Abstract
Lignocellulosic biomass needs attention as an alternative energy source to overcome the adverse impacts of fossil fuels. Diversified Galiyat forests of Lower Himalaya may represent the potential source of lignocellulose degrading microbiota, particularly the lytic bacteria. Therefore, soil and wood samples were collected from different sites of Nathiagali and Thandiani of Galiyat forests. The soil samples collected were clayey, with a pH between 6.7 and 7.0, and with an organic matter of 2.8%–2.9% in Nathiagali and 2.1%–2.2% in Thandiani. The soils were enriched with more diversified cultivable bacteria (9 Log CFU/g) than the respective wood samples (7.4–8.6 Log CFU/g). Out of 90 bacteria, 22 isolates were efficient for cellulose degradation, 14 for xylanase activity, and 10 for laccase production. Cluster analysis showed that lignocellulolytic bacteria were grouped based on the sample medium (soil–wood) rather than the sampling site (Thandiani–Nathiagali). Efficient bacteria were also sequenced, and we found that cellulase production was prevalent in Pseudomonas spp. while laccase activity was diverse among taxonomically varied bacteria. Moreover, Stenotrophomonas sp. TS2B1 performed the best for corncob xylose degradation. Overall, the results suggest that Galiyat forests represent diverse lignocellulolytic microbial populations which should be further evaluated for applications in lignocellulosic waste management and for potential consequent fuel production.
1. Introduction
The upsurge in consumption of conventional energy sources is causing adverse impacts on the quality of the environment. It requires renewable and sustainable alternative energy resources to tackle the energy crisis and reduce the impacts of non-renewable energy sources on the environment [1]. In the last few decades, interest in transforming lignocellulosic biomass into biofuels has increased. The first generation of bioethanol (produced from direct fermentation) has been widely used particularly in the US and Brazil. Among the resources for second-generation bioenergy, lignocellulose is favored over the others because it is abundant and relatively easy to process with enzymatic catalysis [2,3].
Lignocellulosic biomass (LCB) is the most suitable alternative to fossil resources as it is composed of agricultural and forest residues, industrial and municipal wastes, and crops dedicated to producing bioenergy (particularly bioethanol). The main composition of lignocellulose is cellulose and hemicellulose backbone, which is bound by lignin moieties [4]. Notably, different components of lignocellulosic biomass have a variety of applications, e.g., glucose—from cellulose—to acquire bioethanol [5]; xylitol—from hemicellulose—as a food additive and pharmaceutics [6]; humic acids and cresol—obtained from lignin—as solvents and cleaners [7,8]. The bonding within and between cellulose, hemicellulose, and lignin forms a composite biopolymer structure in plant biomass [9]. This composite structure is a hindrance to the saccharification process [9]. There are different pre-treatment processes, such as physical, chemical, and biological pre-treatments, that disrupt the composite polymer structure of lignocellulosic biomass [10]. In this regard, biological pre-treatment is considered a sustainable method that does not pollute the environment. This method is comprised of certain microorganisms which can degrade the recalcitrant plant cell wall by releasing hydrolytic enzymes [2].
Until now, numerous bacterial strains have been studied, such as the Actinomycetes taxon employed for the active decomposition of grasses [11,12]. Several other studies showed that bacteria can break cellulosic biomass by producing cellulolytic and xylanolytic enzymes under aerobic and anaerobic conditions [13,14]. In nature, fungi and bacteria are the most important players in plant biomass degradation as they produce enzymes for lignocellulose decomposition [15]. The use of such enzymes from bacteria is preferred over fungi because bacteria are comparatively more cosmopolitan, can adapt to diverse pH and temperature conditions, and have a short generation time compared to fungi. A recent analysis of a microbial community showed that bacteria have significant lignocellulolytic enzyme activity [16].
The lignocellulolytic properties of some bacterial genera, such as Cellulomonas, Cellvibrio, Pseudomonas, Bacillus, and Micrococcus, were reported earlier [17]. The biofuel production from lignocellulosic biomass has some potential, but effective enzymatic hydrolysis is a barrier because the bioconversion requires efficient synergetic actions among released enzymes, i.e., cellulases, xylanases, and laccases [17,18,19]. Additionally, microbes in the conventional conversion processes need high enzyme loading and more incubation time; optimum conditions are thus required in a cost-efficient process for smooth conversions [20].
In this context, the optimum growth conditions of lignocellulolytic organisms and their performance are of prime importance. Biological pre-treatment of biomass and subsequent fermentation is, though, a promising technology, but the process efficiency needs further investigation. One of the strategies could be the isolation of comparatively more efficient organisms and establishing their cooperative mixtures to recruit the performer organisms in more realistic conditions. It is, therefore, more likely that lignocellulosic biomass-enriched ecosystems would provide the microbiota satisfying these prerequisites of biomass pre-treatment. In this regard, forests of the Galiyat region, Abbottabad Pakistan—Lower Himalaya—represent a potential ecosystem with huge floral diversity producing lignocellulosic material. The Himalayan region has recently been reported as a hotspot for diversified microbiota of varied ecosystems, with fluctuating microbial diversity at different sample altitudes [21]. The particular region comprises pristine habitats for the exploration of microorganisms capable of a multitude of functioning. The plant waste—of diversified nature—is continuously recycled in these forests by native microbial strains producing a thick layer of organic matter, which represents an excellent opportunity for the presence of efficient lytic organisms [22], potentially better than the already reported ones in the literature. A pristine environment of the Pangi–Chamba Himalayan (PCH) region was sampled for bioresource exploration and for bacterial diversity investigation to degrade cellulose. Their isolated bacteria belonged to 28 genera representing four different phyla, i.e., Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria [23]. Soil bacterial diversity from other geographical regions of the Himalayan mountains was also studied [24] through culture-independent and culturable approaches. Soils in these Himalayan foothills were dominated by varied bacterial genera, including Exiguobacterium, Arthrobacter, Cedecea, Pseudomonas, Erwinia, and Bacillus. Some of these bacterial genera showed novel hydrolytic enzyme activities as well [24]. Such diversified bacteria—with proficient cellulolytic activity—provide an opportunity for developing a bioprocess for efficient pre-treatment of lignocellulosic biomass.
Furthermore, the presence of efficient lignocellulolytic microbes in such samples [25] is obvious because of the complex biomass degradation therein. In this context, the present study aims to isolate and characterize the microbial strains from Lower Himalayan sampling sites as well as from decaying pine substrates of the region with comparatively better lignocellulose degradation potential. The particular focus is the isolation of bacterial strains from naturally diversified environments of the Lower Himalayas, which is mainly the novelty of this work.
