Harvest Residue Decomposition from Eucalyptus sp. Plantations in Temperate Climate: Indicators and Contribution to Nutrient Cycling
Abstract
1. Introduction
- (a)
- To identify and quantify the characteristics of each species (E. dunnii, E. grandis and E. globulus) that affect the decomposition rates of the different harvest residues, as well as the indicators that can explain the process.
- (b)
- To quantify the potential recycling of N, P, K, Ca and Mg to the soil from the decomposition of these residues and assess the quantitative and qualitative differences between the species evaluated.
2. Materials and Methods
- -
- Location of experimental sites
- -
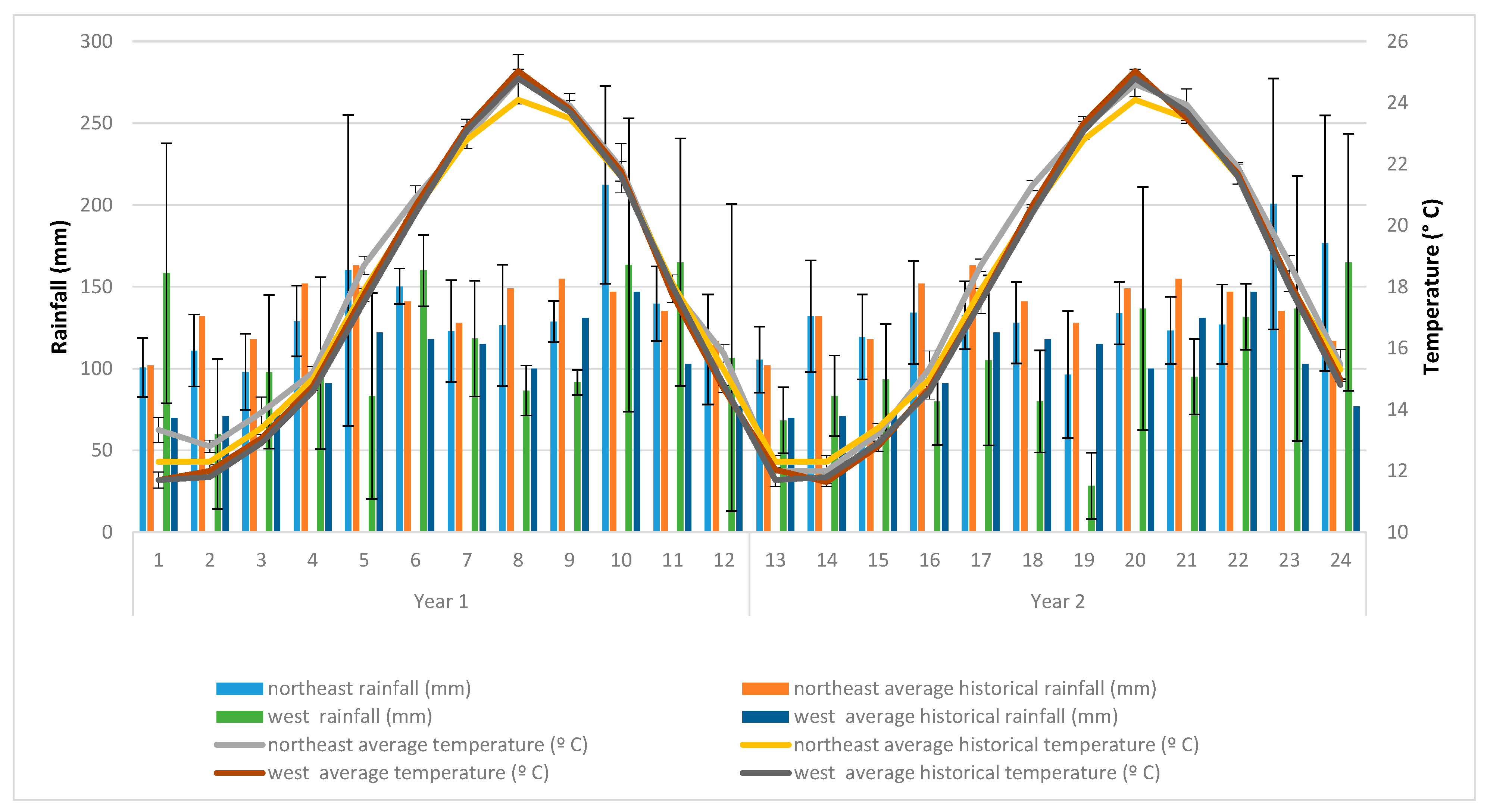
- Climatic characteristics of the study areas
- -
- Plant sampling and chemical analysis
- -
- Calculations and statistical analysis of the information
3. Results
3.1. Amounts of Harvest Residues
3.2. Decomposition of Harvest Residues
3.3. Nutrient Cycling
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Livestock, Agriculture and Fisheries. 2022. Available online: https://www.gub.uy/ministerio-ganaderia-agricultura-pesca/datos-y-estadisticas/datos/resultados-cartografia-forestal2021#:~:text=La%20cartograf%C3%ADa%20cuantific%C3%B3%20una%20superficie,efectivas%20destinadas%20al%20uso%20forestal (accessed on 8 September 2022).
