Features of Scots Pine Mortality Due to Incursion of Pine Bark Beetles in Symbiosis with Ophiostomatoid Fungi in the Forest-Steppe of Central Siberia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Instrumental In Situ Measurements
2.3. Dendrochronological Measurements and Seasonal Growth
2.4. Beetles and Fungi Monitoring
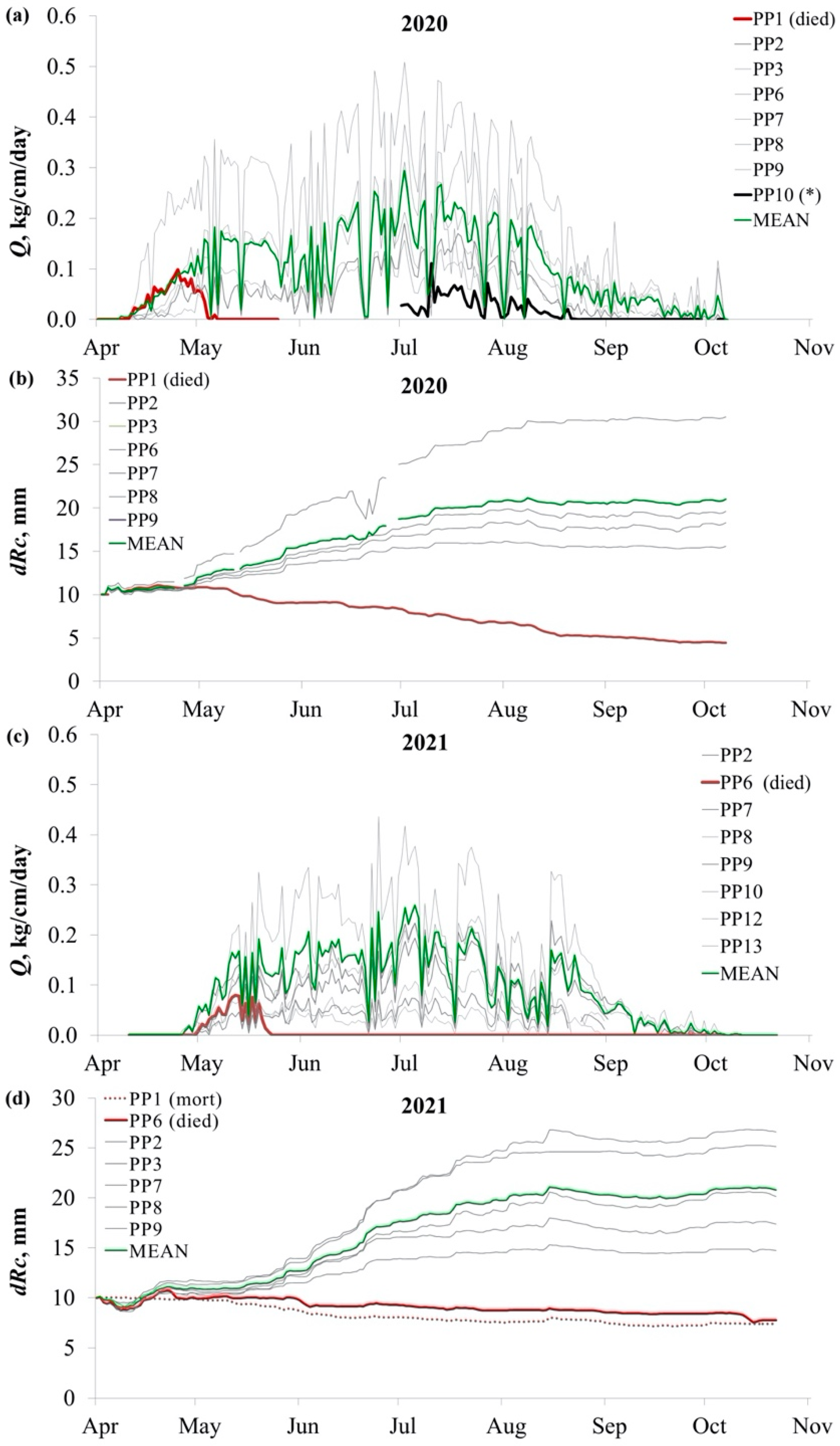
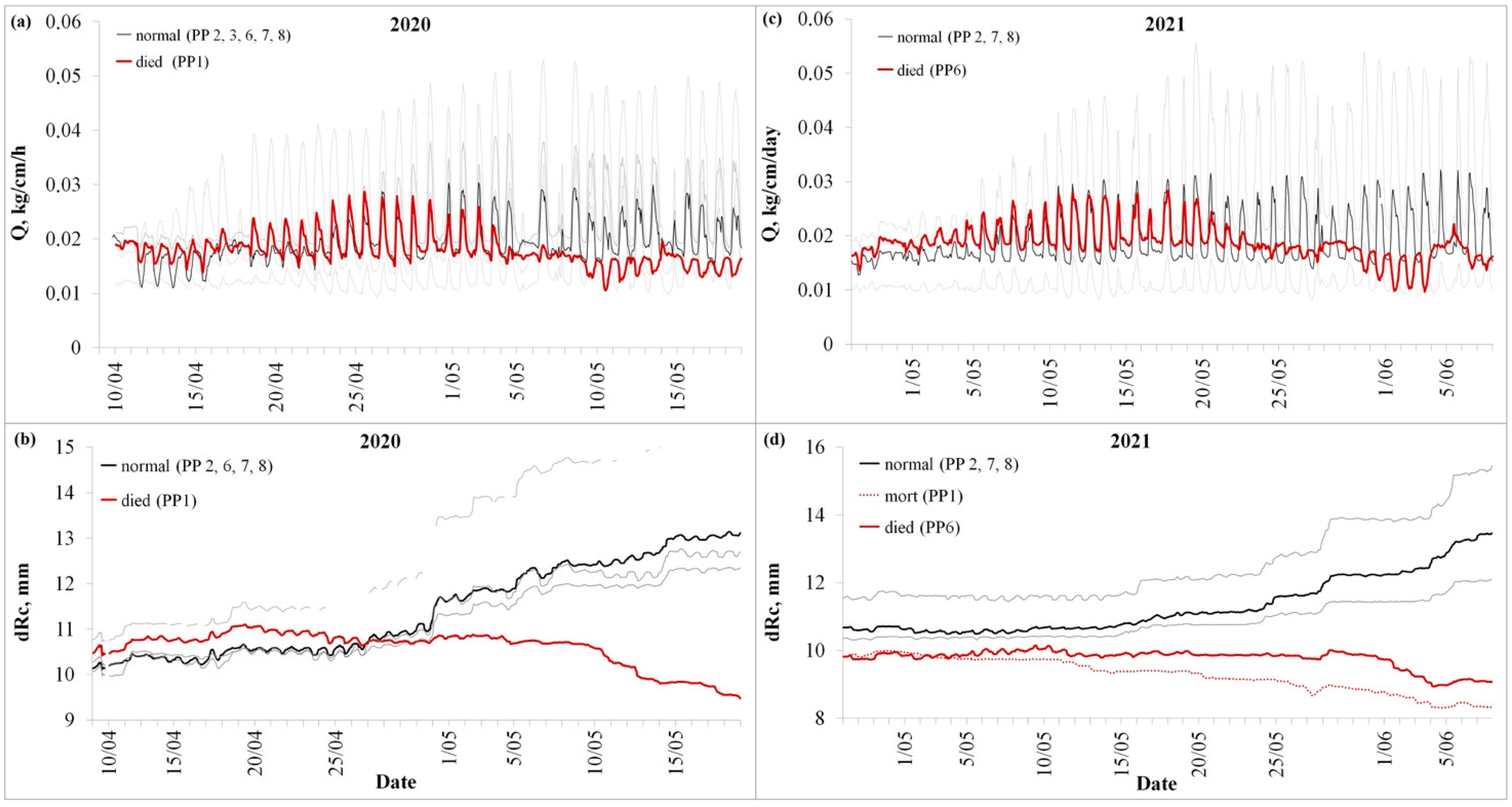
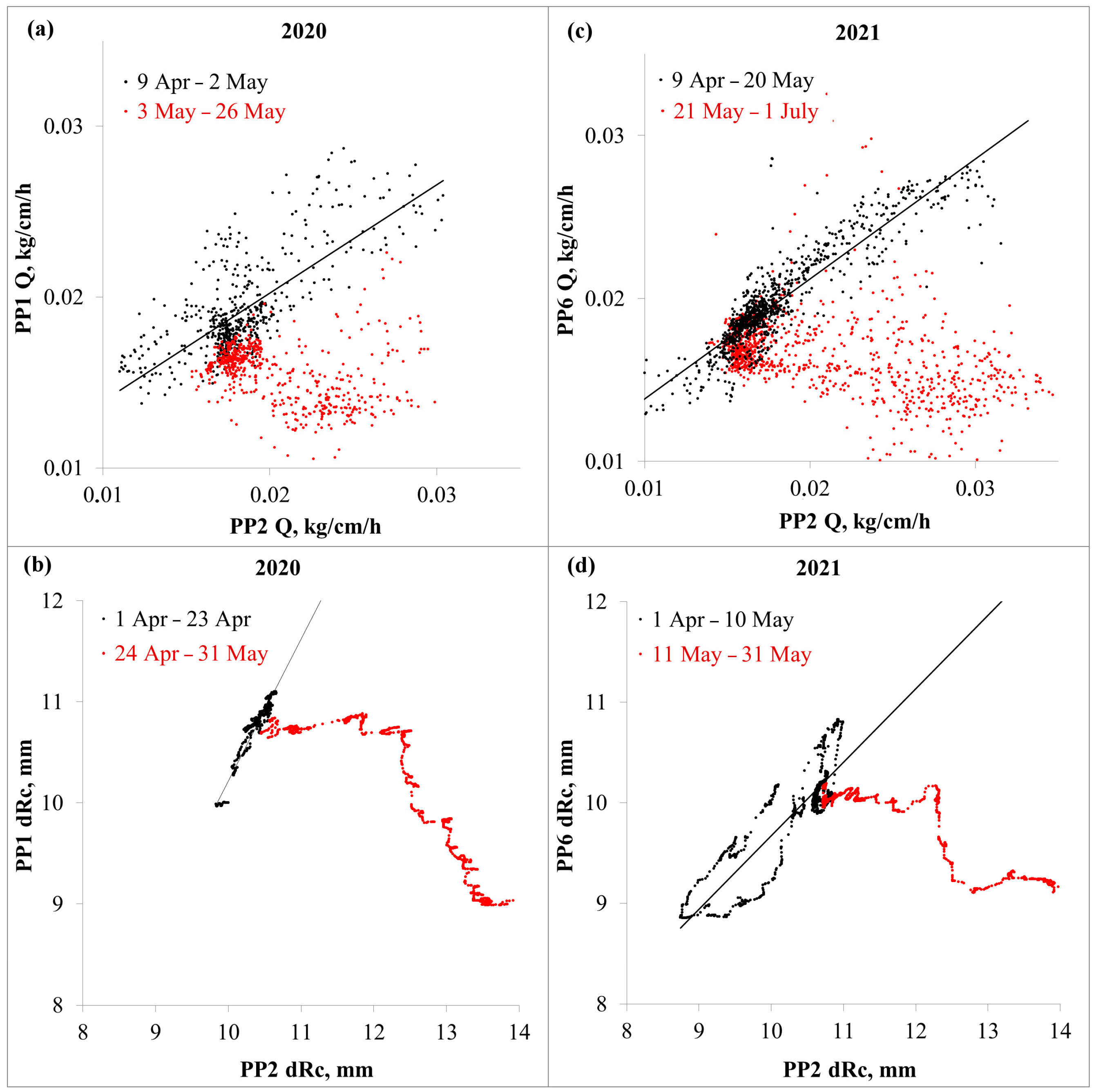
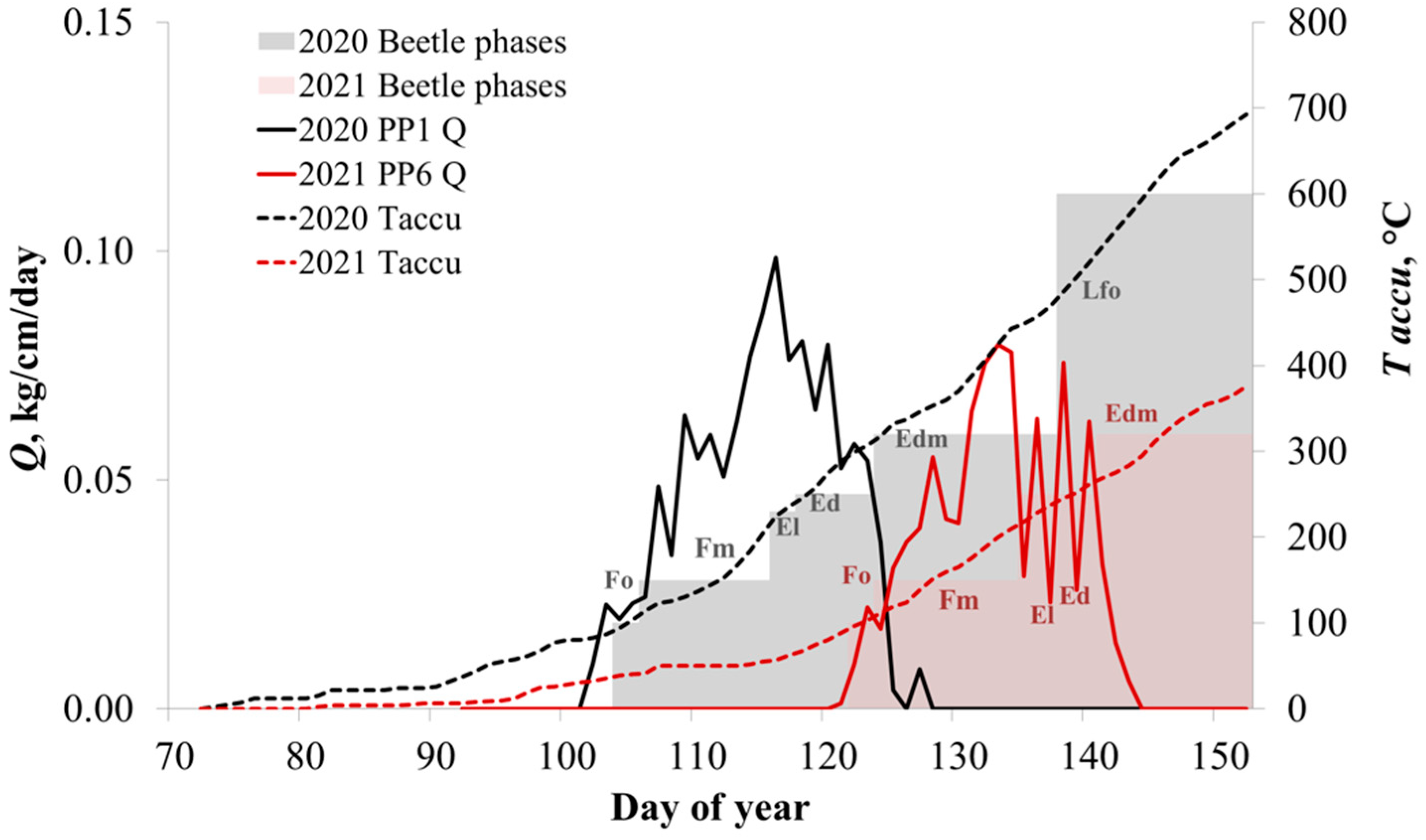
3. Results
3.1. Stem Sap Flow and Radial Size Changes
3.2. Development of Beetles and Fungi
3.3. Wood Formation and Climate Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.Z.; Schepaschenko, D.G. Boreal Forest Health and Global Change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Giles-Hansen, K.; Spencer, S.A.; Ge, X.; Onuchin, A.; Li, Q.; Burenina, T.; Ilintsev, A.; Hou, Y. Forest Harvesting and Hydrology in Boreal Forests: Under an Increased and Cumulative Disturbance Context. For. Ecol. Manag. 2022, 522, 120468. [Google Scholar] [CrossRef]
- Bytnerowicz, A.; Omasa, K.; Paoletti, E. Integrated Effects of Air Pollution and Climate Change on Forests: A Northern Hemisphere Perspective. Environ. Pollut. 2007, 147, 438–445. [Google Scholar] [CrossRef]
- Allen, C.D. Climate-Induced Forest Dieback: An Escalating Global Phenomenon? Unasylva 2009, 60, 43–49. [Google Scholar]
- Kharuk, V.I.; Im, S.T.; Ranson, K.J.; Yagunov, M.N. Climate-Induced Northerly Expansion of Siberian Silkmoth Range. Forests 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Kharuk, V.I.; Im, S.T.; Soldatov, V.V. Siberian Silkmoth Outbreaks Surpassed Geoclimatic Barrier in Siberian Mountains. J. Mt. Sci. 2020, 17, 1891–1900. [Google Scholar] [CrossRef]
