Mixed Plantations Improve Soil Bacterial Similarity by Reducing Heterogeneous Environmental Selection
Abstract
1. Introduction
2. Methods
2.1. Study Site
2.2. Experimental Design
2.3. Soil Sampling and Biogeochemical Analyses
2.4. DNA Extraction and MiSeq Sequencing of 16S rRNA Gene Amplicons
2.5. Sequence Analyses
2.6. Statistical Analyses
3. Results
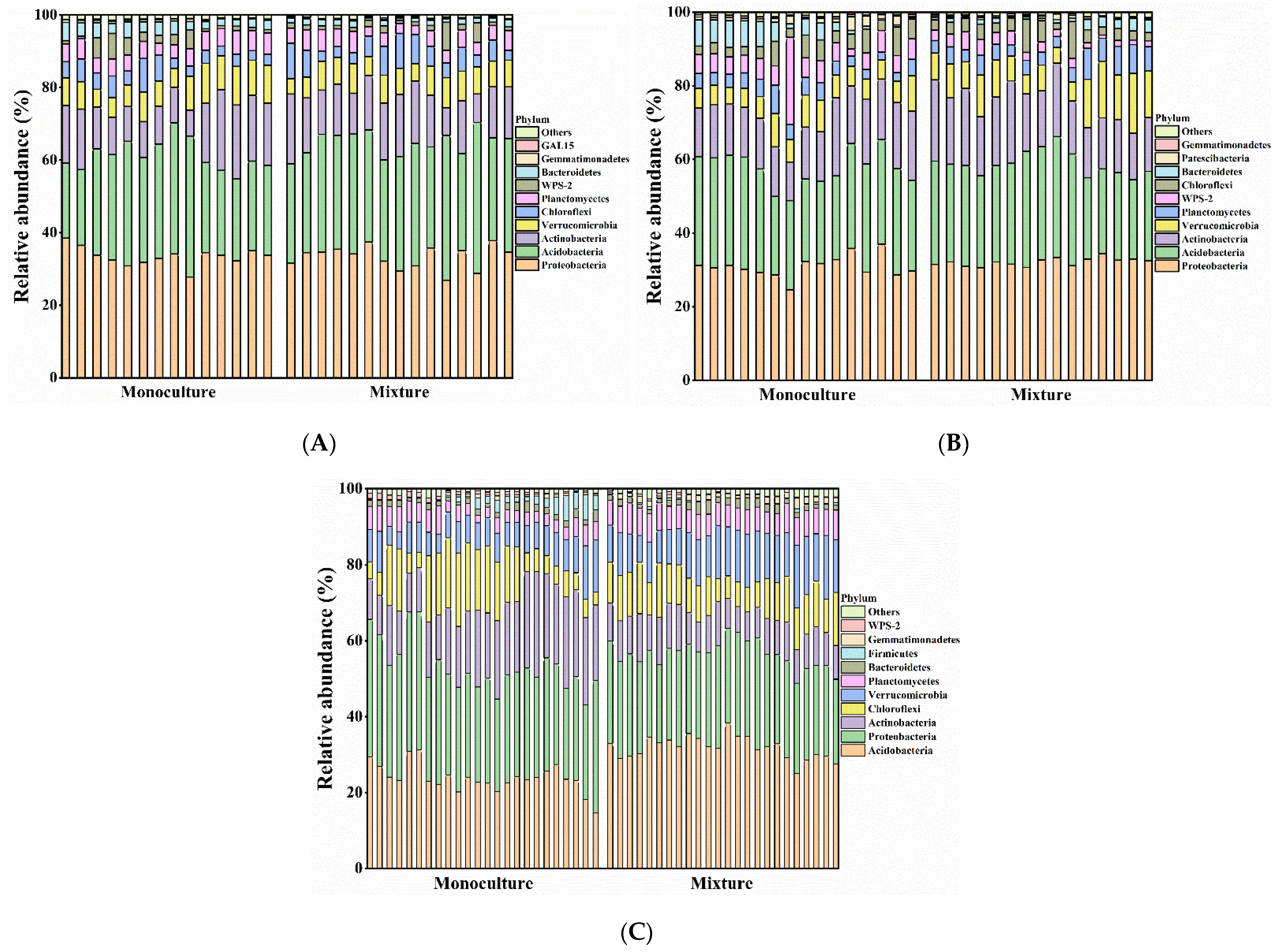
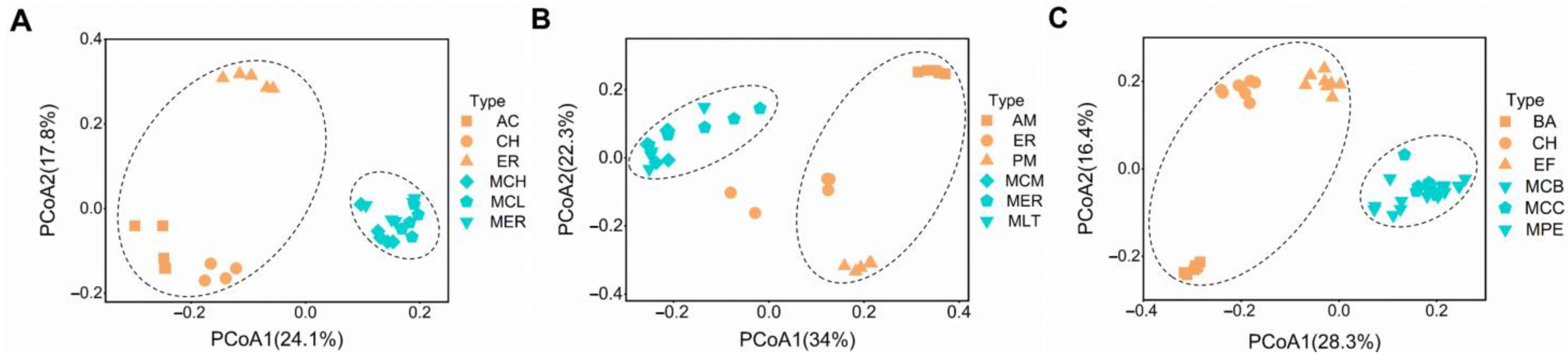
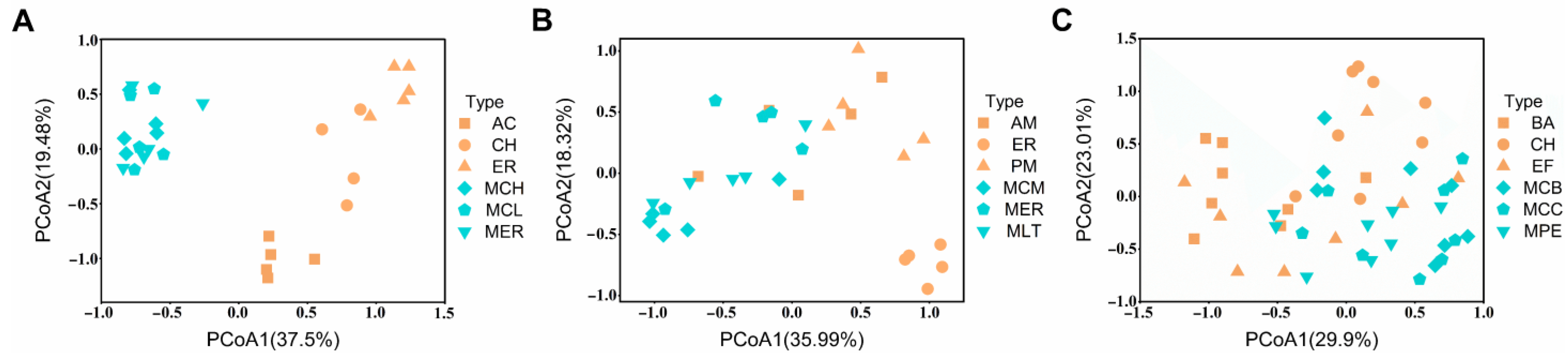
3.1. Effects of Monocultures and Mixed Plantations on Bacterial Community Similarity
3.2. Different Community Assembly Processes of Bacterial Communities among Monocultures and Mixed Plantations
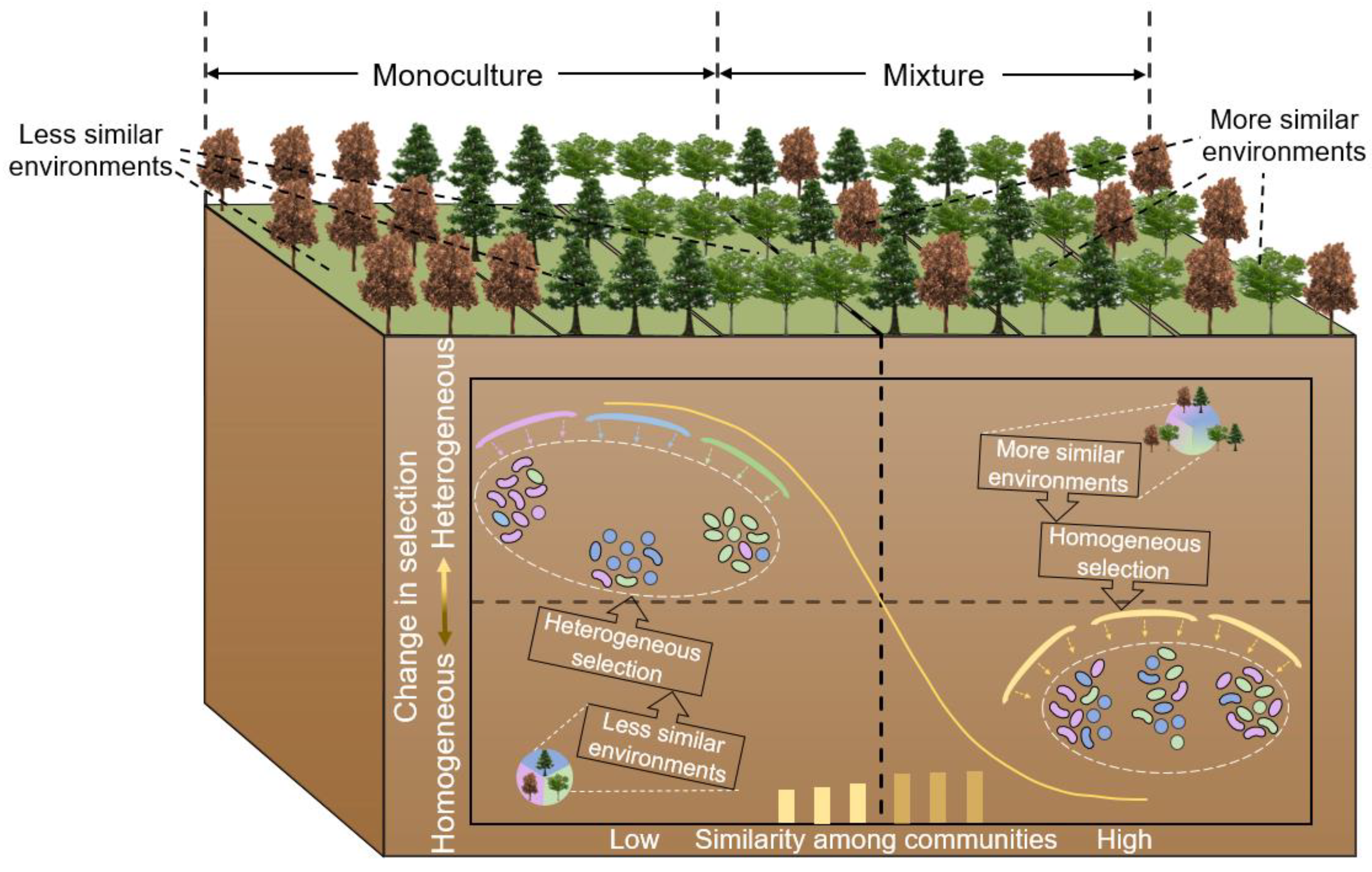
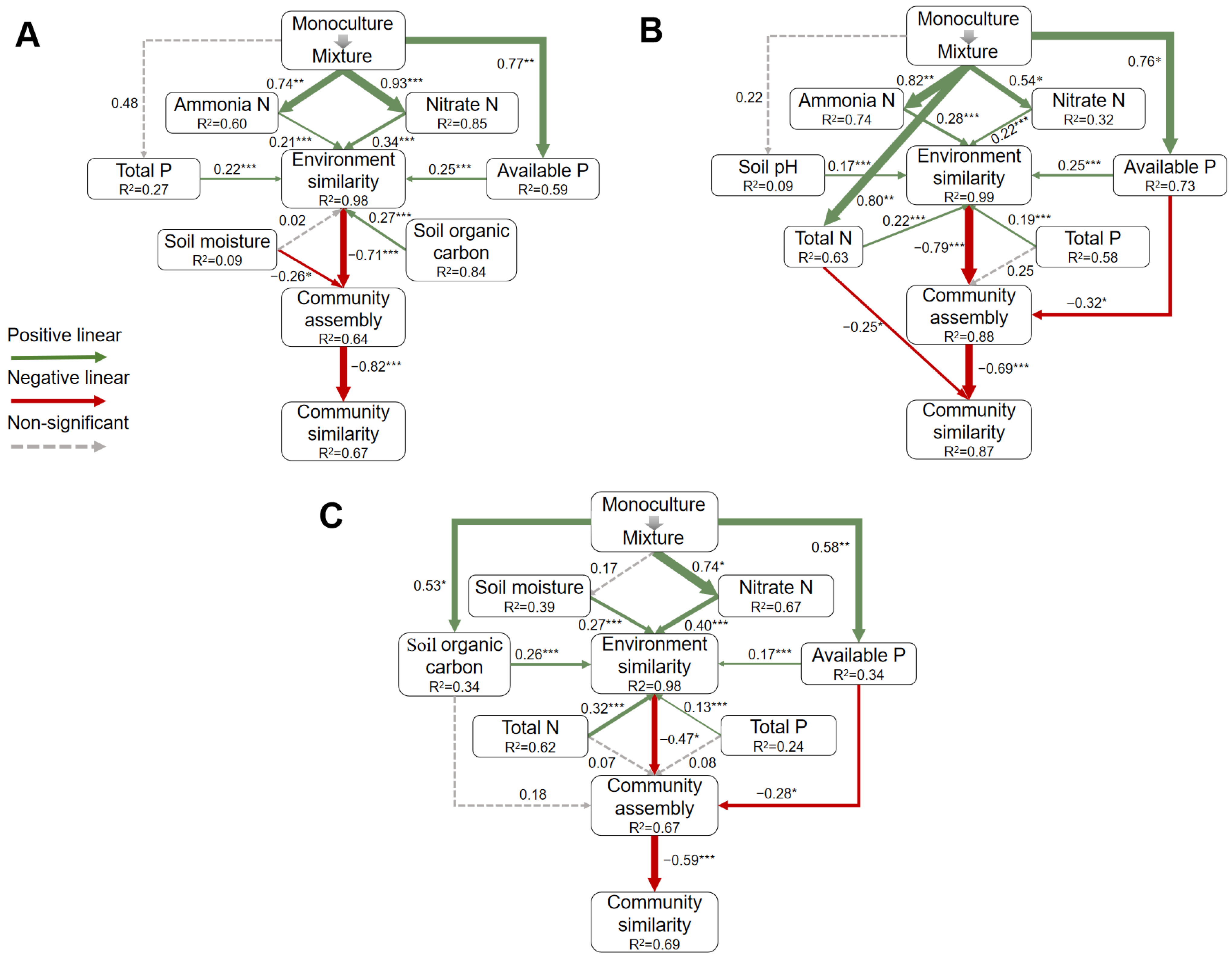
3.3. Mechanism of the Influence of Mixed Plantations on Soil Bacterial Community Similarity
4. Discussion
4.1. Tree Species Mixture Result in More Similar Soil Bacterial Communities Than Monocultures
4.2. Soil Bacterial Similarity among Monoculture and Mixed Plantations Is Driven by Different Community Assembly Processes
4.3. Driving Mechanism of Soil Bacterial Similarity among Monocultures and Mixed Plantations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.-L.; Hu, H.-W.; Yan, Z.-Z.; Li, C.-Y.; Nguyen, B.-A.T.; Sun, A.-Q.; Zhu, Y.-G.; He, J.-Z. Deterministic selection dominates microbial community assembly in termite mounds. Soil Biol. Biochem. 2021, 152, 108073. [Google Scholar] [CrossRef]
- Vellend, M. Conceptual synthesis in community ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.M.; Stegen, J.C.; Kim, M.; Dong, K.; Adams, J.M.; Lee, Y.K. Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J. 2018, 12, 1072–1083. [Google Scholar] [CrossRef]
- Barnett, S.E.; Youngblut, N.D.; Buckley, D.H. Soil characteristics and land-use drive bacterial community assembly patterns. FEMS Microbiol. Ecol. 2020, 96, fiz194. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Konopka, A.E.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, S.P.; Borda-de-Água, L. The Unified Neutral Theory of Biodiversity and Biogeography; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- An, J.; Liu, C.; Wang, Q.; Yao, M.; Rui, J.; Zhang, S.; Li, X. Soil bacterial community structure in Chinese wetlands. Geoderma 2019, 337, 290–299. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Zhang, W.; Wang, C.; Wang, P.; Niu, L.; Wu, H. Homogeneous selection dominates the microbial community assembly in the sediment of the Three Gorges Reservoir. Sci. Total Environ. 2019, 690, 50–60. [Google Scholar] [CrossRef]
