Identification of Eight Pterocarpus Species and Two Dalbergia Species Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging (HSI)
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Equipment and Spectra Acquisition
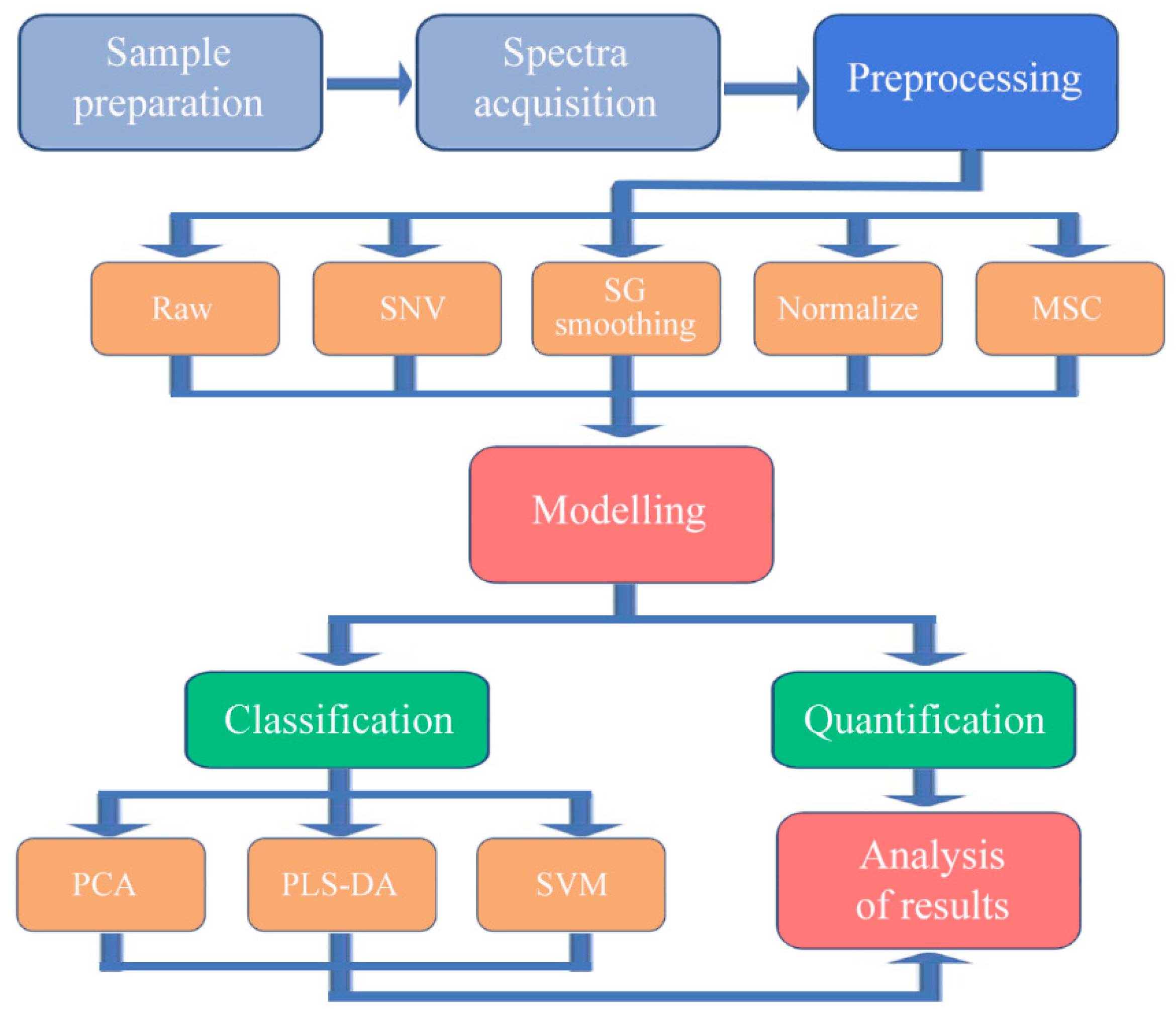
2.3. Model Development
3. Results
3.1. Spectroscopic Characterization
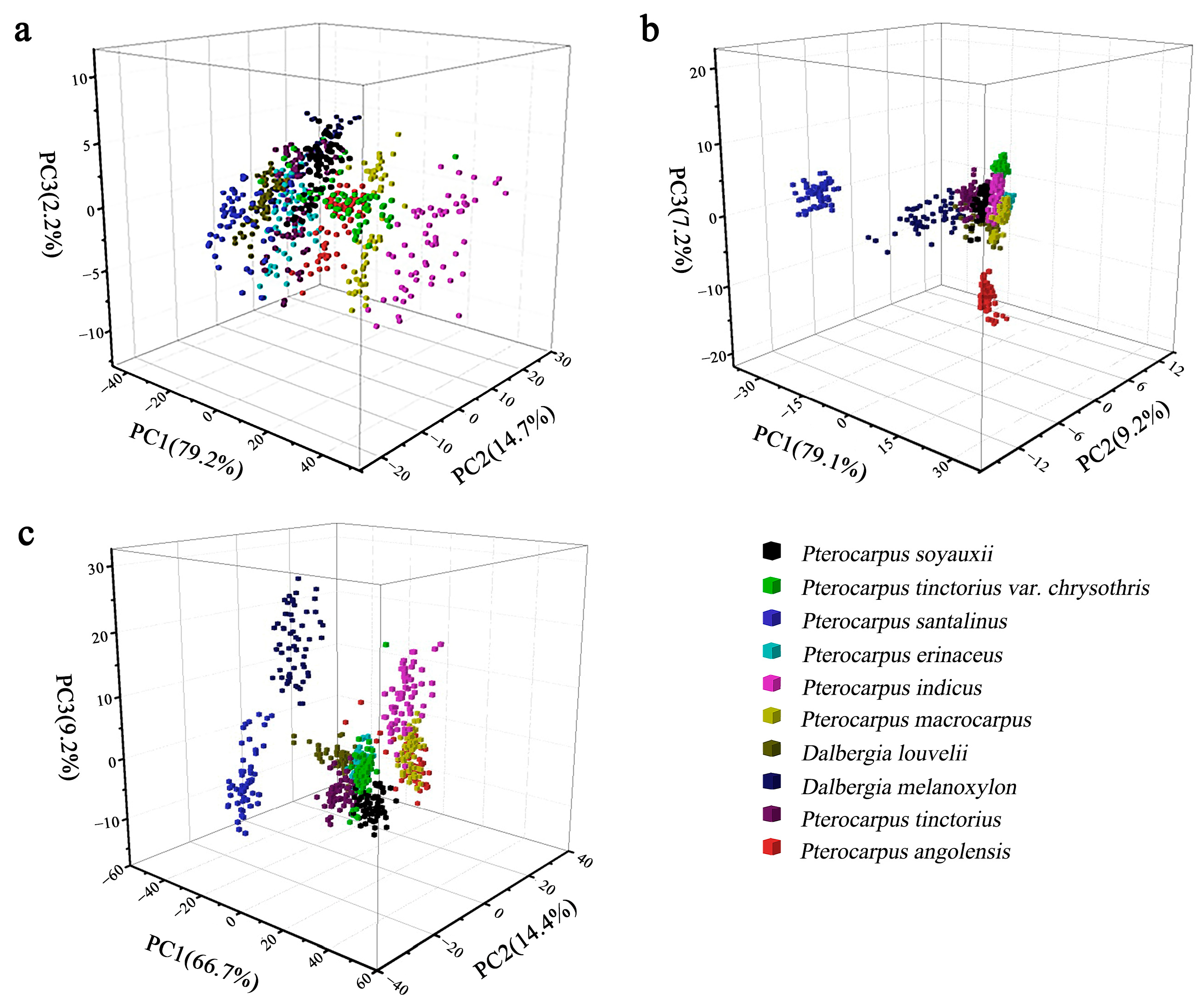
3.2. Principal Component Analysis
3.3. Results Using PLS-DA
3.4. Results Using SVM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karthikeyan, A.; Arunprasad, T. Growth Response of Pterocarpus santalinus Seedlings to Native Microbial Symbionts (Arbuscular mycorrhizal Fungi and Rhizobium aegyptiacum) under Nursery Conditions. J. For. Res. 2021, 32, 225–231. [Google Scholar] [CrossRef]
- Soundararajan, V. A Review on red sanders (Pterocarpus santalinus linn.)-Phyto-Chemistry and pharmacological importance. World J. Pharm. Pharm. Sci. 2016, 5, 667–689. [Google Scholar]
- Kukrety, S.; Dwivedi, P.; Jose, S.; Alavalapati, J.R.R. Stakeholders’ Perceptions on Developing Sustainable Red Sanders (Pterocarpus santalinus L.) Wood Trade in Andhra Pradesh, India. For. Policy Econ. 2013, 26, 43–53. [Google Scholar] [CrossRef]
- Prakash, E.; Sha Valli Khan, P.S.; Sreenivasa Rao, T.J.V.; Meru, E.S. Micropropagation of Red Sanders (Pterocarpus santalinus L.) Using Mature Nodal Explants. J. Res. 2006, 11, 329–335. [Google Scholar] [CrossRef]
- Arunkumar, A.N.; Joshi, G. Pterocarpus santalinus (Red Sanders) an Endemic, Endangered Tree of India: Current Status, Improvement and the Future. J. Trop. For. Environ. 2014, 4, 1–10. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Martin, S.; Perinbam, K.; Arockianathan, P.M.; Beatrice, V. Free Radical Scavenging Activity and HPTLC Finger Print of Pterocarpus santalinus L.—An in Vitro Study. Indian J. Sci. Technol. 2008, 1, 1–3. [Google Scholar] [CrossRef]
- Jiao, L.; Yu, M.; Wiedenhoeft, A.C.; He, T.; Li, J.; Liu, B.; Jiang, X.; Yin, Y. DNA Barcode Authentication and Library Development for the Wood of Six Commercial Pterocarpus Species: The Critical Role of Xylarium Specimens. Sci. Rep. 2018, 8, 1945. [Google Scholar] [CrossRef]