- Pavlov, I.N.; Litovka, Y.A.; Golubev, D.V.; Astapenko, S.A.; Chromogin, P.V.; Usoltseva, Y.V.; Makolova, P.V.; Petrenko, S.M. Mass Reproduction of Polygraphus proximus Blandford in Fir Forests of Siberia Infected with Root and Stem Pathogens: Monitoring, Patterns, and Biological Control. Contemp. Probl. Ecol. 2020, 13, 71–84. [Google Scholar] [CrossRef]
- Krivets, S.A.; Bisirova, E.M.; Kerchev, I.A.; Pats, E.N.; Chernova, N.A. Transformation of Taiga Ecosystems in the Western Siberian Invasion Focus of Four-Eyed Fir Bark Beetle Polygraphus proximus Blandford (Coleoptera: Curculionidae, Scolytinae). Russ. J. Biol. Invasions 2015, 6, 94–108. [Google Scholar] [CrossRef]
- Pashenova, N.V.; Kononov, A.V.; Ustyantsev, K.V.; Blinov, A.G.; Pertsovaya, A.A.; Baranchikov, Y.N. Ophiostomatoid Fungi Associated with the Four-Eyed Fir Bark Beetle on the Territory of Russia. Russ. J. Biol. Invasions 2018, 9, 63–74. [Google Scholar] [CrossRef]
- Linnakoski, R.; de Beer, Z.W.; Ahtiainen, J.; Sidorov, E.; Niemelä, P.; Pappinen, A.; Wingfield, M.J. Ophiostoma Spp. Associated with Pine- and Spruce-Infesting Bark Beetles in Finland and Russia. Persoonia Mol. Phylogeny Evol. Fungi 2010, 25, 72–93. [Google Scholar] [CrossRef]
- Mandelshtam, M.Y.; Selikhovkin, A.V. Bark and Ambrosia Beetles (Coleoptera, Curculionidae: Scolytinae) of Northwest Russia: History of the Study, Composition and Genesis of the Fauna. Entomol. Rev. 2020, 100, 800–826. [Google Scholar] [CrossRef]
- Preisler, Y.; Tatarinov, F.; Grünzweig, J.M.; Yakir, D. Seeking the “Point of No Return” in the Sequence of Events Leading to Mortality of Mature Trees. Plant. Cell Environ. 2020, 44, 1315–1328. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Im, S.T.; Oskorbin, P.A.; Petrov, I.A.; Ranson, K.J. Siberian Pine Decline and Mortality in Southern Siberian Mountains. For. Ecol. Manag. 2013, 310, 312–320. [Google Scholar] [CrossRef]
- De Beer, Z.W.; Seifert, K.A.; Wingfield, M.J. A Nomenclator for Ophiostomatoid Genera and Species in the Ophiostomatales and Microascales. Biodivers. Ser. 2013, 12, 245–322. [Google Scholar]