- Zhou, J.; Ning, D. Stochastic community assembly: Does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef]
- Huber, P.; Metz, S.; Unrein, F.; Mayora, G.; Sarmento, H.; Devercelli, M. Environmental heterogeneity determines the ecological processes that govern bacterial metacommunity assembly in a floodplain river system. ISME J. 2020, 14, 2951–2966. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Konopka, A.E. Estimating and mapping ecological processes influencing microbial community assembly. Front. Microbiol. 2015, 6, 370. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Wu, Y.; Soininen, J.; Stegen, J.C.; He, J.; Liu, X.; Zhang, L.; Zhang, E. Phylogenetic beta diversity in bacterial assemblages across ecosystems: Deterministic versus stochastic processes. ISME J. 2013, 7, 1310–1321. [Google Scholar] [CrossRef]
- Chen, F.; Zheng, H.; Zhang, K.; Ouyang, Z.; Lan, J.; Li, H.; Shi, Q. Changes in soil microbial community structure and metabolic activity following conversion from native Pinus massoniana plantations to exotic Eucalyptus plantations. For. Ecol. Manag. 2013, 291, 65–72. [Google Scholar] [CrossRef]
- Schlatter, D.C.; Bakker, M.G.; Bradeen, J.M.; Kinkel, L.L. Plant community richness and microbial interactions structure bacterial communities in soil. Ecology 2015, 96, 134–142. [Google Scholar] [CrossRef]
- Uroz, S.; Buee, M.; Deveau, A.; Mieszkin, S.; Martin, F. Ecology of the forest microbiome: Highlights of temperate and boreal ecosystems. Soil Biol. Biochem. 2016, 103, 471–488. [Google Scholar] [CrossRef]
- Li, H.; Ye, D.; Wang, X.; Settles, M.L.; Wang, J.; Hao, Z.; Zhou, L.; Dong, P.; Jiang, Y.; Ma, Z.S. Soil bacterial communities of different natural forest types in Northeast China. Plant Soil 2014, 383, 203–216. [Google Scholar] [CrossRef]
- Prober, S.M.; Leff, J.W.; Bates, S.T.; Borer, E.T.; Firn, J.; Harpole, W.S.; Lind, E.M.; Seabloom, E.W.; Adler, P.B.; Bakker, J.D. Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol. Lett. 2015, 18, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, A.; Steffens, C.; Seven, J.; Jacob, A.; Hertel, D.; Leuschner, C.; Gleixner, G. Effects of tree identity dominate over tree diversity on the soil microbial community structure. Soil Biol. Biochem. 2015, 81, 219–227. [Google Scholar] [CrossRef]
