Abstract
Widespread mortality of eastern hemlock (Tsuga canadensis [L.] Carr.) has been occurring due to the introduction of hemlock woolly adelgid (Adelges tsugae Annand) (HWA), threatening millions of hectares of hemlock-dominated forests in the eastern United States. HWA feeds at the base of needles and removes stored carbohydrates, which can impact leaf-level physiology, contributing to the decline of the tree. However, these physiological mechanisms in HWA-infested hemlocks are still not clearly understood. We investigated hemlock leaf physiology year-round at three forested sites with various degrees of infestation. At each site, half the trees were treated with imidacloprid (Merit® 2 F, Bayer, Kansas City, MO, USA) while the rest were left untreated. Imidacloprid is widely used to control HWA but can itself have phytotoxic effects. After one growing season, there was an increase in photosynthetic rates (7.5%, p = 0.0163) and stomatal conductance (7.1%, p = 0.0163) across sites in the trees treated with imidacloprid. After two years, the imidacloprid treatment also increased bud break from 22.5% to 88.7% at Fishburn (the most severely impacted site) and from 22.7% to 58.9% at Mountain Lake (the least impacted site), and slightly increased chlorophyll fluorescence for treated trees at Fishburn. Chemical treatment also slightly increased water use efficiency at Mountain Lake. These results suggest that HWA is causing tree mortality largely through a reduction in leaf area caused by decreasing bud break and also by a slight, but significant, reduction in leaf-level photosynthesis and stomatal conductance.
1. Introduction
Non-native pests are a primary threat to natural forests and constitute a major type of disturbance with severe socioeconomic impacts. One such exotic pest, hemlock woolly adelgid (Adelges tsugae Annand) (HWA), has led to severe impacts on ecosystem productivity [1,2,3,4]. Given the large spatial scale of the invasion, it is likely to have a great impact on forest ecosystem health in North America [5]. With no natural predators in its non-native range of the eastern U.S. [6], and its spread limited only by suitable hosts and very low temperatures in the North, HWA poses a critical threat to many forest ecosystems. Currently, 20 states and two Canadian provinces have been affected by HWA, and over 2 million m2 Tsuga spp. basal area are at risk [5,7]. Since the extent of the invasion is so widespread, eradication of the insect is virtually impossible [8]. HWA is highly mobile, as it is distributed by wind and animals [9]. Because of this mobility, any attempt to halt the spread along the front of the invasion would be very difficult [8].
Quantifying the economic losses associated with HWA is necessary to formulate policies and management strategies to deal with this invasive species [10]. In the case of hemlock, for which there is generally a low timber demand, the greatest losses incurred are in nonmarket goods such as recreation, wildlife habitat, and aesthetics [10]. In addition to the economic costs associated with HWA infestation, biotic disturbances can also significantly impact forest C dynamics by causing a decrease in net primary productivity (NPP) through defoliation and consequential tree mortality [4] as well as impacting forest fuels, and fire frequency and intensity [11]. Such dynamics between infestation and C cycling have a strong temporal component, as the stage of decline, dieback, or recovery, will strongly influence whether the ecosystem is acting as a C source or sink [5].
Development of C budget models may be used to predict whether an ecosystem will serve as a C source or sink as well as their overall health; therefore, they can be used to predict the relationships between pest invasions and ecosystem function. The major limitation to the development of such models is a lack of data on the dynamics among pest invasions, tree physiological processes, climate, and abiotic disturbances such as drought. The effects of pests on seasonal leaf physiological properties such as photosynthesis, stomatal conductance, transpiration, and specific leaf area have not been quantified or studied in great detail. Thus, predicting how HWA will impact hemlock decline and ecosystem C dynamics remains uncertain [5]. Although there are some papers examining how HWA impacts hemlock leaf gas exchange measurements [12,13,14,15], data have not been collected over an entire growing season.
Imidacloprid is a systemic neonicotinoid insecticide widely used and effective for controlling HWA [16,17] and was selected for use to control HWA in this study. However it is known to be phytotoxic [18,19] and can change leaf optical properties in a manner that would reduce photosynthetic potential [17]; therefore, this study is not examining the isolated effects of HWA on leaf gas exchange, but rather the effect of HWA control using imidacloprid. The specific objectives of this study were to: examine initial changes in needle physiology (leaf-level photosynthesis, conductance, water use efficiency) and bud break in response to HWA control with imidacloprid and examine the impact of imidacloprid control on hemlock chlorophyll fluorescence at sites with various levels of decline due to HWA.
2. Materials and Methods
2.1. Sites
Three sites at different stages of canopy decline, ranging from slightly impacted to severely impacted, were selected for the study. Site one (Fishburn) is located in the Valley and Ridge physiographic province in Montgomery County, Virginia, USA (elevation 610 m). It is considered severely impacted with an average crown class score of 2.1 (where 1 = dead, 2 ≤ 25% live crown, 3 = 25%–50% live crown, 4 = 50%–75% live crown, 5 ≥ 75% live crown) at the beginning of the study. Site two (Mountain Lake), located in the Valley and Ridge physiographic province in Giles County, VA, USA (elevation 1170 m), was overall the least impacted site with an average crown class of 3.1. Site three (Twin Falls) is in the Appalachian Plateau physiographic province (elevation 610 m) in Wyoming County, WV, USA, and was less impacted than Fishburn but more so than Mountain Lake, with a beginning crown class score of 2.5.