- Mendham, D.; Ogden, G.; Short, T.; O’Connell, T.; Grove, T.; Rance, S. Repeated harvest residue removal reduces E. globulus productivity in the 3rd rotation in southwestern Australia. For. Ecol. Manag. 2014, 329, 279–286. [Google Scholar] [CrossRef]
- Epron, D.; Nouvellon, Y.; Deleporte, P.; Ifo, S.; Kazotti, G.; Thongo M’Bou, A.; Mouvondy, W.; Andre, L.; Roupsard, O.; Jourdan, C.; et al. Soil carbon balance in a clonal Eucalyptus plantation in Congo: Effects of logging on carbon inputs and soil CO2 efflux. Glob. Chang. Biol. 2006, 12, 1021–1031. [Google Scholar] [CrossRef]
- Versini, A.; Zeller, B.; Derrien, D.; Mazoumbou, J.; Mareschal, L.; Saint-André, L.; Ranger, J.; Laclau, J. The role of harvest residues to sustain tree growth and soil nitrogen stocks in a tropical Eucalyptus plantation. Plant Soil. 2013, 376, 245–260. [Google Scholar] [CrossRef]
- Gonçalves, J.; Alvares, C.; Higa, A.; Silva, L.; Alfenas, A.; Stahl, J.; Ferraz, S.; Lima, W.; Brancalion, P.; Hubner, A.; et al. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For. Ecol. Manag. 2013, 301, 6–27. [Google Scholar] [CrossRef]
- Hernández, J. Dinámica de los Nutrientes y la Materia Orgánica del Suelo en los Sistemas Forestales. Tesis Doctorado en Ciencias Agrarias, Facultad de Agronomía, Montevideo, Uruguay, 2016; 190p. [Google Scholar]
- Rocha, J.; Marques, E.; Gonçalves, J.; Hübner, A.; Brandani, C.; Ferraz, A.; Moreira, R. Decomposition rates of forest residues and soil fertility after clear-cutting of Eucalyptus grandis stands in response to site management and fertilizer application. Soil Use Manag. 2016, 32, 289–302. [Google Scholar] [CrossRef]
- Spangenberg, A.; Grimm, U.; Silva, J.; Fölster, H. Nutrient store and export rates of Eucalyptus urograndis plantations in eastern Amazonia (Jari). For. Ecol. Manag. 1996, 80, 225–234. [Google Scholar] [CrossRef]
- Rezende, J.; Garcia, Q.; Scotti, M. Laboratory decomposition of Dalbergia nigra All. ex Benth and Eucalyptus grandis W. Hill ex Maiden leaves in forest and eucalypt plantation soils. Acta Botánica Bras. 2001, 15, 305–312. [Google Scholar] [CrossRef]
- Sánchez, G. Descomposición de Restos de Cosecha de EUCALYPTUS sp. y Pinus Taeda en Condiciones Controladas de Humedad y Temperatura. Tesis de Maestría en Ciencias Agrarias, Facultad de Agronomía, Montevideo, Uruguay, 2011; 89p. [Google Scholar]