2. Materials and Methods
2.1. Study Area
The samples for this study were collected from the forest sites of the Lower Himalayan region, Nathiagali and Thandiani of District Abbottabad, Pakistan (Figure 1). In Nathiagali, the selected sites were within Ayubia National Park (ANP) forest—reserved under International Union for Conservation of Nature (IUCN)—which is stretched from 33°51 to 34°83 at North latitude and 73°8 to 73°57 at East longitude. ANP is located at 2520 m elevation, which is mostly covered with coniferous species of Pinus wallichiana, Cedrus deodara, and deciduous and medicinal plants. Thandiani is located at 2700 m elevation to the north side of Nathiagali, stretched as 34°17–34°10 N and 73°23–73°17 E, which is mostly covered with Pinus wallianchiana, Pinus ruxburgii, and other natural flora. Specifically, the samples, including degrading wood and adjacent surface soil (up to 5 cm depth), were collected in sterile zip-lock plastic bags maintaining aseptic conditions and were brought to the laboratory for further use and analyses. The sample codes, elevation, and geographical coordinates are given in Table 1.
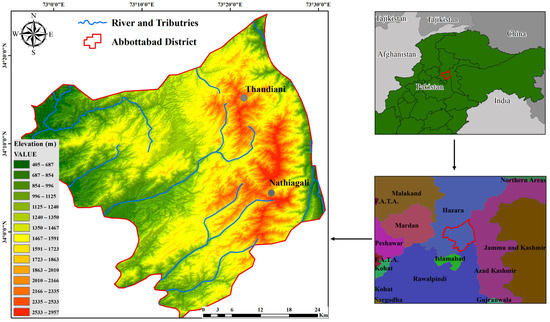
Figure 1.
Geographical map showing the sites in the Lower Himalayan region (Abbottabad–Pakistan) from where the soil and decaying wood samples were collected for lignocellulolytic bacterial isolation.

Table 1.
Description of samples collected in this study from the Lower Himalayan region, Abbottabad, Pakistan.
2.2. Chemical Reagents and Culture Media
All the chemicals used during this work (e.g., CMC-Na, Xylan, and ABTS – Sigma Aldrich, Beijing China) were of analytical grade. The culture media used were nutrient broth (Jinan Biotech, Jinan China): 0.3% beef extract, 0.5% peptone, 12% agar, pH 7.0–7.5; and Luria–Bertani broth (LB, LCC Lahore, Pakistan): yeast extract 5 g/L, peptone 5 g/L, NaCl 10 g/L, pH 6.5–7.0. M9, minimal salts medium (Sigma–Aldrich, Beijing China) composition (g/L) was KH2PO4, 15; NaCl, 2.5; Na2HPO4, 33.9 and NH4Cl, 5. The 100× Stock solution of trace elements included 1100 mg/L FeCl3, 50 mg/L CuSO4, 200 mg/L HBO3, 200 mg/L MnSO4·H2O, 80 mg/L Na2MoO4·2H2O, 60 mg/L CoCl2·6H2O, 90 mg/L ZnSO4·7H2O, and 10 mg/L Na2SeO4. The media and chemical reagents were autoclaved at 121 °C and 15 psi for 15 min before their use for different tests [26].
2.3. Physicochemical Characteristics of Samples
In order to get a uniform soil sample, the larger substances (such as stones and twigs) were removed following air drying. Samples were then ground with a mortar and passed through a 2 mm sieve. The pH of the samples was measured in 1:5 mixtures of soil and deionized water with a standardized pH meter (PHS-550). Soil solutions were shaken for 30 min and then kept static for 5 min before pH measurements. The soil texture (amount of sand, silt, and clay) was evaluated as per previously reported testing protocols [27]. The decaying wood samples were smashed with mortar to harmonize the contents for maximum availability of the microorganisms in subsequent analyses.
2.4. Bacterial Isolation, Screening, and Preservation
Ten grams of homogenized sample (soil or decaying wood) were suspended within 90 mL of sterile 0.85% NaCl solution by stirring for 1 h at 120 rpm in an orbital shaker. Then, the samples were serially diluted (up to 10−6) and plated on nutrient agar (Jinan Biotech, China) with the following composition: yeast extract 2.0 g/L, peptone 5.0 g/L, sodium chloride 5.0 g/L, and agar 15.0 g/L at pH 7. The bacterial growth plates were incubated at 30 °C for 24 h. After the appearance of microbial growth, bacterial colonies were evaluated on the basis of their morphology, such as color, shape, and edge of the colony. In order to get a pure bacterial culture, the single colonies were streaked on nutrient agar plates to get a uniform colony morphology [28]. For a long time storage and further use in different microbiological analyses, the fresh overnight-grown bacterial cultures were mixed as 1:1 with glycerol (50% w/v—DaeJung Chemicals, Siheung-si Republic of Korea) as a −80 °C stock [29].
2.5. Characterization of Isolated Bacterial Strains
Crude enzyme production: To screen for the potential lytic enzyme producers, fresh cultures of bacterial isolates were incubated in LB broth for bacterial growth at 30 °C on shaking (speed 120 rpm) for 24 h. Alternatively, potential lignocellulolytic bacteria were grown in minimal media supplemented (for carbon as an energy source) with 1% CMC, cob corn xylan, and ABTS as inducers for cellulase, xylanase, and laccase enzyme production. Subsequent to the optimal growth, the cell-free culture supernatants were obtained by centrifugation of growth media at 10,000 rpm for 10 min at 4 °C. These culture supernatants were then used as crude extracellular enzyme sources for different enzymatic assays [30], as described in the following section.
Cellulase assay: Cellulase activity within the culture of respective bacterial isolates was evaluated from a 1:1 mixture of crude enzyme and 1% carboxymethyl cellulose (CMC) (prepared in 50 mM sodium phosphate buffer (pH 7.0) (DaeJung Chemicals, Siheung-si Republic of Korea) [17]. The mixture was incubated for 30 min at 35 °C. The released amount of glucose in this mixture was measured by adding 1 mL of this mixture to 3 mL of DNS (3,5-dinitrosalicylic acid, Sigma–Aldrich, Beijing China) following 15 min boiling. The color change in DNS was measured by transferring 250 µL of each enzyme-substrate sample into a 96-well microtiter plate reader, and the absorbance values were recorded at 540 nm wavelength by using a UV-VIS spectrophotometer (FLUO star Omega) [31]. The optical density of the samples was measured against a blank having all the reagents with no enzyme. A standard curve was made by mixing 1 mL of (serially diluted concentration from 0 to 20 mg/mL) glucose with 3 mL DNS and boiling for 15 min. Statistical models were used to infer the concentration of lytic enzyme [32]. The absorbance of these standards was also measured through a UV-VIS spectrophotometer (wavelength 540 nm). One unit of cellulase enzyme activity meant the amount of enzyme reducing 1 μmol of the sugar in 1 min (IU/mL) at pH 7.0 and 35 °C.