- Kirisits, T. Fungal Associates of European Bark Beetles with Special Emphasis on the Ophiostomatoid Fungi. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Springer: Dordrecht, The Netherlands, 2007; pp. 181–236. [Google Scholar]
- Salle, A.; Ye, H.; Yart, A.; Lieutier, F. Seasonal Water Stress and the Resistance of Pinus yunnanensis to a Bark-Beetle-Associated Fungus. Tree Physiol. 2008, 28, 679–687. [Google Scholar] [CrossRef]
- Steppe, K.; von der Crone, J.S.; De Pauw, D.J.W. TreeWatch.Net: A Water and Carbon Monitoring and Modeling Network to Assess Instant Tree Hydraulics and Carbon Status. Front. Plant Sci. 2016, 7, 993. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, R.; Granda, V.; Molowny-Horas, R.; Mencuccini, M.; Steppe, K.; Martínez-Vilalta, J. SAPFLUXNET: Towards a Global Database of Sap Flow Measurements. Tree Physiol. 2016, 36, 1449–1455. [Google Scholar] [CrossRef]
- Zweifel, R.; Etzold, S.; Basler, D.; Bischoff, R.; Braun, S.; Buchmann, N.; Conedera, M.; Fonti, P.; Gessler, A.; Haeni, M.; et al. TreeNet–The Biological Drought and Growth Indicator Network. Front. For. Glob. Chang. 2021, 4, 776905. [Google Scholar] [CrossRef]
- Rubtsov, A.; Arzac, A.; Knorre, A.; Shashkin, A.; Benkova, V.; Vaganov, E. Stem Growth and Stem Sap Flow Measurements of Three Conifer Tree Species in Siberia. IOP Conf. Ser. Earth Environ. Sci. 2020, 611, 012028. [Google Scholar] [CrossRef]
- Biondi, F.; Hartsough, P.C.; Estrada, I.G. Daily Weather and Tree Growth at the Tropical Treeline of North America. Arctic Antarct. Alp. Res. 2005, 37, 16–24. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; McLaughlin, S.B.; Ayres, M.P. High-Resolution Analysis of Stem Increment and Sap Flow for Loblolly Pine Trees Attacked by Southern Pine Beetle. Can. J. For. Res. 2004, 34, 2387–2393. [Google Scholar] [CrossRef]
