Floristic Composition, Structure, and Aboveground Biomass of the Moraceae Family in an Evergreen Andean Amazon Forest, Ecuador
Abstract
1. Introduction
2. Materials and Methods
2.1. Geographic Setting
2.2. Geographical Distribution of the Moraceae Family
2.3. Floristic Composition, Structure and Carbon Stored in the Biomass of the Species of the Moraceae Family
2.4. Data Analysis
2.4.1. Floristic Composition
2.4.2. Horizontal and Vertical Structure
2.4.3. Ecologically Important Value Index
2.4.4. Floristic Richness
2.4.5. Species Diversity
2.4.6. Aboveground Biomass Carbon
3. Results and Discussion
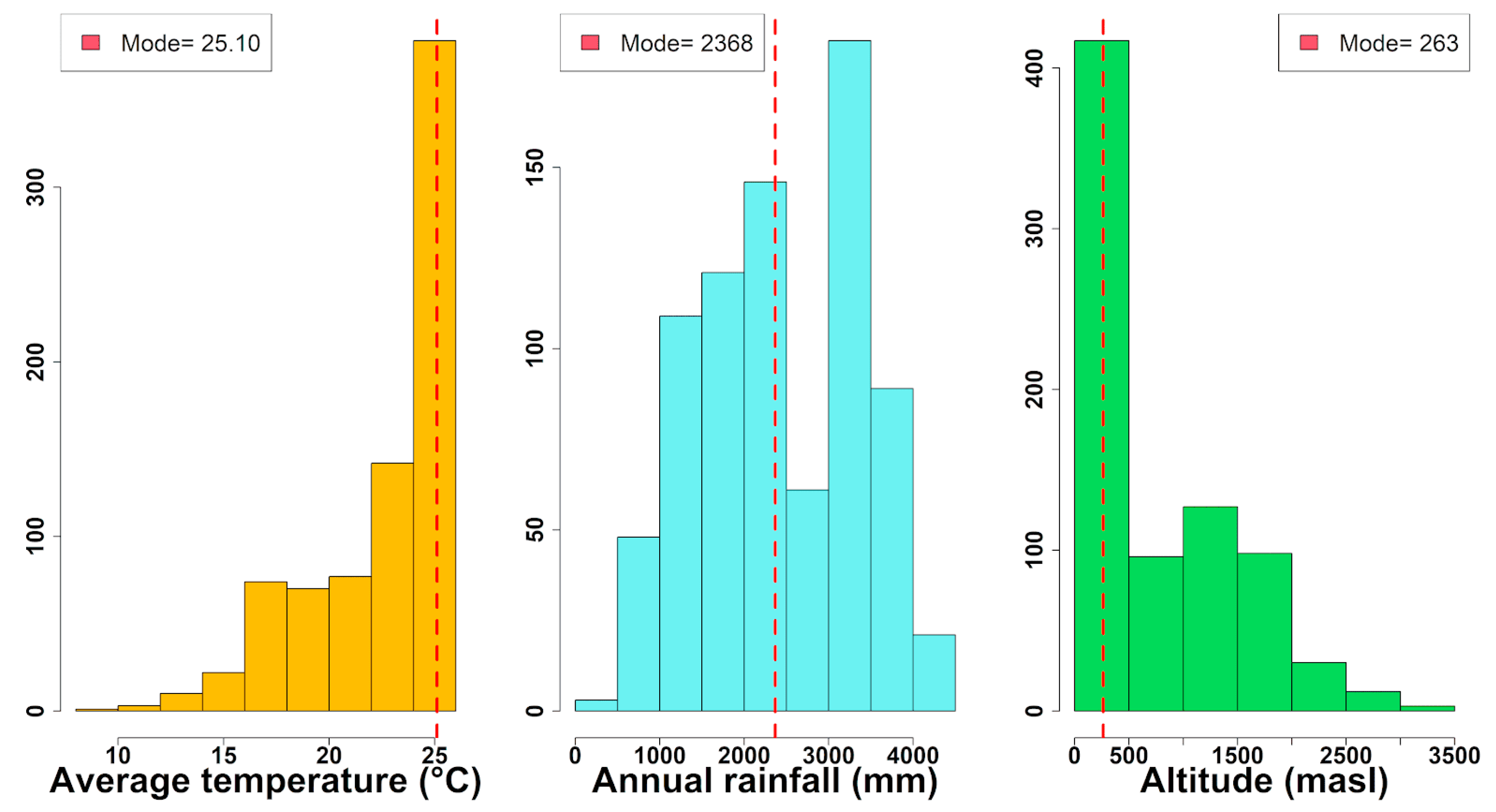
3.1. Geographical Distribution and Environmental Preferences of the Moraceae Family in Continental Ecuador
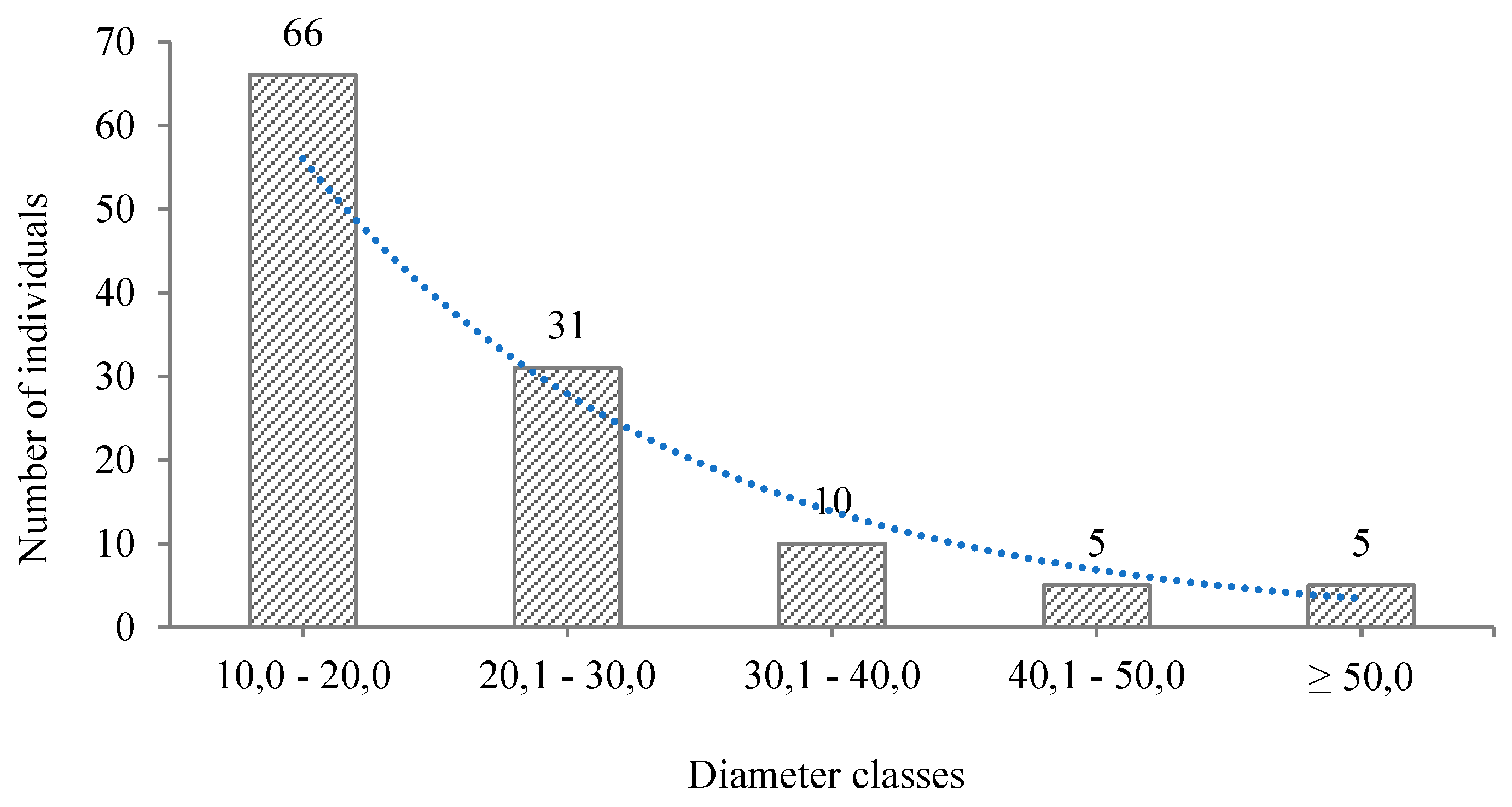
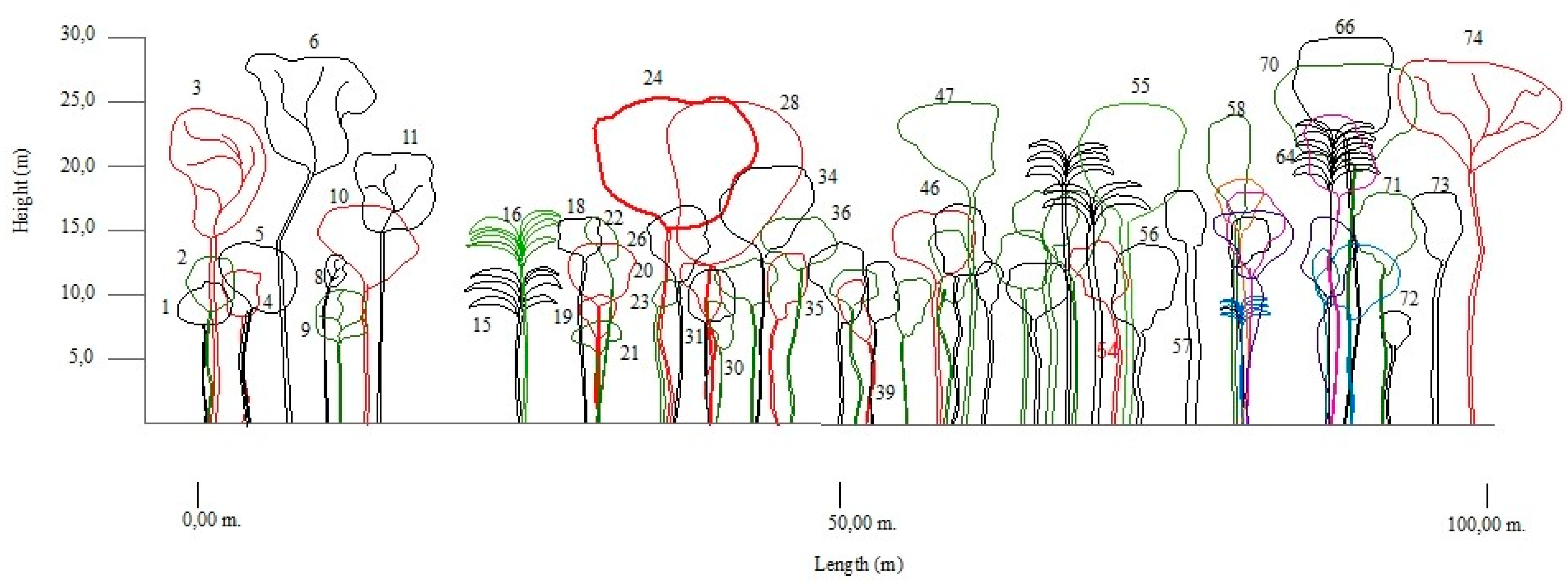
3.2. Composition and Structure (Horizontal and Vertical)
3.3. Ecological Importance Value Index (IVI)
3.4. Floristic Wealth
3.4.1. Relative Abundance
3.4.2. Dominance
3.5. Diversity Indices
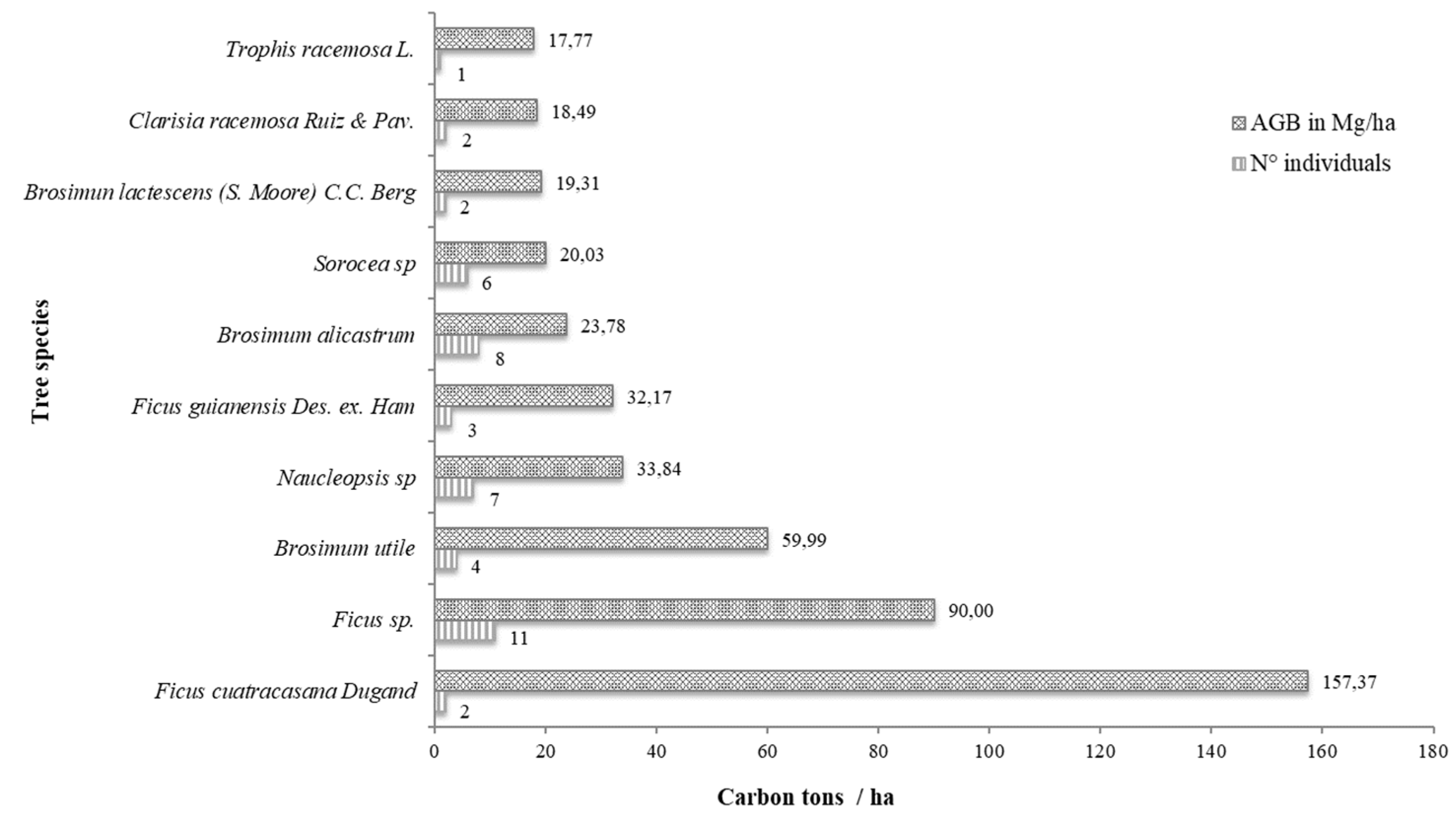
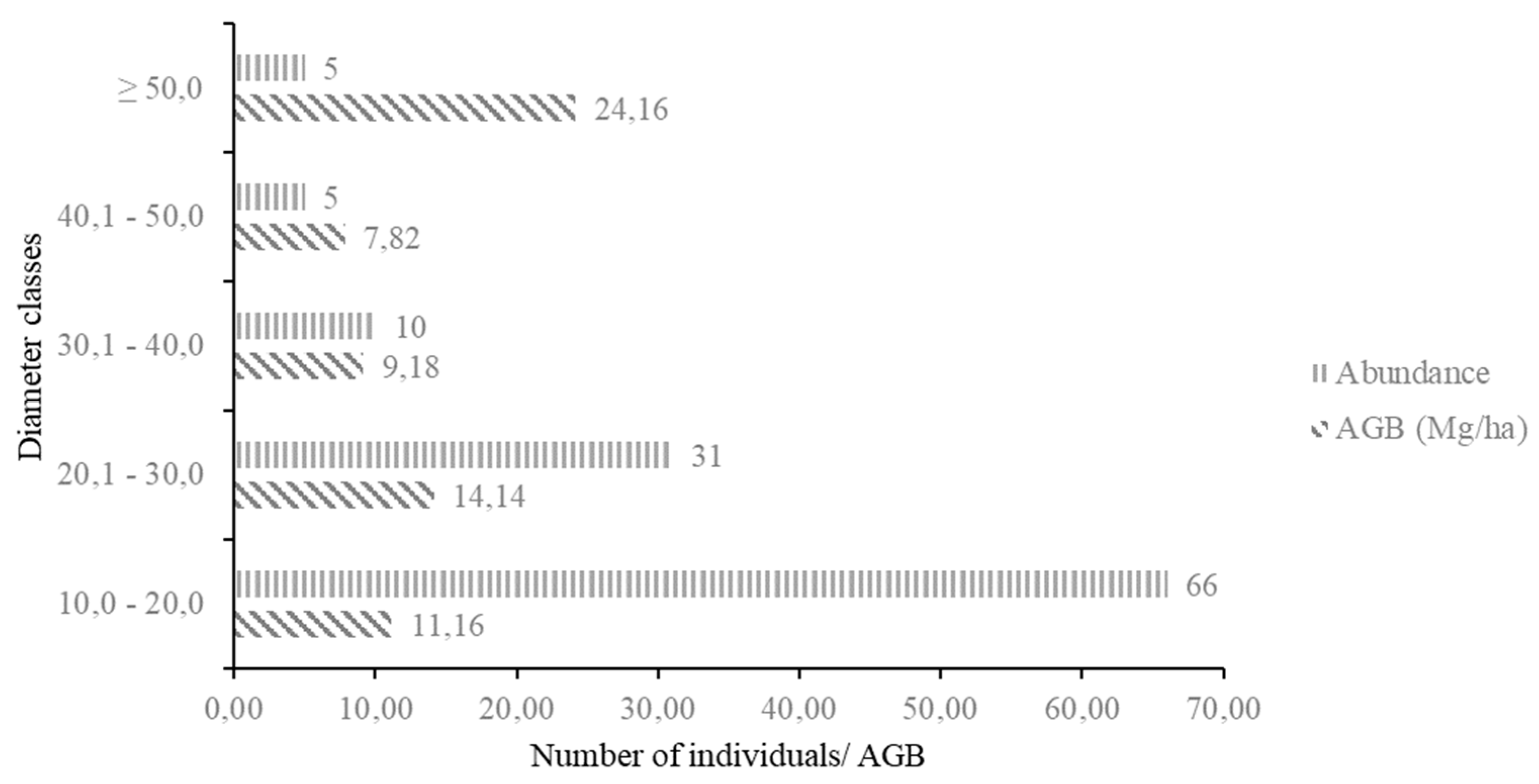
3.6. Accumulated Aboveground Biomass of the Moraceae Family
3.7. Limitations and Future Research
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noroozi, J.; Talebi, A.; Doostmohammadi, M.; Rumpf, S.B.; Linder, H.P.; Schneeweiss, G.M. Hotspots within a Global Biodiversity Hotspot-Areas of Endemism Are Associated with High Mountain Ranges. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Raven, P.H.; Gereau, R.E.; Phillipson, P.B.; Chatelain, C.; Jenkins, C.N.; Ulloa Ulloa, C. The Distribution of Biodiversity Richness in the Tropics. Sci. Adv. 2020, 6, eabc6228. [Google Scholar] [CrossRef] [PubMed]
- Ulloa Ulloa, C.; Acevedo-Rodríguez, P.; Beck, S.; Belgrano, M.J.; Bernal, R.; Berry, P.E.; Brako, L.; Celis, M.; Davidse, G.; Forzza, R.C. An Integrated Assessment of the Vascular Plant Species of the Americas. Science 2017, 358, 1614–1617. [Google Scholar] [CrossRef] [PubMed]
- Ter Steege, H.; Pitman, N.C.A.; Sabatier, D.; Baraloto, C.; Salomão, R.P.; Guevara, J.E.; Phillips, O.L.; Castilho, C.V.; Magnusson, W.E.; Molino, J.-F. Hyperdominance in the Amazonian Tree Flora. Science 2013, 342, 1243092. [Google Scholar] [CrossRef] [PubMed]
- Guevara Andino, J.E.; Pitman, N.C.A.; Ulloa Ulloa, C.; Romoleroux, K.; Fernández-Fernández, D.; Ceron, C.; Palacios, W.; Neill, D.A.; Oleas, N.; Altamirano, P. Trees of Amazonian Ecuador: A Taxonomically Verified Species List with Data on Abundance and Distribution. Ecology 2019, 100, e02894. [Google Scholar] [CrossRef]
- Ter Steege, H.; Prado, P.I.; de Lima, R.A.F.; Pos, E.; de Souza Coelho, L.; de Andrade Lima Filho, D.; Salomão, R.P.; Amaral, I.L.; de Almeida Matos, F.D.; Castilho, C.V. Biased-Corrected Richness Estimates for the Amazonian Tree Flora. Sci. Rep. 2020, 10, 10130. [Google Scholar] [CrossRef]
