Natural Hybridization between Bursera bicolor × B. glabrifolia (Burseraceae) Complex: Molecular and Chemical Evidence
Abstract
:1. Introduction
2. Materials and Methods
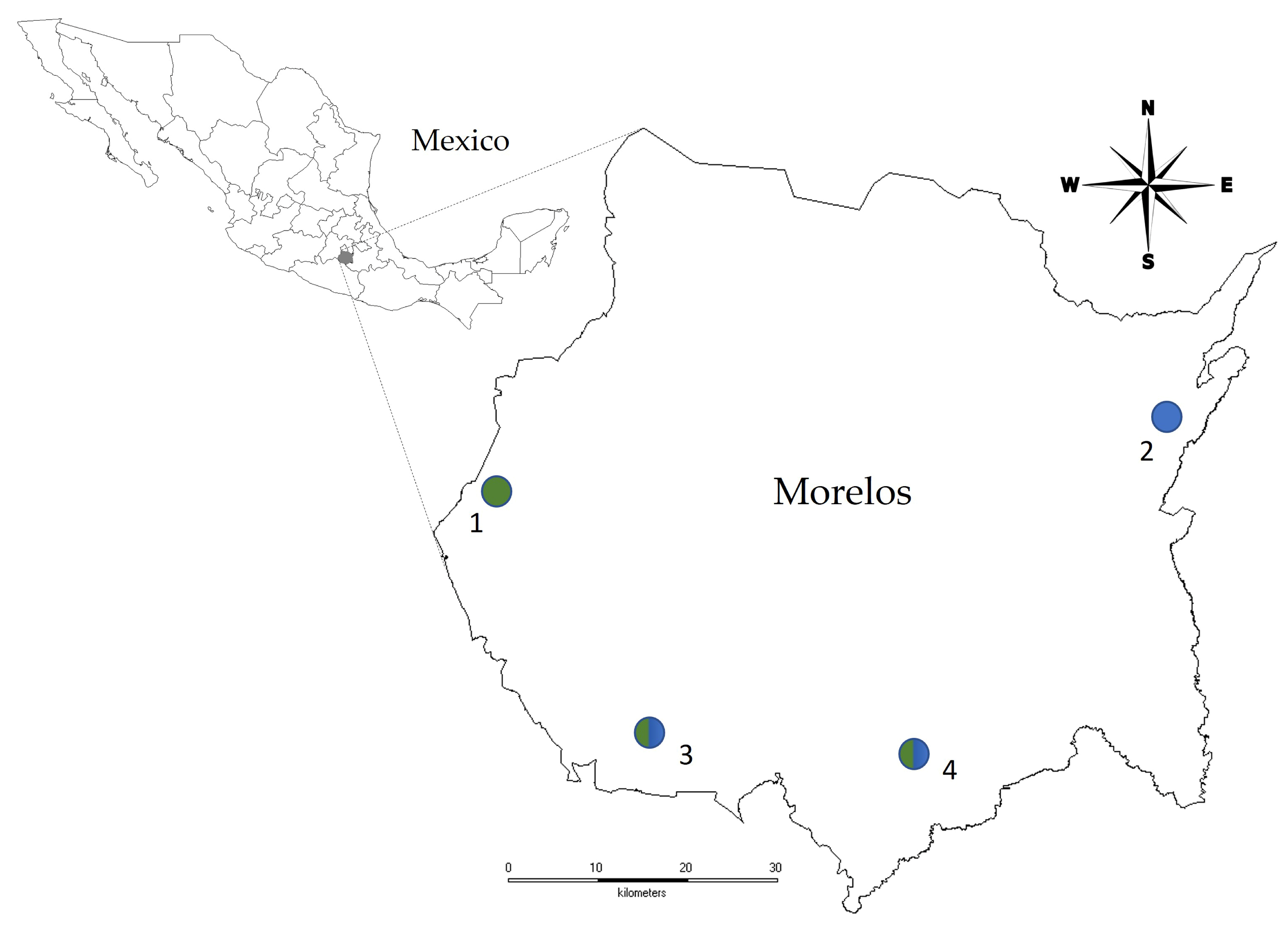
2.1. Study Sites
2.2. Study Species
2.3. Population Sampling
2.4. Molecular Data
2.5. Genetic Analysis
2.6. Plant Material
2.7. Obtaining n-hexane Extracts
2.8. Analysis of the Extracts by GC/MS
2.9. Phytochemical Analysis
3. Results
3.1. Assignment of Parental Categories
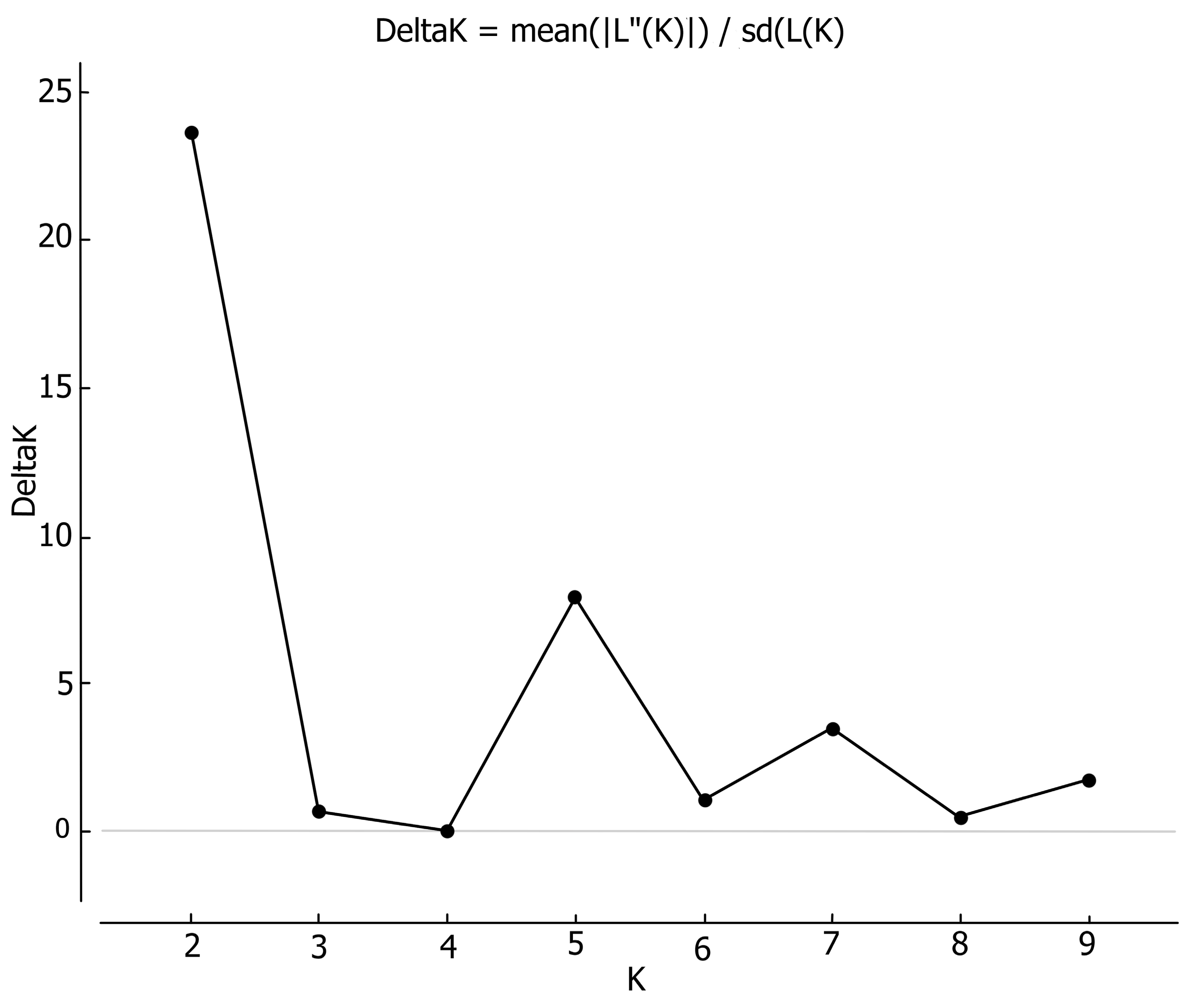
3.1.1. Genetic Data
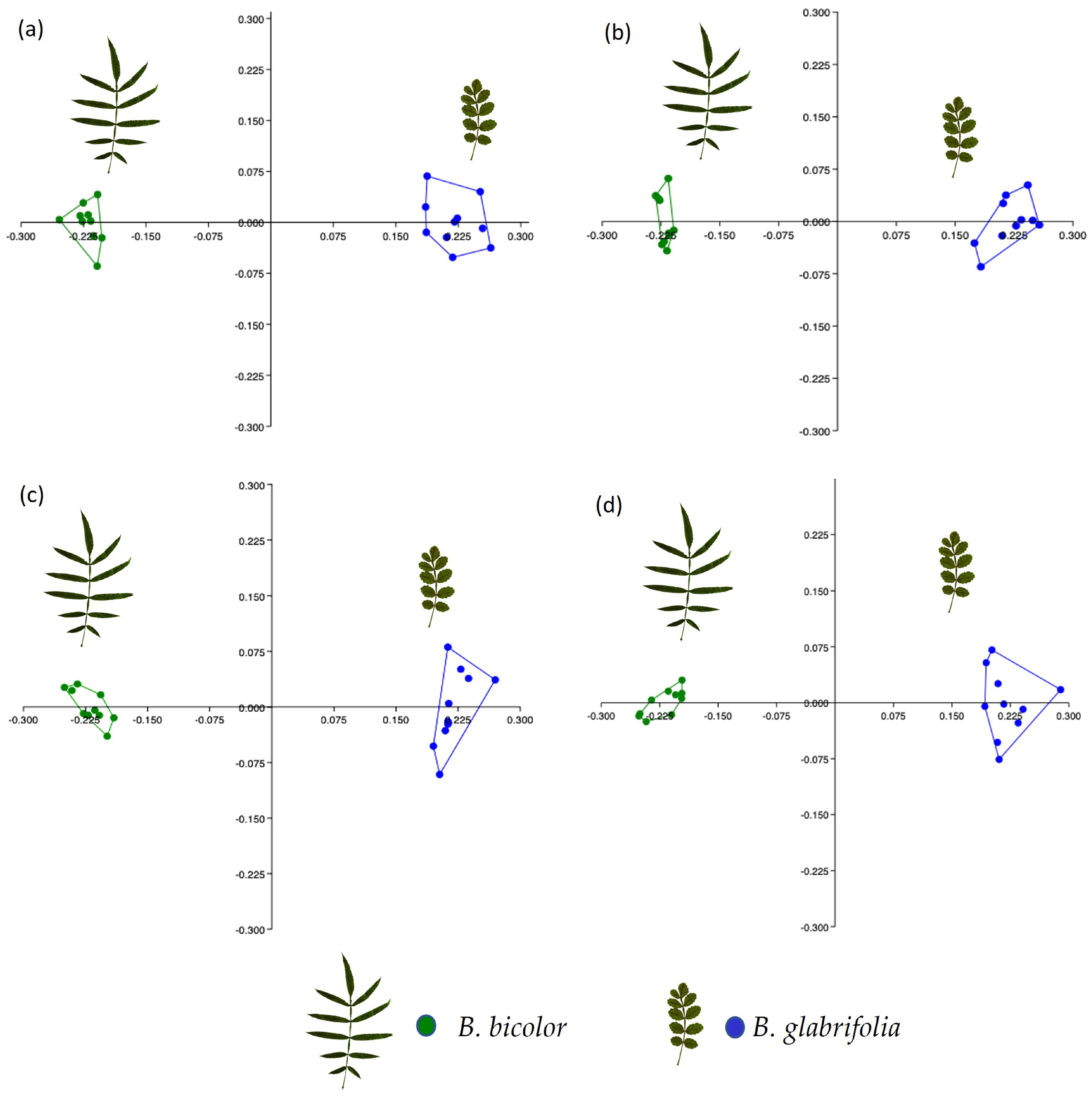
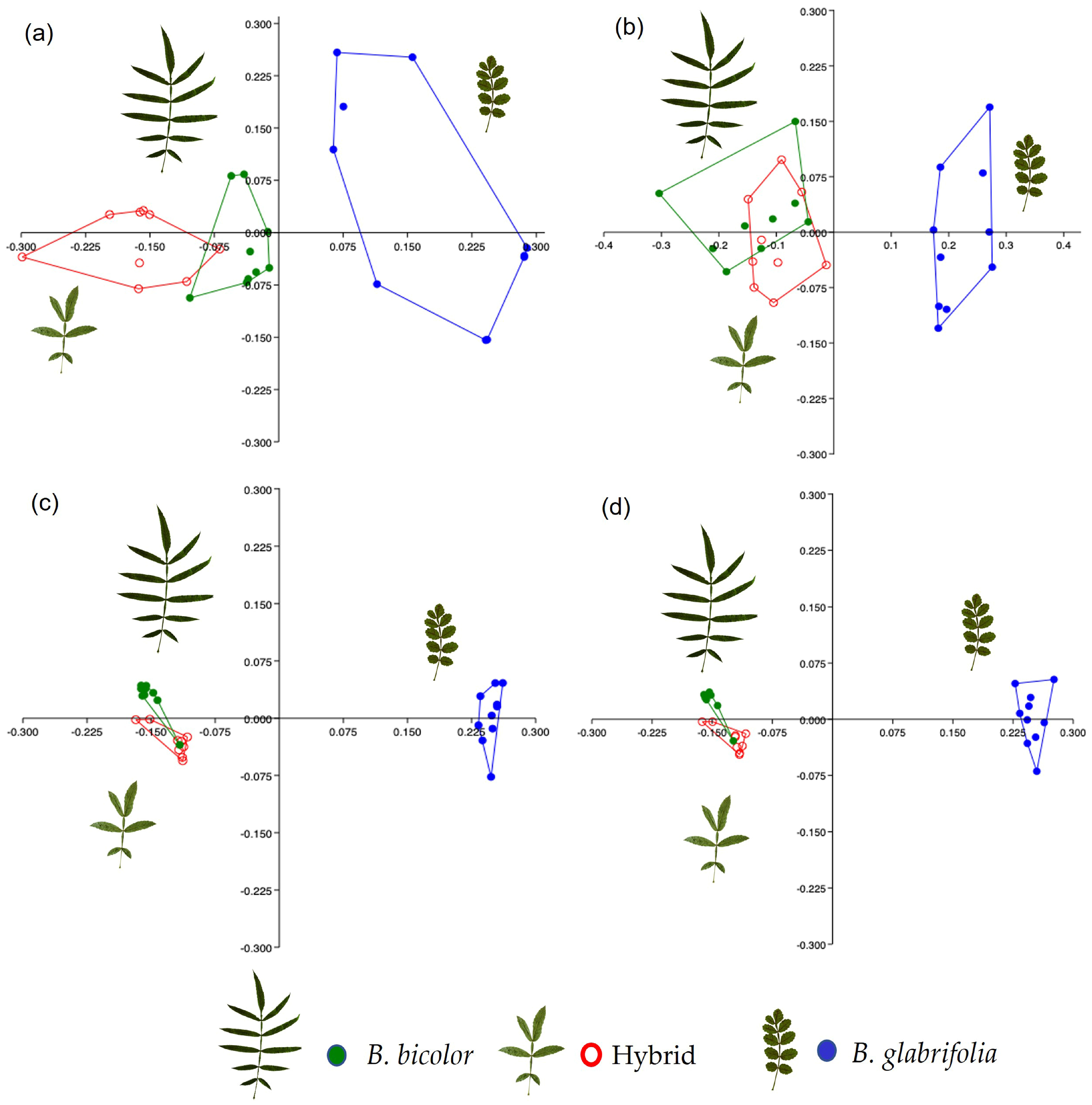
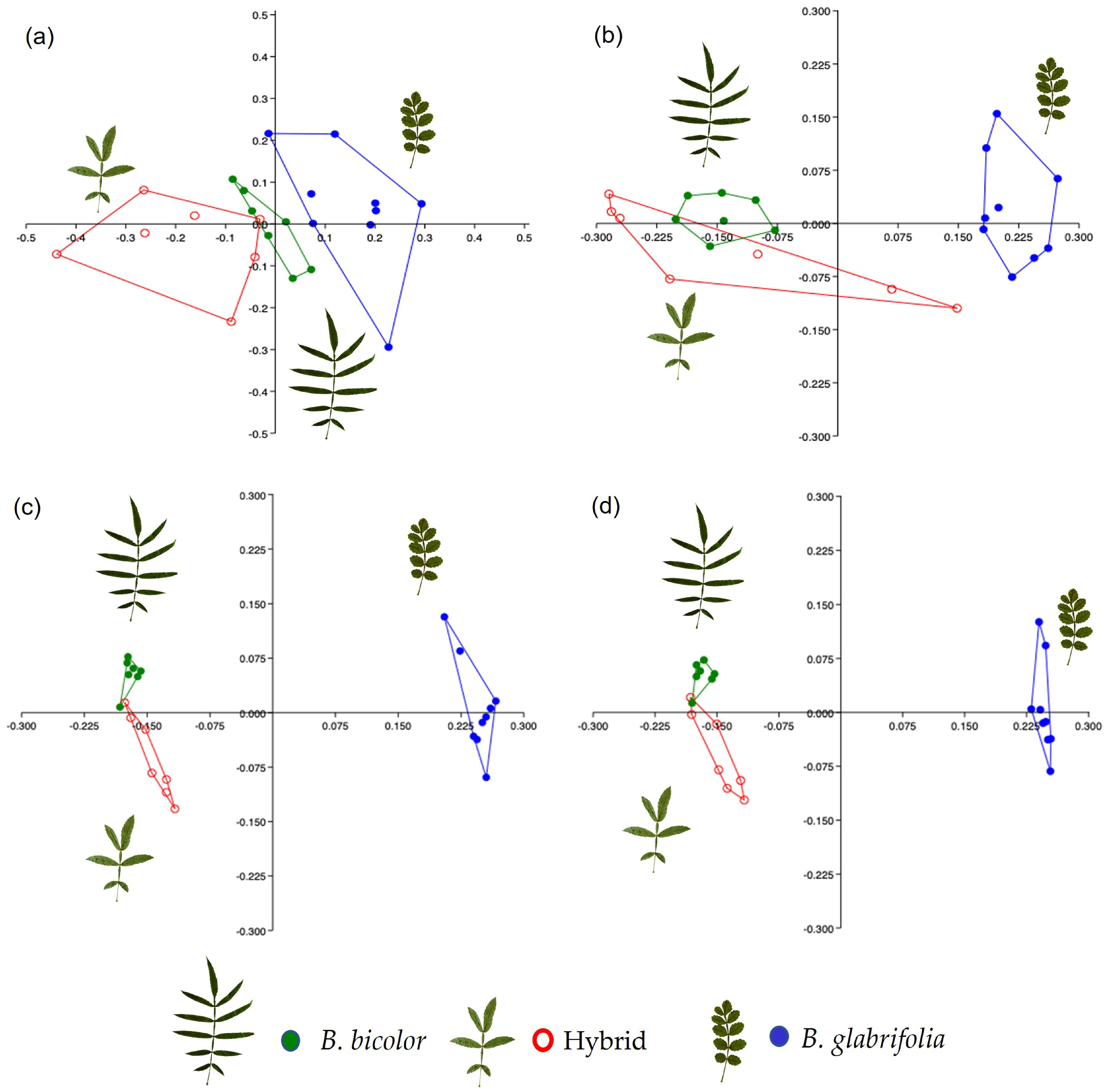
3.1.2. Chemical Data
3.2. Assignment of Hybrid Categories
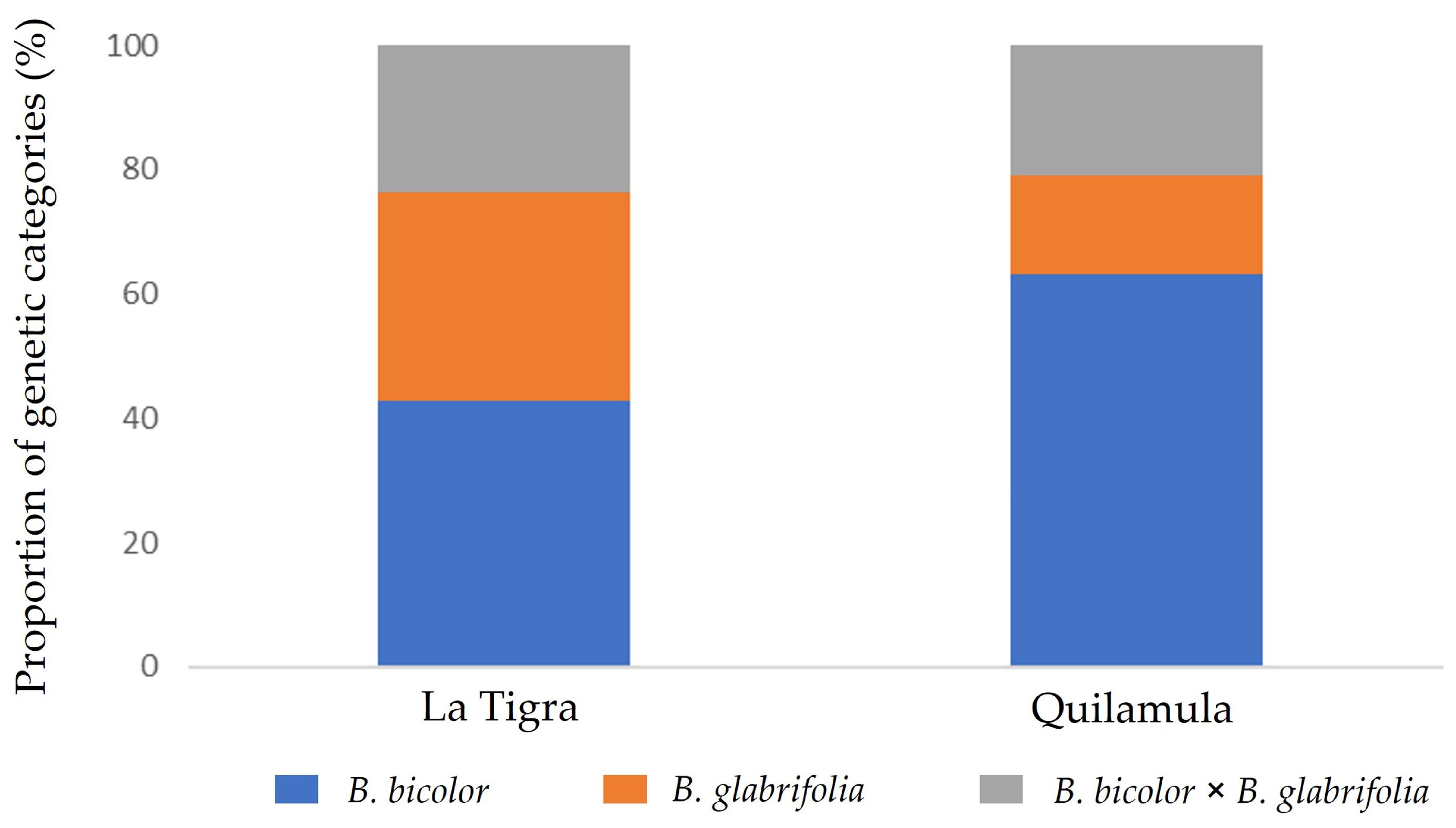
3.2.1. Genetic Data
3.2.2. Chemical Data
4. Discussion
4.1. Genetic and Chemical Assignment of Bursera bicolor and B. glabrifolia at Allopatric Sites