- Baietto, A.; Hernández, J.; del Pino, A. Comparative dynamics of above ground litter production and decomposition from Eucalyptus grandis Hill ex Maiden and Pinus taeda L., and their contribution to the soil organic carbon. Forests 2021, 12, 349. [Google Scholar] [CrossRef]
- Bradford, M.; Warren, R.; Baldrian, P.; Crowther, T.; Maynard, D.; Oldfield, E.; Wieder, W.; Wood, S.; King, J. Climate fails to predict wood decomposition at regional scales. Nat. Clim. Chang. 2014, 4, 625–630. [Google Scholar] [CrossRef]
- Ferreira, G.; Soares, E.; Oliveira, F.; Silva, I.; Dungait, J.; Souza, I.; Vergütz, L. Nutrient release from decomposing Eucalyptus harvest residues following simulated management practices in multiple sites in Brazil. For. Ecol. Manag. 2016, 370, 1–11. [Google Scholar] [CrossRef]
- De Souza, I. Decomposição de Residuos da Colheita e Transferência de Carbono Para o Solo em Plantações de Eucalipto. Tesis de Maestría, Universidad de Viçosa, Viçosa, Brazil, 2012; 80p. [Google Scholar]
- Corbeels, M.; O’Connell, A.; Grove, T.; Mendham, D.; Rance, S. Nitrogen release from eucalypt leaves and legume residues as influenced by their biochemical quality and degree of contact with soil. Plant Soil 2003, 250, 15–28. [Google Scholar] [CrossRef]
- Ambus, P.; Jenssen, E. Crop residue management strategies to reduce N losses—Interaction with crop N supply. Comm. Soil Sci. Plant Anal. 2007, 32, 981–996. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin/Heidelberg, Germany, 2003; ISBN: 978-3-540-74922-6 (Print), 978-3-540-74923-3 (Online); 338p. [Google Scholar]
- Guo, B.; Sims, R. Eucalypt litter decomposition and nutrient release under a short rotation forest regime and effluent irrigation treatments in New Zealand: II. internal effects. Soil Biol. Bioch. 2002, 34, 913–922. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Hernández, J.; del Pino, A.; Salvo, L.; Arrarte, G. Nutrient export and harvest residue decomposition patterns of a Eucalyptus dunnii Maiden plantation in temperate climate of Uruguay. For. Ecol. Managt. 2009, 258, 92–99. [Google Scholar] [CrossRef]
- González, A.; Hernández, J.; del Pino, A. Extracción y reciclaje de nutrientes por cosecha de Eucalyptus globulus en Uruguay. Bosque 2016, 37, 175–186. [Google Scholar] [CrossRef]
- Inumet. Dirección Nacional de Meteorología, Montevideo, Uruguay. 2022. Available online: https://www.inumet.gub.uy/clima/estadisticas-climatologicas/caracteristicas-climaticas (accessed on 8 July 2022).