- Cazzolla Gatti, R.; Reich, P.B.; Gamarra, J.G.P.; Crowther, T.; Hui, C.; Morera, A.; Bastin, J.-F.; De-Miguel, S.; Nabuurs, G.-J.; Svenning, J.-C. The Number of Tree Species on Earth. Proc. Natl. Acad. Sci. USA 2022, 119, e2115329119. [Google Scholar] [CrossRef]
- da Rosa, C.M.; Marques, M.C.M. How Are Biodiversity and Carbon Stock Recovered during Tropical Forest Restoration? Supporting the Ecological Paradigms and Political Context Involved. J. Nat. Conserv. 2021, 65, 126115. [Google Scholar] [CrossRef]
- Koch, A.; Kaplan, J.O. Tropical Forest Restoration under Future Climate Change. Nat. Clim. Chang. 2022, 12, 279–283. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Harris, N.L.; Gibbs, D.A.; Baccini, A.; Birdsey, R.A.; De Bruin, S.; Farina, M.; Fatoyinbo, L.; Hansen, M.C.; Herold, M.; Houghton, R.A. Global Maps of Twenty-First Century Forest Carbon Fluxes. Nat. Clim. Chang. 2021, 11, 234–240. [Google Scholar] [CrossRef]
- Armstrong McKay, D.I.; Staal, A.; Abrams, J.F.; Winkelmann, R.; Sakschewski, B.; Loriani, S.; Fetzer, I.; Cornell, S.E.; Rockström, J.; Lenton, T.M. Exceeding 1.5 C Global Warming Could Trigger Multiple Climate Tipping Points. Science 2022, 377, eabn7950. [Google Scholar] [CrossRef] [PubMed]
- Fahey, T.J.; Knapp, A.K. Principles and Standards for Measuring Primary Production; Oxford University Press: Oxford, UK, 2007; ISBN 0198037724. [Google Scholar]
- Zhang, Y.; Liang, S.; Yang, L. A Review of Regional and Global Gridded Forest Biomass Datasets. Remote Sens. 2019, 11, 2744. [Google Scholar] [CrossRef]
- Chapman, M.; Walker, W.S.; Cook-Patton, S.C.; Ellis, P.W.; Farina, M.; Griscom, B.W.; Baccini, A. Large Climate Mitigation Potential from Adding Trees to Agricultural Lands. Glob. Chang. Biol. 2020, 26, 4357–4365. [Google Scholar] [CrossRef]
- Ali, A.; Lin, S.; He, J.; Kong, F.; Yu, J.; Jiang, H. Big-sized Trees Overrule Remaining Trees’ Attributes and Species Richness as Determinants of Aboveground Biomass in Tropical Forests. Glob. Chang. Biol. 2019, 25, 2810–2824. [Google Scholar] [CrossRef] [PubMed]
- Duncanson, L.; Armston, J.; Disney, M.; Avitabile, V.; Barbier, N.; Calders, K.; Carter, S.; Chave, J.; Herold, M.; Crowther, T.W. The Importance of Consistent Global Forest Aboveground Biomass Product Validation. Surv. Geophys. 2019, 40, 979–999. [Google Scholar] [CrossRef]
- Li, Y.; Brando, P.M.; Morton, D.C.; Lawrence, D.M.; Yang, H.; Randerson, J.T. Deforestation-Induced Climate Change Reduces Carbon Storage in Remaining Tropical Forests. Nat. Commun. 2022, 13, 1964. [Google Scholar] [CrossRef]