- Braga, J.W.B.; Pastore, T.C.M.; Coradin, V.T.R.; Camargos, J.A.A.; Silva, A.R. da The Use of near Infrared Spectroscopy to Identify Solid Wood Specimens of Swietenia Macrophylla0 (Cites Appendix II). IAWA J. 2011, 32, 285–296. [Google Scholar] [CrossRef]
- MacLachlan, I.R.; Gasson, P. PCA of Cites Listed Pterocarpus santalinus (Leguminosae) Wood. IAWA J. 2010, 31, 121–138. [Google Scholar] [CrossRef]
- Gasson, P. How Precise Can Wood Identification Be? Wood Anatomy’s Role in Support of the Legal Timber Trade, Especially Cites. IAWA J. 2011, 32, 137–154. [Google Scholar] [CrossRef]
- Ravindran, P.; Thompson, B.J.; Soares, R.K.; Wiedenhoeft, A.C. The XyloTron: Flexible, Open-Source, Image-Based Macroscopic Field Identification of Wood Products. Front. Plant Sci. 2020, 11, 1015. [Google Scholar] [CrossRef] [PubMed]
- Brunswick, P.; Cuthbertson, D.; Yan, J.; Chua, C.C.; Duchesne, I.; Isabel, N.; Evans, P.D.; Gasson, P.; Kite, G.; Bruno, J.; et al. A Practical Study of CITES Wood Species Identification by Untargeted DART/QTOF, GC/QTOF and LC/QTOF Together with Machine Learning Processes and Statistical Analysis. Environ. Adv. 2021, 5, 100089. [Google Scholar] [CrossRef]
- Sun, X.; Li, H.; Yi, Y.; Hua, H.; Guan, Y.; Chen, C. Rapid Detection and Quantification of Adulteration in Chinese Hawthorn Fruits Powder by Near-Infrared Spectroscopy Combined with Chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 250, 119346. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Z.; Xu, L.-L.; Tang, G.-Q.; Song, Q.-Q.; Feng, Q.-X. Rapid Detection of Surface Color of Shatian Pomelo Using Vis-NIR Spectrometry for the Identification of Maturity. Food Anal. Methods 2016, 9, 192–201. [Google Scholar] [CrossRef]
- da Silva, V.A.G.; Talhavini, M.; Peixoto, I.C.F.; Zacca, J.J.; Maldaner, A.O.; Braga, J.W.B. Non-Destructive Identification of Different Types and Brands of Blue Pen Inks in Cursive Handwriting by Visible Spectroscopy and PLS-DA for Forensic Analysis. Microchem. J. 2014, 116, 235–243. [Google Scholar] [CrossRef]
- Wu, D.; He, Y.; Nie, P.; Cao, F.; Bao, Y. Hybrid Variable Selection in Visible and Near-Infrared Spectral Analysis for Non-Invasive Quality Determination of Grape Juice. Anal. Chim. Acta 2010, 659, 229–237. [Google Scholar] [CrossRef]
