the level of anthropogenic impact. The highest values for the volume weight of soils in the O horizon were recorded in the areas with the highest levels of anthropogenic impact (CL), with an average total value of 0.147 ± 0.05 g/cm3. This value was followed by the site with the middle level of anthropogenic pressures (SLCC), where the value was 0.146 + 0.02 cm3. The lowest value was observed in the control area (VF)—0.103 ± 0.03 g/cm3. In the mineral horizon of soils (E) the picture is somewhat different. An increase in this indicator to 1.50 ± 0.19 g/cm3 was noted in the CL site, whereas in the soils of the control site (VA) and the SLCC, the values were close and amounted to 1.30 ± 0.20 g/cm3 and 1.27 ± 0.49 g/cm3, respectively. In the deeper layers of soils, the data values are close to each other. Statistical analysis (ANOVA) showed that there were no statistically significant differences in the volume weight of soils from the different sites.
3.2. Stocks of Carbon, Nitrogen and Potassium in Soils
Table 3 shows the change in carbon stocks in different soil horizons. We have established that the largest carbon reserves are in the upper soil horizon (O); in the underlying mineral horizons (E and BF), the element reserves are much lower and account for up to 3% of the reserves of the upper horizon. Table 3 also shows the reserves of carbon, nitrogen and potassium in different soil horizons, depending on anthropogenic impact.

Table 3.
Stock of organic carbon and elements of the mineral nutrition of plants in soils after logging: controlling cutting areas (SLCC), conventional logging areas (CL) and under virgin forest areas control (VF).
Stocks of carbon in the O layer were arranged in ascending order as follows: at the control (VF), SLCC and CL sites were 32.26 ± 2.30 t ha−1, 37.06 ± 2.42 t ha−1 and 38.167 ± 1.88 t ha−1 of carbon, respectively. In the podzolic (E) and (BF) soil horizons, there was a general trend for changes in this indicator: the lowest value was recorded at the control site (VF) (0.97 ± 0.11 and 0.68 ± 0.17 t ha−1) and at the SLCC (in the E horizon 1.32 ± 0.19 and BF 0.88 ± 0.15 t ha−1) and CL sites (1.27 ± 0.18 and 1.14 ± 0 t ha−1) the values were higher. Statistical analysis (ANOVA) showed statistically significant differences in carbon stocks in the E horizons of soils.
Nitrogen stocks in the different soil horizons from the sites with different levels of anthropogenic impact are shown in Table 3. It was revealed that the largest nitrogen reserves were in the upper soil horizons (O); in the underlying mineral horizons (E, BF), reserves are much lower and account for up to 7% of the reserves of the upper soil horizons. Table 3 also provides data on the impact of logging equipment on the stocks of the element biophiles. Nitrogen stocks in the upper (O) layer of soils were arranged in ascending order as follows: VF, SLCC and CL were 1.21 ± 0.04, 1.24 ± 0.03 and 1.34 ± 0.03 t ha−1, respectively. In the podzolic (E) soil horizon, the highest value was at the SLCC site (0.09 ± 0.02 t ha−1); the values at the control (VF) and CL sites were close to 0.06 ± 0.02 and 0.05 ± 0.01 t ha−1, respectively. In the horizon (BF) of soils, the values at the CL sites were lower (0.01 ± 0.005 t ha−1), whereas the control (VF) and SLCC sites were equal and higher (0.04 ± 0.01 t ha−1). Statistical analysis (ANOVA) showed statistically significant differences in nitrogen reserves in the lower (E and BF) soil horizons.
Table 3 also provides data on the impact of logging equipment on the stocks of the elements. The trend for higher potassium values in the O, E and BF horizons was revealed at the SLCC site, where reserves were 0.096 ± 0.19, 0.035 ± 0.0006 and 0.0027 ± 0.0001 t ha−1, respectively. Potassium stocks in the upper (O) horizon at the control and CL sites were distributed unevenly: the lowest value was in the control (0.059 ± 0.004 t ha−1) and the highest in the CL (0.096 ± 0.019 t ha−1). Stocks of potassium at the control in the mineral (E and BF) horizons were unequal and amounted to 0.0015 ± 0.0004 t ha−1. These indicators were lower than at the CL site in the mineral (E and BF) horizons and amounted to 0.0021 ± 0.0005 and 0.00155 ± 0.0001 t ha−1, respectively. Statistical analysis (ANOVA) did not show statistically significant differences in potassium reserves.
3.3. Changes in the Ratio of Stratification of Organic Carbon, Nitrogen and Potassium in the Soil
SR concentrations of C, N and K were ranked as SR3 < SR1 < SR2 at all sites (CL, SLCC and VF) (Figure 3).