- Kiorapostolou, N.; Camarero, J.J.; Carrer, M.; Sterck, F.; Brigita, B.; Sangüesa-Barreda, G.; Petit, G. Scots Pine Trees React to Drought by Increasing Xylem and Phloem Conductivities. Tree Physiol. 2020, 40, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.; Rossi, S.; Campelo, F.; Nabais, C. Are Neighboring Trees in Tune? Wood Formation in Pinus Pinaster. Eur. J. For. Res. 2014, 133, 41–50. [Google Scholar] [CrossRef]
- Mäkinen, H.; Seo, J.-W.; Nöjd, P.; Schmitt, U.; Jalkanen, R. Seasonal Dynamics of Wood Formation: A Comparison between Pinning, Microcoring and Dendrometer Measurements. Eur. J. For. Res. 2008, 127, 235–245. [Google Scholar] [CrossRef]
- Cocozza, C.; Palombo, C.; Tognetti, R.; La Porta, N.; Anichini, M.; Giovannelli, A.; Emiliani, G. Monitoring Intra-Annual Dynamics of Wood Formation with Microcores and Dendrometers in Picea abies at Two Different Altitudes. Tree Physiol. 2016, 36, 832–846. [Google Scholar] [CrossRef]
- Ziaco, E.; Biondi, F.; Rossi, S.; Deslauriers, A. Environmental Drivers of Cambial Phenology in Great Basin Bristlecone Pine. Tree Physiol. 2016, 36, 818–831. [Google Scholar] [CrossRef]
- Urban, J.; Rubtsov, A.V.; Urban, A.V.; Shashkin, A.V.; Benkova, V.E. Canopy Transpiration of a Larix Sibirica and Pinus Sylvestris Forest in Central Siberia. Agric. For. Meteorol. 2019, 271, 64–72. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Zeitschrift 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Čermák, J.; Kučera, J.; Nadezhdina, N. Sap Flow Measurements with Some Thermodynamic Methods, Flow Integration within Trees and Scaling up from Sample Trees to Entire Forest Stands. Trees 2004, 18, 529–546. [Google Scholar] [CrossRef]
- Trcala, M.; Čermák, J. Improvement of the Trunk Heat Balance Method Including Measurement of Zero and Reverse Sap Flows. Agric. For. Meteorol. 2012, 166–167, 120–126. [Google Scholar] [CrossRef]