4.2. Genetic and Chemical Evidence of Hybridization between Bursera bicolor and B. glabrifolia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitney, K.D.; Ahern, J.R.; Campbell, L.G.; Albert, L.P.; King, M.S. Patterns of hybridization in plants. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 175–182. [Google Scholar] [CrossRef]
- Beddows, I.; Rose, L.E. Factors determining hybridization rate in plants: A case study in Michigan. Perspect. Plant Ecol. Evol. Syst. 2018, 34, 51–60. [Google Scholar] [CrossRef]
- Mitchell, N.; Campbell, L.G.; Ahern, J.R.; Paine, K.C.; Giroldo, A.B.; Whitney, K.D. Correlates of hybridization in plants. Evol. Lett. 2019, 3, 570–585. [Google Scholar] [CrossRef] [Green Version]
- Rieseberg, L.H.; Wendel, J.F. Introgression and its consequences in plants. In Hybrid Zones and the Evolutionary Process; Harrison, R.G., Ed.; Oxford University Press: New York, NY, USA, 1993; pp. 70–109. [Google Scholar]
- Valencia-Cuevas, L.; Piñero, D.; Mussali-Galante, P.; Valencia-Ávalos, S.; Tovar-Sánchez, E. Effect of a red oak species gradient on genetic structure and diversity of Quercus castanea (Fagaceae) in Mexico. Tree Genet. Genomes 2014, 10, 641–652. [Google Scholar] [CrossRef]