- He, K.; Zhong, M.; Li, Z.; Liu, J. Near-Infrared Spectroscopy for the Concurrent Quality Prediction and Status Monitoring of Gasoline Blending. Control Eng. Pract. 2020, 101, 104478. [Google Scholar] [CrossRef]
- Jingyan, L.I.; Xiaoli, C.H.U.; Pu, C.; Songbai, T. Application of Spectral Automatic Retrieval Algorithm on the Rapid Establishment of Gasoline Spectral Database. Acta Pet. Sin. (Pet. Process. Sect.) 2017, 33, 131. [Google Scholar]
- Lin, Z.D.; Wang, Y.B.; Wang, R.J.; Wang, L.S.; Lu, C.P.; Zhang, Z.Y.; Song, L.T.; Liu, Y. Improvements of the Vis-NIRS Model in the Prediction of Soil Organic Matter Content Using Spectral Pretreatments, Sample Selection, and Wavelength Optimization. J. Appl. Spectrosc. 2017, 84, 529–534. [Google Scholar] [CrossRef]
- Mishra, P.; Herrmann, I.; Angileri, M. Improved Prediction of Potassium and Nitrogen in Dried Bell Pepper Leaves with Visible and Near-Infrared Spectroscopy Utilising Wavelength Selection Techniques. Talanta 2021, 225, 121971. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, C.; Takeda, F. Nondestructive Detection and Quantification of Blueberry Bruising Using Near-Infrared (NIR) Hyperspectral Reflectance Imaging. Sci. Rep. 2016, 6, 35679. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.A.; Conceição, D.G.; Viana, M.B.; de J. Silva, G.; Santos, L.S.; Ferrão, S.P.B. NIR and MIR Spectroscopy for Quick Detection of the Adulteration of Cocoa Content in Chocolates. Food Chem. 2021, 349, 129095. [Google Scholar] [CrossRef] [PubMed]
- Lakeh, M.A.; Karimvand, S.K.; Khoshayand, M.R.; Abdollahi, H. Analysis of Residual Moisture in a Freeze-Dried Sample Drug Using a Multivariate Fitting Regression Model. Microchem. J. 2020, 154, 104516. [Google Scholar] [CrossRef]
- Amaral, E.A.; dos Santos, L.M.; Hein, P.R.G.; Costa, E.V.S.; Rosado, S.C.S.; Trugilho, P.F. Evaluating Basic Density Calibrations Based on NIR Spectra Recorded on the Three Wood Faces and Subject to Different Mathematical Treatments. N. Z. J. For. Sci. 2021, 51. [Google Scholar] [CrossRef]
- Blaschek, M.; Roudier, P.; Poggio, M.; Hedley, C.B. Prediction of Soil Available Water-Holding Capacity from Visible near-Infrared Reflectance Spectra. Sci. Rep. 2019, 9, 12833. [Google Scholar] [CrossRef]