- Murphy, J.; Riley, J. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Nelson, D.; Sommers, L. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3: Chemical Methods; Page, A.L., Helmke, P.A., Loeppert, R.H., Sparks, D.L., Eds.; John Wiley & Sons: Madison WI, USA, 1996; pp. 961–1010. [Google Scholar] [CrossRef]
- Schwanninger, M.; Hinterstoisser, B. Klason lignin: Modifications to improve the precision of the standardized determination. Holzforschung 2002, 56, 161–166. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. J. Enol. Vitic. 1965, 16, 144–148. [Google Scholar] [CrossRef]
- Olsen, J. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Conover, W. Practical Nonparametric Statistics, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Bentancor, L. Extracción de Nutrientes por Eucalyptus Dunnii Maiden de 4 Años con Destino a la Producción de Biomasa Para Energía y Celulosa. Tesis Maestría en Ciencias Agrarias, Facultad de Agronomía, Montevideo, Uruguay, 2017; 109p. [Google Scholar]
- Aguirre, N.; Filippi, C.; Zaina, G.; Rivas, J.; Acuña, C.; Villalba, P.; García, M.; González, S.; Rivarola, M.; Martínez, M.; et al. Optimizing ddRADseq in Non-Model Species: A case Study in Eucalyptus dunnii Maiden. Agronomy 2019, 9, 484. [Google Scholar] [CrossRef]
- Shammas, K.; O’Connell, A.; Grove, T.; McMurtrie, R.; Damon, P.; Rance, S. Contribution of decomposing harvest residues to nutrient cycling in a second rotation Eucalyptus globulus plantation in south-western Australia. Biol. Fert. Soils 2003, 38, 228–235. [Google Scholar]
- González, A.; Hernández, J.; del Pino, A.; Hirigoyen, A. Nutrient use efficiency in commercial eucalypt plantations in different soils under temperate climate. South. For. 2022, 84, 123–135. [Google Scholar] [CrossRef]
- Jones, H.; Madeira, M.; Herraez, L.; Dighton, J.; Fabiao, A.; González-Rio, F.; Fernandez, M.; Gomez, C.; Tomé, M.; Feith, H.; et al. The effect of organic-matter management on the productivity of Eucalyptus globulus stands in Spain and Portugal: Tree growth and harvest residue decomposition in relation to site and treatment. For. Ecol. Manag. 1999, 122, 73–86. [Google Scholar] [CrossRef]