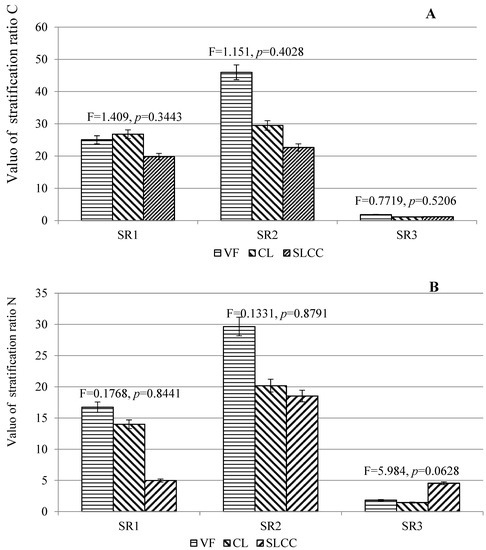
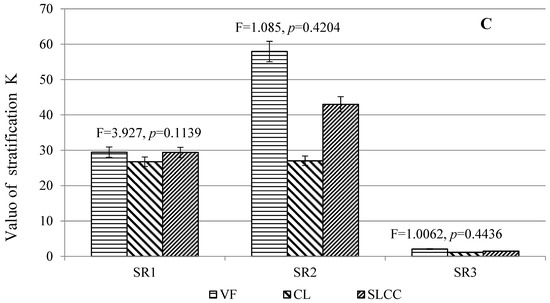
Figure 3.
Coefficient of stratification of carbon content (A) and plant nutrition elements (B,C) in soils affected by logging (controlling cutting areas (SLCC), conventional logging areas (CL) and virgin forest areas as control (VF)). The vertical bars represent standard deviations. The values of F and p for soils are obtained on the basis of ANOVA.
There was a general trend for unidirectional changes in SR carbon and nitrogen indices in soils: SR1 VF > SR1 (CL, SLCC), SR2 VF > SR2 (CL, SLCC). For potassium, such a general trend was not observed for SR1. For the SR2 indicator, a sharp increase was noted in the control area. The SR C ranged from 28.20 to 33.57, from 34.03 to 53.21 and from 1.14 to 1.56 for SR1 (O-E), SR2 (O-BF) and SR3 (E-BF), respectively, among the three experience options. The lowest SR values in all soil horizons were noted in the SLCC site, the highest were at the control site (VF). The SR N ranged from 14.00 to 25.11, from 29.97 to 48.28 and from 1.33 to 2.15 for SR1 (O-E), SR2 (O-BF) and SR3 (E-BF), respectively, among the three experience options. The lowest SR values for the studied soils were noted at the SLCC site, the highest at the VF site. The SR K ranged from 28.17 to 44.61, from 40.12 to 67.01 and from 1.44 to 1.78 for SR1 (O-E), SR2 (O-BF) and SR3 (E-BF), respectively, among the three experience options. The lowest values of SR in the studied soil horizons were noted at the CL site, the highest at the control (VF) site. One-sided ANOVA analysis does not show significant differences for the studied indicator in the soil in the different studied territories.
3.4. Cellulolytic Activity of Soils
The cellulolytic activity of soils decreased with the depth of the soil in the horizon order O > E > BF, from 53.7 to 69.0 in the O horizon, from 27.3 to 32.7 in the E horizon and from 12.0 to 15.6 in the BF horizon. A marked increase in the cellulolytic activity of soils was noted at the CL site in the upper (O) and underlying (E) horizons, which amounted to 69.0 and 32.7, respectively. The lowest values of the studied indicator (12%) were found in the BF horizon. The ternary graph (Figure 4) shows a greater convergence in the cellulolytic activity of the soils of the upper (O) and mineral (E) horizons (red color), whereas the cellulolytic activity of the alpha-humus (BF) horizon (orange color) has a different location on the graph (Figure 4).
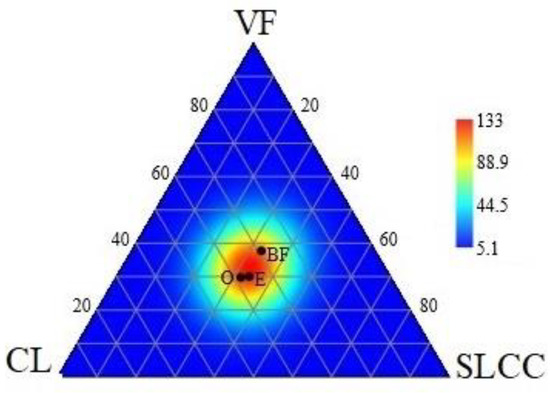
Figure 4.
Ternary graph showing changes in the cellulolytic capacity of soils under control conditions or affected by logging equipment.
4. Discussion
Soil is one of the most important components of terrestrial ecosystems, providing a cycle of nutrients and being a source of moisture for plants [2,21]. Logging technology affects all soil properties: morphological, physicochemical and biological, as well as the development of plant root systems (Figure 5).
Due to the mixing and indentation of horizons, the usual rhythms of destruction of organic matter and the work of the biodestructive block are disrupted [17,42,43]. At the same time, it is noted that under the influence of logging equipment, soil mixing occurs more intensively in wetter soils than dry ones [34]. The compaction of soils leads to an increase in volume weight [44]. A similar tendency to increase the volume weight of soils against the background of the impact of technology was noted in [12]. As noted above, as a result of cutting down a stand, the temperature regime of soils changes [45], as do the volume weight [46] and the rate of development of root systems [13]. The latter, being the “conductors” of microbiological activity, changes the structural and functional organization of soils and possibly causes deformation of soil microbiocenosis. Disruption of the composition of microbiocenosis causes changes in the physicochemical properties of soils, the circulation of element biophiles and the formation of a different macro- and microelement pool of soils [47,48]. The areas that were studied 15 years after logging did not show a sharp change in the studied soil properties compared with virgin forests. The results showed that after cutting down the stand, no significant changes in the physical properties of the soils were detected. The results of the volume weight of soils in areas experiencing anthropogenic impact (CL and SLCC) showed higher values in the upper horizons of soils compared with the control (Table 2). In the upper horizon of soils (O), the volume weight of soils decreases in the series CL > SLCC > control, in horizon E it is CL > control > SLCC and in BF it is SLCC > control > VF. The absence of pronounced soil compaction may be due to the light granulometric composition of the studied soils. The sandy soil base is less susceptible to compaction, which allows it, in a humid climate, to recover faster after mechanical damage by logging equipment. Higher rates of compaction of the upper soil horizons compared with the lower ones against the background of harvesting equipment were also noted in separate works [17]. The authors noted that soils of heavier granulometric composition were more compacted compared with light sandy soils under the actions of heavy machinery [21].