- Arzac, A.; Tabakova, M.A.; Khotcinskaia, K.; Koteneva, A.; Kirdyanov, A.V.; Olano, J.M. Linking Tree Growth and Intra-Annual Density Fluctuations to Climate in Suppressed and Dominant Pinus sylvestris L. Trees in the Forest-Steppe of Southern Siberia. Dendrochronologia 2021, 67, 125842. [Google Scholar] [CrossRef]
- Arzac, A.; Tychkov, I.; Rubtsov, A.; Tabakova, M.A.; Brezhnev, R.; Koshurnikova, N.; Knorre, A.; Büntgen, U. Phenological Shifts Compensate Warming-Induced Drought Stress in Southern Siberian Scots Pines. Eur. J. For. Res. 2021, 140, 1487–1498. [Google Scholar] [CrossRef]
- Grissino-Mayer, H.D. Evaluating Crossdating Accuracy: A Manual and Tutotial for the Computer Program COFECHA. Tree-Ring Res. 2001, 57, 205–221. [Google Scholar]
- Cook, E.R.; Holmes, R. Guide for Computer Program ARSTAN. In The International Tree-Ring Data Bank Program Library Version 2.0; Grissino-Mayer, H.D., Holmes, R.L., Fritts, H.C., Eds.; Laboratory of Tree-Ring Research: Tucson, AZ, USA, 1996; pp. 75–87. [Google Scholar]
- Thornthwaite, D.W. An Approach toward a Rational Classification of Climate. Geogr. Rev. 1948, 38, 55–94. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Menardi, R. Trephor: A New Tool for Sampling Microcores from Tree Stems. IAWA J. 2006, 27, 89–97. [Google Scholar] [CrossRef]
- Yakovenko, A.I. Phenological Features of Pine Shoot Beetles (Tomicus piniperda L. and T. Minor Hart.) at the Moscow Region. For. Bull. 2014, 18, 154–163. (In Russian) [Google Scholar]
- Jankowiak, R. Fungi Associated with Tomicus Minor on Pinus sylvestris in Poland and Their Succession into the Sapwood of Beetle-Infested Windblown Trees. Can. J. For. Res. 2008, 38, 2579–2588. [Google Scholar] [CrossRef]
- Borkowski, A. Spatial Distribution of Fallen Shoots of Scots Pine Pruned by Pine Shoot Beetles (Tomicus Spp.), and Evaluation of Methods of Shoot Collection in Central Poland. J. For. Res. 2007, 12, 358–364. [Google Scholar] [CrossRef]
