Ammoniacal Zinc Borate for Wood Protection against Fungi and Insects
Abstract
1. Introduction
2. Materials and Methods
2.1. Preservative Suspension
2.2. Strains and Culture Conditions
2.3. Wood Decay Test: Conditioning and Impregnation
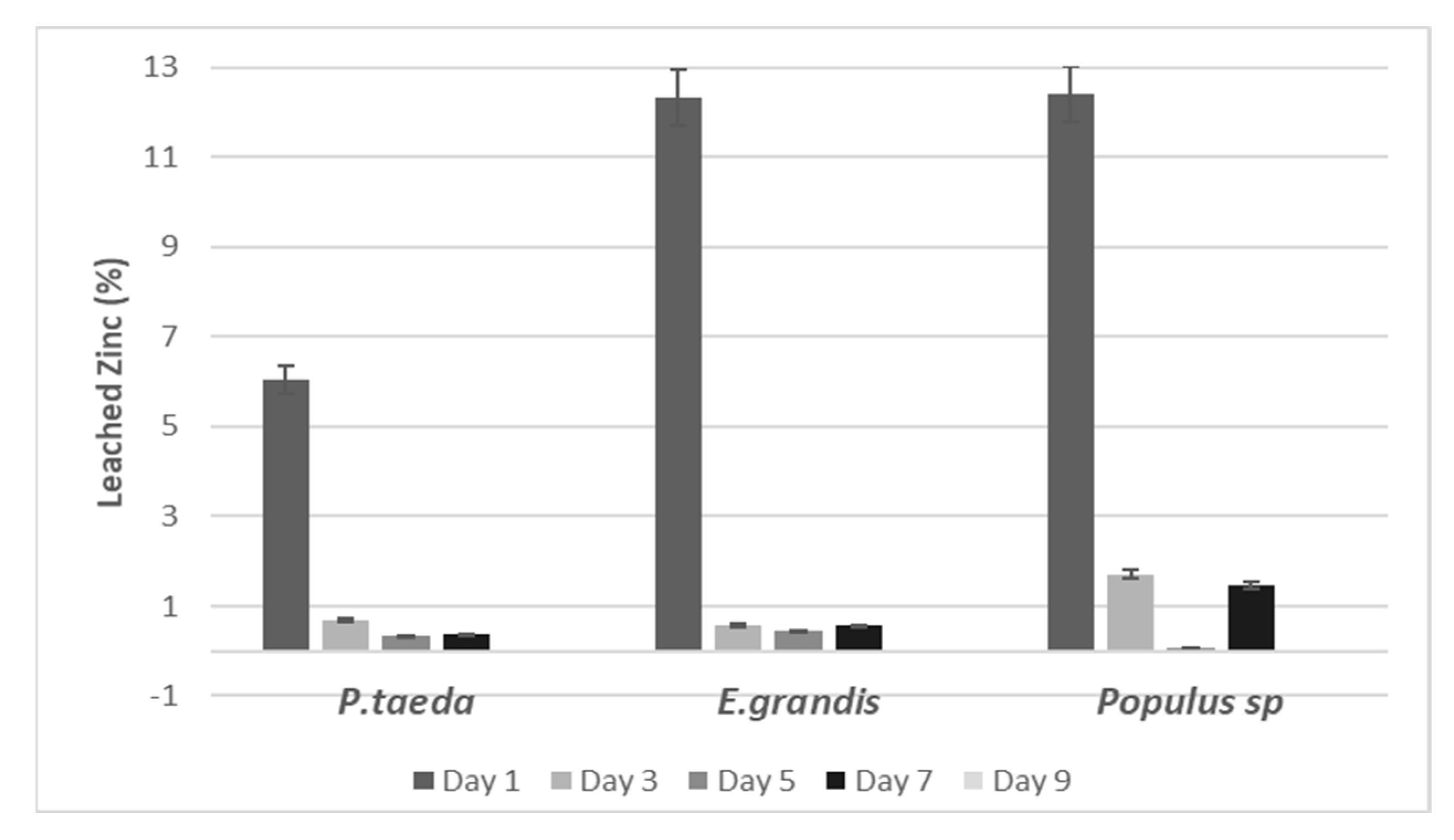
2.4. Leaching Test
2.5. Quantification of Leached Zinc
2.6. Insect Larvae
2.7. Resistance Test to Anobium punctatum
2.8. SEM Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. ZnB in the Wood
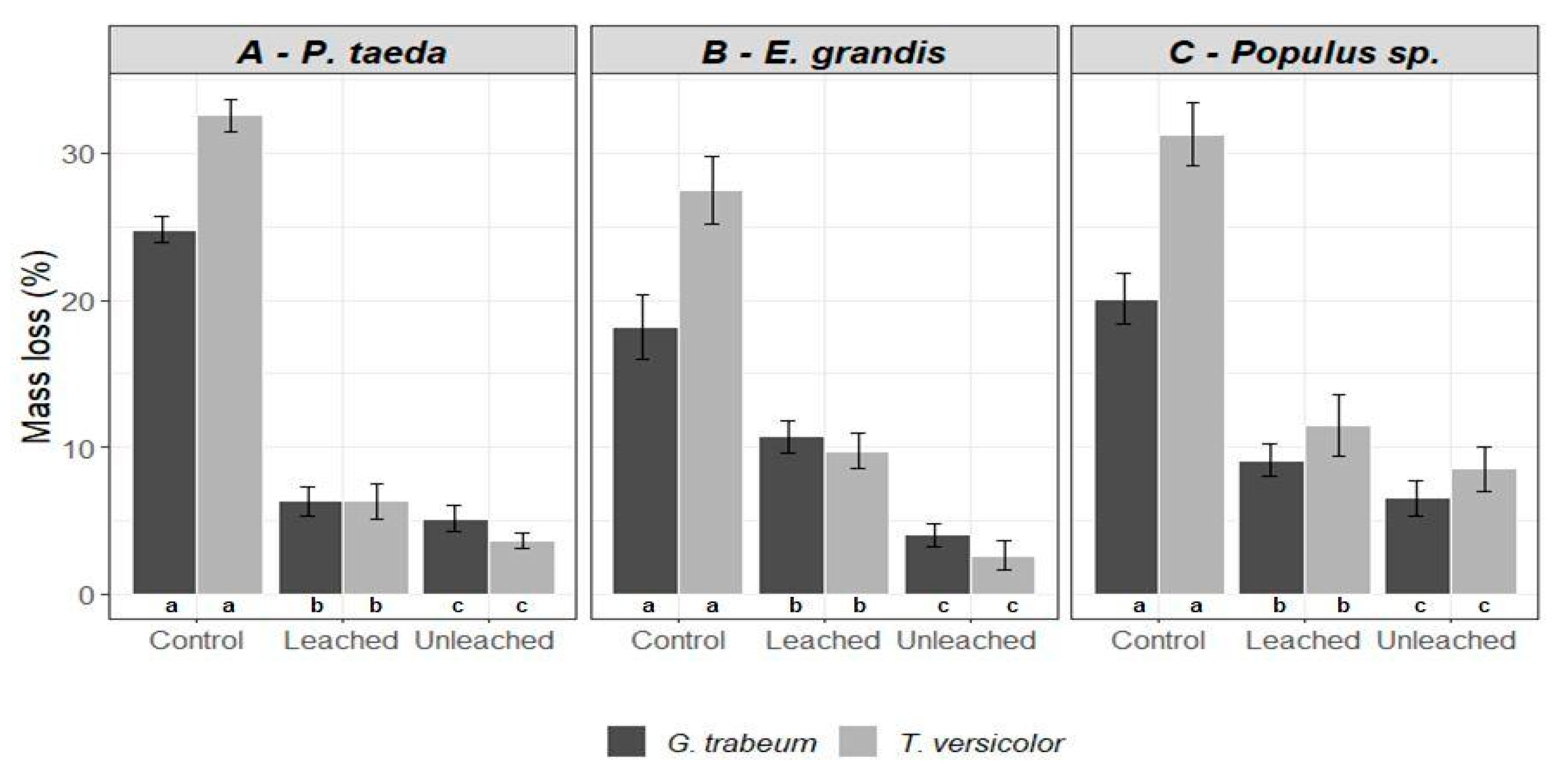
3.2. Wood Decay Test
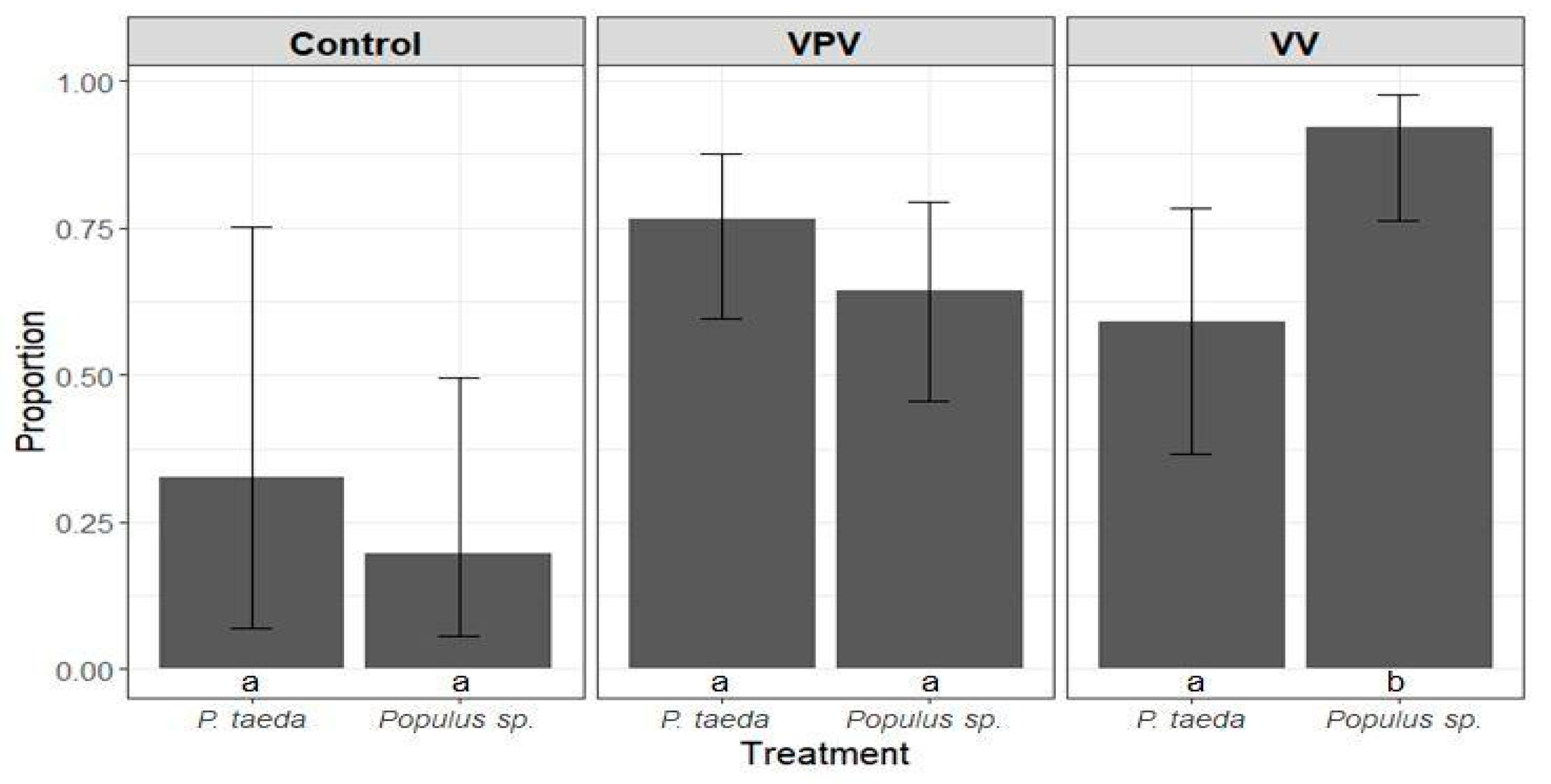
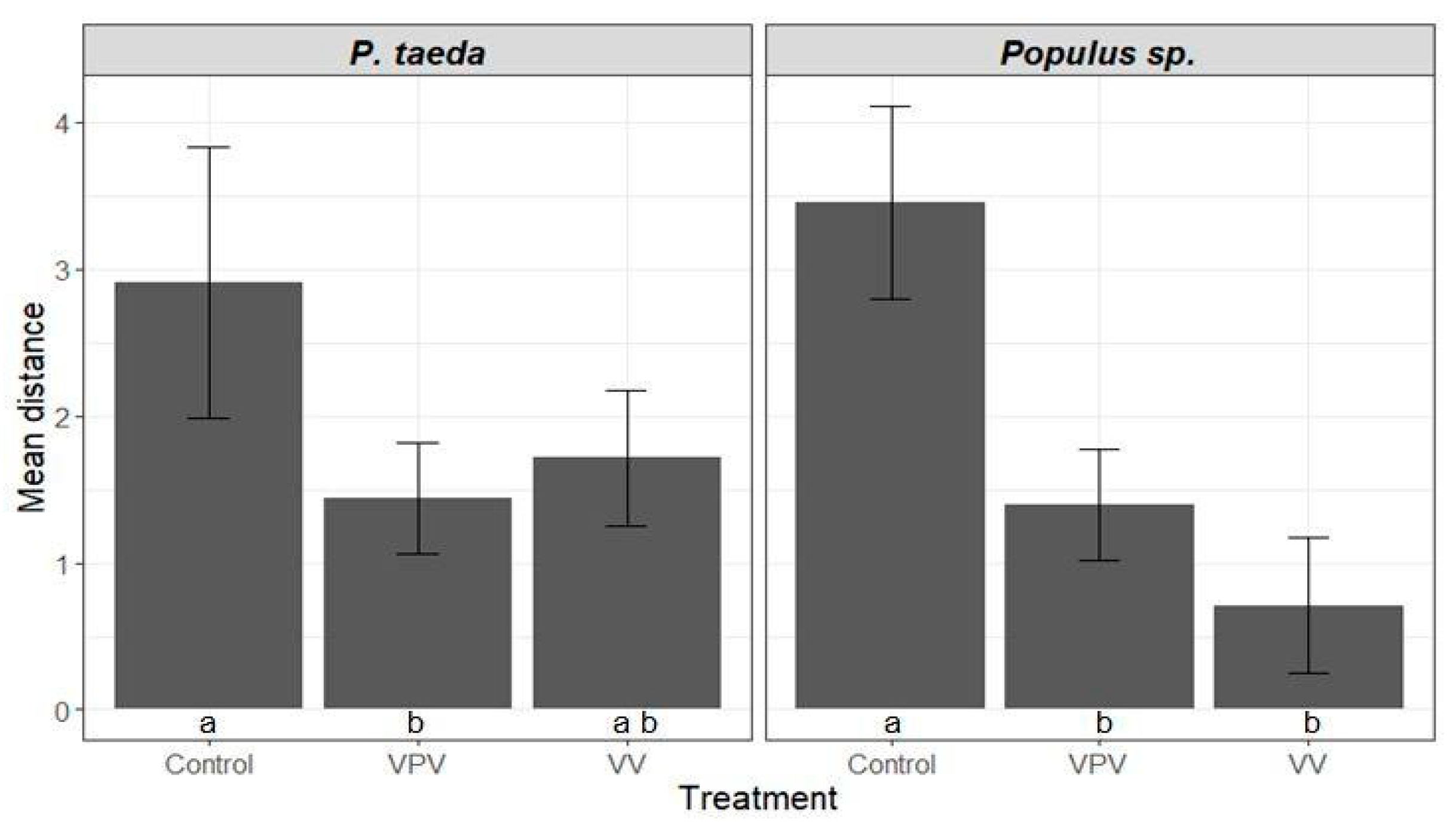
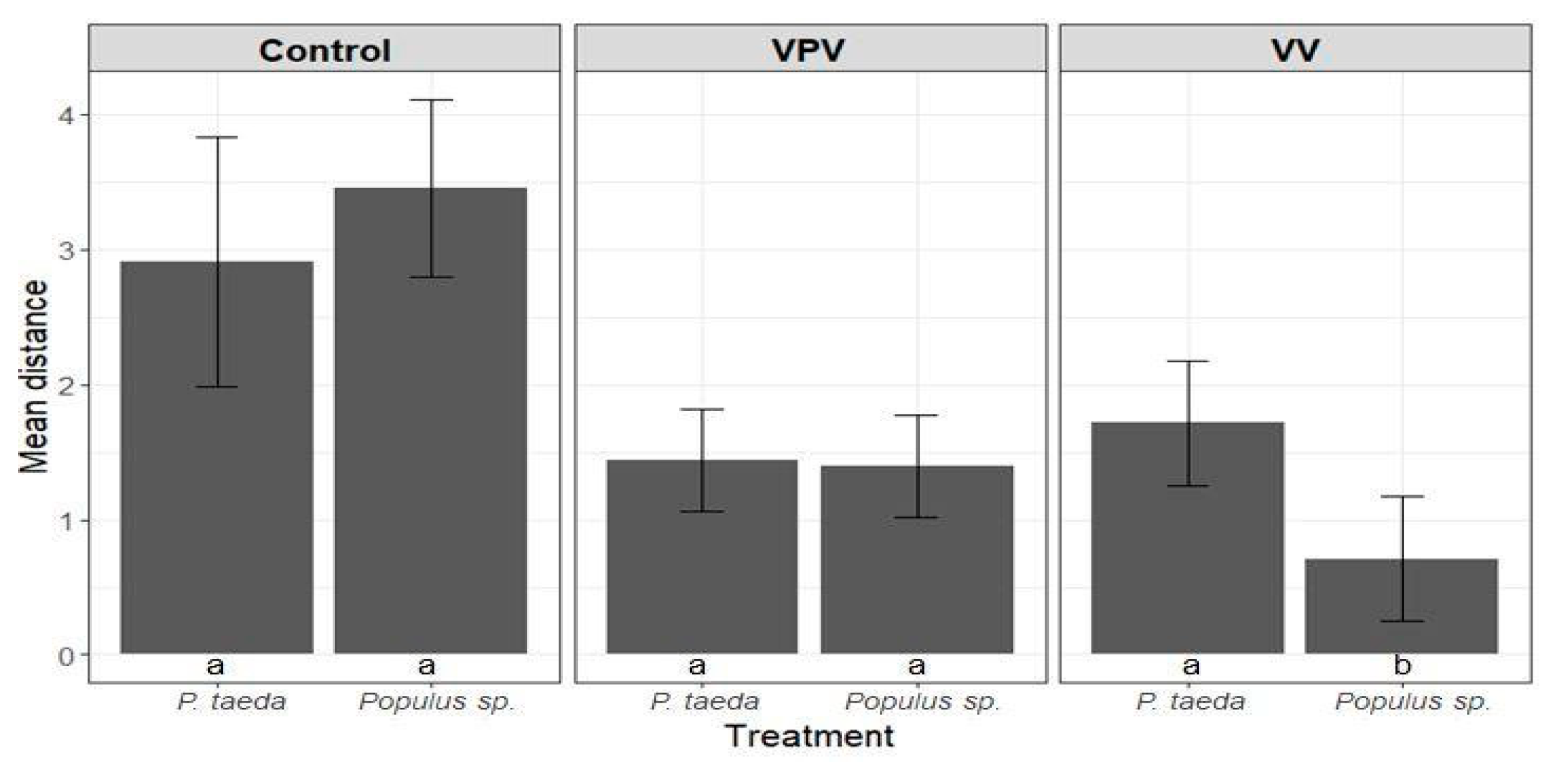
3.3. Anobium punctatum Resistance Test
3.3.1. Larvae Mortality Analysis
3.3.2. Length of the Gallery in Longitudinal Direction Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zabel, R.; Morrell, J. Wood Microbiology: Decay and Its Prevention, 2nd ed.; Elsevier: San Diego, CA, USA, 2020; p. 476. [Google Scholar]
- Goodell, B.; Winandy, J.E.; Morrell, J.J. Fungal Degradation of Wood: Emerging Data, New Insights and Changing Perceptions. Coatings 2020, 10, 1210. [Google Scholar] [CrossRef]
- Marais, B.N.; Brischke, C.; Militz, H. Wood durability in terrestrial and aquatic environments–A review of biotic and abiotic influence factors. Wood Mater. Sci. Eng. 2022, 17, 82–105. [Google Scholar] [CrossRef]
- Blanchette, R. A Guide to Wood Deterioration Caused by Microorganisms and Insects. In The Structural Conservation of Panel Paintings: Proceedings of a Symposium at the Journal of Paul Getty Museum; Dardes, K., Andrea, R., Eds.; Getty Conservation Institute: Los Angeles, CA, USA, 1995. [Google Scholar]