At all three sites, a series of plots were established with half the trees treated with imidacloprid and half the trees left untreated. A minimum distance of 15 m was used between plots in order to ensure no contamination of chemicals in control plots, and all trees in a plot were within approximately 30.5 m of each other. At Fishburn and Mountain Lake, 12 plots (six treated and six control) were installed in a completely randomized design with a split plot with imidacloprid treatment as the whole plot factor and tree size class as the split plot factor. The size classes used were based on the diameter at breast height (DBH) and consisted of small (<5 cm), medium (10–15 cm), and large (20–35 cm) trees with one tree of each class in each plot. At Twin Falls, 10 plots were installed (five treated and five control) and no tree size classes were used. The study design was completely randomized.
An analysis of the initial DBH distribution revealed that there were no significant differences in size between trees selected to be treated and control trees in any of the three size classes at Fishburn and Mountain Lake, and no differences between treated and control trees at Twin Lakes (Figure 1). The large and medium-sized trees from each plot were cored at their bases using an increment borer to determine tree age. The mean tree ages for treated and control trees across all sites were 50.9 and 48.5 years, respectively.
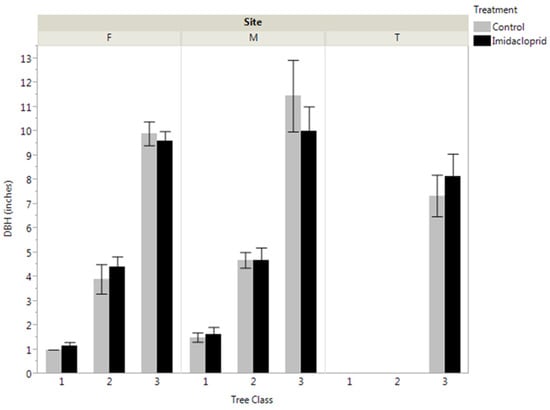
Figure 1.
Average eastern hemlock tree size distributions at the three study site locations: Fishburn (F), Mountain Lake (M), and Twin Falls (T). 1, 2, and 3 represent small, medium, and large trees, respectively. No tree size classes were investigated at Twin Falls.
Plots were randomly chosen to be treated with imidacloprid (Merit® 2 F, Bayer, Kansas City, MO, USA) at a rate of 1.2 g (AI)/2.5 cm dbh with Kioritz® soil injectors (Kioritz Corporation, Tokyo, Japan). Treatments were applied in August 2011 at Fishburn, November 2011 at Mountain Lake and October 2011 at Twin Falls.
2.2. Gas Exchange
Needle gas exchange measurements, including light-saturated photosynthesis (Amax) and stomatal conductance (gs), and transpiration (Tr) were collected using a LI-COR 6400 portable open path gas exchange system (LI-COR Inc, Lincoln, NE, USA). Chamber conditions were set to a reference CO2 of 385 ppm, quantum flux was saturated at 1200 µmol m−2 s−1, and temperature and relative humidity were held to near ambient. A flow rate of 200 was used, as it was determined to provide a measurable difference between reference and sample CO2 concentrations. Measurements were taken in a 2–3 h period encompassing solar noon and were recorded after A and gs stabilized, typically within 2 min.
Measurements were taken on attached branch sections of one-year-old foliage at Fishburn and Mountain Lake. Small segments (approximately 2 cm) on a randomly selected branch of the lower crown were tagged and a small amount (approximately 1 cm) of neighboring foliage on that twig was removed to isolate the segment of foliage so it would fit cleanly, and seal tightly into the cuvette with no needles trapped in the cuvette gaskets. When possible, the newest mature growth on the tree was selected and prepared for measurements. Periodically, when it was obvious that needles were beginning to decline, branch segments were harvested to determine leaf area and newer mature branch segments were prepared for the next month’s measurements. Visible flushing of shoot segments, after they had been prepared and repeatedly measured, indicated that handling foliage in this manner did not cause additional stress to the branch.
At Twin Falls, not all trees had low-hanging branches on which measurements could be taken; therefore, a clipped branch segment was used for all trees at this site. A randomly selected branch was clipped, prepared as described above and measured immediately. It was determined that clipped branches have stable photosynthetic rates for approximately 15 min and all measurements on clipped branches were taken well within this time frame. For use as a variable in regression analysis, soil moisture (across 0 to 12 cm) was recorded at the base of each tree using a soil moisture meter (Hydrosense TM, Campbell Scientific, Inc., Logan, UT, USA). The leaf area of sampled branch segments was determined using a LI-COR 3100 area meter (LI-COR Inc., Lincoln, NE, USA). Needles were then oven dried (60 °C) to constant weight for determination of specific leaf area (SLA, m2/g).