- Solheim, H.; Krokene, P.; Långström, B. Effects of Growth and Virulence of Associated Blue-Stain Fungi on Host Colonization Behaviour of the Pine Shoot Beetles Tomicus Minor and T. Piniperda. Plant Pathol. 2001, 50, 111–116. [Google Scholar] [CrossRef]
- Krokene, P.; Solheim, H. Pathogenicity of Four Blue-Stain Fungi Associated with Aggressive and Nonaggressive Bark Beetles. Phytopathology 1998, 88, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; Zhu, X.; Yue, Y.; Xue, J.; Shi, L. How Permafrost Degradation Threatens Boreal Forest Growth on Its Southern Margin? Sci. Total Environ. 2021, 762, 143154. [Google Scholar] [CrossRef]
- Tabakova, M.; Arzac, A.; Martínez, E.; Kirdyanov, A. Climatic Factors Controlling Pinus sylvestris Radial Growth along a Transect of Increasing Continentality in Southern Siberia. Dendrochronologia 2020, 62, 125709. [Google Scholar] [CrossRef]
- Lieutier, F.; Yart, A.; Salle, A. Stimulation of Tree Defenses by Ophiostomatoid Fungi Can Explain Attack Success of Bark Beetles on Conifers. Ann. For. Sci. 2009, 66, 801. [Google Scholar] [CrossRef]
- Rosner, S.; Hannrup, B. Resin Canal Traits Relevant for Constitutive Resistance of Norway Spruce against Bark Beetles: Environmental and Genetic Variability. For. Ecol. Manag. 2004, 200, 77–87. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and Chemical Defenses of Conifer Bark against Bark Beetles and Other Pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef] [PubMed]
- Valor, T.; Hood, S.M.; Piqué, M.; Larrañaga, A.; Casals, P. Resin Ducts and Bark Thickness Influence Pine Resistance to Bark Beetles after Prescribed Fire. For. Ecol. Manag. 2021, 494, 119322. [Google Scholar] [CrossRef]
- DeRose, R.J.; Bekker, M.F.; Long, J.N. Traumatic Resin Ducts as Indicators of Bark Beetle Outbreaks. Can. J. For. Res. 2017, 47, 1168–1174. [Google Scholar] [CrossRef]