- Van Acker, J.; Palanti, S. 5.3 Durability. In Performance of Bio-Based Building Materials, 1st ed.; Jones, D., Brischke, C., Eds.; Elsevier: Duxford, UK, 2017; pp. 257–277. [Google Scholar]
- Unger, A.; Schniewind, A.P.; Unger, W. Conservation of Wood Artifacts: A Handbook, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2001; p. 595. [Google Scholar]
- Falck, R. Apparent Destruction of Wood by Larvae of Anobium. Cellul. Chem. 1930, 11, 128–129. [Google Scholar]
- Spiller, D. Digestion of α- cellulose by larvae of Anobium punctatum de Geer. Nature 1951, 168, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Kazimir, K. Documentary study of the bio-ecology of the xylophagous beetle Anobium punctatum De Geer 1774, pest of the patrimonial wooden objects. Rom. J. Plant Prot. 2018, 11, 64–68. [Google Scholar]
- Biebl, S.; Querner, P. Transportation of Wood Boring Beetles in Wooden Transport Boxes, Wooden Pallets, and Newly Bought Wood in Museums. Stud. Conserv. 2020, 66, 44–50. [Google Scholar] [CrossRef]
- Auer, J.; Opitz, C.; Kassel, A. A new biological method to control Anobium punctatum, by using the parasitoid wasp Spathius exarator. J. Appl. Entomol. 2020, 145, 73–81. [Google Scholar] [CrossRef]
- Balasoiu, C.F.; Zagury, G.J.; Deschenes, L. Partitioning and speciation of chromium, copper, and arsenic in CCA- contaminated soils: Influence of soil composition. Sci. Total Environ. 2001, 280, 239–255. [Google Scholar] [CrossRef]
- Khademibami, L.; Bobadilha, G.S. Recent Developments Studies on Wood Protection Research in Academia: A Review. Front. For. Glob. Change 2022, 5, 793177. [Google Scholar] [CrossRef]
- Eaton, R.A.; Hale, M.D.C. Wood: Decay, Pests and Protection, 1st ed.; Chapman y Hall: London, UK, 1993. [Google Scholar]
