Radial Growth–Climate Relationship Varies with Spatial Distribution of Schima superba Stands in Southeast China’s Subtropical Forests
Abstract
1. Introduction
2. Materials and Methods
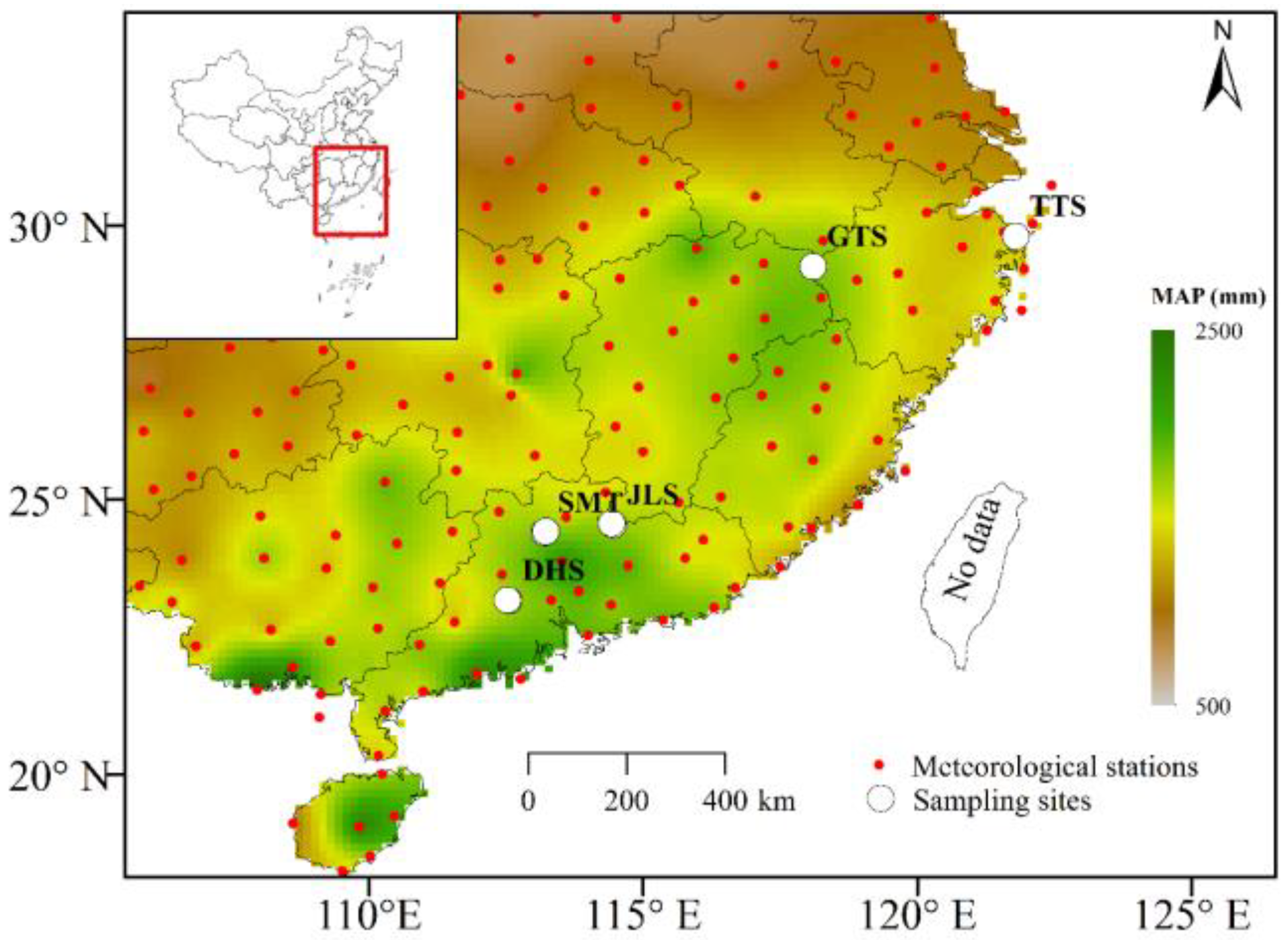
2.1. Study Area
2.2. Field Sampling of Tree-Ring Cores
2.3. Building of Site-Specific Tree-Ring Width Residual Chronologies
2.4. Site-Specific Climate Data
2.5. Statistical Analysis
3. Results
3.1. Statistics of Tree-Ring Width Series and Residual Chronologies
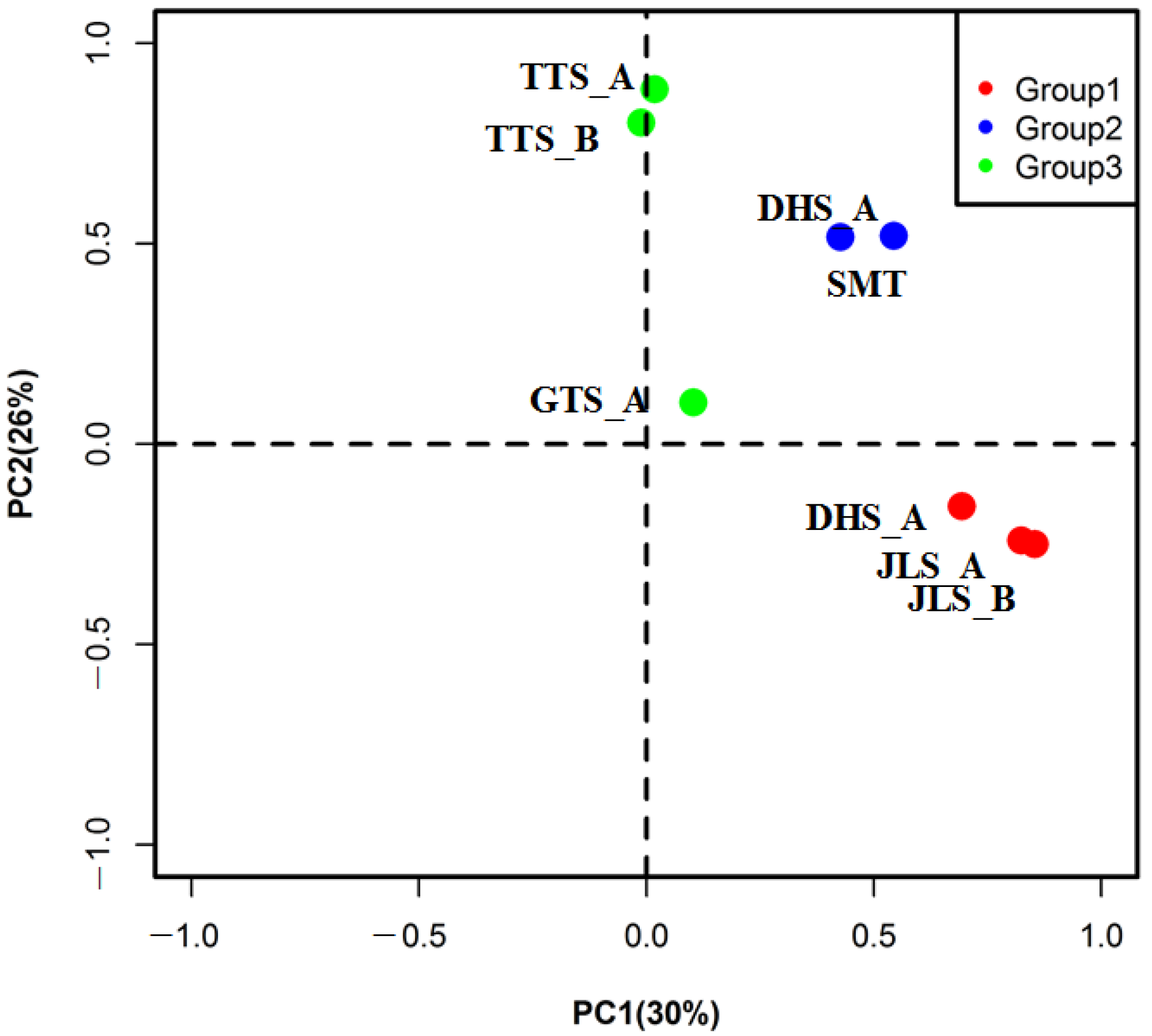
3.2. Spatial Difference and Grouping in Radial Growth
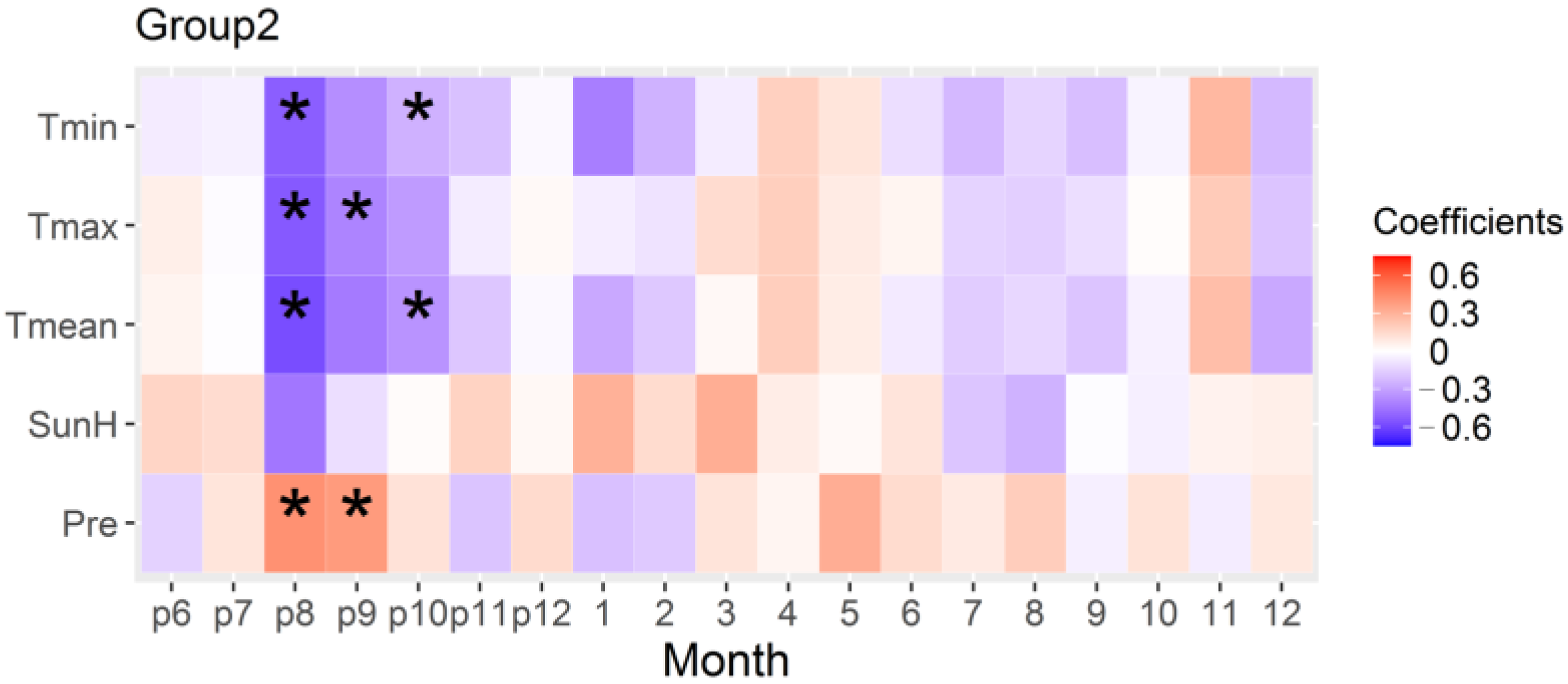
3.3. Relationship of Radial Growth of High-Elevation Stands in the Southern Margin and Climate
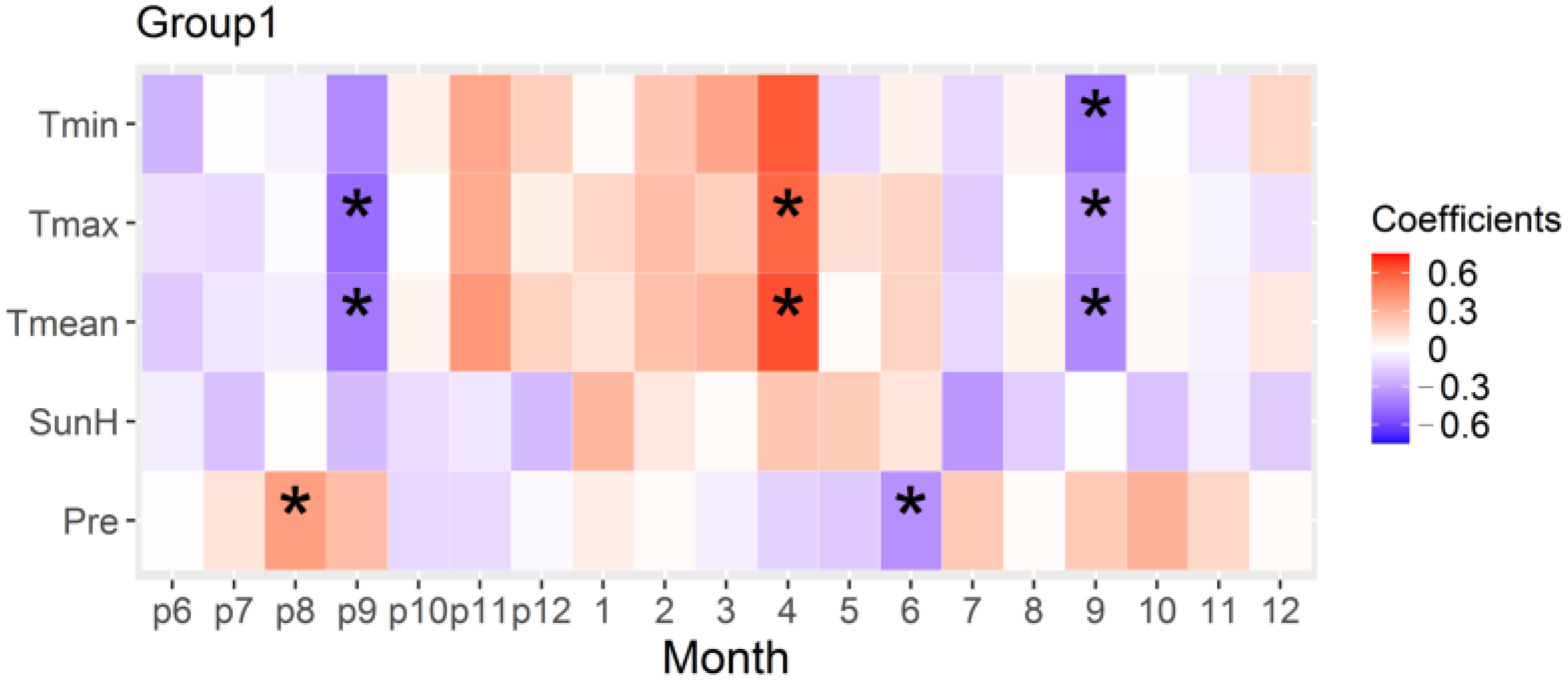
3.4. Relationship of Radial Growth of Low-Elevation Stands in the Southern Margin and Climate
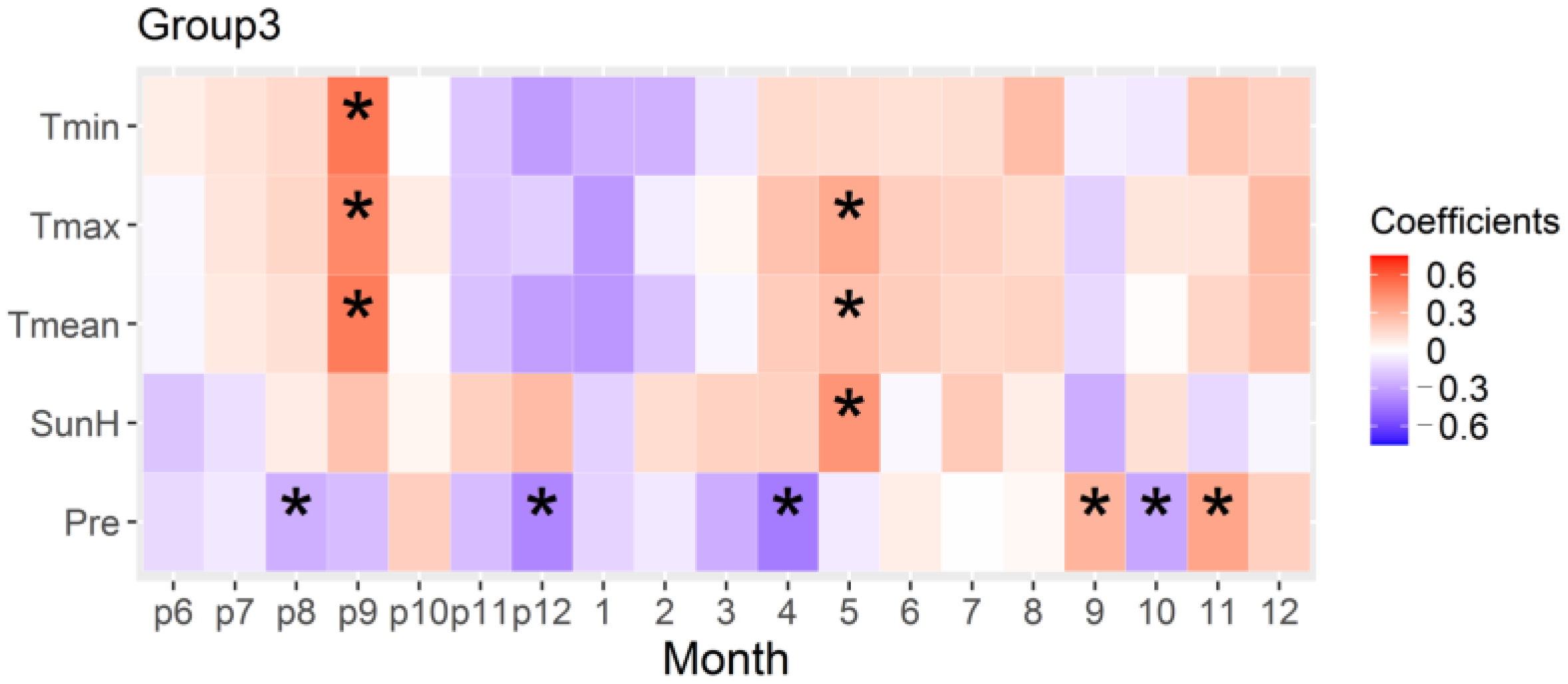
3.5. Relationship of Radial Growth of Stands in the Northern Margin and Climate
4. Discussion
4.1. The Positive Effect of Warm Spring on the Radial Growth of Stands in the Northern Margin and High-Elevation Stands in the Southern Margin
4.2. The Negative Effect of High Temperatures in the Late Rainy Season on the Radial Growth of Stands in the Southern Margin
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Site | Latitude | Longitude | Elevation | Pre | Tmean | Tmax | Tmin | SunH |
|---|---|---|---|---|---|---|---|---|
| DHS_A | 23.16 | 112.55 | 77 | 1653.28 | 22.15 | 26.49 | 19.06 | 1619.18 |
| DHS_B | 23.17 | 112.53 | 577 | 1649.15 | 19.13 | 23.49 | 16.02 | 1618.95 |
| SMT_A | 24.40 | 113.20 | 244 | 1775.58 | 19.59 | 24.53 | 16.22 | 1581.64 |
| SMT_B | 24.42 | 113.23 | 225 | 1769.07 | 19.69 | 24.62 | 16.33 | 1581.96 |
| JLS-A | 24.57 | 114.43 | 490 | 1666.01 | 18.46 | 23.65 | 14.94 | 1599.04 |
| JLS_B | 24.54 | 114.46 | 500 | 1674.18 | 18.45 | 23.66 | 14.91 | 1600.68 |
| GTS_A | 29.25 | 118.10 | 471 | 1802.69 | 15.27 | 20.39 | 11.49 | 1676.38 |
| TTS_A | 29.80 | 121.79 | 83 | 1432.84 | 16.92 | 21.08 | 13.83 | 1761.66 |
| TTS_B | 29.80 | 121.79 | 83 | 1432.84 | 16.92 | 21.08 | 13.83 | 1761.66 |
| Site | Latitude | Longitude | Elevation | Tmax | Tmin | Tmean | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 7 | 9 | 1 | 4 | 7 | 9 | 1 | 4 | 7 | 9 | ||||
| DHS_A | 23.16 | 112.55 | 77 | 17.94 | 26.01 | 33.11 | 31.45 | 10.77 | 19.57 | 25.46 | 23.97 | 13.74 | 22.26 | 28.61 | 27.14 |