- Melito, M.; Arroyo-Rodríguez, V.; Metzger, J.P.; Cazetta, E.; Rocha-Santos, L.; Melo, F.P.L.; Santos, B.A.; Magnago, L.F.S.; Hernández-Ruedas, M.A.; Faria, D. Landscape Forest Loss Decreases Aboveground Biomass of Neotropical Forests Patches in Moderately Disturbed Regions. Landsc. Ecol. 2021, 36, 439–453. [Google Scholar] [CrossRef]
- Qin, Y.; Xiao, X.; Wigneron, J.-P.; Ciais, P.; Brandt, M.; Fan, L.; Li, X.; Crowell, S.; Wu, X.; Doughty, R. Carbon Loss from Forest Degradation Exceeds That from Deforestation in the Brazilian Amazon. Nat. Clim. Chang. 2021, 11, 442–448. [Google Scholar] [CrossRef]
- Vashum, K.T.; Jayakumar, S. Methods to Estimate Above-Ground Biomass and Carbon Stock in Natural Forests-a Review. J. Ecosyst. Ecography 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Poorter, L.; van der Sande, M.T.; Thompson, J.; Arets, E.J.M.M.; Alarcón, A.; Álvarez-Sánchez, J.; Ascarrunz, N.; Balvanera, P.; Barajas-Guzmán, G.; Boit, A. Diversity Enhances Carbon Storage in Tropical Forests. Glob. Ecol. Biogeogr. 2015, 24, 1314–1328. [Google Scholar] [CrossRef]
- Duffy, J.E.; Godwin, C.M.; Cardinale, B.J. Biodiversity Effects in the Wild Are Common and as Strong as Key Drivers of Productivity. Nature 2017, 549, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Ali, A. Forest Stand Structure and Functioning: Current Knowledge and Future Challenges. Ecol. Indic. 2019, 98, 665–677. [Google Scholar] [CrossRef]
- Herold, M.; Carter, S.; Avitabile, V.; Espejo, A.B.; Jonckheere, I.; Lucas, R.; McRoberts, R.E.; Næsset, E.; Nightingale, J.; Petersen, R. The Role and Need for Space-Based Forest Biomass-Related Measurements in Environmental Management and Policy. Surv. Geophys. 2019, 40, 757–778. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, H.Y.H.; Bork, E.W.; Carlyle, C.N.; Chang, S.X. Carbon Accumulation in Agroforestry Systems Is Affected by Tree Species Diversity, Age and Regional Climate: A Global Meta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1817–1828. [Google Scholar] [CrossRef]
- Panwar, P.; Mahalingappa, D.G.; Kaushal, R.; Bhardwaj, D.R.; Chakravarty, S.; Shukla, G.; Thakur, N.S.; Chavan, S.B.; Pal, S.; Nayak, B.G. Biomass Production and Carbon Sequestration Potential of Different Agroforestry Systems in India: A Critical Review. Forests 2022, 13, 1274. [Google Scholar] [CrossRef]
- Araujo, E.C.G.; Sanquetta, C.R.; Dalla Corte, A.P.; Pelissari, A.L.; Orso, G.A.; Silva, T.C. Global Review and State-of-the-Art of Biomass and Carbon Stock in the Amazon. J. Environ. Manag. 2023, 331, 117251. [Google Scholar] [CrossRef]