- Garau, G.; Morillas, L.; Roales, J.; Castaldi, P.; Mangia, N.P.; Spano, D.; Mereu, S. Effect of monospecific and mixed Mediterranean tree plantations on soil microbial community and biochemical functioning. Appl. Soil Ecol. 2019, 140, 78–88. [Google Scholar] [CrossRef]
- Pereira, A.P.; Durrer, A.; Gumiere, T.; Gonçalves, J.L.; Robin, A.; Bouillet, J.-P.; Wang, J.; Verma, J.P.; Singh, B.K.; Cardoso, E.J. Mixed Eucalyptus plantations induce changes in microbial communities and increase biological functions in the soil and litter layers. For. Ecol. Manag. 2019, 433, 332–342. [Google Scholar] [CrossRef]
- Fierer, N.; Morse, J.L.; Berthrong, S.T.; Bernhardt, E.S.; Jackson, R.B. Environmental controls on the landscape-scale biogeography of stream bacterial communities. Ecology 2007, 88, 2162–2173. [Google Scholar] [CrossRef]
- Ampoorter, E.; Baeten, L.; Koricheva, J.; Vanhellemont, M.; Verheyen, K. Do diverse overstoreys induce diverse understoreys? Lessons learnt from an experimental–observational platform in Finland. For. Ecol. Manag. 2014, 318, 206–215. [Google Scholar] [CrossRef]
- Feng, Y.; Schmid, B.; Loreau, M.; Forrester, D.I.; Fei, S.; Zhu, J.; Tang, Z.; Zhu, J.; Hong, P.; Ji, C. Multispecies forest plantations outyield monocultures across a broad range of conditions. Science 2022, 376, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, S.; Huang, Y.; Fu, S.; Wang, J.; Ming, A.; Li, X.; Yao, M.; Li, H. Tree species mixture inhibits soil organic carbon mineralization accompanied by decreased r-selected bacteria. Plant Soil 2018, 431, 203–216. [Google Scholar] [CrossRef]
- Wang, J.; Ren, H.; Yang, L.; Duan, W.J. Establishment and early growth of introduced indigenous tree species in typical plantations and shrubland in South China. For. Ecol. Manag. 2009, 258, 1293–1300. [Google Scholar] [CrossRef]
- Mo, Q.; Li, Z.; Zhu, W.; Zou, B.; Li, Y.; Yu, S.; Ding, Y.; Chen, Y.; Li, X.; Wang, F. Reforestation in southern China: Revisiting soil N mineralization and nitrification after 8 years restoration. Sci. Rep. 2016, 6, 19770. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chen, Y.; Zhao, J.; Fu, S.; Li, Z.; Xia, H.; Zhou, L. Temperature sensitivity of total soil respiration and its heterotrophic and autotrophic components in six vegetation types of subtropical China. Sci. Total Environ. 2017, 607–608, 160–167. [Google Scholar] [CrossRef] [PubMed]