- Wang, Q.; Li, I.J.; Winandy, E.J. Chemical mechanism of fire retardance of boric acid on wood. J. Wood Sci. 2004, 38, 375–389. [Google Scholar] [CrossRef]
- Simsek, H.; Baysal, E.; Peker, H. Some mechanical properties and decay resistance of wood impregnated with environmentally-friendly borates. Constr. Build Mater. 2010, 24, 2279–2284. [Google Scholar] [CrossRef]
- Hakki, I.; Deveci, I.; Baysal, E.; Turkoglu, T.; Toker, H.; Peker, H. Thermal analysis of Oriental beech wood treated with some borates as fire retardants. Maderas. Cienc. Tecnol. 2016, 18, 293–304. [Google Scholar] [CrossRef]
- Nasir, M.A.; Roszaini, K.; Salmiah, U.; Tumirah, K.; Zaihan, J. Durability of selected malaysian wood treated with disodium octaborate tetrahydrate used under hazard class 2 condition. J. Trop. For. Sci. 2019, 31, 43–49. [Google Scholar]
- Kartal, N.; Terzi, E.; Yoshimura, T. Performance of fluoride and boron compounds against drywood and subterranean termites and decay and mold fungi. J. For. Res. 2020, 31, 1425–1434. [Google Scholar] [CrossRef]
- Bolt, H.; Duydu, Y.; Basaran, N.; Golka, K. Boron and its compounds: Current biological research activities. Arch. Toxicol. 2017, 91, 2719–2722. [Google Scholar] [CrossRef] [PubMed]
- Bolt, H.; Başaran, N.; Duydu, Y. Effects of boron compounds on human reproduction. Arch. Toxicol. 2020, 94, 717–724. [Google Scholar] [CrossRef]
- Ramos, A.M.; Caldeira Jorge, F.; Botelho, C. Boron fixation in wood: Studies of fixation mechanisms using model compounds and maritime pine. Holz. Roh. Werkst. 2006, 64, 445–450. [Google Scholar] [CrossRef]