At each site, gas exchange measurements were taken at approximately 4 week intervals throughout the year beginning in January the year following imidacloprid applications. For days in which weather conditions caused poor water data (i.e., damp foliage over estimating transpiration rates), water-related data for the entire day was discarded. This was conducted by determining typical value ranges for each parameter and examining the data set for unreasonable water exchange rates.
2.3. Chlorophyll Fluorescence
The year following imidacloprid treatment, at the Fishburn and Mountain Lake sites, chlorophyll fluorescence was measured monthly from June through October 2012 for each tree using a Handy PEA fluorometer (Hansatech Instruments Ltd., King’s Lynn, UK). Since low-hanging branches were not present on all trees at Twin Falls, fluorescence measurements were not collected at this site. Randomly selected needles were dark-acclimated for 15 min using dark adaptor clips. Mature needles were placed in the clips to maximize the amount of needle area in the clip. Leaves were then subjected to a 1-s flash of saturating light (3000 µmol/m2 s) using a high-intensity LED centered at a wavelength of 650 nm. Fo (represents emissions by chlorophyll a molecules in PSII) is determined by generating a best-fit line between data points 4–16 recorded from the onset of illumination and extrapolating to time zero. Maximum fluorescence (Fm) is determined at the peak of variable fluorescence (Fv = Fm − Fo) over Fm and this measure is independent of leaf area.
2.4. Bud Break
In May 2012, 2013, and 2015, the percentage of flushing buds was determined on five randomly selected branches in the lower crown by counting the total number of buds and flushing buds. Percent bud break was calculated as the total number of flushing buds divided by the total number of buds.
2.5. Statistical Analysis
Day-to-day treatment differences in gas exchange were analyzed using an analysis of variance separately for each measurement day. At Fishburn and Mountain Lake, a two-way ANOVA was used to test the effect of chemical treatment, tree size class, and the interaction of treatment and size class. The Estimated Mean Squares (EMS) method was used to analyze the split plot. At Twin Falls, a 1-way ANOVA was used to test the effect of chemical treatment only.
Annual averages in leaf gas exchange and SLA were analyzed using two-way ANOVA with the date as the blocking factor to test the effect of chemical treatment and tree size class. Since no significant interaction was detected for gas exchange and tree size class, the interaction was removed from the model.
Models for gas exchange were also developed using environmental variables collected from nearby meteorological stations and a stepwise regression procedure. Weather data were collected from the National Ocean and Atmospheric Administration (http://www.noaa.gov, accessed in April 2013) website, as well as from the Mountain Lake Biological Station Meteorological Data website (http://www.mlbs.virginia.edu/meteorological-data, accessed in April 2013). Model selection was conducted with a stepwise regression procedure for the initial screening of variables followed by an examination of best R2 values. A simple model with three parameters containing leaf temperature, relative humidity, and soil moisture was selected for both photosynthesis and stomatal conductance. Using this model, treatment effects (imidacloprid and tree class) on parameter estimates (slopes and intercepts) were examined using an analysis of covariance. This analysis was performed to determine whether any of the treatments modified how needles were responding to the environment. The effects of the growing season and imidacloprid treatment were investigated with two-way ANOVA. The days of years were divided into growing seasons and winter seasons and the pooled results were analyzed in each site.
Photosynthetic rates in each site were regressed on stomatal conductance and the effects of imidacloprid treatment on the slope were tested, indicating the effect of chemical treatment on water use efficiency. Day-to-day differences in chlorophyll fluorescence were analyzed using a t-test separately for each measurement day. Then a linear regression was performed to test the effects of the day of the year and chemical treatment. Bud break was analyzed using a t-test each year for each site.
All analyses were conducted using JMP Pro 10 software (SAS Institute Inc., Cary, NC, USA). Direct statistical comparisons were not made between study locations, although quantitative comparisons will be made.
3. Results
3.1. Annual Average Leaf-Level Physiology and Specific Leaf Area
In the year following imidacloprid treatment at Fishburn, annual average photosynthetic rates and annual average stomatal conductance increased by 6.9% (p = 0.0190, Table 1) and 12.9% (p < 0.0001), respectively. Treated trees showed significantly higher photosynthetic rates on day 121 (by 31.8%, p = 0.0224, Figure 2a), 265 (by 31.9%, p = 0.0159) and 321 (by 24.6%, p = 0.0463). Similarly, significantly higher stomatal conductance rates were found in treated hemlocks on day 265 (by 42.2%, p = 0.0048, Figure 2b), 299 (by 31.1%, p = 0.0357), 321 (by 42.7%, p = 0.0035) and 349 (by 199%, p = 0.0238). Tree class slightly influenced photosynthetic rates at Fishburn (p = 0.0780, Table 1) but did not affect stomatal conductance (p = 0.2862).

Table 1.
Eastern hemlock seasonal mean (±SE) photosynthetic rates, stomatal conductance, and specific leaf area (SLA) as influenced by treatment and tree class at each site 1.