| DHS_B | 23.17 | 112.53 | 577 | 14.93 | 23.01 | 30.12 | 28.46 | 7.73 | 16.55 | 22.44 | 20.93 | 10.71 | 19.25 | 25.59 | 24.11 |
| SMT_A | 24.40 | 113.20 | 244 | 14.46 | 23.97 | 32.68 | 30.23 | 6.77 | 17.02 | 24.15 | 21.50 | 9.79 | 19.94 | 27.60 | 25.04 |
| SMT_B | 24.42 | 113.23 | 225 | 14.53 | 24.07 | 32.79 | 30.33 | 6.85 | 17.12 | 24.28 | 21.61 | 9.87 | 20.04 | 27.73 | 25.16 |
| JLS_A | 24.57 | 114.43 | 490 | 14.15 | 23.17 | 31.68 | 29.01 | 5.59 | 15.73 | 22.57 | 20.23 | 8.96 | 18.83 | 26.25 | 23.78 |
| JLS_B | 24.54 | 114.46 | 500 | 14.24 | 23.18 | 31.62 | 28.98 | 5.59 | 15.72 | 22.49 | 20.19 | 9.00 | 18.82 | 26.18 | 23.74 |
| GTS_A | 29.25 | 118.10 | 471 | 7.49 | 20.41 | 31.79 | 27.37 | 0.22 | 11.12 | 22.62 | 18.30 | 3.21 | 15.09 | 26.71 | 22.16 |
| TTS_A | 29.80 | 121.79 | 83 | 9.38 | 19.97 | 32.42 | 27.83 | 2.90 | 11.74 | 24.94 | 21.33 | 5.66 | 15.27 | 27.98 | 24.13 |
| TTS_B | 29.80 | 121.79 | 83 | 9.38 | 19.97 | 32.42 | 27.83 | 2.90 | 11.74 | 24.94 | 21.33 | 5.66 | 15.27 | 27.98 | 24.13 |
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. Large and persistent carbon sin in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sleen, P.; Groenendijk, P.; Vlam, M.; Anten, N.P.; Boom, A.; Bongers, F.; Pons, T.L.; Terburg, G.; Zuidema, P.A. No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat. Geosci. 2015, 8, 24–28. [Google Scholar] [CrossRef]
- Groenendijk, P.; van der Sleen, P.; Vlam, M.; Bunyavejchewin, S.; Bongers, F.; Zuidema, P.A. No evidence for consistent long-term growth stimulation of 13 tropical tree species: Results from tree-ring analysis. Glob. Change Biol. 2015, 21, 3762–3776. [Google Scholar] [CrossRef]
- Cabon, A.; Kannenberg, S.A.; Arain, A.; Babst, F.; Baldocchi, D.; Belmecheri, S.; Delpierre, N.; Guerrieri, R.; Maxwell, J.T.; McKenzie, S.; et al. Cross-biome synthesis of source versus sink limits to tree growth. Science 2022, 376, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Chen, Z.; Piao, S.; Peng, C.; Ciais, P.; Wang, Q.; Li, X.; Zhu, X. High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsoon region. Proc. Natl. Acad. Sci. USA 2014, 111, 4910–4915. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Z.; Chen, D.; Zhao, S.; Zhou, F.; Cao, X.; Fang, K. Growth decline of Pinus Massoniana in response to warming induced drought and increasing intrinsic water use efficiency in humid subtropical China. Dendrochronologia 2019, 57, 125609. [Google Scholar] [CrossRef]