- Freeman, M.H.; Mcintyre, C.R.; Jackson, D. A Critical and Comprehensive Review of Boron. Wood Preserv. Proc. Am. Wood Prot. Assoc. 2008, 105, 279–294. [Google Scholar]
- Akhtari, M.; Nicholas, D. Evaluation of particulate zinc and copper as wood preservatives for termite control. Eur. J. Wood Prod. 2013, 71, 395–396. [Google Scholar] [CrossRef]
- Civardi, C.; Schwarze, F.; Wick, P. Micronized copper wood preservative: An efficiency and potential health risk assessment for copper-based nanoparticles. Environ. Pollut. 2015, 2000, 126–132. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Leach, R. Micronized Wood Preservative Formulations Comprising Boron Compounds. US Patent 8168304 B2, 1 May 2012. [Google Scholar]
- Lenox, J.; Miller, N.; Rossi, M. Amino Acid-Solubilized Borate, Silicate and Zinc Compositions and Methods for Treating Wood Products. US Patent US20080069978 A1, 30 December 2008. [Google Scholar]
- Schubert, D.M. Boric Oxide, Boric Acid, and Borates. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–32. [Google Scholar]
- Schubert, D.M. Hydrated Zinc Borates and Their Industrial Use. Molecules 2019, 24, 2419. [Google Scholar] [CrossRef] [PubMed]
- Tascioglu, C.; Yoshimura, T.; Tsunoda, K. Biological performance of wood–plastic composites containing zinc borate: Laboratory and 3-year field test results. Compos. Part B 2013, 51, 185–190. [Google Scholar] [CrossRef]
- Tascioglu, C.; Umemura, K.; Yoshimura, T. Seventh-year durability evaluation of zinc borate incorporated wood-plastic composites and particleboard. Compos. Part B Eng. 2018, 137, 123–128. [Google Scholar] [CrossRef]
- Altuntas, E.; Yılmaz, E.; Salan, T.; Alma, M.H. Combined Effect of Zinc Borate and Coupling Agent against Brown and White Rot Fungi in Wood-Plastic Composites. BioResources 2017, 12, 7056–7068. [Google Scholar] [CrossRef]