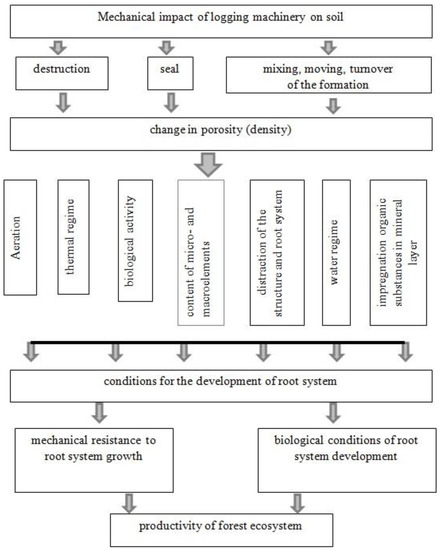
Figure 5.
Diagram of the consequences of the mechanical impact of logging machinery on the soil affecting the productivity of forest ecosystems (adaptation [48]).
The studied soils are acidic. The changes occur from 2.7 to 4.7 in the upper (O) horizon, from 3.0 to 3.3 in the podzolic (E) horizon and from 4.5 to 4.7 in the lower (BF) horizon (Table 1). The high acidity of the soils is due to the plants growing in this area. Spruce and pine stands are widespread in this taiga territory [33]. According to our data, the litter of spruce plants entering the soil has an acidity of up to 2.7, so the acidity of the upper horizons of the soils is high. The high acidity of the litter has an inhibitory effect on the processes of mineralization of organic matter and creates conditions for the formation of forest litter and carbon accumulation [49,50,51]. As is known, the rate of chemical reactions doubles when the temperature is increased by 10 °C (Van ’t Hoff reaction). When cutting down a stand, it is possible to change the thermal regime of soils due to the “opening” of the territory from the forest canopy, which can directly and indirectly enhance the microbial transformation of organic matter [15,20]. In this regard, a question also arises regarding the need to optimize the state of the microbiota so that carbon losses into the atmosphere are optimal and the forest can perform its ecosystem functions of maintaining the carbon balance in the ecosystem.
Logging equipment can have an impact on carbon stocks and plant nutrition elements. Organic matter is associated with the properties of soils, as it is an accumulator of nutrients and determines the temperature and hydrothermal regimes of soils [52,53]. Despite the fact that the changes in carbon stocks in the soil were at the level of a trend, the studied sites formed series in descending order in the upper (O) horizon of CL > SLCC > control, in the E horizon of SLCC > CL > control and in the BF horizon of CL > SLCC > control. Lower carbon stock values were noted in the control area, which was not affected by anthropogenic factors. It can be assumed that the higher values for carbon reserves in these areas are due to the arrival of the remains of parts of woody plants, which appear in large amounts when cutting down the stand. It may also be due to the drying of the litter in the summer and a decrease in the microbiological activity of soils; in this regard, the processes of destruction of the lignified mass may be inhibited. As is known, undisturbed forests are a source of more litter than disturbed ones [41,54]. Apparently, in CL plots, the growth of deciduous trees leads to the arrival of birch litter, the acidity of which (up to pH 4.2) is lower compared with spruce litter (pH up to 3.7). In the composition of forest litter, the structure is determined by many factors [5]. Among them, we can single out the stress of trees that are located close to the CL site: the destruction of root systems could lead to insufficient moisture uptake and, as a consequence, the dumping of a larger mass of coniferous litter into the ecosystem [42]. An interesting conclusion was made that the composition of forest litter (horizon O) of soils formed during logging does not always differ from the control [55]. The authors suggested that this is due to the fall coming from undisturbed areas, which once again indirectly confirms the existence of a spatial continuum in forest ecosystems.
Proper and rational forest management can regulate the supply of nitrogen, which is the basis for the functioning of terrestrial ecosystems. Nitrogen reserves in soils have a similar distribution trend across plots. In the upper horizon of soils, nitrogen reserves decrease in the following orders: in the O horizon CL > SLCC > VF, in the E horizon SLCC > VF ≈ CL and in the BF horizon VF ≈ SLCC > CL. Higher nitrogen values in the upper horizons of soils (O and BF) of anthropogenically disturbed sites (SLCC and CL), proving higher mineralization of plant residues and favorable conditions for the development of microbiota. As is known, oligonitrophils can carry out non-symbiotic nitrogen fixation, thereby creating conditions for a complex of cellulolytic organisms [56,57,58]. Nitrogen is known to be necessary for the functional activity of these microorganisms. The arrival of deciduous plant litter (birch litter pH 4.2) can also have a positive effect on the nitrogen cycle and consequently improve the forest-growing properties of soils.