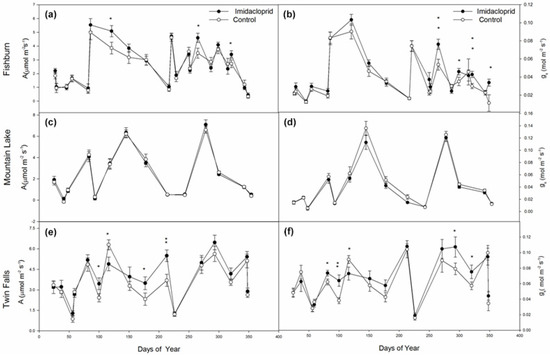
Figure 2.
Eastern hemlock photosynthesis (left panels) and stomatal conductance (right panels) for all measurement days at Fishburn (a,b), Mountain Lake (c,d), and Twin Falls (e,f). Points show mean ± SE. Single asterisk denotes difference at α = 0.05; double asterisk denotes a significant difference at α = 0.01.
There were no significant treatment effects detected at Mountain Lake for photosynthetic rates (Table 1, Figure 2c). However, imidacloprid treatment significantly reduced annual average stomatal conductance by 5.19% (p = 0.0115, Table 1). Tree class affected neither photosynthetic rates nor stomatal conductance at Mountain Lake.
Similar to Fishburn, annual photosynthetic rates and annual stomatal conductance rates of chemically treated trees at Twin Falls were significantly higher than control by 11.3% (p = 0.0074, Table 1) and 7.7% (p = 0.0147), respectively. Significantly higher photosynthetic rates were found in treated trees on days 81 (p = 0.0228), 100 (p = 0.0038), 293 (p = 0.0394), and 320 (p = 0.0472, Figure 2e). Likewise, on days 100, 177, and 213, significantly higher leaf conductance rates were observed in imidacloprid-treated trees (p = 0.0333, p = 0.0447, and p = 0.0043, respectively, Figure 2f). Nevertheless, on day 116, treated trees had lower photosynthetic rates (p = 0.020) and stomatal conductance (p = 0.0420) compared to control trees.
Treatment significantly reduced SLA by 7.5% (p < 0.0001) at Fishburn, while it increased SLA by 4.2% (p = 0.0040) at Mountain Lake (Table 1). At Twin Falls, however, there were no differences between chemical-treated hemlock and control for SLA (p = 0.9246). Tree class significantly influenced SLA at Mountain Lake (p = 0.0059) but showed no influence at the other two sites. There was no interaction between treatment and tree class at any sites for SLA.
3.2. Models and Seasonal Patterns of Leaf Gas Exchange
Imidacloprid treatment increased photosynthetic rates by 7.5% (p = 0.0163, Table 2), and stomatal conductance by 7.1% (p = 0.0160) compared to control trees, with leaf temperature (p < 0.0001), relative humidity (p < 0.0001), and soil moisture (p < 0.0001) as covariates and sites (p < 0.0001) as blocking factor. Neither the chemical treatment nor tree class influenced the photosynthetic rates and stomatal conductance. None of the interactions between chemical treatment and the environmental variables was significant.

Table 2.
p-values for the treatment models of eastern hemlock photosynthetic rates and stomatal conductance, with soil moisture content (%), relative humidity of leaf (%), and temperature of leaf (°C) as covariates and site as blocking factor 1.
Photosynthetic rates during the growing season were 83.2 (p < 0.0001), 159.8 (p < 0.0001), and 19.7% (p = 0.0024) higher than during winter seasons at Fishburn, Mountain Lake, and Twin Falls, respectively (Figure 3). During the growing season, imidacloprid treatment significantly increased photosynthetic rates by 9.3 and 13.7% at Fishburn and Twin Falls, respectively. Nevertheless, no differences in photosynthetic rates were found between the treated group and the control group during the winter season. Although seasonal variations for photosynthetic rates were observed at Mountain Lake, there were no treatment effects during the growth season or winter season.
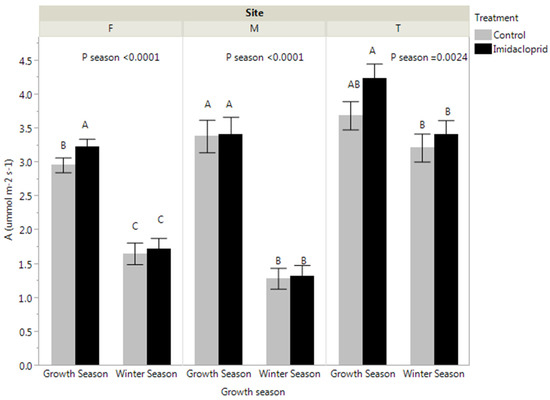
Figure 3.
Eastern hemlock photosynthesis (leaf panels) during growth season and winter season at Fishburn (F), Mountain Lake (M), and Twin Falls (T). Bars show mean ± SE. Different letters indicate significant differences between treatments within a site (p < 0.05).