- Wu, G.; Liu, H.; Hua, L.; Luo, Q.; Lin, Y.; He, P.; Feng, S.; Liu, J.; Ye, Q. Differential responses of stomata and photosynthesis to elevated temperature in two co-occurring subtropical forest tree species. Front. Plant Sci. 2018, 9, 467. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Peng, C.; Li, Y.; Liu, S.; Zhang, Q.; Tang, X.; Liu, J.; Yan, J.; Zhang, D.; Chu, G. A climate change-induced threat to the ecological resilience of a subtropical monsoon evergreen broad-leaved forest in Southern China. Glob. Change Biol. 2013, 19, 1197–1210. [Google Scholar] [CrossRef]
- Zhou, G.; Houlton, B.Z.; Wang, W.; Huang, W.; Xiao, Y.; Zhang, Q.; Liu, S.; Cao, M.; Wang, X.; Wang, S.; et al. Substantial reorganization of China’s tropical and subtropical forests: Based on the permanent plots. Glob. Change Biol. 2014, 20, 240–250. [Google Scholar] [CrossRef]
- Shi, J.; Cook, E.R.; Lu, H.; Li, J.; Wright, W.E.; Li, S. Tree-ring based winter temperature reconstruction for the lower reaches of the Yangtze River in southeast China. Clim. Res. 2010, 41, 169–175. [Google Scholar] [CrossRef]
- He, D.; Jiang, M.X.; Wei, X.Z. A dendroclimatic investigation of radial growth–climate relationships for the riparian species Cercidiphyllum japonicum in the Shennongjia area, central China. Trees 2012, 26, 503–512. [Google Scholar] [CrossRef]
- Zheng, Y.; Shao, X.; Lu, F.; Li, Y. February–May temperature reconstruction based on tree-ring widths of Abies fargesii from the Shennongjia area in central China. Int. J. Biometeorol. 2016, 60, 1175–1181. [Google Scholar] [CrossRef]
- Wang, B.; Gao, P. Relationship of tree ring width of Cinnamomum camphora with climate factors in Southern China. Afr. J. Agric. Res. 2012, 7, 2297–2303. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.; Wei, W.; Yu, S.; Wang, H. Tree-ring response of subtropical tree species in southeast China on regional climate and sea-surface temperature variations. Trees 2015, 29, 17–24. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, Q.B.; Lv, L.; Zhang, C. Regional-scale winter-spring temperature variability and chilling damage dynamics over the past two centuries in southeastern China. Clim. Dynam. 2012, 39, 919–928. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.J.; Wei, W.S.; Yu, S.L.; Zhang, T.W. Tree ring-based winter temperature reconstruction for Changting, Fujian, subtropical region of Southeast China, since 1850: Linkages to the Pacific Ocean. Theor. Appl. Climatol. 2012, 109, 141–151. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.J.; Yu, S.L.; Zhang, T.W. Influence of climate warming and resin collection on the growth of Masson pine (Pinus massoniana) in a subtropical forest, southern China. Trees 2015, 29, 1423–1430. [Google Scholar] [CrossRef]