Potassium is necessary for plant growth and determines the productive capacity of forest ecosystems. According to the potassium content, the soils are arranged in the following orders: in the upper (O) horizon SLCC ≈ SL > control, in the E horizon SLCC > CL > control and in the BF horizon SLCC > CL > control. According to this ranking, the highest indicators for potassium for all soil horizons were noted for the SLCC site. For mineral soil horizons, a general trend for a higher potassium reserve in the CL site compared with the control (VF) was noted. As is known, forest litter accumulates potassium, which plants and microorganisms can use for their constructive purposes. An increase in potassium reserves in the soil creates favorable conditions for the development of plant root systems, allowing them to adapt to new environmental conditions [59]. It should be emphasized that changes in potassium reserves in the soil can affect carbon and nitrogen reserves and vice versa [60]. In this regard, it is necessary to know about the peculiarities of the formation of pools of macroelements for implementation by forest ecosystems as carbon reservoirs.
The effect of the anthropogenic impact on the stratification coefficients was shown in Figure 2. It should be noted that this integral indicator for assessing the distribution of element stocks in the soil is a good indicator of their condition, since it reflects the differences in the formation inherent to soils in different natural and climatic zones [25,29,59]. The concentrations of C, N and K in the soil had a general tendency to change. It was established that SR1 and SR2 were >2, whereas SR3 < 2. This indicates the features of forest soils: biogenic accumulation of C, N and K in the upper horizons of soils and a sharp decrease in the mineral thickness [61].
The change in this indicator for all soils was wide for SR1 and SR2, whereas for SR3 this indicator was narrower. The two opposite values for the SR indicate the accumulation of nutrients in the upper horizons of soils, which is associated with the climatic features of the territory [36,38]. At the same time, it was found that for potassium, there was the largest range of changes in SR1 and SR2; for nitrogen and carbon it was narrower. For SR3, the largest ranges of changes for this indicator were noted for carbon and potassium; for nitrogen the range was smaller. The general trend of higher values for the SR measurement range for potassium is associated with its properties, specifically its high migration ability. Carbon and nitrogen are elements that can accumulate, forming a sustainability pool in the soil; this results in their indicators being less variable.
In this paper, three SR values were calculated (SR1, SR2 and SR3). The influence of logging equipment on the SR indicators was established at individual sites (Figure 2). Generally, the SRC values were higher at the control site, which indicates that the soil quality there was higher than in areas that had experienced an anthropogenic impact. The SRN value was higher in the VF sites, whereas this indicator was lower in the SLCC and CL sites. In the latter case, we can talk about the unfavorable nitrogen regime of soils in the area experiencing the greatest anthropogenic impact. The SRK value was also higher at the control (VF) site, indicating more favorable conditions for mineral nutrition.
The common tendency for the accumulation of carbon, nitrogen and potassium at the SLCC site indicates the favorable conditions that are formed at the site where selective logging took place. It is possible that this is determined by the microclimatic conditions where the selective removal of trees took place: a small illumination, the creation of more illuminated micro-conditions, an increase in soil temperature and a change in humidity can contribute to improving the forest-growing properties of soils and the development of a stand [12,62,63]. There is no doubt that despite the fact that the data obtained had the same direction of changes, as noted above, further work is needed to verify their validity and the possibility of using them in other natural and climatic conditions [6].
Against the background of changes in the physicochemical properties of soils, a change in the biotic component is also possible [12,64,65]. To determine the spatiotemporal changes in the carbon cycle in the soil, a good indicator is the determination of the cellulose activity of soils in a model field experiment (Figure 4). This cellulose decomposition test makes it possible to determine the state of the hydrolytic prokaryotic complex of the microbioma that is involved in the initial states of the conversion of carbon-containing compounds. This test is used in monitoring studies and can be a reliable indicator of soil quality. An increase in the rate of decomposition of cellulose in the O, E and BF horizons of soils in the studied areas (CL and SLCC) indicates an increase in the carbon cycle and the activity of destructors. Cellulose decomposition is carried out by a cellulolytic complex of microorganisms that produce cellulases and act synergistically on cellulose [66]. In this regard, the change in the rate of decomposition of cellulose indirectly indicates a change in the complex of cellulolytics and their functional activity [65]. When organic substances are decomposed in soils, carbon dioxide is formed and can migrate into deep-lying layers or enter the atmosphere [67].
The processes change under anthropogenic influences, which leads to an increase in the carbon load on forest ecosystems. It is appropriate to note an interesting conclusion that in compacted soils, an increase in CO2 emissions is not always associated with an increase in biological activity but may be due to a decrease in aeration [19]. Deforestation is one of the most powerful types of anthropogenic impact on soils. The impact of logging equipment on various levels of the organization of forest ecosystems is complex and the change is complex [68,69,70]. In this regard, the natural restoration of soil properties occurs gradually, whereas various indicators can be used to diagnose their changes [26,71]. In further studies, it is necessary to take into account the state of the soil microbiota, as well as to determine the biochemical parameters.