- Schubert, D.M. Zinc Borate Hydrolysis. Molecules 2022, 27, 5768. [Google Scholar] [CrossRef]
- Dev, I.; Bagga, J.K.; Kumar, S. Termite resistance and permanency tests on zinc borate an environmental friendly preservative. J. Timb. Dev. Assoc. India 1997, 42, 10–15. [Google Scholar]
- Ibáñez, C.M.; Camargo, A.; Mantero, C.; Faccio, R.; Malanga, A.; Rabinovich, M. Effectiveness of Micronizing Zinc Borate to Improve Its Fungicidal Properties. BioResources 2019, 14, 6231–6246. [Google Scholar] [CrossRef]
- Tascioglu, C.; Umemura, K.; Yoshimura, T.; Tsunoda, K. Biological performance of zinc borate-incorporated particleboard: Effects of leaching on efficacy. Compos. Part B Eng. 2014, 57, 31–34. [Google Scholar] [CrossRef]
- Mantanis, G.; Terzi, E.; Kartal, N.; Papadopoulos, A. Evaluation of mold, decay and termite resistance of pine wood treated with zinc- and cooper-based nanocompounds. Int. Biodeterior. Biodegrad. 2014, 90, 140–144. [Google Scholar] [CrossRef]
- Lykidis, C.; Bak, M.; Mantanis, G.; Németh, R. Biological resistance of pine wood treated with nano-sized zinc oxide and zinc borate against brown-rot fungi. Eur. J. Wood 2016, 74, 909–911. [Google Scholar] [CrossRef]
- Ibáñez, C.M.; Mantero, C.; Ibarra, A.; Rabinovich, M. Ammoniacal Zinc Borate as Solid Wood Preservative (IRG/WP 14-30659); International Research Group on Wood Preservation: Stockholm, Sweden, 2014. [Google Scholar]
- EN 84, European Norm 84; Wood Preservatives. Accelerated Ageing of Treated Wood Prior to Biological Testing. Leaching Procedure. European Committee for Standardization: Bruselas, Bélgica, 2020.