- Cai, Q.; Liu, Y.; Wang, Y.; Ma, Y.; Liu, H. Recent warming evidence inferred from a tree-ring-based winter-half year minimum temperature reconstruction in northwestern Yichang, South Central China, and its relation to the large-scale circulation anomalies. Int. J. Biometeorol. 2016, 60, 1885–1896. [Google Scholar] [CrossRef]
- Yuan, S.; Zheng, Y.; Wu, S.; Xu, P.; Zhang, F.; Kong, F.; Qi, Y.; Wang, D. March SST reconstruction in the South China Sea based on Pinus massoniana tree-ring widths from Changting, Fujian, in Southeast China since 1893 CE. Mar. Micropaleontol. 2018, 145, 21–27. [Google Scholar] [CrossRef]
- Wang, L.; Fang, K.; Chen, D.; Dong, Z.; Zhou, F.; Li, Y.; Zhang, P.; Ou, T.; Guo, G.; Cao, X.; et al. Intensified variability of the El Niño-Southern Oscillation enhances its modulations on tree growths in southeastern China over the past 218 years. Int. J. Climatol. 2018, 38, 5293–5304. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.J.; Wei, W.S.; Yu, S.L.; Zhang, T.W. Reconstructed temperature for Yong’an, Fujian, Southeast China: Linkages to the Pacific Ocean climate variability. Glob. Planet. Change 2012, 86, 11–19. [Google Scholar] [CrossRef]
- Cai, Q.; Liu, Y.; Liu, H.; Sun, C.; Wang, Y. Growing-season precipitation since 1872 in the coastal area of subtropical southeast China reconstructed from tree rings and its relationship with the East Asian summer monsoon system. Ecol. Indic. 2017, 82, 441–450. [Google Scholar] [CrossRef]
- Su, H.; Axmacher, J.C.; Yang, B.; Sang, W. Differential radial growth response of three coexisting dominant tree species to local and large-scale climate variability in a subtropical evergreen broad-leaved forest of China. Ecol. Res. 2015, 30, 745–754. [Google Scholar] [CrossRef]
- Liang, H.; Huang, J.G.; Ma, Q.; Li, J.; Wang, Z.; Guo, X.; Zhu, H.; Jiang, S.; Zhou, P.; Yu, B.; et al. Contributions of competition and climate on radial growth of Pinus massoniana in subtropics of China. Agric. For. Meteorol. 2019, 274, 7–17. [Google Scholar] [CrossRef]