- Asner, G.P.; Powell, G.V.N.; Mascaro, J.; Knapp, D.E.; Clark, J.K.; Jacobson, J.; Kennedy-Bowdoin, T.; Balaji, A.; Paez-Acosta, G.; Victoria, E. High-Resolution Forest Carbon Stocks and Emissions in the Amazon. Proc. Natl. Acad. Sci. USA 2010, 107, 16738–16742. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, D.; Li, G.; Wang, G.; Chen, Q.; Liu, L.; Li, D. Comparative Analysis of Modeling Algorithms for Forest Aboveground Biomass Estimation in a Subtropical Region. Remote Sens. 2018, 10, 627. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; Hartig, F.; Latifi, H.; Berger, C.; Hernández, J.; Corvalán, P.; Koch, B. Importance of Sample Size, Data Type and Prediction Method for Remote Sensing-Based Estimations of Aboveground Forest Biomass. Remote Sens. Environ. 2014, 154, 102–114. [Google Scholar] [CrossRef]
- González-Jaramillo, V.; Fries, A.; Zeilinger, J.; Homeier, J.; Paladines-Benitez, J.; Bendix, J. Estimation of above Ground Biomass in a Tropical Mountain Forest in Southern Ecuador Using Airborne LiDAR Data. Remote Sens. 2018, 10, 660. [Google Scholar] [CrossRef]
- Torres, B.; Vasseur, L.; López, R.; Lozano, P.; García, Y.; Arteaga, Y.; Bravo, C.; Barba, C.; García, A. Structure and above Ground Biomass along an Elevation Small-Scale Gradient: Case Study in an Evergreen Andean Amazon Forest, Ecuador. Agrofor. Syst. 2020, 94, 1235–1245. [Google Scholar] [CrossRef]
- Torres, B.; Bravo, C.; Torres, A.; Tipán-Torres, C.; Vargas, J.C.; Herrera-Feijoo, R.J.; Heredia-R, M.; Barba, C.; García, A. Carbon Stock Assessment in Silvopastoral Systems along an Elevational Gradient: A Study from Cattle Producers in the Sumaco Biosphere Reserve, Ecuadorian Amazon. Sustainability 2023, 15, 449. [Google Scholar] [CrossRef]
- Maza, B.; Rodes-Blanco, M.; Rojas, E. Aboveground Biomass Along an Elevation Gradient in an Evergreen Andean–Amazonian Forest in Ecuador. Front. For. Glob. Chang. 2022, 5, 738585. [Google Scholar] [CrossRef]
- García Cox, W.O. Composición Florística de la Familia Moraceae, Como Fuente de Carbono Aéreo en la Gradiente Altitudinal de un Bosque Siempreverde, Piemontano de la Amazonia Ecuatoriana, Año 2018. Master’s Thesis, Universidad Técnica Estatal de Quevedo, Quevedo, Ecuador, 2019. [Google Scholar]
- Clement, W.L.; Weiblen, G.D. Morphological Evolution in the Mulberry Family (Moraceae). Syst. Bot. 2009, 34, 530–552. [Google Scholar] [CrossRef]
- Berg, C.C. Moraceae Diversity in a Global Perspective. In Proceedings of the Symposium on Plant Diversity and Complexity Patterns—Local, Regional and Global Dimensions, International Symposium Held at the Royal Danish Academy of Sciences and Letters in Copenhagen, Copenhagen, Denmark, 25–28 May 2003; Det Kongelige Danske Videnskabernes Selskab: Copenhagen, Denmark, 2005; pp. 423–440. [Google Scholar]
- Zerega, N.J.C.; Clement, W.L.; Datwyler, S.L.; Weiblen, G.D. Biogeography and Divergence Times in the Mulberry Family (Moraceae). Mol. Phylogenet. Evol. 2005, 37, 402–416. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, I.; Verma, P.K.; Singh, B.J.; Singh, R.; Upadhyay, S.K. A Study on Diversity and Distribution of Ficus L. (Dicotyledonae: Moraceae) Species at Forest Research Institute (FRI), Dehradun (Uttarakhand), India. J. Appl. Nat. Sci. 2021, 13, 552–560. [Google Scholar] [CrossRef]