- FAO. World reference base for soil resources 2006. In World Soil Resources Reports; FAO: Rome, Italy, 2006. [Google Scholar]
- He, J.; Tedersoo, L.; Hu, A.; Han, C.; He, D.; Wei, H.; Jiao, M.; Anslan, S.; Nie, Y.; Jia, Y.; et al. Greater diversity of soil fungal communities and distinguishable seasonal variation in temperate deciduous forests compared with subtropical evergreen forests of eastern China. FEMS Microbiol. Ecol. 2017, 93, fix069. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Wang, J.; Huang, Y.; Freedman, Z.; Fu, S.; Liu, K.; Wang, H.; Li, X.; Yao, M. Local community assembly mechanisms shape soil bacterial β diversity patterns along a latitudinal gradient. Nat. Commun. 2020, 11, 5428. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Li, X.; Wang, J.; Ding, Q.; Wang, H.; Tian, C.; Yao, M.; An, J.; Huang, Y. Changes of soil prokaryotic communities after clear-cutting in a karst forest: Evidences for cutting-based disturbance promoting deterministic processes. FEMS Microbiol. Ecol. 2016, 92, fiw026. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Waste not, want not: Why rarefying microbiome data is inadmissible. PLoS Comput. Biol. 2014, 10, e1003531. [Google Scholar] [CrossRef] [PubMed]
- Lefcheck, J.S. PIECEWISESEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Shigyo, N.; Umeki, K.; Hirao, T. Plant functional diversity and soil properties control elevational diversity gradients of soil bacteria. FEMS Microbiol. Ecol. 2019, 95, fiz025. [Google Scholar] [CrossRef]
- Yamamura, T.; Schwendenmann, L.; Lear, G. Tree species identity has little impact on the structure of soil bacterial communities in a 10-year-old tropical tree plantation. Biol. Fertil. Soils 2013, 49, 819–828. [Google Scholar] [CrossRef]
- Gunina, A.; Smith, A.R.; Godbold, D.L.; Jones, D.L.; Kuzyakov, Y. Response of soil microbial community to afforestation with pure and mixed species. Plant Soil 2017, 412, 357–368. [Google Scholar] [CrossRef]