- Wagner, F.; Rossi, V.; Aubry-Kientz, M.; Bonal, D.; Dalitz, H.; Gliniars, R.; Stahl, C.; Trabucco, A.; Hérault, B. Pan-tropical analysis of climate effects on seasonal tree growth. PLoS ONE 2014, 9, e92337. [Google Scholar] [CrossRef]
- Vlam, M.; Baker, P.J.; Bunyavejchewin, S.; Zuidema, P.A. Temperature and rainfall strongly drive temporal growth variation in Asian tropical forest trees. Oecologia 2014, 174, 1449–1461. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, M.; Braeuning, A. Tree radial growth is projected to decline in South Asian moist forest trees under climate change. Glob. Planet. Change 2018, 170, 106–119. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, M.; Gebrekirstos, A.; Bräuning, A. Trends in tree growth and intrinsic water-use efficiency in the tropics under elevated CO2 and climate change. Trees 2019, 33, 623–640. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.G.; Tardif, J.C.; Liang, H.; Jiang, S.; Zhu, H.; Zhou, P. Spatially heterogeneous responses of tree radial growth to recent El Niño southern-oscillation variability across East Asia subtropical forests. Agric. For. Meteorol. 2020, 287, 107939. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Wigley, T.M.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Appl. Meteorol. Clim. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Pebesma, E.J. Multivariable geostatistics in S: The gstat package. Comput. Geosci. 2004, 30, 683–691. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. Treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Zeng, A.; Zhou, F.; Li, W.; Bai, Y.; Zeng, C. Tree-ring indicators of winter-spring temperature in Central China over the past 200 years. Dendrochronologia 2019, 58, 125634. [Google Scholar] [CrossRef]
- Liu, S.; Gao, P.; Liu, P.; Niu, X.; Wang, B. The Response of Chinese Fir Forest Tree Ring Growth to Climate Change in China’s Dagangshan Region. Pol. J. Environ. Stud. 2019, 28, 2371–2379. [Google Scholar]
- Li, Y.; Liu, J.; Zhou, G.; Huang, W.; Duan, H. Warming effects on photosynthesis of subtropical tree species: A translocation experiment along an altitudinal gradient. Sci. Rep. 2016, 6, 24895. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Li, Y.; Wu, T.; Zhou, G.; Liu, S.; Meng, Y.; Wang, J.; Ling, L.; Liu, J. Warming effects on morphological and physiological performances of four subtropical montane tree species. Ann. For. Sci. 2020, 77, 2. [Google Scholar] [CrossRef]