Xylanase Assay: All the isolates were screened for xylanase activity using 1% solubilized cob corn xylan as substrate. Microbial culture supernatant containing extracellular enzyme mixture of respective bacteria was let for reaction with xylan substrate in a 1:1 ratio. This mixture (1 mL) was further added to 1.5 mL of DNS reagent (pH 5.5) and incubated at 37 °C for 30 min in a water bath [33]. The absorbance was measured at 540 nm through a UV-VIS spectrophotometer (FLUO star Omega) [31]. The standard graph was established using the analytical grade xylose (0–20 mg/mL). One unit of xylanase activity was considered the amount of enzyme liberating 1 μmol of reducing sugar (equivalent to xylose) per minute from 5.0 mg/mL of xylan at pH 5.5 and 37 °C.
Laccase assay: For laccase activity determination of the isolated bacterial strains, 2, 2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was used as a substrate, prepared in 0.1 M sodium phosphate buffer (pH 6). Five hundred microliter (500 µL) of 0.5 mM ABTS was reacted (for potential oxidation) with 500 µL crude laccase enzyme at room temperature (≈25 °C). The absorbance values were measured at 420 nm wavelength in a microplate reader (FLUO star Omega). For the standard curve, different ABTS concentrations (0–5 mM at pH 6) were mixed (as 1:1) with the enzyme at 25 °C, and the change in absorbance at 420 nm was measured at 1 min intervals until the absorbance became constant. A plot of the final absorbance vs. ABTS concentration in the cuvette was made. One unit of laccase enzyme activity (IU) is the amount of laccase that catalyzes the oxidation of 1 µM ABTS per minute at standard assay conditions, mentioned above [34].
2.6. Phylogenetic Analysis of the Lignocellulose-Degrading Bacterial Isolates
Identification of bacterial strains: Genomic DNA of lignocellulolytic bacteria was extracted as previously reported [35] from selected lignocellulolytic bacteria grown in LB medium. Universal primers for 16S rRNA gene—27-F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492-R (5′-TACGGTTACCTTGTTACGACTT-3′)—were used for amplification of the target gene. The PCR reaction mixture (25 µL) was composed of 0.25 µL of DNA (approximately 15 ng), 2 µL of 2.5 mmol dNTP mixture, 0.5 µL of forward and reverse primer (10 nM) each, 0.25 µL of Taq DNA polymerase (GenScript, Nanjing China), 2.5 µL of buffer solution (10× buffer), and 19 µL of ddH2O. The PCR reaction conditions were: 95 °C denaturation step for 3 min, followed by 25 cycles of 95 °C for 30 s, 50 °C for 30 s, and 72 °C for 1 min, followed by 72 °C for 8 min to allow final extension [36]. The amplified PCR products were observed on 0.8% agarose gel. The purified PCR products were then sent to RCEES-CAS (Beijing, China) for sequencing. The obtained sequences were evaluated with Chromas (version 2.6.6) and subsequently used for alignment, identity inferences, and phylogenetic tree formation using MEGA (version XII). Furthermore, the sequences of these Lower Himalayan lignocellulolytic bacterial isolates were deposited in a public database of NCBI under the accession numbers OR085338–OR085368.
Phylogeny: The obtained sequences were compared with deposited sequences in GenBank using the BLAST program. A phylogenetic tree was also constructed using the neighbor-joining method with MEGA software (version XII). The 16S rRNA gene stretch used to construct this phylogenetic tree covered particularly the v5–v9 regions of heterogeneity. The maximum composite likelihood model was used with the ‘Bootstrap method’ using the replications as 1000, while the values below 50 were not included in the final tree.
2.7. Statistical Analyses
All the data were collected with three biological replicates (at least), and the standard deviations were calculated accordingly. One-way ANOVA using Dunnett’s multiple comparisons tests was performed [32] with Origin2022 to find the microbes with significant enzymatic activity. PCoA analysis was performed for clustering the efficient bacterial isolates into groups using the Vegan library in R.
3. Results
3.1. Physical Characteristics
By determining the percentages of soil particle types (sand, silt, and clay), it was found that all soil samples have clay texture—clayey soil and clayey loam soil (Table 1). There was no significant difference in the pH (p > 0.05) of all soil samples, as the pH values ranged from 6.7 to 7.0. The concentration of organic matter was significantly higher (p < 0.05) in Nathiagali soils (2.8%–2.9%) compared to Thandiani soils (2.1%–2.2%), while the two sampled soils of each sampling site (p > 0.05) did not show any significant difference.
3.2. Enrichment of Lignocelluzlolytic Lower Himalayan Bacterial Strains
The abundance and community diversity of culturable bacteria were evaluated in samples collected from the Lower Himalayan forest sites of Nathiagali and Thandiani (Figure 2). The collected soil samples showed relatively high bacterial numbers (9 Log CFU/g) for both sites. In contrast, the decaying wood samples showed relatively less abundance of culturable bacteria (7.4–8.6 Log CFU/g) particularly for the Thandiani wood samples (Figure 2).
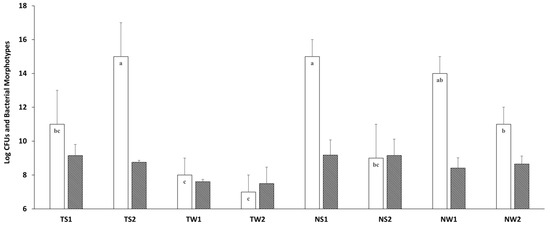
Figure 2.
Bacterial diversity (morphotypes per sample) (white, empty bars) and abundance (Log CFU/g) (shaded grey bars) depicted from different samples of the Lower Himalayan region, Abbottabad, Pakistan. Letters in white bars (a,b,c) indicate different statistical groups for bacterial morphotypes. For strain codes on the x-axis: TS, Thandiani soil; TW, Thandiani wood; NS, Nathiagali soil; NW, Nathiagali wood.
Statistically, collected samples did not show any significant difference in bacterial abundance. The highest bacterial population was observed (Log 9.16 ± 0.07 CFU/g) in TS1, while the lowest bacterial numbers were Log 7.41 ± 0.17 CFU/g in TW1. Interestingly, the cultivable bacterial diversity was found to be significantly different in different samples collected from these sites. Particularly, TS2 and NS1 showed higher bacterial diversity up to 19 bacterial morphotypes, while TW2 showed the least number of bacterial morphotypes 7 (Figure 2), probably because of niche selectivity. Statistically, the bacterial diversity distribution was significantly different at the Thandiani site, both in soil and wood samples.