- Ramírez, F.C.; Castillo, G.A.; Flores, Y.; Galván, O.F.; Riveros, L.; Sáenz, L.H. Composición, Estructura e Importancia Ecológica de Las Moraceae En Un Bosque Residual de Ucayali, Perú. Rev. For. Perú 2021, 36, 247–260. [Google Scholar] [CrossRef]
- Gardner, E.M.; Garner, M.; Cowan, R.; Dodsworth, S.; Epitawalage, N.; Arifiani, D.; Baker, W.J.; Forest, F.; Maurin, O.; Zerega, N.J.C. Repeated Parallel Losses of Inflexed Stamens in Moraceae: Phylogenomics and Generic Revision of the Tribe Moreae and the Reinstatement of the Tribe Olmedieae (Moraceae). Taxon 2021, 70, 946–988. [Google Scholar] [CrossRef]
- Vásquez, R.; Rojas, R.; Monteagudo, A.M.; Valenzuela, L.G.; Huamantupa, I. Catalogo de Los Arboles Del Perú. Q’ueña Rev. De La Soc. Botánica Cusco 2018, 9, 1. [Google Scholar]
- Daïnou, K.; Laurenty, E.; Mahy, G.; Hardy, O.J.; Brostaux, Y.; Tagg, N.; Doucet, J. Phenological Patterns in a Natural Population of a Tropical Timber Tree Species, Milicia Excelsa (Moraceae): Evidence of Isolation by Time and Its Interaction with Feeding Strategies of Dispersers. Am. J. Bot. 2012, 99, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Daïnou, K.; Mahy, G.; Duminil, J.; Dick, C.W.; Doucet, J.-L.; Donkpégan, A.S.L.; Pluijgers, M.; Sinsin, B.; Lejeune, P.; Hardy, O.J. Speciation Slowing down in Widespread and Long-Living Tree Taxa: Insights from the Tropical Timber Tree Genus Milicia (Moraceae). Heredity 2014, 113, 74–85. [Google Scholar] [CrossRef]
- Nair, N.; Yadav, S.; Biharee, A.; Prathap, V.M.; Majeed, J. Updated Ethnobotanical Notes, Phytochemistry and Phytopharmacology of Plants Belonging to the Genus Morus (Family: Moraceae). Phytomed. Plus 2021, 2, 100120. [Google Scholar]
- Shi, Y.; Mon, A.M.; Fu, Y.; Zhang, Y.; Wang, C.; Yang, X.; Wang, Y. The Genus Ficus (Moraceae) Used in Diet: Its Plant Diversity, Distribution, Traditional Uses and Ethnopharmacological Importance. J. Ethnopharmacol. 2018, 226, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Alegría Meneses, D.O. Influencia de la Disponibilidad de Frutos (Familia Moraceae) en las Dinámicas de Fisión-Fusión de Ateles Chamek (Humboldt, 1812) en el Parque Nacional de Manu. Master’s Thesis, Universidad Nacional Agraria La Molina, Lima, Peru, 2019. [Google Scholar]
- Sánchez, M.D. World Distribution and Utilization of Mulberry, Potential for Animal Feeding. In Proceedings of the FAO Electronic Conference on Mulberry for Animal Production (Morus1-L) 1, Citeseer. 2000, Volume 111. Available online: https://www.cabdirect.org/globalhealth/abstract/20023111035 (accessed on 20 March 2023).