- Keel, S.G.; Schädel, C. Expanding leaves of mature deciduous forest trees rapidly become autotrophic. Tree Physiol. 2010, 30, 1253–1259. [Google Scholar] [CrossRef]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Souto-Herrero, M.; Rozas, V.; Garcia-Gonzalez, I. Chronologies of earlywood vessels and latewood width disentangle climate drivers of oak growth in a mild oceanic region. Dendrochronologia 2018, 51, 40–53. [Google Scholar] [CrossRef]
- Olson, M.E.; Anfodillo, T.; Rosell, J.A.; Martínez-Méndez, N. Across climates and species, higher vapour pressure deficit is associated with wider vessels for plants of the same height. Plant Cell Environ. 2020, 43, 3068–3080. [Google Scholar] [CrossRef]
- Eilmann, B.; Zweifel, R.; Buchmann, N.; Fonti, P.; Rigling, A. Drought-induced adaptation of the xylem in Scots pine and pubescent oak. Tree Physiol. 2009, 29, 1011–1020. [Google Scholar] [CrossRef]
- Camarero, J.J.; Collado, E.; Martínez-de-Aragón, J.; De-Miguel, S.; Büntgen, U.; Martinez-Peña, F.; Martín-Pinto, P.; Ohenoja, E.; Romppanen, T.; Salo, K.; et al. Associations between climate and earlywood and latewood width in boreal and Mediterranean Scots pine forests. Trees 2021, 35, 155–169. [Google Scholar] [CrossRef]
- Rosas, T.; Mencuccini, M.; Barba, J.; Cochard, H.; Saura-Mas, S.; Martínez-Vilalta, J. Adjustments and coordination of hydraulic, leaf and stem traits along a water availability gradient. New Phytol. 2019, 223, 632–646. [Google Scholar] [CrossRef]
- Trugman, A.T.; Anderegg, L.D.L.; Sperry, J.S.; Wang, Y.; Venturas, M.; Anderegg, W.R.L. Leveraging plant hydraulics to yield predictive and dynamic plant leaf allocation in vegetation models with climate change. Glob. Change Biol. 2019, 25, 4008–4021. [Google Scholar] [CrossRef]
- Trugman, A.T.; Anderegg, L.D.L.; Wolfe, B.T.; Birami, B.; Ruehr, N.K.; Detto, M.; Bartlett, M.K.; Anderegg, W.R.L. Climate and plant trait strategies determine tree carbon allocation to leaves and mediate future forest productivity. Glob. Change Biol. 2019, 25, 3395–3405. [Google Scholar] [CrossRef]
- Way, D.A.; Oren, R. Differential responses to changes in growth temperature between trees from different functional groups and biomes: A review and synthesis of data. Tree Physiol. 2010, 30, 669–688. [Google Scholar] [CrossRef]
- Liang, J.; Xia, J.; Liu, L.; Wan, S. Global patterns of the responses of leaf-level photosynthesis and respiration in terrestrial plants to experimental warming. J. Plant Ecol. 2013, 6, 437–447. [Google Scholar] [CrossRef]