- EN 113-1, European Norm 113; Wood Preservatives. Test Method for Determining the Protective Effectiveness against Wood Destroying Basidiomycetes. Determination of the Toxic Values. European Committee for Standardization: Bruselas, Bélgica, 2020.
- Anzano, J.; Gónzalez, P. Determination of iron and copper in peanuts by flame atomic absorption spectrometry using acid digestion. Microchem. J. 2000, 64, 141–145. [Google Scholar] [CrossRef]
- EN 48, Wood Preservatives; Determination of Eradicant Action against Larvae of Anobium punctatum (De Geer) (Laboratory Method). British Standards Institution (BSI): London, UK, 2005.
- Luke, S.G. Evaluating significance in linear mixed-effects models in R. Behav. Res. 2017, 49, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 9 February 2023).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.5.2-1. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 5 November 2022).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Terzi, E.; Sutcu, H.; Pişkin, S.; Kartal, N. Thermal Behavior of Zinc Borate-Treated Wood. IRG/WP 09-30511; International Research Group on Wood Preservation: Stockholm, Sweden, 2009. [Google Scholar]
- Camargo, A.; Marquéz, A.; Passarella, D.N.; Hernandez, L.; Reyes, A.L.; Ibáñez, C.M. Structural assessment and EDS analysis of uruguayan wood impregnated with zinc borate (ZnB). Microsc. Microanal. 2020, 26, 157–158. [Google Scholar] [CrossRef]
- Obanda, D.N.; Shupe, T.F.; Barnes, H.M. Reducing leaching of boron-based wood preservatives—A review of research. Bioresour. Technol. 2008, 99, 7312–7322. [Google Scholar] [CrossRef] [PubMed]