- Driebe, E.M.; Whitham, T.G. Cottonwood hybridization affects tannin and nitrogen content of leaf litter and alters decomposition. Oecologia 2000, 123, 99–107. [Google Scholar] [CrossRef]
- Arnold, M.L. Evolution through Genetic Exchange; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Centeno-Betanzos, L.Y.; López-Caamal, A.; Cortés Rendon, N.; León Santiago, M.; Osorio, E.; Bastida-Armengol, J.; Cano-Santana, Z.; Reyes-Chilpa, R.; Tovar-Sánchez, E. Microsatellites, morphological, and alkaloids characterization of Zephyranthes fosteri and Z. alba (Amaryllidaceae): Allopatric populations. Biochem. Syst. Ecol. 2022, 101, 104398. [Google Scholar] [CrossRef]
- López-Caamal, A.; Reyes-Chilpa, R.; Tovar-Sánchez, E. Hybridization between Tithonia tubaeformis and T. rotundifolia (Asteraceae) evidenced by nSSR and secondary metabolites. Plant Syst. Evol. 2018, 304, 313–326. [Google Scholar] [CrossRef]
- Zobel, B. Oleoresin composition as a determinant of Pine hybridity. Bot. Gaz. 1951, 113, 221–227. [Google Scholar] [CrossRef]
- Castillo-Mendoza, E.; Salinas-Sánchez, D.; Valencia-Cuevas, L.; Zamilpa, A.; Tovar-Sánchez, E. Natural hybridisation among Quercus glabrescens, Q. rugosa and Q. obtusata (Fagaceae): Microsatellites and secondary metabolites markers. Plant Biol. 2019, 21, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Cheng, D.; Vrieling, K.; Klinkhamer, P.G. The effect of hybridization on secondary metabolites and herbivore resistance: Implications for the evolution of chemical diversity in plants. Phytochem. Rev. 2011, 10, 107–117. [Google Scholar] [CrossRef] [Green Version]
- López-Caamal, A.; Mussali-Galante, P.; Valencia-Cuevas, L.; Ramírez, J.J.; Flores, K.V.; Tovar-Sánchez, E. Transgressive character expression in hybrid zones between the native invasives Tithonia tubaeformis and Tithonia rotundifolia (Asteraceae) in Mexico. Plant Syst. Evol. 2013, 299, 1781–1792. [Google Scholar] [CrossRef]