- Alvares, C.; Stape, J.; Sentelhas, P.; Goncalves, J.; Sparovek, G. Koppen’s climate classification map for Brazil. Meteorol. Zeitschrift. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, A.; Mendham, D. Impact of N and P fertilizer application on nutrient cycling in jarrah (Eucalyptus marginata) forests of southwestern Australia. Biol. Fert. Soils 2004, 40, 136–143. [Google Scholar] [CrossRef]
- Goya, J.; Frangi, J.; Dalla Tea, F.; Marco, M.; Larocca, F. Biomasa, productividad y contenido de nutrientes en plantaciones de Eucalyptus grandis en el noreste de la Provincia de Entre Ríos. In XII Jornadas Forestales de Entre Ríos; Concordia University: Montréal, QC, Canada, 1997; Volume III, pp. 1–18. [Google Scholar]
- Alvarez, E.; Fernandez, M.; Torrado, V.; Fernandez Sanjurjo, M. Dynamics of macronutrients during the first stages of litter decomposition from forest species in a temperate area (Galicia, NW Spain). Nutr. Cycl. Agroecosyst. 2008, 80, 243–256. [Google Scholar] [CrossRef]
- Trinsoutrot, S.; Recous, B.; Linères, M.; Chèneby, D.; Nicolardot, B. Biochemical quality of crop residues and nitrogen mineralization kinetics under nonlimiting nitrogen conditions. Soil Sci. Soc. Am. J. 2000, 64, 918–926. [Google Scholar] [CrossRef]
- O’Connell, A.; Grove, T. Influence of nitrogen and phosphorus fertilizers on amount and nutrient content of litterfall in a regrowth eucalypt forest. New For. 1993, 7, 33–47. [Google Scholar] [CrossRef]
- Marshner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2003; pp. 229–299. [Google Scholar]
- Laclau, J.; Ranger, J.; Goncalves, J.; Maquere, V.; Krusche, A.; M’Bou, A.; Nouvellon, Y.; Saint-Andre’, L.; Bouillet, J.; Cassia Piccolo, M.; et al. Biogeochemical cycles of nutrients in tropical Eucalyptus plantations: Main features shown by intensive monitoring in Congo and Brazil. For. Ecol. Manag. 2010, 259, 1771–1785. [Google Scholar] [CrossRef]

| Coordinates | Species | Soil Taxonomy | A Horizon Depth | pH (H2O) | Clay | P (†) | TB | ECEC | OC | |
|---|---|---|---|---|---|---|---|---|---|---|
| cm | g kg−1 | mg kg−1 | cmolc kg−1 | g kg−1 | ||||||
| 32° 25′ 56′′ S | 57°17′40′′ W | E. dunnii * | Alfic Argiudoll | 33 | 5.3 | 184 | 3 | 6.35 | 7.12 | 9.8 |
| 31° 52′ 55′′ S | 57°30′35′′ W | E. grandis | Abruptic Argiudoll | 33 | 5.2 | 187 | 3 | 6.36 | 7.05 | 9.9 |
| 33° 25’ 18′′ S | 57°48′25′′ W | E. globulus ** | Typic Hapludert | 30 | 5.4 | 199 | 4 | 6.43 | 7.01 | 12.5 |
| 31° 08′ 44′′ S | 55°37′22′′ W | E. grandis | Humic Hapludult | 57 | 4.5 | 118 | 3 | 2.53 | 3.57 | 6.3 |
| 31° 45′ 40′′ S | 56°05′35′′ W | E. globulus | Humic Hapludult | 49 | 4.7 | 122 | 3 | 2.59 | 3.54 | 6.5 |