- Gentry, A.H. Changes in Plant Community Diversity and Floristic Composition on Environmental and Geographical Gradients. Ann. Missouri Bot. Gard. 1988, 75, 1–34. [Google Scholar] [CrossRef]
- Ter Steege, H.; Sabatier, D.; Castellanos, H.; Van Andel, T.; Duivenvoorden, J.; De Oliveira, A.A.; Ek, R.; Lilwah, R.; Maas, P.; Mori, S. An Analysis of the Floristic Composition and Diversity of Amazonian Forests Including Those of the Guiana Shield. J. Trop. Ecol. 2000, 16, 801–828. [Google Scholar] [CrossRef]
- Schöngart, J.; Wittmann, F.; Worbes, M.; Piedade, M.T.F.; Krambeck, H.-J.; Junk, W.J. Management Criteria for Ficus Insipida Willd. (Moraceae) in Amazonian White-Water Floodplain Forests Defined by Tree-Ring Analysis. Ann. For. Sci. 2007, 64, 657–664. [Google Scholar] [CrossRef]
- Terborgh, J.; Andresen, E. The Composition of Amazonian Forests: Patterns at Local and Regional Scales. J. Trop. Ecol. 1998, 14, 645–664. [Google Scholar] [CrossRef]
- Burn, M.J.; Mayle, F.E. Palynological Differentiation between Genera of the Moraceae Family and Implications for Amazonian Palaeoecology. Rev. Palaeobot. Palynol. 2008, 149, 187–201. [Google Scholar] [CrossRef]
- Phillips, O. Ficus Insipida (Moraceae): Ethnobotany and Ecology of an Amazonian Anthelmintic. Econ. Bot. 1990, 44, 534–536. [Google Scholar]
- Nascimento, M.T.; Proctor, J.; Villela, D.M. Forest Structure, Floristic Composition and Soils of an Amazonian Monodominant Forest on Maracá Island, Roraima, Brazil. Edinburgh J. Bot. 1997, 54, 1–38. [Google Scholar] [CrossRef]
- Bravo, A.; Harms, K.E. The Biogeography of Sodium in Neotropical Figs (Moraceae). Biotropica 2017, 49, 18–22. [Google Scholar] [CrossRef]
- Rezende, G.M.; Vieira, D.L.M. Forest Restoration in Southern Amazonia: Soil Preparation Triggers Natural Regeneration. For. Ecol. Manag. 2019, 433, 93–104. [Google Scholar] [CrossRef]
- Coomes, D.A.; Grubb, P.J. Amazonian Caatinga and Related Communities at La Esmeralda, Venezuela: Forest Structure, Physiognomy and Floristics, and Control by Soil Factors. Vegetatio 1996, 122, 167–191. [Google Scholar] [CrossRef]
- Caranqui, J. Composición y Diversidad de Especies Arbóreas En Transectos de Localidades Del Bosque Siempreverde de Tierras Bajas Del Ecuador. Enfoque UTE 2015, 6, 96–105. [Google Scholar] [CrossRef]
- Mejía, E.; Pacheco, P. Forest Use and Timber Markets in the Ecuadorian Amazon; CIFOR: Bogor, Indonesia, 2014; Volume 111, ISBN 6021504143. [Google Scholar]
- MAATE Interactive Map of MAATE. Available online: http://ide.ambiente.gob.ec/mapainteractivo/ (accessed on 23 February 2023).