- Georgescu, L.; Stefanakis, M.K.; Kokkini, S.; Katerinopoulos, H.E.; Pirintsos, S.A. Chemical and genetic characterization of Phlomis species and wild hybrids in Crete. Phytochemistry 2016, 122, 91–102. [Google Scholar] [CrossRef]
- López-Caamal, A.; Tovar-Sánchez, E. Genetic, morphological, and chemical patterns of plant hybridization. Rev. Chil. Hist. Nat. 2014, 87, 16. [Google Scholar] [CrossRef]
- De-Nova, J.A.; Medina-Lemos, R.; Montero, J.C.; Weeks, A.; Rosell, J.A.; Olson, M.E.; Eguiarte, L.E.; Magallon, S. Insights into the historical construction of species-rich Mesoamerican seasonally dry tropical forests: The diversification of Bursera (Burseraceae, Sapindales). New Phytol. 2012, 193, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Rzedowski, J.; Medina-Lemos, R.; Calderón de Rzedowski, G. Inventario del conocimiento taxonómico, así como de la diversidad y del endemismo regionales de las especies mexicanas de Bursera (Burseraceae). Acta Bot. Mex. 2005, 70, 85–111. [Google Scholar] [CrossRef] [Green Version]
- Becerra, J.X. Timing the origin and expansion of the Mexican tropical dry forest. Proc. Natl. Acad. Sci. USA 2005, 102, 10919–10923. [Google Scholar] [CrossRef]
- Gámez, N.; Escalante, T.; Espinosa, D.; Eguiarte, L.E.; Morrone, J.J. Temporal dynamics of areas of endemism under climate change: A case study of Mexican Bursera (Burseraceae). J. Biogeogr. 2014, 41, 871–881. [Google Scholar] [CrossRef]
- Quintero-Melecio, E.; Rico, Y.; Lira-Noriega, A.; González-Rodríguez, A. Molecular evidence and ecological niche modeling reveal an extensive hybrid zone among three Bursera species (Section Bullockia). PLoS ONE 2021, 16, e0260382. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Manzur, C.A. El Género Bursera (Burseraceae) en el Estado de Guerrero. Tesis de Licenciatura, Facultad de Ciencias, Universidad Nacional Autónoma de México, México City, Mexico, 1982. [Google Scholar]
- INEGI. Clima. 2022. Available online: https://cuentame.inegi.org.mx/monografias/informacion/mor/territorio/clima.aspx?tema=me&e=17 (accessed on 22 November 2022).