3.3. Water Use Efficiency, and Photosystem Efficiency
Photosynthetic rates were significantly correlated with stomatal conductance at all three sites (p < 0.0001, Figure 4), and imidacloprid treatment did not significantly influence this relationship, implying water use efficiency was not affected by treatment.
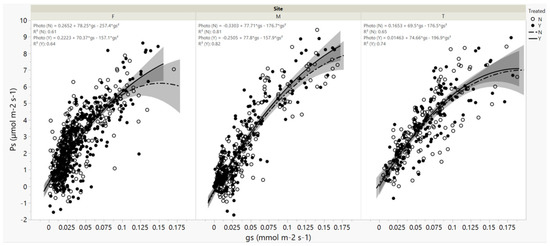
Figure 4.
Eastern hemlock photosynthetic rates (Ps) vs. stomatal conductance (Gs) as influenced by imidacloprid treatment at Fishburn (F), Mountain Lake (M), and Twin Falls (T). Shadows indicate 95% confidence interval. N denotes control (open circle) and Y denotes imidacloprid treatment (closed circle). Treatment did not affect the intercept or the slope at α = 0.05 level.
Both Fishburn and Mountain Lake showed an overall decrease in chlorophyll fluorescence (Fv/Fm) day of year increased likely due to decreasing temperatures (p < 0.0001, Figure 5). There was a significant imidacloprid and day of year interaction at Fishburn (p = 0.0292). By day of year 299 at Fishburn, treated trees had a significantly higher Fv/Fm than control trees (p < 0.01). The chlorophyll fluorescence at Mountain Lake generally did not differ due to chemical treatment except that on day of year 243, treated trees had a significantly lower Fv/Fm than control trees (p < 0.01).
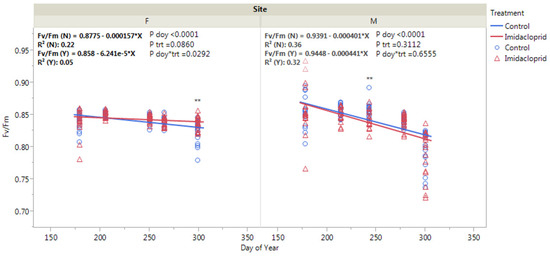
Figure 5.
Eastern hemlock chlorophyll fluorescence (Fv/Fm) as affected by imidacloprid treatment at Fishburn (F) and Mountain Lake (M). Double asterisk denotes a significant difference at α = 0.01. Blue open circle denotes control group and red open triangle denotes imidacloprid treatment.
3.4. Bud Break
Imidacloprid treatment had a significant effect on bud break (Table 3). After one year of treatment, Fishburn treated trees averaged significantly higher bud break relative to control trees (13.4 and 5.8%, respectively), although all bud break was very low. At Mountain Lake, the average bud break was nearly identical between treated and control trees (46.6 and 46.9%, respectively). After two years of treatment, the average bud break increased for all trees at Fishburn but increased to over 88% for treated trees. At Mountain Lake, the average bud break of treated trees increased to 58.9% and was significantly greater than that of control trees, which dropped to 24.5%. Three years after treatment, average bud break no longer differed between treatments and notably control trees showed a large increase in bud break.

Table 3.
Mean (±SE) eastern hemlock percent bud break as affected by imidacloprid treatment at Fishburn and Mountain Lake 1.
4. Discussion
In comparison to several past studies [14,15,20,21] which found a large decrease in photosynthesis and stomatal conductance in HWA-infected hemlock, we found only a small (but still significant) decrease in both variables in control trees compared to imidacloprid-treated trees. This reduction was modeled using all data across all HWA-influenced sites (Table 2). The differences in our study may be due to the larger gas exchange data set which covered an entire growing season at three different sites. It may also be due to differences in initial tree health at the start of the study and larger differences may have developed with more years of recovery. It is possible that the phytotoxic effects of imidacloprid [18,19] are working antagonistically against improvements in health due to beetle control reducing any improvements in needle gas exchange. However, similar to our study, Preston et al. [22] found significant, but only slight improvements in gas exchange in hemlock where HWA was controlled with Laricobus nigrinus beetles and not imidacloprid.
One objective of the study was to compare how three sites with contrasting tree health compared in foliar level gas exchange. Our modeling of leaf-level photosynthesis found that the site significantly impacted model parameters, even with temperature, humidity and soil moisture already included in the model. The effects of imidacloprid treatment varied among sites with different levels of infestation. At the severely impacted site (Fishburn) and medium impacted site (Twin Falls), annual photosynthetic rates and annual stomatal conductance in imidacloprid-treated trees were significantly higher than in control trees, though there were only a few dates with significant differences. Nevertheless, at the least impacted and coldest of the sites (Mountain Lake), photosynthetic rates were not affected by imidacloprid treatment, but stomatal conductance was lowered by imidacloprid treatment, indicating increased water use efficiency.