- Rachid, C.T.; Balieiro, F.D.C.; Peixoto, R.S.; Pinheiro, Y.; Piccolo, M.D.C.; Chaer, G.M.; Rosado, A.S. Mixed plantations can promote microbial integration and soil nitrate increases with changes in the N cycling genes. Soil Biol. Biochem. 2013, 66, 146–153. [Google Scholar] [CrossRef]
- Koutika, L.-S.; Fiore, A.; Tabacchioni, S.; Aprea, G.; Pereira, A.P.; Bevivino, A. Influence of Acacia mangium on Soil Fertility and Bacterial Community in Eucalyptus Plantations in the Congolese Coastal Plains. Sustainability 2020, 12, 8763. [Google Scholar] [CrossRef]
- Schmid, M.W.; Moorsel, S.J.V.; Hahl, T.; Luca, E.D.; Deyn, G.B.D.; Wagg, C.; Niklaus, P.A.; Schmid, B. Effects of plant community history, soil legacy and plant diversity on soil microbial communities. J. Ecol. 2021, 109, 3007–3023. [Google Scholar] [CrossRef]
- Cong, W.F.; van Ruijven, J.; Mommer, L.; De Deyn, G.B.; Berendse, F.; Hoffland, E. Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J. Ecol. 2014, 102, 1163–1170. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Wang, J.; Shen, C.; He, J.-Z.; Ge, Y. Plant evenness modulates the effect of plant richness on soil bacterial diversity. Sci. Total Environ. 2019, 662, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Lamb, E.G.; Kennedy, N.; Siciliano, S.D. Effects of plant species richness and evenness on soil microbial community diversity and function. Plant Soil 2011, 338, 483–495. [Google Scholar] [CrossRef]
- Arnillas, C.A.; Cadotte, M.W. Experimental dominant plant removal results in contrasting assembly for dominant and non-dominant plants. Ecol. Lett. 2019, 22, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Dassen, S.; Cortois, R.; Martens, H.; de Hollander, M.; Kowalchuk, G.A.; van der Putten, W.H.; De Deyn, G.B. Differential responses of soil bacteria, fungi, archaea and protists to plant species richness and plant functional group identity. Mol. Ecol. 2017, 26, 4085–4098. [Google Scholar] [CrossRef]
- Puettmann, K.J.; Coates, K.D.; Messier, C. A Critique of Silviculture: Managing for Complexity; Island Press: Washington, DC, USA, 2008. [Google Scholar]
- Kou, Y.P.; Wei, K.; Li, C.N.; Wang, Y.S.; Tu, B.; Wang, J.M.; Li, X.Z.; Yao, M.J. Deterministic processes dominate soil methanotrophic community assembly in grassland soils. Geoderma 2020, 359, 114004. [Google Scholar] [CrossRef]