5. Conclusions
Logging operations can lead to changes at all levels of the organization of forest ecosystems, including soils. The change in soil properties against the background of a negative impact depends on the natural and climatic features, the type of soil and the recovery time for biogeocenosis, as well as the anthropogenic load itself. Unfortunately, insufficient attention is paid to this problem in Northern European. A way to reduce the negative impact is to conduct more extensive research, conducting studies of soil changes of various types in contrasting environmental conditions. The results of the current study show that there were no sharp changes in the studied soil properties at different levels of anthropogenic impact (CL and SLCC sites) 15 years after logging. Changes in soil acidity, volume weight and the stratification coefficients (SRs) of elements are at the trend level. Stratification coefficients (SRs) reflect the specifics of soils of the alpha-humus genesis when there is a biogenic accumulation of carbon, nitrogen and potassium in the upper horizons of soils and a sharp decrease in their contents with increasing depth. The general trend for decreasing SRC, SRN and SRC in soils in the area experiencing the greatest anthropogenic impact (CL) indicates the influence of logging in the past. Higher values for cellulose decomposition in CL and SLC soils compared with the control site (VF) prove positive changes and improvement in the forest-growing properties of soils during the 15 years since the anthropogenic disturbance. In addition, data on the rate of decomposition of cellulose also indicate positive changes in the biogeocenosis, i.e., its restoration when the anthropogenic impact is removed. It is necessary to continue the study in order to further establish the trajectory of the development of forest eco-systems, for a more perfect plan for managing forest resources in the context of sustainable development, preserving their diversity, as well as performing their carbon-depositing function in the biosphere [72].
Author Contributions
Conceptualization, M.V.M.; Methodology, M.V.M. and V.A.; Validation, V.A.; Formal analysis, V.A.; Investigation, M.V.M. and V.A.; Data curation, M.V.M. and V.A.; Visualization, M.V.M. and V.A. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out according to the state assignment of the Forest Institute of the Karelian Scientific Center of the Russian Academy of Sciences.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The State of the World’s Forests 2020; Forests, biodiversity and people; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Enescu, R.; Dincă, L.; Vasile, D.; Vlad, R. Does the slope aspect influence the soil organic matter concentration in forest soils? Forests 2022, 13, 1472. [Google Scholar] [CrossRef]
- Watros, A.; Tkaczyk, P.; Lipińska, H.; Lipiński, W.; Krzyszczak, J.; Baranowski, P.; Brodowska, M. Mineral nitrogen content in soils depending on land use and agronomic category. Appl. Ecol. Environ. Res. 2019, 17, 5663–5675. [Google Scholar] [CrossRef]
- da Silva, W.B.; Périco, E.; Dalzochio, M.S.; Santos, M.; Cajaiba, R.L. Are litterfall and litter decomposition processes indicators of forest regeneration in the Neotropics? Insights from a case study in the Brazilian Amazon. For. Ecol. Manag. 2018, 429, 189–197. [Google Scholar] [CrossRef]
- Jasinska, J.; Sewer’niak, P.; Puchałka, R. Litterfall in a scots pine forest on inland dunes in Central Europe: Mass, Seasonal Dynamics and Chemistry. Forests 2020, 11, 678. [Google Scholar] [CrossRef]
- Kezik, U.; Acar, H. The potential ecological effects of forest harvesting on forest soil. Eur. J. For. Eng. 2016, 2, 89–97. [Google Scholar]
- Riutta, T.; Kho, L.K.; Teh, Y.A.; Ewers, R.; Majalap, N.; Malhi, Y. Major and persistent shifts in below-ground carbon dynamics and soil respiration following logging in tropical forests. Glob. Chang. Biol. 2021, 27, 2225–2240. [Google Scholar] [CrossRef] [PubMed]
- Ares, A.; Terry, T.; Miller, R.; Anderson, H.; Flaming, B. Ground-based forest harvesting effects on soil physical properties and Douglas-fir growth. Soil Sci. Soc. Am. 2005, 69, 1822–1832. [Google Scholar] [CrossRef]
- Berg, S.; Schweier, J.; Brüchert, F.; Poissonnet, M.; Pizzirani, S.; Varet, A.; Sauter, U. Towards assessing the sustainability of European logging operations. Eur. J. For. Res. 2012, 131, 81–94. [Google Scholar] [CrossRef]
- Tan, X.; Chang, S.; Kabzems, R. Soil compaction and forest floor removal reduced microbial biomass and enzyme activities in a boreal aspen forest soil. Biol. Fert. Soils 2008, 44, 471–479. [Google Scholar] [CrossRef]
- Leinonen, T.; Kolström, T. Model forests in Russia: Experience and prospects for the future. In Proceedings of the International Workshop Held in Petrozavodsk, Russia, Petrozavodsk, Russia, 28 June 1999. [Google Scholar]
- Cambi, M.; Certini, G.; Neri, F.; Marchi, E. The impact of heavy traffic on forest soils: A review. For. Ecol. Manag. 2015, 338, 124–138. [Google Scholar] [CrossRef]
- Gebauer, R.; Martinková, M. Effects of pressure on the root systems of Norway spruce plants (Picea abies [L.] Karst.). J. For. Sci. 2005, 51, 268–275. [Google Scholar] [CrossRef]
- Howard, R.; Singer, M.; Frantz, G. Effects of soil properties, water-content, and compactive effort on the compaction of selected California forest and range soils. Soil Sci. Soc. Am. J. 1981, 45, 231–236. [Google Scholar] [CrossRef]
- Cazzolla, G.; Castaldi, S.; Lindsell, J.; Coomes, D.; Marchetti, M.; Maesano, M.; Di Paola, A.; Paparella, F.; Valentini, R. The impact of selective logging and clearcutting on forest structure, tree diversity and above-ground biomass of African tropical forests. Ecol. Res. 2015, 30, 119–132. [Google Scholar] [CrossRef]
- Karvinen, S.; Välkky, E.; Torniainen, T.; Gerasimov, Y. Northwest Russian Forestry in a Nutshell. Working Papers of the Finnish Forest Research Institute 30. Joensuu, MELTA, 2006; 98p. Available online: http://www.metla.fi/julkaisut/workingpapers/2006/mwp030.pdf (accessed on 1 March 2020).