- Pace, J.-H.C.; de F. Latorraca, J.-V.; Hein, P.; da Silva, C.-E.S. Wood Species Identification from Atlantic Forest by near Infrared Spectroscopy. For. Syst. 2019, 28, 3. [Google Scholar]
- Xue, X.; Chen, Z.; Wu, H.; Gao, H. Identification of Guiboutia Species by NIR-HSI Spectroscopy. Sci. Rep. 2022, 12, 11507. [Google Scholar] [CrossRef]
- Ziyang, W.; Shikui, Y.I.N.; Ying, L.I.; Yaoxiang, L.I. Identification of common wood species in northeast China using Vis/NIR spectroscopy. J. Zhejiang AF Univ. 2019, 36, 162–169. [Google Scholar]
- Zhao, P.; Li, Z.-Y.; Wang, C.-K. Wood Species Recognition Based on Visible and Near-Infrared Spectral Analysis Using Fuzzy Reasoning and Decision-Level Fusion. J. Spectrosc. 2021, 2021, e6088435. [Google Scholar] [CrossRef]
- Sandak, J.; Sandak, A.; Zitek, A.; Hintestoisser, B.; Picchi, G. Development of Low-Cost Portable Spectrometers for Detection of Wood Defects. Sensors 2020, 20, 545. [Google Scholar] [CrossRef]
- Palacios-Morillo, A.; Jurado, J.M.; Alcázar, A.; Pablos, F. Differentiation of Spanish Paprika from Protected Designation of Origin Based on Color Measurements and Pattern Recognition. Food Control 2016, 62, 243–249. [Google Scholar] [CrossRef]
- Andrés, S.; Murray, I.; Navajas, E.A.; Fisher, A.V.; Lambe, N.R.; Bünger, L. Prediction of Sensory Characteristics of Lamb Meat Samples by near Infrared Reflectance Spectroscopy. Meat Sci. 2007, 76, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Emsley, A.M.; Herman, H.; Heywood, R.J. Spectroscopic Studies of the Ageing of Cellulosic Paper. Polymer 2001, 42, 2893–2900. [Google Scholar] [CrossRef]
- Núñez-Sánchez, N.; Martínez-Marín, A.L.; Polvillo, O.; Fernández-Cabanás, V.M.; Carrizosa, J.; Urrutia, B.; Serradilla, J.M. Near Infrared Spectroscopy (NIRS) for the Determination of the Milk Fat Fatty Acid Profile of Goats. Food Chem. 2016, 190, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Badaró, A.T.; Morimitsu, F.L.; Ferreira, A.R.; Clerici, M.T.P.S.; Fernandes Barbin, D. Identification of Fiber Added to Semolina by near Infrared (NIR) Spectral Techniques. Food Chem. 2019, 289, 195–203. [Google Scholar] [CrossRef]