Hemlock carbon assimilation was reduced during the winter months compared to the growth season across all the sites, as previous studies suggested [23,24]. The stands either became a carbon source during the winter season as a mixed coniferous stand, including Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco), silver fir (Abies amabalis (Douglas ex Louden) Douglas ex Forbes), Scots pine (Pinus sylvestris L.), and Norway spruce (Picea abies (L.) Karst. [24] or showed small amounts of carbon assimilation during mild winter, as indicated in a study of red spruce [23]. Although positive photosynthetic rates were found at all sites during winter months, chemical treatment did not influence photosynthetic rates during winter months but significantly increased photosynthetic rates during the growing season. HWA might be killed or have very little activity during winter, which could explain why there was no difference between the treated group and the untreated group during winter. Gonda-King et al. [21] found a large reduction in photosynthesis and leaf conductance in HWA-infested hemlock seedlings in October, but little difference in September. This may be a result of HWA being dormant in September and breaking dormancy in October [25].
Domec et al. [20] found large decreases in leaf conductance as well as less negative delta 13C tissue values in the adelgid declining eastern and Carolina hemlocks (Tsuga caroliniana Engelm.), suggesting that infestation increased water use efficiency, foliar N content, and leaf hydraulic conductance. However, in this study, chlorophyll fluorescence, photosynthetic rates/stomatal conductance correlation, and specific leaf area were investigated, and the results were not consistent among sites. Most of the results conflicted with the conclusion of Domec et al. [20] who found a large decrease in modeled carbon uptake. Chlorophyll fluorescence measurements indicated that HWA did not affect the photosynthetic capacity at Mountain Lake and slightly decreased the photosynthetic capacity at Fishburn. The quantum efficiency of Photosystem II was at or near optimal across most measurement days for both treatments, indicating that photosynthetic performance was not being severely hindered. At both sites, chlorophyll fluorescence declined with days of year, probably due to increasingly lowered temperatures. Photosynthetic rates/stomatal conductance ratio (A/Gs) indicated instantaneous water use efficiency at the leaf level. Past studies found that across species and biomes, higher SLA correlated with higher leaf Nmass within a species and also with a higher WUE [26,27]. At Mountain Lake, the least infested site, both SLA and A/Gs were increased by chemical treatment, implying that HWA reduced water use efficiency. At Twin Falls, chemical treatment did not cause a significant difference in water use efficiency. At Fishburn, the most infested site, A/Gs measurements suggested that water use efficiency was not affected by chemical treatment. However, SLA suggested that water use efficiency was decreased by chemical treatment, which was the only result of ours to agree with Domec et al.’s [20] conclusion that HWA induced compensating photosynthesis through increasing water use efficiency. The higher SLA of control trees may, therefore, indicate that those needles have higher Nmass compared to needles of treated trees. This may be a mechanism by which declining trees allocate resources to rebuild their crown, which subsequently allows greater light capture and photosynthate production during an infestation. An alternative explanation may be that needles of control trees lack starch from adelgid feeding and thus weigh less than needles of treated trees. Whether the observed relationship between non-treatment and SLA is a cause or effect of infestation remains unknown and is a topic for further investigation.
Given the only slight change in leaf-level photosynthetic rates over the course of a growing season, other factors must play a role in the decline of hemlock in response to HWA. Bud break increased significantly at both sites for trees treated with imidacloprid, indicating that leaf area development may play a large role. Webb et al. [28] also found that even trees in severe decline had the capacity to uptake chemical from the soil and deliver it to the foliage in order to kill HWA. In that same study, Webb et al. [28] also found little to no new growth on control trees, while treated trees experience significant biomass gains. As trees transported the chemical systemically, adelgid populations decreased and the tree could recover by putting on new growth. Similarly, Miniat et al. [13] found that hemlock trees released from competition also responded by producing greater shoot growth. Released trees fixed twice the carbon and grew nine times more [13] regardless of infestation suggesting whole tree carbon fixation rather than leaf-level physiological changes is critical to maintaining hemlock health. The observed increases in bud break for both treated and control trees at Fishburn indicated a period of recovery from adelgid infestation. In contrast, the observed decrease in bud break of control trees at Mountain Lake indicated a period of decline from infestation. These results also suggest that mortality and tree decline caused by HWA are largely due to a decline in leaf area, rather than a reduction in needle physiological capacity. Stadler et al. [29] found little to no new growth on heavily-infested trees. Furthermore, heavily-infested trees shed 48% more needle biomass (g/m2) compared to uninfested or lightly-infested trees. They found that heavily-infested trees had 24,140 1-year-old shoots compared to 139,680 for uninfested trees. While the photosynthetic ability per unit area may not be greatly affected, the loss of needle area causes a reduction in whole-tree carbon assimilation that eventually leads to mortality.
5. Conclusions
The results of this extensive leaf-level physiological study suggest that HWA is causing tree mortality through a reduction in leaf area as a consequence of HWA feeding. Some evidence of the altered leaf-level physiological capability of eastern hemlock was observed over the course of an entire growing season, but in comparison to changing leaf area as a result of decreased bud break, it seems rather minor. Photosystem efficiency and water use efficiency were also increased by treatment at some sites. The significant regression models incorporating several environmental parameters show that hemlock physiology tracks environmental parameters closely and suggests that hemlock growth may be predictable using process-based models [30].