- Serdjukova, I.R.; Toskina, I.N. Some characters of biology and physiology of the common furniture beetle Anobium punctatum DeGeer (Coleoptera Anobiidae). Russ. Entomol. J. 1996, 4, 35–43. [Google Scholar]
- Toper Kaygin, A.; Yildiz, Y.; Yildiz, U.C.; Yildiz, S.; Onat, S.M.; Özkazanç, N.K.; Bülent Kaygin, B.; Çelikyay, S. An Important Wood Destroying Beetle: Anobium Punctatum (De Geer) (Coleoptera: Anobiidae) and Distribution of Western Black Sea Region. IRG/WP 08-10666; International Research Group on Wood Preservation: Stockholm, Sweden, 2008. [Google Scholar]
- De Jonge, J.T. The Efficacy of Boron Preparations. (IRG/WP 3400); International Research Group on Wood Preservation: Stockholm, Sweden, 1987. [Google Scholar]


| Retention Level (kg m−3) | |||
|---|---|---|---|
| Decay Test | Insect Test | ||
| Specimens | Specimens | ||
| VPV | VPV | VV | |
| P. taeda | 23.4 (0.9) | 28.9 (1.6) | 15.2 (0.8) |
| Populus sp. | 24.1 (1.1) | 26.9 (1.1) | 14.9 (0.6) |
| E. grandis | 21.9 (1.5) | ||
| df | LRT | p-Value | |
|---|---|---|---|
| Fixed effects | |||
| genus | 1 | 0.331 | 0.5649 |
| treatment | 2 | 14.875 | 0.0006 |
| genus by treatment | 2 | 7.871 | 0.0195 |
| Random effect | |||
| wood sample | 1 | 6.115 | 0.0020 |
| A—Genus | ||
|---|---|---|
| Contrast | OR | p-Value |
| P. taeda | ||
| Control/VPV | 0.149 | 0.1488 |
| Control/VV | 0.334 | 0.5484 |
| VPV/VV | 2.249 | 0.3857 |
| Populus sp. | ||
| Control/VPV | 0.135 | 0.0371 |
| Control/VV | 0.021 | 0.0002 |
| VPV/VV | 0.156 | 0.0342 |
| B—Treatments | ||
| Contrast | OR | p-Value |
| Control | ||
| Populus sp./P. taeda | 0.505 | 0.5621 |
| VPV | ||
| Populus sp./P. taeda | 0.556 | 0.2915 |
| VV | ||
| Populus sp./P. taeda | 8.033 | 0.0091 |
| df | LRT | p-Value | |
|---|---|---|---|
| Fixed effects | |||