- Kane, J.M.; Kolb, T.E. Importance of Resin Ducts in Reducing Ponderosa Pine Mortality from Bark Beetle Attack. Oecologia 2010, 164, 601–609. [Google Scholar] [CrossRef]
- Ferrenberg, S.; Kane, J.M.; Mitton, J.B. Resin Duct Characteristics Associated with Tree Resistance to Bark Beetles across Lodgepole and Limber Pines. Oecologia 2014, 174, 1283–1292. [Google Scholar] [CrossRef]
- Heiniger, U.; Theile, F.; Rigling, A.; Rigling, D. Blue-Stain Infections in Roots, Stems and Branches of Declining Pinus sylvestris Trees in a Dry Inner Alpine Valley in Switzerland. For. Pathol. 2011, 41, 501–509. [Google Scholar] [CrossRef]
- Jaime, L.; Batllori, E.; Ferretti, M.; Lloret, F. Climatic and Stand Drivers of Forest Resistance to Recent Bark Beetle Disturbance in European Coniferous Forests. Glob. Chang. Biol. 2022, 28, 2830–2841. [Google Scholar] [CrossRef]
- Gaylord, M.L.; Kolb, T.E.; Pockman, W.T.; Plaut, J.A.; Yepez, E.A.; Macalady, A.K.; Pangle, R.E.; McDowell, N.G. Drought Predisposes Piñon–Juniper Woodlands to Insect Attacks and Mortality. New Phytol. 2013, 198, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Rubtsov, A.V.; Shashkin, A.V.; Benkova, V.E. Growth, Transpiration and Water Use Efficiency of Larix sibirica, Larix gmelinii and Pinus sylvestris Forest in Siberia. Acta Hortic. 2018, 1222, 125–131. [Google Scholar] [CrossRef]
- Lévesque, M.; Saurer, M.; Siegwolf, R.; Eilmann, B.; Brang, P.; Bugmann, H.; Rigling, A. Drought Response of Five Conifer Species under Contrasting Water Availability Suggests High Vulnerability of Norway Spruce and European Larch. Glob. Chang. Biol. 2013, 19, 3184–3199. [Google Scholar] [CrossRef] [PubMed]
- Guada, G.; Camarero, J.J.; Sánchez-Salguero, R.; Cerrillo, R.M.N. Limited Growth Recovery after Drought-Induced Forest Dieback in Very Defoliated Trees of Two Pine Species. Front. Plant Sci. 2016, 7, 418. [Google Scholar] [CrossRef] [PubMed]