| Sample Type | E. dunnii | E. grandis | E. globulus | E. dunnii | E. grandis | E. globulus |
|---|---|---|---|---|---|---|
| Mg ha−1 year−1 | % | |||||
| Bark | 3.0 (A) | 1.9 (B) | 1.8 (B) | 33.3 | 36.6 | 46.2 |
| Leaves | 1.3 (A) | 1.0 (B) | 0.6 (C) | 14.5 | 19.2 | 15.4 |
| Thin branches | 2.0 (A) | 0.9 (B) | 0.5 (C) | 22.2 | 17.3 | 12.8 |
| Thick branches | 2.7 (A) | 1.4 (B) | 1.0 (C) | 30.0 | 26.9 | 25.6 |
| Total residues | 9.0 (A) | 5.2 (B) | 3.9 (C) | 100.0 | 100.0 | 100.0 |
| Species | Sample Type | Biomass Loss | K | r2 | Half-Life |
|---|---|---|---|---|---|
| (%) | (year−1) | years | |||
| E. dunnii | Bark | 22 | 0.12 | 0.91 | 5.62 (A) |
| Leaves | 83 | 0.74 | 0.93 | 0.94 (G) | |
| Thin branches | 35 | 0.19 | 0.95 | 3.61 (DE) | |
| Thick branches | 33 | 0.18 | 0.95 | 3.75 (CD) | |
| E. grandis | Bark | 38 | 0.21 | 0.89 | 3.30 (E) |
| Leaves | 57 | 0.43 | 0.98 | 1.62 (F) | |
| Thin branches | 31 | 0.17 | 0.95 | 4.09 (CD) | |
| Thick branches | 29 | 0.16 | 0.87 | 4.32 (BC) | |
| E. globulus | Bark | 32 | 0.17 | 0.86 | 4.04 (CD) |
| Leaves | 68 | 0.51 | 0.94 | 1.37 (FG) | |
| Thin branches | 30 | 0.16 | 0.97 | 4.25 (BCD) | |
| Thick branches | 27 | 0.14 | 0.97 | 4.90 (B) |
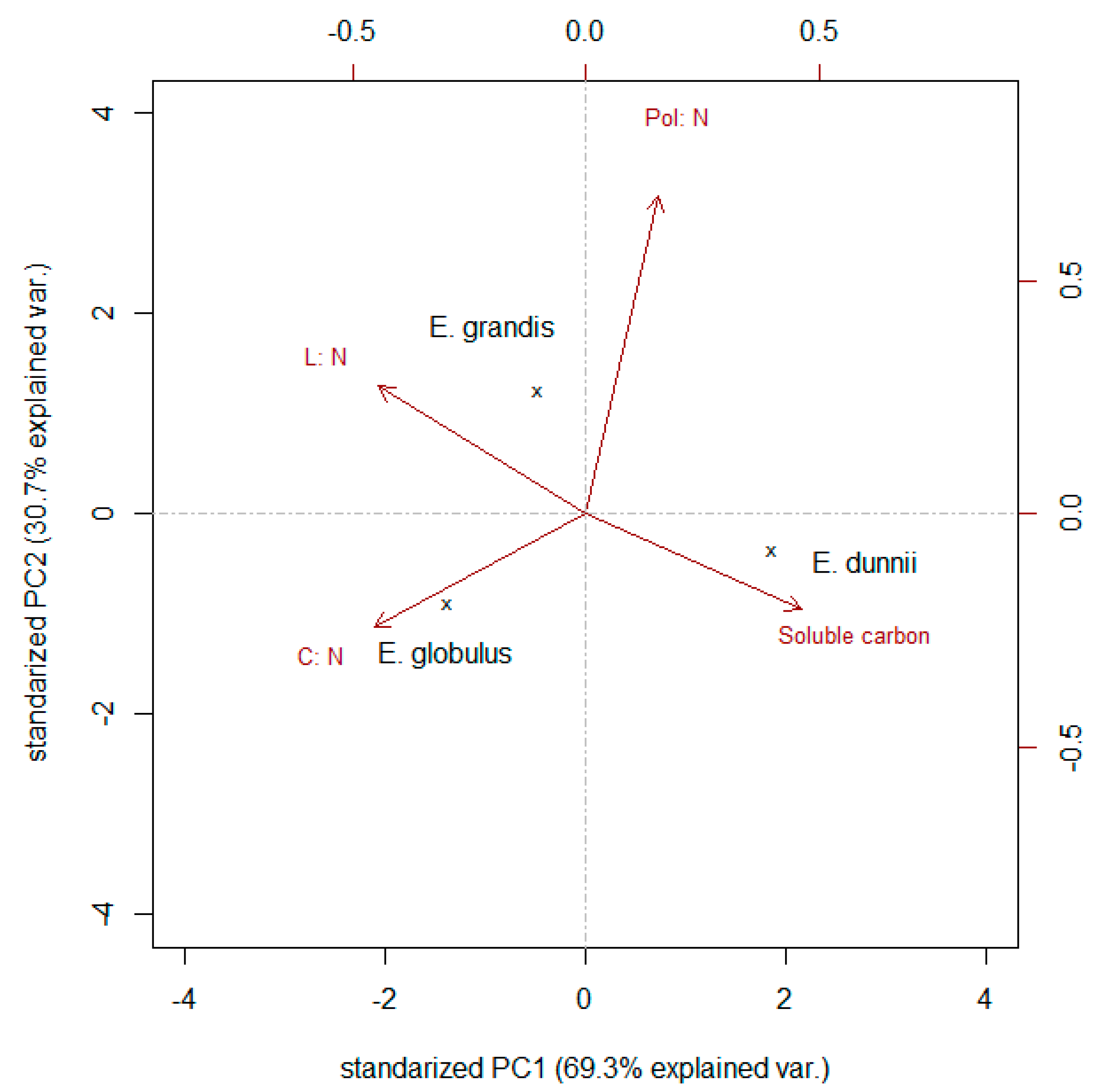
| Sample Type | Total C | Soluble C | Polyphenols | Lignin | N | C:N Ratio | Pol:N Ratio | L:N Ratio |
|---|---|---|---|---|---|---|---|---|
| E. dunnii | g kg−1 | |||||||
| Bark | 413 | 39 | 76 | 359 | 2.9 | 142 | 26 | 123 |
| Leaves | 505 | 179 | 122 | 330 | 16.3 | 31 | 7 | 20 |
| Thin branches | 457 | 68 | 83 | 322 | 4.4 | 104 | 19 | 73 |
| Thick branches | 454 | 30 | 25 | 332 | 2.5 | 182 | 10 | 133 |
| E. grandis | ||||||||
| Bark | 411 | 54 | 55 | 334 | 2.6 | 160 | 21 | 130 |
| Leaves | 436 | 142 | 117 | 322 | 18.3 | 24 | 6 | 18 |
| Thin branches | 402 | 60 | 70 | 285 | 3.0 | 135 | 24 | 96 |
| Thick branches | 378 | 34 | 18 | 294 | 1.4 | 262 | 13 | 204 |
| E. globulus | ||||||||
| Bark | 462 | 74 | 41 | 362 | 2.4 | 190 | 17 | 149 |
| Leaves | 569 | 143 | 91 | 306 | 13.4 | 43 | 7 | 23 |
| Thin branches | 517 | 46 | 67 | 285 | 4.2 | 124 | 16 | 68 |
| Thick branches | 493 | 28 | 26 | 244 | 1.3 | 394 | 21 | 195 |
| Variables | Half-Life (Years) |
|---|---|
| Correlation Coefficient | |
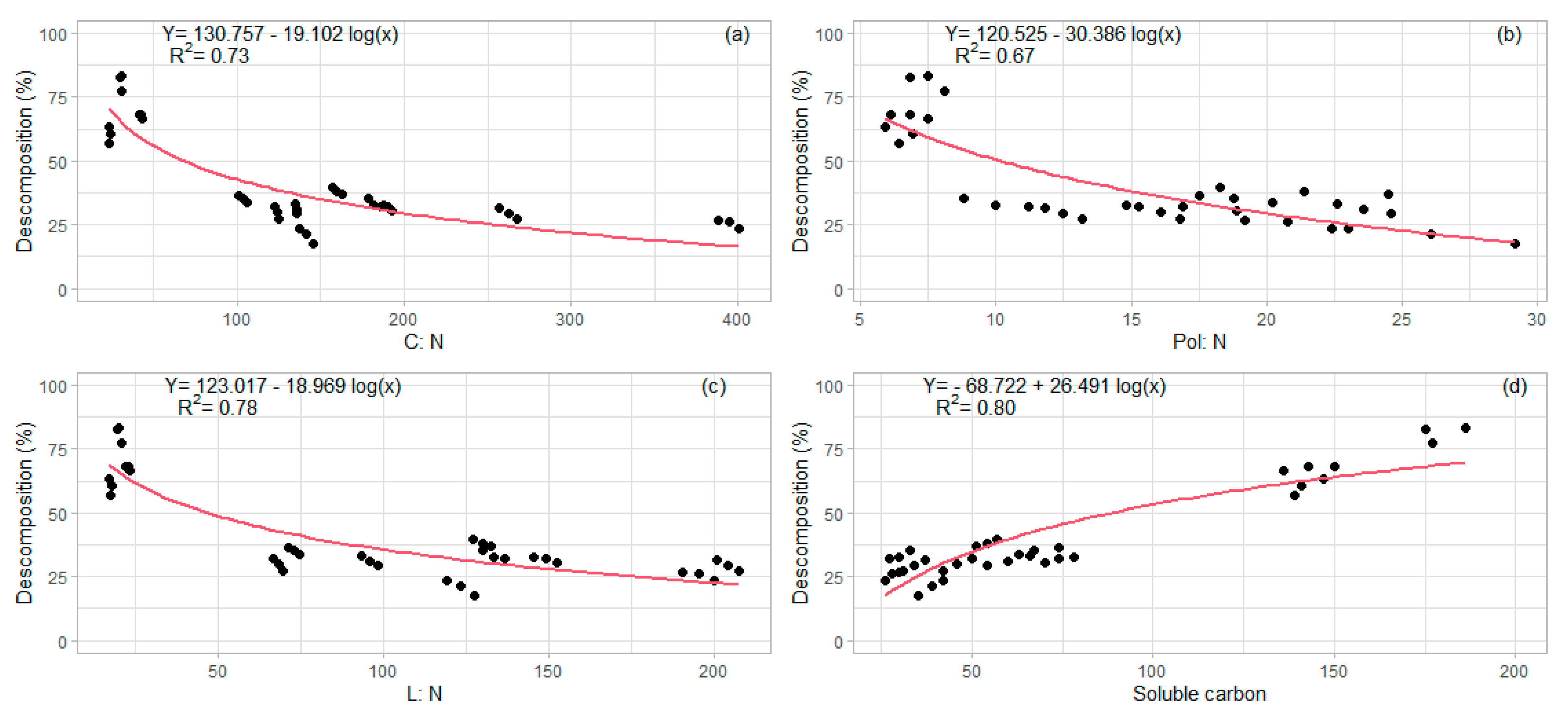
| C:N ratio (36) | 0.69 (<0.01) |
| Pol:N ratio (36) | 0.69 (<0.01) |