- Cao, P.; Wang, J.-T.; Hu, H.-W.; Zheng, Y.-M.; Ge, Y.; Shen, J.-P.; He, J.-Z. Environmental filtering process has more important roles than dispersal limitation in shaping large-scale prokaryotic beta diversity patterns of grassland soils. Microb. Ecol. 2016, 72, 221–230. [Google Scholar] [CrossRef]
- Dini-Andreote, F.; Stegen, J.C.; van Elsas, J.D.; Salles, J.F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci. USA 2015, 112, E1326–E1332. [Google Scholar] [CrossRef]
- Xiao, F.; Bi, Y.; Li, X.; Huang, J.; Yu, Y.; Xie, Z.; Fang, T.; Cao, X.; He, Z.; Juneau, P. The impact of anthropogenic disturbance on bacterioplankton communities during the construction of Donghu Tunnel (Wuhan, China). Microb. Ecol. 2019, 77, 277–287. [Google Scholar] [CrossRef]
- Logares, R.; Deutschmann, I.M.; Giner, C.R.; Krabberød, A.K.; Schmidt, T.S.; Rubinat-Ripoll, L.; Mestre, M.; Salazar, G.; Ruiz-González, C.; Sebastián, M. Different processes shape prokaryotic and picoeukaryotic assemblages in the sunlit ocean microbiome. bioRxiv 2018, 20, 37–49. [Google Scholar]
- Heino, J.; Melo, A.S.; Siqueira, T.; Soininen, J.; Valanko, S.; Bini, L.M. Metacommunity organisation, spatial extent and dispersal in aquatic systems: Patterns, processes and prospects. Freshw. Biol. 2015, 60, 845–869. [Google Scholar] [CrossRef]
- Xue, R.; Zhao, K.; Yu, X.; Stirling, E.; Liu, S.; Ye, S.; Ma, B.; Xu, J. Deciphering sample size effect on microbial biogeographic patterns and community assembly processes at centimeter scale. Soil Biol. Biochem. 2021, 156, 108218. [Google Scholar] [CrossRef]
- Allen, R.; Hoffmann, L.J.; Larcombe, M.J.; Louisson, Z.; Summerfield, T.C. Homogeneous environmental selection dominates microbial community assembly in the oligotrophic South Pacific Gyre. Mol. Ecol. 2020, 29, 4680–4691. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, M.; Petropoulos, E.; Zhang, J.; Nie, J.; Liao, Y.; Li, Z.; Lin, X.; Feng, Y. Responses of paddy soil bacterial community assembly to different long-term fertilizations in southeast China. Sci. Total Environ. 2019, 656, 625–633. [Google Scholar] [CrossRef]
- Ofiţeru, I.D.; Lunn, M.; Curtis, T.P.; Wells, G.F.; Criddle, C.S.; Francis, C.A.; Sloan, W.T. Combined niche and neutral effects in a microbial wastewater treatment community. Proc. Natl. Acad. Sci. USA 2010, 107, 15345–15350. [Google Scholar] [CrossRef]