- Coloma-Santos, A. Parque Nacional Sumaco Napo-Galeras. In Guía Patrimonio Areas Naturales Protegidas Ecuador; Ecol. y MAE, Ed.; Ministerio del Ambiente: Quito, Ecuador, 2007; pp. 219–224. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Torres, B.; Günter, S.; Acevedo-Cabra, R.; Knoke, T. Livelihood Strategies, Ethnicity and Rural Income: The Case of Migrant Settlers and Indigenous Populations in the Ecuadorian Amazon. For. Policy Econ. 2018, 86, 22–34. [Google Scholar] [CrossRef]
- Trew, B.T.; Maclean, I.M.D. Vulnerability of Global Biodiversity Hotspots to Climate Change. Glob. Ecol. Biogeogr. 2021, 30, 768–783. [Google Scholar] [CrossRef]
- Noh, J.K.; Echeverria, C.; Gaona, G.; Kleemann, J.; Koo, H.; Fürst, C.; Cuenca, P. Forest Ecosystem Fragmentation in Ecuador: Challenges for Sustainable Land Use in the Tropical Andean. Land 2022, 11, 287. [Google Scholar] [CrossRef]
- Kleemann, J.; Zamora, C.; Villacis-Chiluisa, A.B.; Cuenca, P.; Koo, H.; Noh, J.K.; Fürst, C.; Thiel, M. Deforestation in Continental Ecuador with a Focus on Protected Areas. Land 2022, 11, 268. [Google Scholar] [CrossRef]
- Ferrer Velasco, R.; Lippe, M.; Fischer, R.; Torres, B.; Tamayo, F.; Kalaba, F.K.; Kaoma, H.; Bugayong, L.; Günter, S. Reconciling Policy Instruments with Drivers of Deforestation and Forest Degradation: Cross-Scale Analysis of Stakeholder Perceptions in Tropical Countries. Sci. Rep. 2023, 13, 2180. [Google Scholar] [CrossRef] [PubMed]
- Torres, B.; Fischer, R.; JC, V.; Günter, S. Deforestación en Paisajes Forestales Tropicales del Ecuador: Bases Científicas Para Perspectivas Políticas; Universidad Estatal Amazónica—Instituto Johann Heinrich von Thünen: Puyo, Ecuador, 2020; 172p. [Google Scholar]
- Sierra, R. Patrones y Factores de Deforestación en el Ecuador Continental, 1990–2010. In Y un Acercamiento a Los Próximos 10 Años; Conservacion Internacional Ecuador y Forest Trends: Quito, Ecuador, 2013; 57p. [Google Scholar]
- Villacís, S.; Ochoa, J.; Borja, M.O.; Josse, C.; Finer, M.M. Minería Ilegal en la Amazonía Ecuatoriana; Amazon Conservation Association: Washington, DC, USA, 2022. [Google Scholar]
- Mestanza-Ramón, C.; Cuenca-Cumbicus, J.; D’Orio, G.; Flores-Toala, J.; Segovia-Cáceres, S.; Bonilla-Bonilla, A.; Straface, S. Gold Mining in the Amazon Region of Ecuador: History and a Review of Its Socio-Environmental Impacts. Land 2022, 11, 221. [Google Scholar] [CrossRef]
- Torres, B.; Vasco, C.; Günter, S.; Knoke, T. Determinants of Agricultural Diversification in a Hotspot Area: Evidence from Colonist and Indigenous Communities in the Sumaco Biosphere Reserve, Ecuadorian Amazon. Sustainability 2018, 10, 1432. [Google Scholar] [CrossRef]
- Torres, B.; Espinoza, Í.; Torres, A.; Herrera-Feijoo, R.; Luna, M.; García, A. Livelihood Capitals and Opportunity Cost for Grazing Areas’ Restoration: A Sustainable Intensification Strategy in the Ecuadorian Amazon. Animals 2023, 13, 714. [Google Scholar] [CrossRef] [PubMed]
- MAATE Sistema Nacional de Indicadores Ambientales y Sostenibilidad (SINIAS). Available online: http://sinias.ambiente.gob.ec:8099/proyecto-sinias-web/estadisticasAmbientales.jsf?menu=01 (accessed on 20 March 2023).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Toulkeridis, T.; Zach, I. Wind Directions of Volcanic Ash-Charged Clouds in Ecuador–Implications for the Public and Flight Safety. Geomat. Nat. Hazards Risk 2017, 8, 242–256. [Google Scholar] [CrossRef]
- Lozano, P.; Cabrera, O.; Peyre, G.; Cleef, A.; Toulkeridis, T. Plant Diversity and Composition Changes along an Altitudinal Gradient in the Isolated Volcano Sumaco in the Ecuadorian Amazon. Diversity 2020, 12, 229. [Google Scholar] [CrossRef]
- Guascal, E.; Rojas, S.; Kirby, E.; Toulkeridis, T.; Fuertes, W.; Heredia, M. Application of Remote Sensing Techniques in the Estimation of Forest Biomass of a Recreation Area by UAV and RADAR Images in Ecuador. In Proceedings of the 2020 Seventh International Conference on eDemocracy & eGovernment (ICEDEG), Buenos Aires, Argentina, 22–24 April 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 183–190. [Google Scholar]
- Basantes, J.; Padilla-Almeida, O.; Toulkeridis, T.; Ordoñez, E.; Zapata-Vela, J.; Zapata-Vela, A.C.; Rojas, S. Dasometric Analysis Applying Terrestrial Laser Scanner and Conventional Techniques for the Estimation of Aboveground Biomass in a Forest of the Inter-Andean Valley in Ecuador. In Proceedings of the 2019 Sixth International Conference on eDemocracy & eGovernment (ICEDEG), Quito, Ecuador, 24–26 April 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 334–337. [Google Scholar]
- Herrera-Feijoo, R.J.; Torres, B.; López-Tobar, R.; Tipán-Torres, C.; Toulkeridis, T.; Heredia-R, M.; Mateo, R.G. Modelling Climatically Suitable Areas for Mahogany (Swietenia Macrophylla King) and Their Shifts across Neotropics: The Role of Protected Areas. Forests 2023, 14, 385. [Google Scholar] [CrossRef]
- Zapata, J.; Galarza, J.; Yánez, M.; Toulkeridis, T.; Zapata, A.; Ordoñez, E. Determination of the Natural Plant Coverage of the Eloy Alfaro Canton Based on GIS, NW Ecuador. In Proceedings of the 2020 Seventh International Conference on eDemocracy & eGovernment (ICEDEG), Buenos Aires, Argentina, 22–24 April 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 175–182. [Google Scholar]
- Maitner, B.S.; Boyle, B.; Casler, N.; Condit, R.; Donoghue, J.; Durán, S.M.; Guaderrama, D.; Hinchliff, C.E.; Jørgensen, P.M.; Kraft, N.J.B. The Bien r Package: A Tool to Access the Botanical Information and Ecology Network (BIEN) Database. Methods Ecol. Evol. 2018, 9, 373–379. [Google Scholar] [CrossRef]
- Albani Rocchetti, G.; Armstrong, C.G.; Abeli, T.; Orsenigo, S.; Jasper, C.; Joly, S.; Bruneau, A.; Zytaruk, M.; Vamosi, J.C. Reversing Extinction Trends: New Uses of (Old) Herbarium Specimens to Accelerate Conservation Action on Threatened Species. New Phytol. 2021, 230, 433–450. [Google Scholar] [CrossRef]
- Lang, P.L.M.; Willems, F.M.; Scheepens, J.F.; Burbano, H.A.; Bossdorf, O. Using Herbaria to Study Global Environmental Change. New Phytol. 2019, 221, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Nic Lughadha, E.; Walker, B.E.; Canteiro, C.; Chadburn, H.; Davis, A.P.; Hargreaves, S.; Lucas, E.J.; Schuiteman, A.; Williams, E.; Bachman, S.P. The Use and Misuse of Herbarium Specimens in Evaluating Plant Extinction Risks. Philos. Trans. R. Soc. B 2019, 374, 20170402. [Google Scholar] [CrossRef] [PubMed]
- Cobos, M.E.; Jiménez, L.; Nuñez-Penichet, C.; Romero-Alvarez, D.; Simões, M. Sample Data and Training Modules for Cleaning Biodiversity Information. Biodivers. Inform. 2018, 13, 49–50. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Bivand, R.; van Etten, J.; Forner, K.; Ooms, J.; Pebesma, E. Package ‘Terra’. 2022. Available online: https://github.com/rspatial/terra (accessed on 20 March 2023).