- Krähmer, A.; Engel, A.; Kadow, D.; Ali, N.; Umaharan, P.; Kroh, L.W.; Schulz, H. Fast and Neat—Determination of Biochemical Quality Parameters in Cocoa Using near Infrared Spectroscopy. Food Chem. 2015, 181, 152–159. [Google Scholar] [CrossRef]
- Lequeue, G.; Draye, X.; Baeten, V. Determination by near Infrared Microscopy of the Nitrogen and Carbon Content of Tomato (Solanum lycopersicum L.) Leaf Powder. Sci. Rep. 2016, 6, 33183. [Google Scholar] [CrossRef]
- Bonaccorsi, I.; Cacciola, F.; Utczas, M.; Inferrera, V.; Giuffrida, D.; Donato, P.; Dugo, P.; Mondello, L. Characterization of the Pigment Fraction in Sweet Bell Peppers (Capsicum annuum L.) Harvested at Green and Overripe Yellow and Red Stages by Offline Multidimensional Convergence Chromatography/Liquid Chromatography-Mass Spectrometry. J. Sep. Sci. 2016, 39, 3281–3291. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, B.; Ren, C.; Guo, M.; Wang, J.; Shi, K.; Wu, X.; Feng, Y. Rapid Identification Model Based on Decision Tree Algorithm Coupling with 1H NMR and Feature Analysis by UHPLC-QTOFMS Spectrometry for Sandalwood. J. Chromatogr. B 2020, 1161, 122449. [Google Scholar] [CrossRef]
- Berrueta, L.A.; Alonso-Salces, R.M.; Héberger, K. Supervised Pattern Recognition in Food Analysis. J. Chromatogr. A 2007, 1158, 196–214. [Google Scholar] [CrossRef]
- Lestander, T.A.; Lindeberg, J.; Eriksson, D.; Bergsten, U. Prediction of Pinus Sylvestris Clear-Wood Properties Using NIR Spectroscopy and Biorthogonal Partial Least Squares Regression. Can. J. For. Res. 2008, 38, 2052–2062. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Z.; Han, X.; Hou, H.; Zheng, R. Comparative Investigation of Partial Least Squares Discriminant Analysis and Support Vector Machines for Geological Cuttings Identification Using Laser-Induced Breakdown Spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2014, 102, 52–57. [Google Scholar] [CrossRef]
- Li, Y.; Via, B.K.; Young, T.; Li, Y. Visible-Near Infrared Spectroscopy and Chemometric Methods for Wood Density Prediction and Origin/Species Identification. Forests 2019, 10, 1078. [Google Scholar] [CrossRef]
- Estimation of Pinus Radiata D. Don Tracheid Morphological Characteristics by near Infrared Spectroscopy. Available online: https://www.degruyter.com/document/doi/10.1515/HF.2004.009/html (accessed on 3 May 2023).