| L:N ratio (36) | 0.66 (<0.01) |
| Soluble carbon (36) | −0.59 (<0.01) |
| Nutrient | Sample Type | Month | Nutrient Released from Residue | ||||
|---|---|---|---|---|---|---|---|
| 0 | 12 | 24 | |||||
| E. dunnii | kg ha−1 | % | |||||
| Nitrogen | Bark | 92 | 78 | 124 | −32 | ||
| Leaves | 225 | 114 | 44 | 181 | |||
| Thin branches | 93 | 76 | 70 | 23 | |||
| Thick branches | 72 | 53 | 64 | 8 | |||
| Total | 482 | 321 | 302 | 180 | 37 | ||
| Phosphorus | Bark | 11.1 | 5.8 | 7.0 | 4.1 | ||
| Leaves | 18.8 | 7.3 | 3.1 | 15.7 | |||
| Thin branches | 7.4 | 4.9 | 3.9 | 3.5 | |||
| Thick branches | 4.9 | 5.0 | 3.9 | 1.0 | |||
| Total | 42.2 | 23.0 | 18.4 | 24.3 | 58 | ||
| Potassium | Bark | 127 | 10 | 12 | 115 | ||
| Leaves | 105 | 5 | 3 | 102 | |||
| Thin branches | 95 | 14 | 8 | 87 | |||
| Thick branches | 57 | 20 | 12 | 45 | |||
| Total | 384 | 49 | 35 | 349 | 91 | ||
| Calcium | Bark | 1026 | 772 | 781 | 245 | ||
| Leaves | 183 | 107 | 50 | 133 | |||
| Thin branches | 211 | 189 | 209 | 2 | |||
| Thick branches | 212 | 168 | 167 | 45 | |||
| Total | 1,632 | 1,236 | 1,207 | 425 | 26 | ||
| Magnesium | Bark | 76 | 51 | 39 | 37 | ||
| Leaves | 26 | 8 | 3 | 23 | |||
| Thin branches | 26 | 20 | 9 | 17 | |||
| Thick branches | 32 | 25 | 17 | 15 | |||
| Total | 160 | 104 | 68 | 92 | 58 | ||
| Nutrient | Sample Type | Month | Nutrient Released from Residue | ||||
|---|---|---|---|---|---|---|---|
| 0 | 12 | 24 | |||||
| E. grandis | kg ha−1 | % | |||||
| Nitrogen | Bark | 51 | 42 | 55 | −4 | ||
| Leaves | 181 | 152 | 96 | 85 | |||
| Thin branches | 28 | 22 | 23 | 5 | |||
| Thick branches | 19 | 16 | 20 | −1 | |||
| Total | 279 | 232 | 194 | 85 | 30 | ||
| Phosphorus | Bark | 11.1 | 5.0 | 5.2 | 5.9 | ||
| Leaves | 11.0 | 7.7 | 5.4 | 5.6 | |||
| Thin branches | 3.2 | 2.0 | 1.7 | 1.5 | |||
| Thick branches | 2.6 | 2.9 | 2.4 | 0.2 | |||
| Total | 27.9 | 17.6 | 14.7 | 13.2 | 47 | ||
| Potassium | Bark | 79 | 5 | 5 | 74 | ||
| Leaves | 72 | 6 | 5 | 67 | |||
| Thin branches | 26 | 4 | 2 | 24 | |||
| Thick branches | 18 | 7 | 4 | 14 | |||
| Total | 195 | 22 | 16 | 179 | 92 | ||
| Calcium | Bark | 606 | 443 | 376 | 230 | ||
| Leaves | 87 | 66 | 49 | 38 | |||
| Thin branches | 65 | 59 | 64 | 1 | |||
| Thick branches | 93 | 81 | 85 | 8 | |||
| Total | 851 | 649 | 574 | 277 | 33 | ||
| Magnesium | Bark | 29 | 18 | 9 | 20 | ||
| Leaves | 25 | 14 | 8 | 17 | |||
| Thin branches | 11 | 9 | 4 | 7 | |||