3.3. Enzymatic Characterization of Lower Himalayan Lignocellulolytic Bacterial Strains
From the soil and decaying wood samples of both sampling sites, a total of 90 bacterial strains were isolated (on the basis of identifiable morphology) by using nutrient agar as a bacterial growth medium. This bacterial collection comprised 44 strains (28 from soil and 16 from decaying wood) from Thandiani and 46 strains (26 from soil and 20 from decaying wood) from Nathiagali (Figure 3). To evaluate the enzymatic potential of these Lower Himalayan lignocellulolytic microorganisms, the bacterial strains were grown overnight in LB broth and tested for cellulase, xylanase, and laccase activity. For further evaluation, a generic criterion of 1.5 IU per ml was selected as a benchmark to consider the bacterial strain as ‘proficient’ for that particular enzyme. As per this criterion, half of our bacterial collection ranked as efficient lignocellulolytic enzyme producers. Precisely, 22, 14, and 10 bacterial strains were proficient in cellulase, xylanase, and laccase enzyme activities (Figure 3). Of these 22 proficient cellulase producers, 10 belonged to N-soil, 5 to N-wood, 4 to T-soil, and 3 to T-wood. Similarly, 7, 2, 3, and 2 showed efficient xylanase production, respectively, from N-soil, N-wood, T-soil, and T-wood samples. Meanwhile, the proficient laccase producers were almost equally distributed in both sampling sites, i.e., 4, 1, 4, and 1 from N-soil, N-wood, T-soil, and T-wood, respectively.
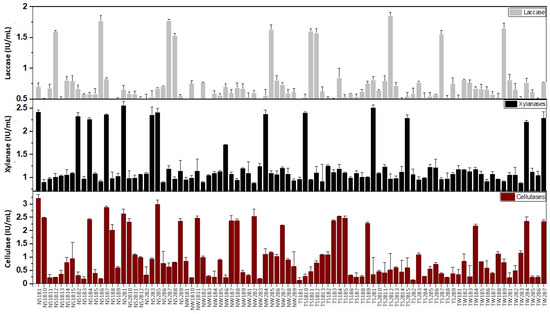
Figure 3.
Cellulase (lower panel), xylanase (middle panel), and laccase (top panel) activities were evaluated within bacterial growth medium (LB) cultivated with bacteria isolated from Lower Himalayan forests of Galiyat Abbottabad, Pakistan.
The PCoA analysis of these lignocellulolytic enzyme-producing bacteria showed five distinguished groups (Figure 4), separated from each other based on sample medium (soil–wood) rather than the sampling site (Thandiani–Nathiagali). A small group on the top right shows three bacterial strains originating from wood, while the top left indicates a group of seven strains from the soil. While both clusters on the bottom are dominated by the bacterial strains originating from the soil, i.e., 10/15 and 8/10 bacteria are from the soil within the bottom left and bottom right group, respectively (Figure 4). The rest of the 55 bacterial strains are clustered in the center of the plot with closeness towards soil and/or wood compared to the site Thandiani and/or Nathiagali.
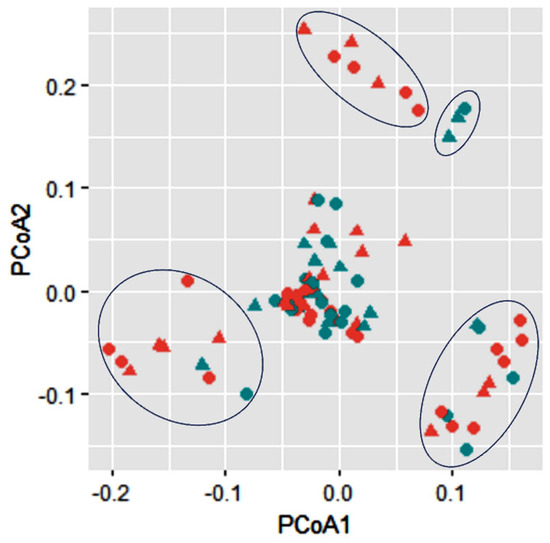
Figure 4.
Cluster analysis (PCoA) for Lower Himalayan bacterial isolates via using the lignocellulytic enzyme potentials. Colors (red and green) indicate the sample mediums (soil and wood, respectively), while the shapes (asterisk and triangle) indicate the sample locations (Nathiagali and Thandiani, respectively).
3.4. Validation of Lignocellulolytic Enzyme Production by Lower Himalayan Bacterial Strains
On the basis of pre-screening results, 46 proficient bacterial strains were re-evaluated for the potential production of lignocellulolytic enzymes within substrate-assisted culture media. A cluster analysis was performed to interrelate the bacterial enzymatic potential with sampling sites and/or the sampling medium (Figure 5). Cellulolytic potentials of the isolated bacterial strains were assayed using the CMC-Na as substrates; 27 strains depicted significant cellulase activity, and most of these efficient bacterial strains were collected from Nathiagali soil. The microbial strain NS1B17 showed the highest cellulase activity (4.99 ± 0.28 IU), followed by NS2B1 (4.57 ± 0.21 IU). To assess the xylanase potential of the isolated bacterial strains, xylan from cob corn was used as substrate. The highest value for xylanase activity was recorded by TS2B1 as 6.66 ± 0.54 IU. The isolates NS1B1 (6.33± 0.32 IU) and NS2B5 (6.31 ± 0.54 IU) were also found to be prominent for xylanase production. Moreover, the ABTS was used as a substrate to determine the laccase production of isolated bacterial strains. The bacterial strains TS1B12 showed the highest laccase activity (2.04 ± 0.23 IU), followed by NS1B6 (1.948 ± 0.09 IU) and NS2B7 (1.947 ± 0.13 IU).
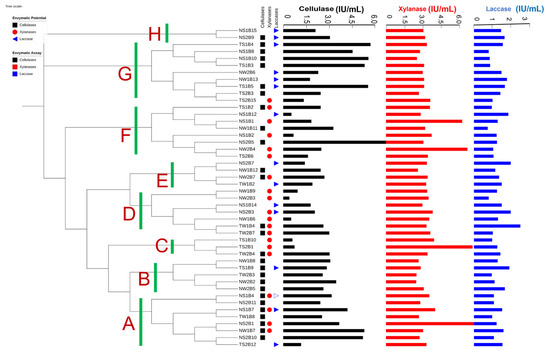
Figure 5.
Cluster analysis of selected proficient Lower Himalayan bacterial isolates using their lignocellulolytic enzyme potentials. A–H are the potential groups based on clustering analysis. Squares, circles, and triangles represent the efficient bacteria for that particular enzyme.