- Gómez-Tuena, A.; Orozco-Esquivel, M.T.; Ferrari, L. Igneous petrogenesis of the Trans-Mexican Volcanic Belt. In Geology of Mexico: Celebrating the Centenary of the Geological Society of Mexico; Alaniz-Álvarez, S.A., Nieto-Samaniego, Á.F., Eds.; Geological Society of America: Boulder, CO, USA, 2007; p. 422. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Wills, D.M.; Hester, M.L.; Liu, A.; Burke, J.M. Chloroplast SSR polymorphisms in the Compositae and the mode of organellar inheritance in Helianthus annuus. Theor. Appl. Genet. 2005, 110, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Bryan, G.J.; McNicoll, J.; Ramsay, G.; Meyer, R.C.; De Jong, W.S. Polymorphic simple sequence repeats markers in chloroplast genomes of Solanaceous plants. Theor. Appl. Genet. 1999, 99, 859–867. [Google Scholar] [CrossRef]
- Paniego, N.; Echaide, M.; Muñoz, M.; Fernández, L.; Torales, S.; Faccio, P.; Fuxan, I.; Carrera, M.; Zandomeni, R.; Suárez, E.Y.; et al. Microsatellite isolation and characterization in sunflower (Helianthus annuus L.). Genome 2002, 45, 34–43. [Google Scholar] [CrossRef]
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1989. [Google Scholar]
- Excoffier, L.; Lischer, H.E.L. Arlequin Suite Ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Lepais, O.; Petit, R.J.; Guichoux, E.; Lavabre, J.E.; Alberto, F.; Kremer, A.; Gerber, S. Species relative abundance and direction of introgression in oaks. Mol. Ecol. 2009, 18, 2228–2242. [Google Scholar] [CrossRef]
- Faith, D.; Minchin, P.; Belbin, L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 1987, 69, 57–68. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Upadhyay, A.; Kadam, U.; Chacko, P.; Aher, L.; Karibasappa, G.M. Microsatellite and RAPD analysis of grape (Vitis spp.) accession and identification of duplicates/misnomers in germplasm collection. Indian J. Hortic. 2010, 67, 8–15. [Google Scholar]
- Chavhan, R.L.; Sable, S.; Narwade, A.V.; Hinge, V.R.; Kalbande, B.B.; Mukherjee, A.K.; Chakrabarty, P.K.; Kadam, U.S. Multiplex molecular marker-assisted analysis of significant pathogens of cotton (Gossypium sp.). Biocatal. Agric. Biotechnol. 2023, 47, 102557. [Google Scholar] [CrossRef]
- Soto, Á.; Rodríguez-Martínez, D.; López De Heredia, U. SIMHYB: A simulation software for the study of the evolution of hybridizing populations. Application to Quercus ilex and Q. suber suggests hybridization could be underestimated. Iforest-Biogeosci. For. 2018, 11, 99. [Google Scholar] [CrossRef] [Green Version]
- Vähä, J.-P.; Primmer, C.R. Efficiency of model-based bayesian methods for detecting hybrid individuals under different hybridization scenarios and with different numbers of Loci: Genetic detection of hybrids. Mol. Ecol. 2005, 15, 63–72. [Google Scholar] [CrossRef]
- Valencia-Cuevas, L.; Mussali-Galante, P.; Piñero, D.; Castillo-Mendoza, E.; Rangel-Altamirano, G.; Tovar-Sánchez, E. Hybridization of Quercus castanea (Fagaceae) across a red oak species gradient in Mexico. Plant Syst. Evol. 2015, 301, 1085–1097. [Google Scholar] [CrossRef]
- Castellanos-Castro, C.; Bonfil, C. Propagación de tres especies de Bursera a partir de estacas. Bot. Sci. 2013, 91, 217–224. [Google Scholar] [CrossRef]
- Siles, P.; Martínez Rayo, J.; Rugama, F.; Molina, L. Diversidad arbórea en cercas vivas y dos fragmentos de bosque en la comunidad de Santa Adelaida, Estelí. Encuentro 2013, 96, 60–76. [Google Scholar] [CrossRef] [Green Version]
- Caseys, C.; Stritt, C.; Glauser, G.; Blanchard, T.; Lexer, C. Effects of hybridization and evolutionary constraints on secondary metabolites: The genetic architecture of phenylpropanoids in European Populus species. PLoS ONE 2015, 10, e0128200. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.; Brüning, G.; Bergmann, J.; Jauch, J. A thin-layer chromatography method for the identification of three different olibanum resins (Boswellia serrata, Boswellia papyrifera and Boswellia carterii, respectively, Boswellia sacra). Phytochem. Anal. 2012, 23, 184–189. [Google Scholar] [CrossRef]
- Zhang, Y.; Ning, Z.; Lu, C.; Zhao, S.; Wang, J.; Liu, B.; Xu, X.; Liu, Y. Triterpenoid resinous metabolites from the genus Boswellia: Pharmacological activities and potential species-identifying properties. Chem. Cent. J. 2013, 7, 153. [Google Scholar] [CrossRef] [Green Version]
- Cole, T.; Gonzalez, A. Filogenia de las Burseraceae (Español). IBONE. 2018. Available online: https://www.researchgate.net/publication/322993034_Filogenia_de_las_Burseraceae_espanol (accessed on 22 November 2022).
- Bangert, R.K.; Lonsdorf, E.V.; Wimp, G.M.; Shuster, S.M.; Fischer, D.; Schweitzer, J.A.; Allan, G.J.; Bailey, J.K.; Whitham, T.G. Genetic structure of a foundation species: Scaling community phenotypes from the individual to the region. Heredity 2008, 100, 121–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangert, R.K.; Turek, R.J.; Rehill, B.; Wimp, G.M.; Schweitzer, J.A.; Allan, G.J.; Bailey, J.K.; Martinsen, G.D.; Keim, P.; Lindroth, R.L.; et al. A genetic similarity rule determines arthropod community structure. Mol. Ecol. 2006, 15, 1379–1391. [Google Scholar] [CrossRef]