- Deslauriers, A.; Huang, J.G.; Balducci, L.; Beaulieu, M.; Rossi, S. The contribution of carbon and water in modulating wood formation in black spruce saplings. Plant Physiol. 2016, 170, 2072–2084. [Google Scholar] [CrossRef]
- Niez, B.; Dlouha, J.; Moulia, B.; Badel, E. Water-stressed or not, the mechanical acclimation is a priority requirement for trees. Trees 2019, 33, 279–291. [Google Scholar] [CrossRef]
- Sperry, J.S.; Alder, N.N.; Eastlack, S.E. The Effect of Reduced Hydraulic Conductance on Stomatal Conductance and Xylem Cavitation. J. Exp. Bot. 1993, 44, 1075–1082. [Google Scholar] [CrossRef]
- Nardini, A.; Salleo, S. Limitation of stomatal conductance by hydraulic traits: Sensing or preventing xylem cavitation? Trees 2000, 15, 14–24. [Google Scholar] [CrossRef]
- Salleo, S.; Nardini, A.; Pitt, F.; Gullo, M.A.L. Xylem cavitation and hydraulic control of stomatal conductance in Laurel (Laurus nobilis L.). Plant Cell Environ. 2000, 23, 71–79. [Google Scholar] [CrossRef]
- Qu, L.; De Boeck, H.J.; Fan, H.; Dong, G.; Chen, J.; Xu, W.; Ge, Z.; Huang, Z.; Shao, C.; Hu, Y. Diverging responses of two subtropical tree species (Schima superba and Cunninghamia lanceolata) to heat waves. Forests 2020, 11, 513. [Google Scholar] [CrossRef]
- Keel, S.G.; Siegwolf, R.; Krner, C. Canopy CO2 enrichment permits tracing the fate of recently assimilated carbon in a mature deciduous forest. New Phytol. 2006, 172, 319–329. [Google Scholar] [CrossRef]
- Kuptz, D.; Fleischmann, F.; Matyssek, R.; Grams, T.E. Seasonal patterns of carbon allocation to respiratory pools in 60-yr-old deciduous (Fagus sylvatica) and evergreen (Picea abies) trees assessed via whole-tree stable carbon isotope labeling. New Phytol. 2011, 191, 160–172. [Google Scholar] [CrossRef]
- Zhao, Z.; Fang, K.; Cao, C.; Chen, D.; Liang, X.; Dong, Z.; Zhang, P. Responses of the radial growth of the endangered species Keteleeria fortunei to climate change in southeastern China. Trees 2019, 33, 977–985. [Google Scholar] [CrossRef]
- Zheng, Z.; Fang, K.; Chen, Y.; Dong, Z.; Zhou, F.; Li, Y. Is the Pinus massoniana Lamb. Tree-Ring Latewood Formation Influenced by the Diurnal Temperature Range in Humid Subtropical China? Forests 2022, 13, 1439. [Google Scholar] [CrossRef]
| Site | DHS | SMT | JLS | GTS | TTS | |||