On the basis of lignocellulolytic enzyme efficiencies, 46 pre-screened bacterial strains were further grouped into eight tight-to-loose clusters (A–H). Clusters A, F, and H are dominated by Nathiagali-soil-originating bacteria; clusters B, D, and E are predominately comprised of Nathiagali-wood-originating bacteria. Cluster C is dominated by the sampling site, i.e., Thandiani (3/3) samples rather than the sampling-medium soil (2/3). On the contrary, cluster G is dominated by 7/9 bacteria from the soil compared to 5/9 from sampling site Thandiani. In terms of multi-enzyme proficiency of the isolated Himalayan bacterial strains, the majority (32/46) of bacteria are good for producing one lytic enzyme. Two bacterial strains (NS1B4, NS1B7) exhibited proficient efficiency for all three tested enzymes. For bi-enzymes, four bacteria (TS1B9, TS1B5, TS1B4, and NS2B9) were found proficient for cellulase and laccase, and seven bacterial strains (NS2B1, NW1B7, TW2B4, TW2B7, TW1B4, NW2B7, and TS1B2) showed activity for cellulase and xylanase. One bacterial strain (NS2B3) was found proficient for xylanase and laccase activity.
3.5. Phylogenetic Analysis of Lignocellulolytic Bacterial Strains
The 16S rDNA sequences of the 36 lignocellulolytic bacterial strains isolated from the Lower Himalayan region were compared with the nearest available sequences in the GenBank database. The closest relative accession number and % identity of these lignocellulolytic bacterial strains are shown in Table 2. The BLASTN and phylogenetic analysis showed that the predominant bacterial genus isolated from the Galiyat region of Lower Himalaya is Pseudomonas, with 36% (13/36) representation in our bacterial collection (Figure 6).

Table 2.
Enzymatic potential and % identity of lignocellulolytic Himalayan bacterial strains with closest relatives’ accession numbers.
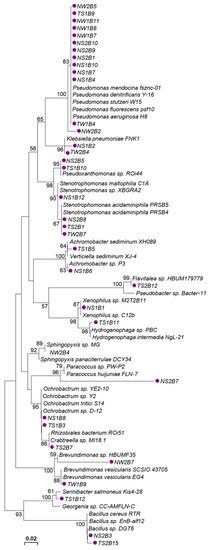
Figure 6.
Phylogenetic analysis of the lignocellulolytic Lower Himalayan bacterial strains, based on their 16 s rRNA gene sequences. The tree is constructed via the neighbor-joining method considering the Maximum Composite Likelihood model with Bootstrap replicates of 1000, and bootstrap values equal to or greater than 50 are shown.
For the phylogenetic clustering, soil—as a sampling medium—served to dominate over the sampling site location, as 7/13 Pseudomonas, 2/2 Pseudoxanthomonas, 3/4 Stenotrophomonas, 2/2 Achromobacter, 2/2 Xenophilus, 2/2 Ochrobactrum, and 2/2 Bacillus strains originated from the soil while one cluster of 2/2 Brevundimonas strains originated from decaying wood of either Nathiagali or Thandiani site (Figure 6).
Particularly, the Pseudomonas species strains were found dominant (12/16) for cellulase enzyme production (Table 2) and activity (e.g., NS1B7) (Table 3). Two Bacillus strains were found proficient for xylanase activity, while the highest performance was noted for Stenotrophomonas sp. TS2B1 (Table 2 and Table 3). Interestingly (and contrary to the cellulase enzyme activity), laccase production was exhibited by the most diverse group of current bacterial collection, i.e., nine different phylogenetic types of bacteria within ten sequenced laccase-positive strains (Table 2), while Flavitalea sp. TS2B12 was found to be the most efficient for laccase activity (Table 3).

Table 3.
Proficient bacterial isolates are capable of lignocellulolytic enzymes’ production by using different substrates.
4. Discussion
The distribution of bacterial community, as a proxy of function in an ecosystem, depends on different factors such as pH, organic matter, soil texture, mean annual temperature, moisture content, amount and type of nutrition, and altitude of the area [22]. Based on our results, lignocellulosic biomass degrading potential bacteria were abundant in the Galiyat forests of Lower Himalaya (Figure 2). Niche differentiation would further have served for inhabiting microorganisms to selectively adopt some of these ecosystem variables [37,38]. Indeed, natural selection might dictate the most special organisms in a specific habitat (‘Origin of species’ theory) as decaying wood in the case of the current study. The data depicts that decaying wood samples have more selectivity and specificity of lignocellulolytic bacteria than the diversity present in nearby soil samples (Figure 3, Figure 4 and Figure 5). Jiménez et al. (2017) proposed that the niche availability and succession dynamics of plant biomass degradation are influenced by spatiotemporal dynamics in fluxes of nutrition, quality, and quantity of available C-substrates, and the ecological interactions of the participating microorganisms. Furthermore, the plant biomass deconstruction and systematic sugar release are controlled directly by microbial activities, which are potentially linked to the numbers (cell biomass and species), metabolic traits, and the level of their interactions [39].
For biological biomass pre-treatment, enzymatic hydrolysis can be a biotransformation strategy functioning via cell-driven microbial suspensions [40]. In this context, acidic medium conditions were reported for the hydrolysis stage of biological biomass pre-treatment [40]. The pH fluctuations were also observed in the incubating medium during the nixtamalized maize pericarp degradation, starting with the initial reduction in pH to less than 6 [41]. In this regard, the acidic nature of our sampled soil indicates the potential enrichment of such lignocellulose degrading microbial community in the Galiyat forests of Lower Himalaya. Wang et al. (2021) observed that the lignocellulolytic microbial consortium showed more efficient degradation of wheat straw at acidic pH compared to neutral growth conditions. Moreover, another study observed slightly acidic conditions as the optimum pH (such as ~6.0) for laccase enzyme to oxidize ABTS and 2, 6-DMP substrates [42].
Due to the inherent heterogeneity and complexity of lignocellulosic biomass, efficient biodegradation thus requires the action of different hydrolytic enzymes. Functional analyses revealed that bacteria were potentially involved in the deconstruction of cellulose, xylan, and lignin-derived chemical compounds. In accordance with our findings, a previous study also showed high cellulase activity among bacterial isolates belonging to the genera Pseudomonas, Achromobacter, and Brevundimonas [43]. Kumar and colleagues (2015) found that Pseudomonas sp. R-28 degraded the filter paper and pure cellulosic waste up to 96% and 95%, respectively. Achromobacter xylosoxidans showed high cellulase activity, due to which it is considered the best candidate for the deinking process [44]. Brevundimonas vesicularis bacterium, isolated from the gut of Dendroctonus armandi, also had cellulolytic potential [45]. In the context of these studies, the bacterial genera found in this study have proficient cellulase enzymatic activities and would also have the potential to degrade lignocellulosic biomass for further use in future applications.