- Wang, C.-K.; Zhao, P.; Li, Z.-Y.; Li, X.-H. Comparison of VIS/NIR Spectral Curves plus RGB Images with Hyperspectral Images for the Identification of Pterocarpus Species. Holzforschung 2022, 76, 579–591. [Google Scholar] [CrossRef]
- Bächle, H.; Zimmer, B.; Wegener, G. Classification of Thermally Modified Wood by FT-NIR Spectroscopy and SIMCA. Wood Sci. Technol. 2012, 46, 1181–1192. [Google Scholar] [CrossRef]
- Bächle, H.; Zimmer, B.; Windeisen, E.; Wegener, G. Evaluation of Thermally Modified Beech and Spruce Wood and Their Properties by FT-NIR Spectroscopy. Wood Sci. Technol. 2010, 44, 421–433. [Google Scholar] [CrossRef]
- Karthick, M.; Parthiban, K.T. Chemical Characterization of Pterocarpus santalinus Wood Using GC-MS. J. Pharm. Phytochem. 2019, 8, 380–382. [Google Scholar]



| Scientific Classification | Calibration Set | Validation Set |
|---|---|---|
| Pterocarpus soyauxii | 60 | 20 |
| Pterocarpus tinctorius var. chrysothris | 60 | 20 |
| Pterocarpus santalinus | 60 | 20 |
| Pterocarpus erinaceus | 60 | 20 |
| Pterocarpus indicus | 60 | 20 |
| Pterocarpus macrocarpus | 60 | 20 |
| Dalbergia louvelii | 60 | 20 |
| Dalbergia melanoxylon | 60 | 20 |
| Pterocarpus tinctorius | 60 | 20 |
| Pterocarpus angolensis | 60 | 20 |
| 400~800 nm | 800~2500 nm | 400~2500 nm | |||||
|---|---|---|---|---|---|---|---|
| Calibration Set (%) | Validation Set (%) | Calibration Set (%) | Validation Set (%) | Calibration Set (%) | Validation Set (%) | ||
| Preprocessing | Raw | 88 | 84.5 | 96.7 | 96.5 | 90.3 | 94 |
| SNV | 79.2 | 76 | 92.8 | 92 | 86 | 88 | |
| SG Smoothing | 88.8 | 85.5 | 96.8 | 96.5 | 90.3 | 94 | |
| Normalization | 88.8 | 85 | 96.5 | 96 | 90.3 | 94 | |
| MSC | 88.8 | 85.5 | 92.7 | 92 | 85.8 | 88.5 | |
| 400~800 nm | 800~2500 nm | 400~2500 nm | |||||
|---|---|---|---|---|---|---|---|
| Calibration Set (%) | Validation Set (%) | Calibration Set (%) | Validation Set (%) | Calibration Set (%) | Validation Set (%) | ||
| Preprocessing | Raw | 96.3 | 94.5 | 99.7 | 99.5 | 99.8 | 99.5 |
| SNV | 93.2 | 92.5 | 97.3 | 99.5 | 95.8 | 98 | |
| SG Smoothing | 96.5 | 95 | 99.7 | 99.5 | 99.8 | 99.5 | |
| Normalization | 96.5 | 95 | 99.7 | 100 | 99.8 | 100 | |
| MSC | 92.2 | 91.5 | 97.3 | 99.5 | 96.7 | 98.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, X.; Chen, Z.; Wu, H.; Gao, H.; Nie, J.; Li, X. Identification of Eight Pterocarpus Species and Two Dalbergia Species Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging (HSI). Forests 2023, 14, 1259. https://doi.org/10.3390/f14061259
Xue X, Chen Z, Wu H, Gao H, Nie J, Li X. Identification of Eight Pterocarpus Species and Two Dalbergia Species Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging (HSI). Forests. 2023; 14(6):1259. https://doi.org/10.3390/f14061259
Chicago/Turabian StyleXue, Xiaoming, Zhenan Chen, Haoqi Wu, Handong Gao, Jiajie Nie, and Xinyang Li. 2023. "Identification of Eight Pterocarpus Species and Two Dalbergia Species Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging (HSI)" Forests 14, no. 6: 1259. https://doi.org/10.3390/f14061259
APA StyleXue, X., Chen, Z., Wu, H., Gao, H., Nie, J., & Li, X. (2023). Identification of Eight Pterocarpus Species and Two Dalbergia Species Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging (HSI). Forests, 14(6), 1259. https://doi.org/10.3390/f14061259