|---|---|---|---|---|---|---|---|---|
| Sampling Stand | A | B | AB | A | B | A | A | B |
| Location | Southern Margin | Northern Margin | ||||||
| Latitude (°N) | 23.16 | 23.17 | 24.40 | 24.57 | 24.54 | 29.25 | 29.80 | 29.80 |
| Longitude (°E) | 112.55 | 112.53 | 113.20 | 114.43 | 114.46 | 118.10 | 121.79 | 121.79 |
| Elevation (m) | 77 | 577 | 225,244 | 490 | 500 | 471 | 83 | 83 |
| Statistics of tree-ring width series | ||||||||
| Sample size (trees/core) | 19/37 | 18/36 | 36/65 | 19/37 | 16/30 | 13/24 | 16/32 | 10/20 |
| Start year with SSS > 0.85 | 1977 | 1983 | 1988 | 1975 | 1980 | 1964 | 1964 | 1963 |
| End year | 2013 | 2013 | 2015 | 2016 | 2016 | 2016 | 2016 | 2016 |
| Mean length of series | 32.5 | 28.5 | 25.2 | 39.4 | 33.3 | 52.6 | 49.2 | 53.3 |
| Correlation with master series | 0.498 | 0.611 | 0.534 | 0.573 | 0.567 | 0.440 | 0.479 | 0.423 |
| Mean sensitivity | 0.463 | 0.327 | 0.246 | 0.264 | 0.266 | 0.228 | 0.224 | 0.273 |
| Common period analysis of residual chronology over 1988–2013 | ||||||||
| Sample size (trees/core) | 16/29 | 15/29 | 13/17 | 19/37 | 16/27 | 13/24 | 16/32 | 10/19 |
| PC1% | 36.3 | 40.8 | 32 | 39 | 32.5 | 27.9 | 32.5 | 28.2 |
| rbar | 0.278 | 0.351 | 0.252 | 0.336 | 0.274 | 0.208 | 0.270 | 0.236 |
| SNR | 11.147 | 15.689 | 5.736 | 18.717 | 10.208 | 6.301 | 11.830 | 6.185 |
| EPS | 0.918 | 0.940 | 0.852 | 0.949 | 0.911 | 0.863 | 0.922 | 0.861 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Guo, X.; Zhao, P.; Liang, H. Radial Growth–Climate Relationship Varies with Spatial Distribution of Schima superba Stands in Southeast China’s Subtropical Forests. Forests 2023, 14, 1291. https://doi.org/10.3390/f14071291
Jiang S, Guo X, Zhao P, Liang H. Radial Growth–Climate Relationship Varies with Spatial Distribution of Schima superba Stands in Southeast China’s Subtropical Forests. Forests. 2023; 14(7):1291. https://doi.org/10.3390/f14071291
Chicago/Turabian StyleJiang, Shaowei, Xiali Guo, Ping Zhao, and Hanxue Liang. 2023. "Radial Growth–Climate Relationship Varies with Spatial Distribution of Schima superba Stands in Southeast China’s Subtropical Forests" Forests 14, no. 7: 1291. https://doi.org/10.3390/f14071291
APA StyleJiang, S., Guo, X., Zhao, P., & Liang, H. (2023). Radial Growth–Climate Relationship Varies with Spatial Distribution of Schima superba Stands in Southeast China’s Subtropical Forests. Forests, 14(7), 1291. https://doi.org/10.3390/f14071291