Xylanases are the major hemicellulose depolymerizing enzymes [46]. Serrano-Gamboa and colleagues (2019) reported that the native microbial community degraded nixtamalized maize pericarp through the production of xylanase and cellulase enzymes, while the highest enzymatic activity (12.45 IU) was noted for xylanase. Microbacterium sp. and Bacillus spp. were the most abundant bacterial species, which showed efficient activity for decomposition processes. The literature showed that Bacillus cereus SaH-05 (isolated from the farmland environment of Fars province, Iran) is an efficient xylanase producer [47]. Klebsiella pneumoniae was isolated from the gut of Bombyx mori and showed xylanase activity with oat spelt xylan as substrate [48]. In our study, we found that Bacillus and Klebsiella were efficient in xylanase production (Table 2). Two novel bacterial strains of Pseudoxanthomonas sp. were found as potential xylanase producers, and so these xylanase-producing bacteria can be used for hemicellulose degradation and the production of different value-added products in the future.
Moreover, we observed that diverse bacterial genera, e.g., Pseudomonas, Rhizobiales, and Stenotrophomonas showed laccase potential. These laccase enzymes oxidize a variety of chemicals (ABTS as well as lignin) and have been potentially found in diverse bacteria [42], including Pseudomonas and Stenotrophomonas (Table 2). The Pseudomonas and Baccillus bacterial strains can also play a role in lignin modification [44]. Díaz-García et al. (2022) identified an association between bacterial taxonomy and functions by establishing an understanding of lignin transformations within soil-derived lignocellulolytic bacteria. They enriched lignin transforming enzymes from three different soil-originated microbial consortia (grown on plant biomass—wheat straw, switchgrass, and corn stover) and found taxonomic affiliation of these enzymes with bacterial families such as Pseudomonadaceae and Xanthomonadaceae. Similarly, the bacterial strains—belonging to these families—are observed in the current study as well for their potential laccase activity in lignin catabolism [49]. In another lignocellulolytic consortium, different novel bacterial taxa, comprising mostly (>90%) Pseudomonas, Pristimantibacillus, and Ochrobactrum, were found with their lignin transformation potential [50].
Although microbes have evolved differently, complementary mechanisms are still used to degrade the lignocellulosic biomass. These microbial mechanisms may comprise intricate and complex processes that may vary in varying ecosystems and/or environmental conditions. The lignocellulolytic microorganisms thus rely on simple-to-complex arrangements of lytic enzymes to execute biomass degradation [51]. The potential of synthetic microbial consortia was examined during a wheat–straw degradation study, which found that monocultures mainly consumed cellulose parts of the tested substrate [52]. Ochrobactrum, Achromobacter, and Stenotrophomonas bacteria were part of the wheat–straw-degrading microbial community [52]. A microbial consortium, as a synthetic community—including Stenotrophomonas sp. and Brevundimonas sp.—was found efficient for lignocellulosic biomass degradation [53]. We found that similar strains were efficient for lytic enzyme production, which depicts their potential for further biomass degradation in solo as well as in combinatorial application fashions.
In mountainous landscapes, the habitats at varied elevations are characterized by areal extent, connectivity, and key biodiversity drivers. Bertuzzo et al. (2016) investigated the role of geomorphology and connectivity of landscape elevation in shaping the species richness patterns. They found that Acidobacteria, Actinobacteria, and Proteobacteria were dominant in high-altitude cold environments, while Firmicutes and Bacteroidetes were observed at lower altitudes of the Himalayas [22]. In our study, the lignocellulolytic bacterial strains, especially Pseudomonas and Bacillus genera, also validate the prevalence of Proteobacteria and Firmicutes in the Himalayan forest habitats of Galiyat Abbottabad. Moreover, forest waste biomass can potentially be used for value-added industrial products by using this renewable material as a production substrate [54]. Biomass pre-treatment of varied lignocellulosic organic material is a mandatory step for improved feedstock biodegradability prior to any downstream process, such as anaerobic digestion (AD) or ethanol production. Ferdes et al. (2020) have recently reviewed various microbial extracellular enzymes for lignocellulose degradation, and our taxonomic associations of lytic bacteria are part of their collection [55].
5. Conclusions
Lower Himalayan Galiyat forest soil and wood samples were collected from two different sites of Nathiagali and Thandiani with respectively clayey and clayey loam soil textures, 6.7 to 7.0 pH, and 2.85% and 2.15% organic matter content. Soil samples were found enriched with diversified cultivable bacteria compared with respective wood samples. Of 90 bacterial isolates, 22 were efficient for cellulase production, 14 for xylanase activity, and 10 for laccase enzyme production. The sample medium (soil–wood) contributed more than the sampling site (Thandiani–Nathiagali) for the clustering of isolated lignocellulolytic bacteria. Cellulase production was found to be prevalent in Pseudomonas spp. while laccase activity was diverse among various taxonomic bacterial species. Moreover, Stenotrophomonas sp. presented the best xylanase activity. Overall, Galiyat forests represent diverse lignocellulolytic microbial populations that require further evaluation for application in potential fuel production and (biomass) waste management.
Author Contributions
Conceptualization, M.O.U.A. and R.N.; methodology, M.O.U.A. and M.I.; software, A.I. and M.I.R.; validation, M.O.U.A., G.O. and R.N.; formal analysis, M.O.U.A.; investigation, M.O.U.A. and U.I.; resources, I.M. and R.N.; data curation, M.O.U.A. and F.H.; writing—original draft preparation, M.O.U.A. and R.N.; writing—review and editing, M.I., R.N. and F.U.; visualization, M.O.U.A. and R.N.; supervision, A.I. and R.N.; project administration, R.N.; funding acquisition, I.M. and R.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Higher Education Commission (HEC) of Pakistan through NRPU 20-3657/R&D/HEC/14/704 for MOUA support and partial experimental assistance.
Data Availability Statement
The data presented in this study were handled by SPSS v25 and Origin 2021 for graphical representation, while the raw data are available upon request from the corresponding author.