- Shorohova, E.; Sinkevich, S.; Kryshen, A.; Vanha-Majamaa, I. Correction to: Variable retention forestry in European boreal forests in Russia. Ecol. Process. 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Katarov, V.K.; Syunev, V.S.; Ratjkova, E.I.; Gerasimov, Y.Y. Impact of wood forwarding on forest soils. Resour. Technol. 2013, 9, 73–81. [Google Scholar] [CrossRef]
- Gaertig, O.T.; Schack-Kirchner, H.; Hildebrand, E.E.; von Wilpert, K. The impact of soil aeration on oak decline in southwestern Germany. For. Ecol. Manag. 2002, 159, 15–25. [Google Scholar] [CrossRef]
- Okuda, T.; Shima, K.; Yamada, T.; Hosaka, T.; Niiyama, K.; Kosugi, Y.; Yoneda, T.; Hashim, M.; Quah, E.S.; Saw, L.G. Spatiotemporal changes in biomass after selective logging in a lowland tropical rainforest in Peninsular Malaysia. Tropics 2021, 30, 11–23. [Google Scholar] [CrossRef]
- Lussetti, D.; Axelsson, E.P.; Ilstedt, U.; Falck, J.; Karlsson, A. Supervised logging and controlling cutting improves stand development:18 years of post-logging data in a tropical rain forest in Borneo. For. Ecol. Manag. 2016, 381, 335–346. [Google Scholar] [CrossRef]
- Navarro-Pedreño, J.; Almendro-Candel, M.B.; Zorpas, A.A. The increase of soil organic matter reduces global warming, myth or reality? Science 2021, 3, 18. [Google Scholar] [CrossRef]
- Klaes, B.; Struck, J.; Schneider, R.; Schüler, G. Middle-Term effects after timber harvesting with heavy machinery on a fine-textured forest soil. Eur. J. For. Res. 2016, 135, 1083–1095. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, Y.; Zhou, C.; Wu, Z.; Zheng, L.; Hu, X.; Chen, H.; Gan, J. Effects of cutting intensity on soil physical and chemical properties in a mixed natural forest in southeastern China. Forests 2015, 6, 4495–4509. [Google Scholar] [CrossRef]
- Yu, P.; Liu, S.; Ding, Z.; Zhang, A.; Tang, X. Changes in storage and the stratification ratio of soil organic carbon under different vegetation types in northeastern China. Agronomy 2020, 10, 290. [Google Scholar] [CrossRef]
- Jobbacy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Santos, C.A.; Rezende, C.P.; Machado, P.E.F.; Pereira, J.M.; Alves, B.J.R.; Urquoaga, S.; Boddey, R.M. Changes in soil carbon stocks after land-use change form native vegetation to pastures in the Atlantic forest region of Brazil. Geoderma 2019, 337, 394–401. [Google Scholar] [CrossRef]
- Reichardt, K.; Timm, L.C. How Plants absorb nutrients from the soil. In Soil, Plant and Atmosphere; Springer: Cham, Switzerland, 2020; pp. 313–330. [Google Scholar]
- Melero, S.; Lopez-Bellido, R.J.; Luis-Bellido, L.; Munoz-Romero, V.; Moren, F.; Murillo, J.M.; Franzluebbers, A.J. Stratification ratios in a rainfed Mediterranean Vertisol in wheat under differnet tillage, rotation and N fertilization rates. Soil Tillage Res. 2012, 119, 7–12. [Google Scholar] [CrossRef]
- Kuzmichev, E.P.; Trushina, I.G.; Lopatin, E.V. Volumes of Illegal Logging of Forest Plantations in the Russian Federation. Forestry Information, 2018. №1. Available online: https://cyberleninka.ru/article/n/obemy-nezakonnyh-rubok-lesnyh-nasazhdeniy-v-rossiyskoy-federatsii (accessed on 2 March 2023). (In Russian).
- Cleophas, F.; Musta, B.; How, P.M.; Bidin, K. Runoff and soil erosion in selectively-logged over forest, Danum Valley Sabah, Malaysia. Trans. Sci. Technol. 2017, 4, 449–459. [Google Scholar]
- Sazonova, T.A.; Pridacha, V.B. Optimization of mineral nutrition in coniferous plants. Agrochemistry 2002, 2, 23–30. Available online: http://elibrary.ru/item.asp?id=21628465 (accessed on 28 June 2023). (In Russian).
- Ananyev, V.A.; Moshnikov, S.A. Structure and Dynamics of the Forest Fund of the Republic of Karelia // News of Universities. Forest Magazine. 2016, №4 (352). Available online: https://cyberleninka.ru/article/n/struktura-i-dinamika-lesnogo-fonda-respubliki-kareliya (accessed on 3 March 2023). (In Russian).