- Evans, S.; Martiny, J.B.; Allison, S.D. Effects of dispersal and selection on stochastic assembly in microbial communities. ISME J. 2017, 11, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Dukunde, A.; Schneider, D.; Schmidt, M.; Veldkamp, E.; Daniel, R. Tree species shape soil bacterial community structure and function in temperate deciduous forests. Front. Microbiol. 2019, 10, 1519. [Google Scholar] [CrossRef]
- Bakker, M.G.; Otto-Hanson, L.; Lange, A.; Bradeen, J.M.; Kinkel, L.L. Plant monocultures produce more antagonistic soil Streptomyces communities than high-diversity plant communities. Soil Biol. Biochem. 2013, 65, 304–312. [Google Scholar] [CrossRef]
- Powell, J.R.; Karunaratne, S.; Campbell, C.D.; Yao, H.; Robinson, L.; Singh, B.K. Deterministic processes vary during community assembly for ecologically dissimilar taxa. Nat. Commun. 2015, 6, 8444. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Tu, B.; Kou, Y.; Li, X. Species pool and local ecological assembly processes shape the β-diversity of diazotrophs in grassland soils. Soil Biol. Biochem. 2021, 160, 108338. [Google Scholar] [CrossRef]
- Karp, D.S.; Rominger, A.J.; Zook, J.; Ranganathan, J.; Ehrlich, P.R.; Daily, G.C. Intensive agriculture erodes β-diversity at large scales. Ecol. Lett. 2012, 15, 963–970. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, K.; Krause, S.M.; Li, S.; Wang, X.; Zhang, Z.; Shen, M.; Yang, Q.; Lian, J.; Wang, X. Changes in assembly processes of soil microbial communities during secondary succession in two subtropical forests. Soil Biol. Biochem. 2021, 154, 108144. [Google Scholar] [CrossRef]
- Dawud, S.M.; Raulund-Rasmussen, K.; Domisch, T.; Finér, L.; Jaroszewicz, B.; Vesterdal, L. Is tree species diversity or species identity the more important driver of soil carbon stocks, C/N ratio, and pH? Ecosystems 2016, 19, 645–660. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, P.; Xiang, W.; Yan, W.; Chen, X. Accumulation of soil organic C and N in planted forests fostered by tree species mixture. Biogeosciences 2017, 14, 3937–3945. [Google Scholar] [CrossRef]
- Lei, P.; Scherer-Lorenzen, M.; Bauhus, J. The effect of tree species diversity on fine-root production in a young temperate forest. Oecologia 2012, 169, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Wedin, D.; Knops, J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 1996, 379, 718–720. [Google Scholar] [CrossRef]