Acknowledgments
The authors acknowledge the technical support from the department during various phases of experimentation. The role of Emilie Widemann is further acknowledged for the English proofreading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bilgen, S. Structure and environmental impact of global energy consumption. In Renewable and Sustainable Energy Reviews; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 38, pp. 890–902. [Google Scholar] [CrossRef]
- Ramos, M.D.N.; Milessi, T.S.; Candido, R.G.; Mendes, A.A.; André Aguiar, A. Enzymatic catalysis as a tool in biofuels production in Brazil: Current status and perspectives. Energy Sustain. Dev. 2022, 68, 103–119. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; Van Zyl, W.H.; Pretorius, I.S. Microbial Cellulose Utilization: Fundamentals and Biotechnology. Bioresour. Technol. 2002, 66, 506–577. [Google Scholar]
- Oke, M.A.; Annuar, M.S.M.; Simarani, K. Mixed Lignocellulosic Biomass Degradation and Utilization for Bacterial Cellulase Production. Waste Biomass Valorization 2017, 8, 893–903. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass—An overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef]
- Huntley, N.F.; Patience, J.F. Xylose: Absorption, fermentation, and post-absorptive metabolism in the pig. J. Anim. Sci. Biotechnol. 2018, 9, 4. [Google Scholar] [CrossRef]
- Jeong, H.J.; Cha, J.-Y.; Choi, J.H.; Jang, K.-S.; Lim, J.; Kim, W.-Y.; Seo, D.-C.; Jeon, J.-R. One-Pot Transformation of Technical Lignins into Humic-Like Plant Stimulants through Fenton-Based Advanced Oxidation: Accelerating Natural Fungus-Driven Humification. ACS Omega 2018, 3, 7441–7453. [Google Scholar] [CrossRef]
- Yu, O.; Kim, K.H. Lignin to materials: A focused review on recent novel lignin applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Shen, P.; Murphy, D.V.; George, S.J.; Lapis-Gaza, H.; Xu, M.; Gleeson, D.B. Increasing the size of the microbial biomass altered bacterial community structure which enhances plant phosphorus uptake. PLoS ONE 2016, 11, e0166062. [Google Scholar] [CrossRef]
- Cragg, S.M.; Beckham, G.T.; Bruce, N.C.; Bugg, T.D.H.; Distel, D.L.; Dupree, P.; Etxabe, A.G.; Goodell, B.S.; Jellison, J.; McGeehan, J.E.; et al. Lignocellulose degradation mechanisms across the Tree of Life. Curr. Opin. Chem. Biol. 2015, 29, 108–119. [Google Scholar] [CrossRef]
- Patel, R.J.; Bhaskaran, L. Screening of novel ascomycetes for the production of laccase enzyme using different lignin model compounds. Int. J. Pharma Bio Sci. 2016, 7, 452–458. [Google Scholar] [CrossRef]
- Wongfaed, N.; O-Thong, S.; Sittijunda, S.; Reungsang, A. Taxonomic and enzymatic basis of the cellulolytic microbial consor tium KKU- MC1 and its application in enhancing biomethane production. Sci. Rep. 2023, 13, 2968. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Mathur, A.S.; Gupta, R.P.; Barrow, C.J.; Tuli, D.K.; Puri, M. Enzyme systems of thermophilic anaerobic bacteria for lignocellulosic biomass conversion. Int. J. Biol. Macromol. 2021, 168, 572–590. [Google Scholar] [CrossRef] [PubMed]
- Bredon, M.; Dittmer, J.; Noël, C.; Moumen, B.; Bouchon, D. Lignocellulose degradation at the holobiont level: Teamwork in a keystone soil invertebrate 06 Biological Sciences 0605 Microbiology. Microbiome 2018, 6, 162. [Google Scholar] [CrossRef]
- Chukwuma, O.B.; Rafatullah, M.; Tajarudine, H.A.; Ismail, N. A review on bacterial contribution to lignocellulose breakdown into useful bio-products. Int. J. Environ. Res. Public Health 2021, 18, 6001. [Google Scholar] [CrossRef]
- Sethi, S.; Datta, A.; Gupta, B.L.; Gupta, S. Optimization of Cellulase Production from Bacteria Isolated from Soil. In International Scholarly Research Notices; Hindawi Publishing Corporation: London, UK, 2013; Volume 2013. [Google Scholar]
- Behera, B.C.; Singdevsachan, S.K.; Mishra, R.; Dutta, S.; Thatoi, H.N. Diversity, mechanism and biotechnology of phosphate solubilising microorganism in mangrove—A review. Biocatal. Agric. Biotechnol. 2014, 3, 97–110. [Google Scholar] [CrossRef]
- Curran, L.L.K.; Pham, L.T.M.; Sale, K.L.; Simmons, B.A. Review of advances in the development of laccases for the valorization of lignin to enable the production of lignocellulosic biofuels and bioproducts. Biotechnol. Adv. 2022, 54, 107809. [Google Scholar] [CrossRef]
- Arora, R.; Behera, S.; Sharma, N.K.; Kumar, S. Bioprospecting thermostable cellulosomes for efficient biofuel production from lignocellulosic biomass. Bioresour. Bioprocess. 2015, 2, 38. [Google Scholar] [CrossRef]
- Breisha, G.Z. Production of 16% ethanol from 35% sucrose. Biomass Bioenergy 2010, 34, 1243–1249. [Google Scholar] [CrossRef]
- Jaggi, V.; Brindhaa, N.T.; Sahgal, M. Microbial diversity in north western Himalayan agroecosystems: Functions and applications. Microbiol. Adv. High. Alt. Agro-Ecosyst. Sustain. 2020, 2020, 135–161. [Google Scholar]
- Thakur, V.; Kumar, V.; Kumar, S.; Singh, D. Diverse culturable bacterial communities with cellulolytic potential revealed from pristine habitat in Indian trans-Himalaya. Can. J. Microbiol. 2018, 64, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, S.; Gowdaman, V.; Prabagaran, S.R. Culturable and Culture-Independent Bacterial Diversity and the Prevalence of Cold-Adapted Enzymes from the Himalayan Mountain Ranges of India and Nepal. Microb. Ecol. 2014, 69, 472–491. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.; Saleem, A.; Malik, A.M. Litter Fall Production and Decomposition in Deodar Forest Ecosystem. Pak. J. Agric. Res. 2019, 32, 441. [Google Scholar] [CrossRef]
- Hayat, W.; Aman, H.; Irshad, U.; Azeem, M.; Iqbal, A.; Nazir, R. Analysis of ecological attributes of bacterial phosphorus solubilizers, native to pine forests of Lower Himalaya. Appl. Soil Ecol. 2017, 112, 51–59. [Google Scholar] [CrossRef]
- Rahim, I.; Ali, S.M.; Aslam, M. GIS Based Landslide Susceptibility Mapping with Application of Analytical Hierarchy Process in District Ghizer, Gilgit Baltistan Pakistan. J. Geosci. Environ. Prot. 2018, 06, 34–49. [Google Scholar] [CrossRef]
- Seo, J.K.; Park, T.S.; Kwon, I.H.; Piao, M.Y.; Lee, C.H.; Ha, J.K. Characterization of cellulolytic and xylanolytic enzymes of Bacillus licheniformis JK7 isolated from the rumen of a native Korean goat. Asian-Australas. J. Anim. Sci. 2013, 26, 50–58. [Google Scholar] [CrossRef]