- Becerra, J.X.; Venable, D.L. Nuclear ribosomal DNA phylogeny and its implications for evolutionary trends in Mexican Bursera (Burseraceae). Am. J. Bot. 1999, 86, 1047–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieseberg, L.H.; Ellstrand, N.C.; Arnold, M. What can molecular and morphological markers tell us about plant hybridization? Crit. Rev. Plant Sci. 1993, 12, 213–241. [Google Scholar] [CrossRef]
- Weeks, A.; Simpson, B.B. Molecular genetic evidence for interspecific hybridization among endemic hispaniolan Bursera (Burseraceae). Am. J. Bot. 2004, 91, 976–984. [Google Scholar] [CrossRef]
- Peñaloza-Ramírez, J.M.; González-Rodríguez, A.; Mendoza-Cuenca, L.; Caron, H.; Kremer, A.; Oyama, K. Interspecific gene flow in a multispecies oak hybrid zone in the sierra Tarahumara of Mexico. Ann. Bot. 2010, 105, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Tovar-Sánchez, E.; Mussali-Galante, P.; Esteban-Jiménez, R.; Piñero, D.; Arias, D.M.; Dorado, O.; Oyama, K. Chloroplast DNA polymorphism reveals geographic structure and introgression in the Quercus crassifolia × Quercus crassipes hybrid complex in Mexico. Botany 2008, 86, 228–239. [Google Scholar] [CrossRef]
- Zhang, R.; Hipp, A.L.; Gailing, O. Sharing of chloroplast haplotypes among red oak species suggests interspecific gene flow between neighboring populations. Botany 2015, 93, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Rzedowski, J.; Ortiz, E. Estudios quimiotaxonómicos de Bursera (Burseraceae). II. Una especie nueva de origen híbrido de la barranca de Tolantongo, estado de Hidalgo. Acta Bot. Mex. 1988, 1, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Rico, Y.; Reyes-Estanislao, L. Pollen viability and germinability of putative Bursera hybrids (Section Bullockia; Burseraceae) in Mexico. Acta Bot. Mex. 2018, 126, 1–10. [Google Scholar] [CrossRef]
- Hinge, V.R.; Shaikh, I.M.; Chavhan, R.L.; Deshmukh, A.S.; Shelake, R.M.; Ghuge, S.A.; Dethe, A.M.; Suprasanna, P.; Kadam, U.S. Assessment of genetic diversity and volatile content of commercially grown banana (Musa spp.) cultivars. Sci. Rep. 2022, 12, 7979. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, W.; Baldwin, I.T. The rapidly evolving associations among herbivore associated elicitor-induced phytohormones in Nicotiana. Plant Signal. Behav. 2015, 10, e1035850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glassmire, A.E.; Jeffrey, C.S.; Forister, M.L.; Parchman, T.L.; Nice, C.C.; Jahner, J.P.; Wilson, J.S.; Walla, T.R.; Richards, L.A.; Smilanich, A.M.; et al. Intraspecific phytochemical variation shapes community and population structure for specialist caterpillars. New Phytol. 2016, 212, 208–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, M.L. Natural hybridization and the evolution of domesticated, pest and disease organisms. Mol. Ecol. 2004, 13, 997–1007. [Google Scholar] [CrossRef]
- Tovar-Sánchez, E.; Oyama, K. Natural hybridization and hybrid zones between Quercus crassifolia and Quercus crassipes (Fagaceae) in Mexico: Morphological and molecular evidence. Am. J. Bot. 2004, 91, 1352–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becerra, J.X.; Venable, D.L.; Evans, P.H.; Bowers, W.S. Interactions between chemical and mechanical defenses in the plant genus Bursera and their implications for herbivores. Am. Zool. 2001, 41, 865–876. [Google Scholar]
- Raffa, K.F. Terpenes tell different tales at different scales: Glimpses into the chemical ecology of conifer-bark beetle-microbial interactions. J. Chem. Ecol. 2014, 40, 1–20. [Google Scholar] [CrossRef] [PubMed]
| Taxa | Locality | N | No. Loci | nh | H | Gd |
|---|---|---|---|---|---|---|
| B. bicolor | Coatlán del Río | 20 | 4 | 13 | 0.539 | 0.275 |
| B. glabrifolia | Tetela del Volcán | 20 | 4 | 11 | 0.412 | 0.201 |
| B. bicolor × B. glabrifolia | La Tigra | 20 | 4 | 14 | 0.750 | 0.314 |
| B. bicolor × B. glabrifolia | Quilamula | 20 | 4 | 15 | 0.846 | 0.379 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocampo-Bautista, F.; Mussali-Galante, P.; Alvarez, L.; Marquina-Bahena, S.; Valencia-Cuevas, L.; Valencia-A, S.; Tovar-Sánchez, E. Natural Hybridization between Bursera bicolor × B. glabrifolia (Burseraceae) Complex: Molecular and Chemical Evidence. Forests 2023, 14, 1382. https://doi.org/10.3390/f14071382
Ocampo-Bautista F, Mussali-Galante P, Alvarez L, Marquina-Bahena S, Valencia-Cuevas L, Valencia-A S, Tovar-Sánchez E. Natural Hybridization between Bursera bicolor × B. glabrifolia (Burseraceae) Complex: Molecular and Chemical Evidence. Forests. 2023; 14(7):1382. https://doi.org/10.3390/f14071382
Chicago/Turabian StyleOcampo-Bautista, Fidel, Patricia Mussali-Galante, Laura Alvarez, Silvia Marquina-Bahena, Leticia Valencia-Cuevas, Susana Valencia-A, and Efraín Tovar-Sánchez. 2023. "Natural Hybridization between Bursera bicolor × B. glabrifolia (Burseraceae) Complex: Molecular and Chemical Evidence" Forests 14, no. 7: 1382. https://doi.org/10.3390/f14071382
APA StyleOcampo-Bautista, F., Mussali-Galante, P., Alvarez, L., Marquina-Bahena, S., Valencia-Cuevas, L., Valencia-A, S., & Tovar-Sánchez, E. (2023). Natural Hybridization between Bursera bicolor × B. glabrifolia (Burseraceae) Complex: Molecular and Chemical Evidence. Forests, 14(7), 1382. https://doi.org/10.3390/f14071382