- Toivio, J.; Helmisaari, H.-S.; Palviainen, M.; Lindeman, H.; Ala-Ilomäki, J.; Sirén, M.; Uusitalo, J. Impacts of timber forwarding on physical properties of forest soils in southern Finland. For. Ecol. Manag. 2017, 405, 22–30. [Google Scholar] [CrossRef]
- Map of climate classifications of Europe and Middle East (JPG). People.eng.unimelbb.edu.au. Retrieved December 31, 2018.
- Yli-Halla, M.; Mokma, D. Classification of soils of Finland according to soil taxonomy. Soil Horiz. 1999, 40, 59. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014: Update 2015, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Word Soil Resources Report 106; Food and Agriculture Organization: Rome, Italy, 2015. [Google Scholar]
- Fedorets, N.G.; Bakhmet, O.N. Ecological features of the soils of the green belt of Fennoscandia. Transactions of Karelian Research Centre of Russian Academy of Science. 2009, №2. Available online: https://cyberleninka.ru/article/n/ekologicheskie-osobennosti-pochv-zelenogo-poyasa-fennoskandii (accessed on 2 March 2023). (In Russian).
- Rautio, P.; Fürst, A.; Stefan, K.; Raitio, H.; Bartels, U. Sampling and analysis of needles and leaves. In Manual and Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects to Air Pollution on Forests; Manual Part XII; UNECE ICP Forest Programme Coordinating Centre: Hamburg, Germany, 2010; p. 19. [Google Scholar]
- Vorobjeva, L. Chemical Analysis of Soils: Textbook; Publishing House of Moscow State University: Moscow, Russia, 1998; p. 272. (In Russian) [Google Scholar]
- Suhaili, N.; Anuar, S.; Wong, W.; Lussetti, D.; Axelsson, E.; Hasselquist, N.; Ilstedt, U.; Awang, B.N. Soil carbon pool and carbon fluxes estimation in 26 years after selective logging tropical forest at Sabah, Malaysia. Forests 2022, 13, 1890. [Google Scholar] [CrossRef]
- Bagheri, I.; Kalhori, S.B.; Akef, M.; Khormali, F. Effect of compaction on physical and micromorphological properties of forest soils. Am. J. Plant Sci. 2012, 3, 159–163. [Google Scholar] [CrossRef]
- Sirén, M.; Ala-Ilomäki, J.; Lindeman, H.; Uusitalo, J.; Kiilo, K.E.K.; Salmivaara, A.; Ryynänen, A. Soil disturbance by cut-to-length machinery on mid-grained soils. Silva Fenn. 2019, 53, 10134. [Google Scholar] [CrossRef]
- Kozlowski, T. Soil compaction and growth of woody plants. Scand. J. For. Res. 1999, 14, 596–619. [Google Scholar] [CrossRef]
- Sadono, R.; Pujiono, E.; Lestari, L. Land cover changes and carbon storage before and after community forestry program in Bleberan Village, Gunungkidul, Indonesia, 1999–2018. For. Sci. Technol. 2020, 16, 134–144. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, J.; Mattson, K.G.; Zhang, W.; Weber, T.A. Sample szes to control error estimates in determining soil bulk density in California forest soils. Soil Sci. Soc. Am. J. 2016, 80, 756–764. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Aziz, C.B.; Saha, M.L. Relationships between soil physico-chemical properties and total viable bacterial counts in sunderban mangrove forests, Bangladesh. Dhaka Univ. J. Biol. Sci. 2012, 21, 169–175. [Google Scholar] [CrossRef]
- Kokieva, G.; Druzynova, V.; Yampilov, S.; Radnaev, D.; Shukhanov, S.; Popova, A. Investigation of the mechanical effect of machinery on the soil. In Networked Control Systems for Connected and Automated Vehicles; Lecture Notes in Networks and Systems, Guda, A., Eds.; Springer: Cham, Switzerland, 2022; Volume 510. [Google Scholar] [CrossRef]
- Ampoorter, E.; Goris, R.; Cornelis, W.; Verheyen, K. Impact of mechanized logging on compaction status of sandy forest soils. For. Ecol. Manag. 2007, 241, 162–174. [Google Scholar] [CrossRef]
- Suhaili, N.; Hatta, S.; James, D.; Hassan, A.; Jalloh, M.; Phua, M.; Besar, N. Soils carbon stocks and litterfall fluxes from the Bornean tropical montane forests, Sabah, Malaysia. Forests 2021, 12, 1621. [Google Scholar] [CrossRef]
- Chen, L.-C.; Guan, X.; Li, H.; Wang, Q.; Zhang, W.; Yang, Q.; Wang, S. Spatiotemporal patterns of carbon storage in forest ecosystems in Hunan Province, China. For. Ecol. Manag. 2019, 432, 656–666. [Google Scholar] [CrossRef]
- Voltr, V.; Menšík, L.; Hlisnikovský, L.; Hruška, M.; Pokorný, E.; Pospíšilová, L. The soil organic matter in connection with soil properties and soil inputs. Agronomy 2021, 11, 779. [Google Scholar] [CrossRef]
- Keen, Y.; Jalloh, M.; Ahmed, O.; Sudin, M.; Besar, N. Soil organic matter and related soil properties in forest, grassland and cultivated land use types. Int. J. Phys. Sci. 2011, 6, 7410–7415. [Google Scholar]