| Thick branches | 13 | 11 | 8 | 5 | |||
| Total | 78 | 52 | 29 | 49 | 63 | ||
| Nutrient | Sample Type | Month | Nutrient Released from Residue | ||||
|---|---|---|---|---|---|---|---|
| 0 | 12 | 24 | |||||
| E. globulus | kg ha−1 | % | |||||
| Nitrogen | Bark | 45 | 37 | 54 | −9 | ||
| Leaves | 77 | 63 | 31 | 46 | |||
| Thin branches | 21 | 18 | 18 | 3 | |||
| Thick branches | 12 | 10 | 13 | −1 | |||
| Total | 155 | 128 | 116 | 39 | 25 | ||
| Phosphorus | Bark | 7.9 | 3.7 | 4.4 | 3.5 | ||
| Leaves | 5.0 | 3.2 | 1.7 | 3.3 | |||
| Thin branches | 1.4 | 0.9 | 0.7 | 0.7 | |||
| Thick branches | 1.3 | 1.3 | 1.0 | 0.3 | |||
| Total | 15.6 | 9.1 | 7.8 | 7.8 | 50 | ||
| Potassium | Bark | 86 | 6 | 7 | 79 | ||
| Leaves | 28 | 2 | 2 | 26 | |||
| Thin branches | 17 | 3 | 2 | 15 | |||
| Thick branches | 26 | 8 | 6 | 21 | |||
| Total | 157 | 19 | 17 | 141 | 90 | ||
| Calcium | Bark | 386 | 274 | 257 | 129 | ||
| Leaves | 63 | 46 | 26 | 37 | |||
| Thin branches | 43 | 40 | 42 | 1 | |||
| Thick branches | 61 | 52 | 57 | 4 | |||
| Total | 553 | 412 | 382 | 171 | 31 | ||
| Magnesium | Bark | 34 | 21 | 15 | 19 | ||
| Leaves | 7 | 4 | 1 | 6 | |||
| Thin branches | 6 | 5 | 2 | 4 | |||
| Thick branches | 8 | 5 | 4 | 4 | |||
| Total | 55 | 35 | 22 | 33 | 60 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, A.; Hernández, J.; Pino, A.d.; Hirigoyen, A.; Ualde, J. Harvest Residue Decomposition from Eucalyptus sp. Plantations in Temperate Climate: Indicators and Contribution to Nutrient Cycling. Forests 2023, 14, 1119. https://doi.org/10.3390/f14061119
González A, Hernández J, Pino Ad, Hirigoyen A, Ualde J. Harvest Residue Decomposition from Eucalyptus sp. Plantations in Temperate Climate: Indicators and Contribution to Nutrient Cycling. Forests. 2023; 14(6):1119. https://doi.org/10.3390/f14061119
Chicago/Turabian StyleGonzález, Alejandro, Jorge Hernández, Amabelia del Pino, Andrés Hirigoyen, and José Ualde. 2023. "Harvest Residue Decomposition from Eucalyptus sp. Plantations in Temperate Climate: Indicators and Contribution to Nutrient Cycling" Forests 14, no. 6: 1119. https://doi.org/10.3390/f14061119
APA StyleGonzález, A., Hernández, J., Pino, A. d., Hirigoyen, A., & Ualde, J. (2023). Harvest Residue Decomposition from Eucalyptus sp. Plantations in Temperate Climate: Indicators and Contribution to Nutrient Cycling. Forests, 14(6), 1119. https://doi.org/10.3390/f14061119