Author Contributions
Conceptualization, K.M.M., J.R.S., S.M.S. and R.J.R.; methodology K.M.M., J.R.S. and S.M.S.; validation, K.M.M., J.R.S. and S.M.S.; formal analysis, K.M.M., J.R.S. and B.W.; data curation, K.M.M. and J.R.S.; writing—original draft preparation, K.M.M. and J.R.S.; writing—review and editing K.M.M., J.R.S., S.M.S., B.W. and R.J.R.; supervision, J.R.S. and S.M.S.; project administration, J.R.S. and S.M.S.; funding acquisition, R.J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the USDA Forest Service, Forest Health Protection Grant #208-11-110A-008-333-1; Mountain Lake Biological Station, University of Virginia, Charlottesville, Virginia, and Twin Falls Resort State Park, Mullins, West Virginia, allowed experimental plot establishment.
Acknowledgments
Special thanks go to John A. Peterson, who provided endless field and technical support, and to Tom McAvoy for assisting with chemical treatment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ellison, A.M.; Orwig, D.A.; Fitzpatrick, M.C.; Preisser, E.L. The past, present, and future of the hemlock woolly adelgid (Adelges tsugae) and its ecological interactions with eastern hemlock (Tsuga canadensis) forests. Insects 2018, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.R.; Vose, J.M. Tsuga canadensis (L.) Carr, mortality will impact hydrologic processes in southern Appalachian forest ecosystems. Ecol. Appl. 2007, 17, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.R.; Elliott, K.J.; Clinton, B.D.; Kloeppel, B.D.; Vose, J.M. Forest dynamics following eastern hemlock mortality in the southern Appalachians. Oikos 2012, 121, 523–526. [Google Scholar] [CrossRef]
- Nuckolls, A.E.; Wurzburger, N.; Ford, C.R.; Hendrick, R.L.; Vose, J.M.; Kloeppel, B.D. Hemlock declines rapidly with hemlock woolly adelgid infestation: Impacts on the carbon cycle of the Southern Appalachian forests. Ecosystems 2009, 12, 179–190. [Google Scholar] [CrossRef]
- Hicke, J.A.; Allen, C.D.; Desai, A.R.; Dietze, M.C.; Hall, R.J.; Ted Hogg, E.H.T.; Kashian, D.M.; Moore, D.; Raffa, K.F.; Sturrock, R.N.; et al. Effects of biotic disturbances on forest carbon cycling in the United States and Canada. Glob. Chang. Biol. 2012, 18, 7–34. [Google Scholar] [CrossRef]
- Wallace, M.S.; Hain, F.P. Field surveys and evaluation of native and established predators of the hemlock woolly adelgid (Homoptera: Adelgidae) in the southeastern United States. Environ. Entomol. 2000, 29, 638–644. [Google Scholar] [CrossRef]
- USDA Forest Service. Hemlock Woolly Adelgid Infestation. Available online: https://www.na.fs.fed.us/fhp/hwa/maps/2015_HWA_Infestation_Map_20160502.pdf (accessed on 16 August 2017).
- Leibhold, A.M.; MacDonald, W.L.; Bergdahl, D.; Mastro, V.C. Invasion by exotic forest pests: A threat to forest ecosystems. For. Sci. 1995, 30, a0001–z0001. [Google Scholar] [CrossRef]
- McClure, M.S. Role of wind, birds, deer, and humans in the dispersal of hemlock woolly adelgid (Homoptera: Adelgidae). Environ. Entomol. 1990, 19, 36–43. [Google Scholar] [CrossRef]
- Holmes, T.P.; Aukema, J.E.; Von Holle, B.; Liebhold, A.; Sills, E. Economic impacts of invasive species in forests: Past, present, and future. Ann. N. Y. Acad. Sci. 2009, 1162, 18–38. [Google Scholar] [CrossRef]
- Khodaee, M.; Hwang, T.; Kim, J.; Norman, S.P.; Robeson, S.M.; Song, C. Monitoring forest infestation and fire disturbance in the southern Appalachians using a time series analysis of landsat imagery. Remote Sens. 2020, 12, 2412. [Google Scholar] [CrossRef]
- Huggett, B.; Savage, J.; Hao, G.-Y.; Preisser, E.; Holbrook, N. Impact of hemlock woolly adelgid (Adelges tsugae) infestation on xylem structure and function and leaf physiology in eastern hemlock (Tsuga canadensis). Funct. Plant Biol. 2017, 45, 501–508. [Google Scholar] [CrossRef]
- Miniat, C.F.; Zietlow, D.R.; Brantley, S.T.; Brown, C.L.; Mayfield, A.E., III; Jetton, R.M.; Rhea, J.R.; Arnold, P. Physiological responses of eastern hemlock (Tsuga canadensis) to light, adelgid infestation, and biological control: Implications for hemlock restoration. For. Ecol. Manag. 2020, 460, 117903. [Google Scholar] [CrossRef]