- Malini, B.; Revathi, M.; Yadav, A.; Sakthivel, N. Purification and Characterization of a Thermophilic Cellulase from a Novel Cellulolytic Strain, Paenibacillus barcinonensis. J. Microbiol. Biotechnol. 2012, 22, 1501–1509. [Google Scholar]
- Khatiwada, P.; Ahmed, J.; Sohag, M.H.; Islam, K.; Azad, A.K. Isolation, Screening and Characterization of Cellulase Producing Bacterial Isolates from Municipal Solid Wastes and Rice Straw Wastes. J. Bioprocess. Biotech. 2016, 6, 4–8. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lee, S.; Garcia, S.; Qian, X. Modeling and Simulation of the Mixed Mode Ventilation Strategies with Heat Recovery and Energy Recovery Wheels for Energy Conservation and IAQ Improvement in the Commercial Buildings. In Recent Researches in Urban Sustainability. Architecture and Structures; Morgan State University: Baltimore, MD, USA, 2013; pp. 209–215. [Google Scholar]
- Kamble, R.D.; Jadhav, A.R. Isolation, purification, and characterization of xylanase produced by a new species of bacillus in solid state fermentation. Int. J. Microbiol. 2012, 2012, 683193. [Google Scholar] [CrossRef]
- Bagewadi, Z.K.; Mulla, S.I.; Ninnekar, H.Z. Optimization of laccase production and its application in delignification of biomass. Int. J. Recycl. Org. Waste Agric. 2017, 6, 351–365. [Google Scholar] [CrossRef]
- Zhang, C.; Kong, F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl. Soil Ecol. 2014, 82, 18–25. [Google Scholar] [CrossRef]
- Woo, H.L.; Hazen, T.C.; Simmons, B.A.; DeAngelis, K.M. Enzyme activities of aerobic lignocellulolytic bacteria isolated from wet tropical forest soils. Syst. Appl. Microbiol. 2014, 37, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Balasundaram, S.V.; Bakkemo, R.I.; Drula, E.; Henrissat, B.; Högberg, N.; Skrede, I. Niche differentiation and evolution of the wood decay machinery in the invasive fungus Serpula lacrymans. ISME J. 2021, 15, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Nuccio, E.E.; Starr, E.; Karaoz, U.; Brodie, E.L.; Zhou, J.; Tringe, S.G.; Malmstrom, R.R.; Woyke, T.; Banfield, J.F.; Firestone, M.K.; et al. Niche differentiation is spatially and temporally regulated in the rhizosphere. ISME J. 2020, 14, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.J.; Dini-Andreote, F.; DeAngelis, K.M.; Singer, S.W.; Falcão Salles, J.; van Elsas, J.D. Ecological Insights into the Dynamics of Plant Biomass Degrading Microbial Consortia. Trends Microbiol. 2017, 25, 788–796. [Google Scholar] [CrossRef]
- Menzel, T.; Neubauer, P.; Junne, S. Role of microbial hydrolysis in anaerobic digestion. Energies 2020, 13, 5555. [Google Scholar] [CrossRef]
- Serrano-Gamboa, J.G.; Rojas-Herrera, R.A.; González-Burgos, A.; Folch-Mallol, J.L.; Jiménez, D.J.; Sánchez-González, M.N. Degradation profile of nixtamalized maize pericarp by the action of the microbial consortium PM-06. AMB Express 2019, 9, 85. [Google Scholar] [CrossRef]
- Berini, F.; Casciello, C.; Marcone, G.L.; Marinelli, F. Metagenomics: Novel enzyme from non- culturable microbes. FEMS Microbiol. Lett 2017, 364, 21. [Google Scholar] [CrossRef]
- Zhang, X.; Borjigin, Q.; Gao, J.L.; Yu, X.F.; Hu, S.P.; Zhang, B.Z.; Sheng-Cai Han, S.C. Community succession and functional prediction of microbial consortium with straw degradation during subculture at low temperature. Sci. Rep. 2022, 12, 20163. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Xian, H.; Zhang, X. Newly isolated cellulose-degrading bacterium achromobacter xylosoxidans L2 has deinking potential. BioResources 2019, 14, 2256–2268. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.; Wang, C.; Chen, H. Cellulolytic Bacteria Associated with the Gut of Dendroctonus armandi Larvae (Coleoptera: Curculionidae: Scolytinae). Forests 2014, 5, 455–465. [Google Scholar] [CrossRef]
- Álvarez, C.; Reyes-Sosa, F.M.; Díez, B. Enzymatic hydrolysis of biomass from wood. Microb. Biotechnol. 2016, 9, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, Y.; Sadeghi, H.; Gholami, A.; Mohkam, M.; Kargar, M. Isolation and Identification of Highly Xylanase Producing Bacterium Sphingobacterium sp. SaH-05 from Soil. Int. J. Sci. Eng. Res. 2014, 5, 205–209. [Google Scholar]
- Anand, A.A.P.; Vennison, S.J.; Sankar, S.G.; Gilwax, D.I.; Vasan, P.T.; Raghuraman, T.; Geoffrey, C.J.; Vendan, S.E. Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose, xylan, pectin and starch and their impact on digestion. J. Insect Sci. 2010, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Berini, F.; Verce, M.; Ausec, L.; Rosini, E.; Tonin, F.; Pollegioni, L.; Mandić-Mulec, I. Isolation and characterization of a heterologously expressed bacterial laccase from the anaerobe Geobacter metallireducens. Appl. Microbiol. Biotechnol. 2018, 102, 2425–2439. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Reis, C.E.; Hu, B. Vinasse from sugarcane ethanol production: Better treatment or better utilization? Front. Energy Res. 2017, 5, 7. [Google Scholar] [CrossRef]
- Bomble, Y.J.; Lin, C.Y.; Amore, A.; Wei, H.; Holwerda, E.K.; Ciesielski, P.N.; Donohoe, B.S.; Decker, S.R.; Lynd, L.R.; Himmel, M.E. Lignocellulose deconstruction in the biosphere. Curr. Opin. Chem. Biol. 2017, 41, 61–70. [Google Scholar] [CrossRef]
- Tolalpa, L.; Salles, J.F.; van Elsas, J.D. Bacterial Synergism in Lignocellulose Biomass Degradation—Complementary Roles of Degraders As Influenced by Complexity of the Carbon Source. Front. Microbiol. 2017, 8, 1628. [Google Scholar] [CrossRef]
- Puentes-Téllez, P.E.; Falcao Salles, J. Construction of Effective Minimal Active Microbial Consortia for Lignocellulose Degradation. Microb. Ecol. 2018, 76, 419–429. [Google Scholar] [CrossRef]
- Azeem, M.; Borg-Karlson, A.K.; Rajarao, G.K. Sustainable bio-production of styrene from forest waste. Bioresour. Technol. 2013, 144, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Ferdes, M.; Dincă, M.N.; Moiceanu, G.; Zăbavă, B.S.; Paraschiv, G. Microorganisms and Enzymes Used in the Biological Pretreatment of the Substrate to Enhance Biogas Production: A Review. Sustainability 2020, 12, 7205. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).