- Rodriguez, E.; Morris, C.S.; Belz, J.E. A Global Assessment of the SRTM Performance. Photogramm. Eng. Remote Sens. 2006, 72, 249–260. [Google Scholar] [CrossRef]
- Gentry, A.H. Patterns of Neotropical Plant Species Diversity. In Evolutionary Biology; Springer: Berlin/Heidelberg, Germany, 1982; pp. 1–84. [Google Scholar]
- Phillips, O.L.; Miller, J. Global Patterns of Forest Diversity: The Dataset of Alwyn H. Gentry. Monogr. Syst. Bot. 2002, 89, 1–319. [Google Scholar]
- Cerón, C.E. Manual de Botánica Ecuatoriana Sistemática y Métodos de Estudio; Universidad Central de Ecuador: Quito, Ecuador, 1993. [Google Scholar]
- Margalef, R. Information Theory in Biology. Gen. Syst. Yearb. 1958, 3, 36–71. [Google Scholar]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T. Tree Allometry and Improved Estimation of Carbon Stocks and Balance in Tropical Forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef]
- García-Quintana, Y.; Arteaga-Crespo, Y.; Torres-Navarrete, B.; Bravo-Medina, C.; Robles-Morillo, M. Biomasa Aérea de Familias Botánicas En Un Bosque Siempreverde Piemontano Sometido a Grados de Intervención. Colomb. For. 2021, 24, 45–59. [Google Scholar] [CrossRef]
- Patiño, J.; Lozano, P.; Tipán, C.; Navarrete, H.; López, R.; Asanza, M.; Torres, B. Composición Florística y Estructura de Un Bosque Siempreverde Piemontano de 600 a 700 m Snm En La Cuenca Del Río Piatúa, Napo, Ecuador. Rev. Amaz. Cienc. Tecnol. 2015, 4, 166–214. [Google Scholar]
- Cano Schütz, A. Diversidad y Composición Florística de Tres Tipos de Bosque En La Estación Biológica Caparú, Vaupés, Colombia. Colomb. Forestal 2009, 12, 63–80. [Google Scholar] [CrossRef]
- Magallanes, A.B.; Rocha, Y.R.; Terán, F.A. Distribución y Abundancia de Las Especies Arbóreas de La Familia Moraceae En La Reserva Ecológica Del Mineral de Nuestra Señora de La Candelaria, Cosalá, Sinaloa. In La Investigación Científica, Tecnológica y Social en La UAS; Universidad Autonoma de Sinaloa: Sinaola, Mexico, 2013. [Google Scholar]
- Calzadilla-Tomianovich, M.H.; Cayola, L. Estructura y Composición Florística de Un Bosque Amazónico de Pie de Monte, Área Natural de Manejo Integrado Madidi, La Paz-Bolivia. Ecol. Boliv. 2006, 41, 117–129. [Google Scholar]
- Estrella Caicedo, L.A. Diversidad Florística, Concentración de Biomasa Aérea y Carbono (c) En Un Bosque Siempre Verde Tierras Bajas de 300 a 400 Msnm., En La Amazonía Ecuatoriana. Master’s Thesis, Universidad Técnica Estatal de Quevedo, Quevedo, Ecuador, 2016. [Google Scholar]



| Protected Área | Extension (ha) | Year of Creation | Overlapping Area in RBS (%) |
|---|---|---|---|
| Cayambe Coca National Park | 403,103 | 17 November 1970 | 8.57 |
| Sumaco National Park | 205,249 | 2 March 1994 | 100 |
| Antisana Ecological Reserve | 120,000 | 21 July 1993 | 2.65 |
| Colonso Chalupas Biological Reserve | 93,163 | 3 April 2014 | 0.51 |
| LLanganates National Park | 219,700 | 18 January 1996 | 8.57 |
| Species | N° Ind | BA | Abund (%) | Dom (%) | IVI | |
|---|---|---|---|---|---|---|
| 1 | Ficus sp. | 11 | 0.96 | 9.40 | 14.56 | 23.96 |
| 2 | Ficus cuatracasana Dugand | 2 | 1.24 | 1.71 | 18.85 | 20.56 |
| 3 | Brosimum lactescens (S. Moore) C.C. Berg | 2 | 0.67 | 1.71 | 10.10 | 11.81 |
| 4 | Naucleopsis sp. | 7 | 0.35 | 5.98 | 5.35 | 11.33 |
| 5 | Brosimum alicastrum | 8 | 0.26 | 6.84 | 3.98 | 10.82 |
| 6 | Sorocea sp. | 6 | 0.20 | 5.13 | 3.10 | 8.22 |
| 7 | Sorocea steinbachii C. C. Berg | 6 | 0.16 | 5.13 | 2.43 | 7.56 |
| 8 | Batocarpus orinocensis Karst. | 5 | 0.19 | 4.27 | 2.87 | 7.14 |
| 9 | Ficus guianensis Des. ex. Ham | 3 | 0.30 | 2.56 | 4.55 | 7.12 |
| 10 | Calliandra carbonaria | 6 | 0.11 | 5.13 | 1.74 | 6.86 |
| Variable | Average by Altitudinal Gradients | ||||
|---|---|---|---|---|---|
| P1 (601–700) | P2 (701–800) | P3 (801–900) | P4 (901–1000) | Average (601–1000) | |
| AGB (Mg ha−1) | 12.34 | 36.21 | 74.63 | 17.06 | 35.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Cox, W.; López-Tobar, R.; Herrera-Feijoo, R.J.; Tapia, A.; Heredia-R, M.; Toulkeridis, T.; Torres, B. Floristic Composition, Structure, and Aboveground Biomass of the Moraceae Family in an Evergreen Andean Amazon Forest, Ecuador. Forests 2023, 14, 1406. https://doi.org/10.3390/f14071406
García-Cox W, López-Tobar R, Herrera-Feijoo RJ, Tapia A, Heredia-R M, Toulkeridis T, Torres B. Floristic Composition, Structure, and Aboveground Biomass of the Moraceae Family in an Evergreen Andean Amazon Forest, Ecuador. Forests. 2023; 14(7):1406. https://doi.org/10.3390/f14071406
Chicago/Turabian StyleGarcía-Cox, Walter, Rolando López-Tobar, Robinson J. Herrera-Feijoo, Aracely Tapia, Marco Heredia-R, Theofilos Toulkeridis, and Bolier Torres. 2023. "Floristic Composition, Structure, and Aboveground Biomass of the Moraceae Family in an Evergreen Andean Amazon Forest, Ecuador" Forests 14, no. 7: 1406. https://doi.org/10.3390/f14071406
APA StyleGarcía-Cox, W., López-Tobar, R., Herrera-Feijoo, R. J., Tapia, A., Heredia-R, M., Toulkeridis, T., & Torres, B. (2023). Floristic Composition, Structure, and Aboveground Biomass of the Moraceae Family in an Evergreen Andean Amazon Forest, Ecuador. Forests, 14(7), 1406. https://doi.org/10.3390/f14071406