- Nelson, L.A.; Dillaway, D.N.; Rieske, L.K. Effect of an exotic herbivore, Adelges tsugae, on photosynthesis of a highly susceptible Tsuga host, with notes on conspecifics. Arthropod-Plant Interact. 2014, 8, 9–15. [Google Scholar] [CrossRef]
- Rubino, L.; Charles, S.; Sirulnik, A.G.; Tuininga, A.R.; Lewis, J.D. Invasive insect effects on nitrogen cycling and host physiology are not tightly linked. Tree Physiol. 2015, 35, 124–133. [Google Scholar] [CrossRef]
- Dilling, C.; Lambdin, P.; Grant, J.; Rhea, R. Spatial and temporal distribution of imidacloprid in Eastern hemlock in the southern Appalachians. J. Econ. Entomol. 2010, 103, 368–373. [Google Scholar] [CrossRef]
- Garris, H.W.; Settle, T.H.; Crossman, J.E.; Grider, S.J.; Michaels, S.L. Combined effects of hemlock woolly adelgid (Adelges tsugae) infestation and treatment with imidacloprid on Eastern hemlock (Tsuga canadensis) Leaf Radiometry. J. For. 2019, 117, 340–350. [Google Scholar] [CrossRef]
- Ebel, R.C.; Wallace, B.; Elkins, C. Phytotoxicity of the systemic insecticide imidacloprid on tomato and cucumber in the greenhouse. HortTechnology 2000, 10, 144–147. [Google Scholar] [CrossRef]
- Ajermoun, N.; Aghris, S.; Ettadili, F.; Tahiri Alaoui, O.; Laghrib, F.; Farahi, A.; Lahrich, S.; Bakasse, M.; Sagrane, S.; El Mhammed, M.A. Phytotoxic effect of the insecticide imidacloprid in Phaseolus vulgaris L. plant and evaluation of its bioaccumulation and translocation by electrochemical methods. Environ. Res. 2022, 214, 113794. [Google Scholar] [CrossRef] [PubMed]
- Domec, J.C.; Rivera, L.N.; King, J.S.; Peszlen, I.; Hain, F.; Smith, B.; Frampton, J. Hemlock woolly adelgid (Adelges tsugae) infestation affects water and carbon relations of eastern hemlock (Tsuga canadensis) and Carolina hemlock (Tsuga caroliniana). New Phytol. 2013, 199, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Gonda-King, L.; Gómez, S.; Martin, J.; Orians, C.; Preisser, E. Tree responses to an invasive sap-feeding insect. Plant Ecol. 2014, 215, 297–304. [Google Scholar] [CrossRef]
- Preston, C.E.; Arneson, A.; Seiler, J.R.; Salom, S.M. The Impact of Predation of Laricobius nigrinus (Coleoptera: Derodontidae) on Adelges tsugae (Hemiptera: Adelgidae) and Tsuga canadensis (Pinales: Pinaceae) Tree Health. Forests 2023, 14, 698. [Google Scholar] [CrossRef]
- Schaberg, P.; Wilkinson, R.; Shane, J.; Donnelly, J.; Cali, P. Winter photosynthesis of red spruce from three Vermont seed sources. Tree Physiol. 1995, 15, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Aubinet, M.; Heinesch, B.; Longdoz, B. Estimation of the carbon sequestration by a heterogeneous forest: Night flux corrections, heterogeneity of the site and inter-annual variability. Glob. Chang. Biol. 2002, 8, 1053–1071. [Google Scholar] [CrossRef]
- McClure, M.S. Evidence of a polymorphic life cycle in the hemlock woolly adelgid, Adelges tsugae (Homoptera: Adelgidae). Ann. Entomol. Soc. Am. 1989, 82, 50–54. [Google Scholar] [CrossRef]
- Pierce, L.L.; Running, S.W.; Walker, J. Regional-scale relationships of leaf area index to specific leaf area and leaf nitrogen content. Ecol. Appl. 1994, 4, 313–321. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S.; Vose, J.M.; Volin, J.C.; Gresham, C.; Bowman, W.D. Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf life-span: A test across biomes and functional groups. Oecologia 1998, 114, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.E.; Frank, J.R.; Raupp, M.J. Eastern hemlock recovery from hemlock woolly adelgid damage following imidacloprid therapy. J. Arbor. 2003, 29, 298–302. [Google Scholar]
- Stadler, B.; Muller, T.; Orwig, D.; Cobb, R. Hemlock woolly adelgid in New England forests: Canopy impacts transforming ecosystem processes and landscapes. Ecosystems 2005, 8, 233–247. [Google Scholar] [CrossRef]
- Zarter, R.C.; Demmig-Adams, B.; Ebbert, V.; Adamska, I.; Adams, W.W., III. Photosynthetic capacity and light harvesting efficiency during the winter-to-spring transition in subalpine conifers. New Phytol. 2006, 172, 283–292. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).