- Zhou, G.; Guan, L.; Wei, X.; Zhang, D.; Zhang, Q.; Yan, J.; Wen, D.; Liu, J.; Liu, S.; Huang, Z. Litterfall production along successional and altitudinal gradients of subtropical monsoon evergreen broadleaved forests in Guangdong, China. Plant Ecol. 2007, 188, 77–89. [Google Scholar] [CrossRef]
- Paudel, E.; Dossa, G.; Xu, J.; Harrison, R. Litterfall and nutrient return along a disturbance gradient in a tropical montane forest. For. Ecol. Manag. 2015, 353, 97–106. [Google Scholar] [CrossRef]
- Andreiuk, E.; Mal’tseva, N. Oligonitrophilic microorganisms and oligonitrophilia. Mikrobiolohichnyi Zhurnal 1978, 40, 173–185. (In Russian) [Google Scholar]
- Perotti, E.; Pidello, A. Plant-Soil-Microorganism Interactions on Nitrogen Cycle: Azospirillum Inoculation. Tech. Eur. 2012, 189–208. [Google Scholar] [CrossRef]
- Baldani, V.; Baldani, J.; Döbereiner, J. Effects of Azospirillum inoculation on root infection and nitrogen incorporation in wheat. Can. J. Microbiol. 1983, 29, 924–929. [Google Scholar] [CrossRef]
- Mouhamad, R.; Atiyah, A.; Iqbal, M. Behavior of potassium in soil: A mini review. Chem. Int. 2016, 2, 47–58. [Google Scholar]
- Yan, T.; Kremenetska, Y.; Zhang, B.; He, S.; Wang, X.; Yu, Z.; Hu, Q.; Liang, X.; Fu, M.; Wang, Z. The relationship between soil particle size fractions, associated carbon distribution and physicochemical properties of historical land-use types in newly formed reservoir buffer strips. Sustainability 2022, 14, 8448. [Google Scholar] [CrossRef]
- Alyabina, I.; Shmatova, A. Al–Fe–Humus Podzols in Russia: Geography of Some Properties. Moscow Univ. Soil Sci. Bull. 2020, 75, 8–18. [Google Scholar] [CrossRef]
- Xiao, W.; Ge, X.; Zeng, L.; Huang, Z.; Lei, J.; Zhou, B.; Li, M. Rates of litter decomposition and soil respiration in relation to soil temperature and water in different-aged Pinus massoniana forests in the three Gorges Reservoir Area, China. PLoS ONE 2014, 9, e101890. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, Z.; Zhao, Y. Stratification ratio of soil organic carbon as an indicator of carbon sequestration and soil quality in ecological restoration: SOC stratification ratio in ecological restoration. Restor. Ecol. 2017, 26, 555–562. [Google Scholar] [CrossRef]
- Beylich, A.; Oberholzer, H.-R.; Schrader, S.; Höper, H.; Wilke, B.-M. Evaluation of soil compaction effects on soil biota and soil biological processes in soils. Soil Tillage Res. 2010, 109, 133–143. [Google Scholar] [CrossRef]
- Grata, K. Determining cellulolytic activity of microorganisms. Chem. Didact. Ecol. Metrol. 2020, 25, 133–143. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Yang, F.; Qu, Y.; Li, X. Isolation and characterization of Achromobacter sp. CX2 from symbiotic Cytophagales, a non-cellulolytic bacterium showing synergism with cellulolytic microbes by producing β-glucosidase. Ann. Microbiol. 2015, 65, 1699–1707. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Meng, D.; Dang, S.; Zhou, J.; Osborne, B.; Ren, Y.; Liang, T.; Yu, K. Effect of soil microorganisms and labile C availability on soil respiration in response to litter inputs in forest ecosystems: A meta-analysis. Ecol. Evol. 2020, 10, 13602–13612. [Google Scholar] [CrossRef]
- Page-Dumroese, D.; Jurgensen, M.; Tiarks, A.; Ponder, J.; Sanchez, F.; Fleming, R. Soil physical property changes at the north American long-term soil productivity study sites: 1 and 5 years after compaction. Can. J. For. Res. 2006, 36, 551–564. [Google Scholar] [CrossRef]
- Demir, M.; Makineci, E.; Yilmaz, E. Investigation of timber harvesting impacts on herbaceous cover, forest floor and surface soil properties on skid road in an oak (Quercus petreae L.) stand. Build. Environ. 2007, 42, 1194–1199. [Google Scholar] [CrossRef]
- Varol, T.; Emir, T.; Akgul, M.; Ozel, H.; Acar, H.; Cetin, M. Impacts of Small-Scale Mechanized Logging Equipment on Soil Compaction in Forests. J. Soil Sci. Plant Nutr. 2020, 20, 953–963. [Google Scholar] [CrossRef]
- Najafi, A.; Solgi, A. Assessing site disturbance using two ground survey methods in a mountain forest. Croat. J. Eng. J. Theory App. For. Eng. 2010, 31, 47–55. Available online: https://hrcak.srce.hr/index.php?show=clanak&id_clanak_jezik=86347 (accessed on 28 June 2023).
- Ananyev, V.; Asikainen, A.; Vialkko, E.; Gerasimov, Y.; Dyomin, K.; Sikanen, L.; Sunyov, V.; Tyukina, O.; Khlustov, V.; Shirnin, Y. Intermediate Yield of Forest in the North-West of Russia; Research Institute of the Forest of Finland: Joensuu, Finland, 2005; 150p. (In Russian) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).