- Hou, E.; Luo, Y.; Kuang, Y.; Chen, C.; Lu, X.; Jiang, L.; Luo, X.; Wen, D. Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat. Commun. 2020, 11, 637. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Binkley, D.; Caldwell, B.A. Effects of dinitrogen-fixing trees on phosphorus biogeochemical cycling in contrasting forests. Soil Sci. Soc. Am. J. 1995, 59, 1452–1458. [Google Scholar] [CrossRef]
- Richards, A.E.; Forrester, D.I.; Bauhus, J.; Scherer-Lorenzen, M. The influence of mixed tree plantations on the nutrition of individual species: A review. Tree Physiol. 2010, 30, 1192–1208. [Google Scholar] [CrossRef]
- Peng, Z.; Wu, Y.; Guo, L.; Yang, L.; Wang, B.; Wang, X.; Liu, W.; Su, Y.; Wu, J.; Liu, L. Foliar nutrient resorption stoichiometry and microbial phosphatase catalytic efficiency together alleviate the relative phosphorus limitation in forest ecosystems. New Phytol. 2023, 238, 1033–1044. [Google Scholar] [CrossRef]
- Treseder, K.K.; Vitousek, P.M. Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 2001, 82, 946–954. [Google Scholar] [CrossRef]
- Liu, Y.; Johnson, N.C.; Mao, L.; Shi, G.; Jiang, S.; Ma, X.; Du, G.; An, L.; Feng, H. Phylogenetic structure of arbuscular mycorrhizal community shifts in response to increasing soil fertility. Soil Biol. Biochem. 2015, 89, 196–205. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, H.; Dong, B.; Yang, Z.; Yuan, Y.; Tan, Y.; Huang, Y.; Zhang, X. Mixed Plantations Improve Soil Bacterial Similarity by Reducing Heterogeneous Environmental Selection. Forests 2023, 14, 1341. https://doi.org/10.3390/f14071341
Dai H, Dong B, Yang Z, Yuan Y, Tan Y, Huang Y, Zhang X. Mixed Plantations Improve Soil Bacterial Similarity by Reducing Heterogeneous Environmental Selection. Forests. 2023; 14(7):1341. https://doi.org/10.3390/f14071341
Chicago/Turabian StyleDai, Handan, Biao Dong, Zhu Yang, Yidan Yuan, Yuhua Tan, Yongtao Huang, and Xiao Zhang. 2023. "Mixed Plantations Improve Soil Bacterial Similarity by Reducing Heterogeneous Environmental Selection" Forests 14, no. 7: 1341. https://doi.org/10.3390/f14071341
APA StyleDai, H., Dong, B., Yang, Z., Yuan, Y., Tan, Y., Huang, Y., & Zhang, X. (2023). Mixed Plantations Improve Soil Bacterial Similarity by Reducing Heterogeneous Environmental Selection. Forests, 14(7), 1341. https://doi.org/10.3390/f14071341