| genus | 1 | 1.627 | 0.2021 |
| treatment | 2 | 25.770 | <0.0001 |
| genus by treatment | 2 | 9.493 | 0.0086 |
| Random effect | |||
| wood sample | 1 | 56.534 | <0.0001 |
| A—Genus | ||
|---|---|---|
| Contrast | Difference | p-Value |
| P. taeda | ||
| Control/VPV | 1.472 | 0.0168 |
| Control/VV | 1.194 | 0.0647 |
| VPV/VV | −0.278 | 0.5972 |
| Populus sp. | ||
| Control/VPV | 2.060 | 0.0001 |
| Control/VV | 2.746 | <0.0001 |
| VPV/VV | 0.686 | 0.0660 |
| B—Treatments | ||
| Contrast | Difference | p-Value |
| Control | ||
| Populus sp.—P. taeda | 0.546 | 0.3242 |
| VPV | ||
| Populus sp.—P. taeda | −0.042 | 0.8713 |
| VV | ||
| Populus sp.—P. taeda | −1.006 | 0.0048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez, C.M.; Katzenstein, G.; Mantero, C.; Benítez, V.; Camargo, A.; Berberian, N.; Bollazzi, M. Ammoniacal Zinc Borate for Wood Protection against Fungi and Insects. Forests 2023, 14, 1152. https://doi.org/10.3390/f14061152
Ibáñez CM, Katzenstein G, Mantero C, Benítez V, Camargo A, Berberian N, Bollazzi M. Ammoniacal Zinc Borate for Wood Protection against Fungi and Insects. Forests. 2023; 14(6):1152. https://doi.org/10.3390/f14061152
Chicago/Turabian StyleIbáñez, Claudia Marcela, Guillermo Katzenstein, Carlos Mantero, Valentina Benítez, Alvaro Camargo, Natalia Berberian, and Martin Bollazzi. 2023. "Ammoniacal Zinc Borate for Wood Protection against Fungi and Insects" Forests 14, no. 6: 1152. https://doi.org/10.3390/f14061152
APA StyleIbáñez, C. M., Katzenstein, G., Mantero, C., Benítez, V., Camargo, A., Berberian, N., & Bollazzi, M. (2023). Ammoniacal Zinc Borate for Wood Protection against Fungi and Insects. Forests, 14(6), 1152. https://doi.org/10.3390/f14061152