- Bakke, A. Ecological Studies on Bark Beetles (Coleoptera: Scolytidae) Associated with Scots Pine (Pinus sylvestris L.) in Norway with Particular Reference to Influence of Temperature. Meddelelser Nor. Skogforsøksves. 1968, 21, 443–602. [Google Scholar]
- Lieutier, F.; Långström, B.; Faccoli, M. The Genus Tomicus. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstette, R.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 371–426. ISBN 9780124171732. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barchenkov, A.; Rubtsov, A.; Safronova, I.; Astapenko, S.; Tabakova, K.; Bogdanova, K.; Anuev, E.; Arzac, A. Features of Scots Pine Mortality Due to Incursion of Pine Bark Beetles in Symbiosis with Ophiostomatoid Fungi in the Forest-Steppe of Central Siberia. Forests 2023, 14, 1301. https://doi.org/10.3390/f14071301
Barchenkov A, Rubtsov A, Safronova I, Astapenko S, Tabakova K, Bogdanova K, Anuev E, Arzac A. Features of Scots Pine Mortality Due to Incursion of Pine Bark Beetles in Symbiosis with Ophiostomatoid Fungi in the Forest-Steppe of Central Siberia. Forests. 2023; 14(7):1301. https://doi.org/10.3390/f14071301
Chicago/Turabian StyleBarchenkov, Alexey, Alexey Rubtsov, Inna Safronova, Sergey Astapenko, Kseniia Tabakova, Kristina Bogdanova, Eugene Anuev, and Alberto Arzac. 2023. "Features of Scots Pine Mortality Due to Incursion of Pine Bark Beetles in Symbiosis with Ophiostomatoid Fungi in the Forest-Steppe of Central Siberia" Forests 14, no. 7: 1301. https://doi.org/10.3390/f14071301
APA StyleBarchenkov, A., Rubtsov, A., Safronova, I., Astapenko, S., Tabakova, K., Bogdanova, K., Anuev, E., & Arzac, A. (2023). Features of Scots Pine Mortality Due to Incursion of Pine Bark Beetles in Symbiosis with Ophiostomatoid Fungi in the Forest-Steppe of Central Siberia. Forests, 14(7), 1301. https://doi.org/10.3390/f14071301





