Abstract
Soil mesofauna plays an important role in decomposing organic matter, recycling nutrients, and increasing nutrient availability. The effects of nitrogen (N) deposition and reduced precipitation on the litter-dwelling mesofaunal community and how this process affects litter decomposition remain poorly understood. Herein, a two-year simulated N deposition and throughfall reduction experiment was carried out in a natural evergreen broad-leaved subtropical forest to examine the effects of N deposition and reduced precipitation on soil mesofauna during litter decomposition. Four treatments were established: control (CK), N deposition (N), reduced precipitation (RP), and combined N deposition and reduced precipitation (N + RP). We collected and identified 19,782 individuals of mesofauna in litterbags during the whole experiment. Mites (Prostigmata, Mesostigmata, and Oribatida) and Collembola comprised almost 90% of the total number of individuals collected and dominated the soil mesofauna in our study. Our results revealed the negative effects of N deposition on the density of Oribatida mites and Collembola and the total density of soil mesofauna. Reduced precipitation significantly increased the density of Collembola and Oribatida mites and the total density of mesofauna and marginally significantly increased the density of Mesostigmata mites but decreased the diversity of mesofauna. The interaction effects of N deposition and reduced precipitation significantly affected the density of Prostigmata mites, Oribatida mites, Collembola, and the diversity of mesofauna. N deposition combined with reduced precipitation significantly inhibited litter decomposition, whereas no significant interaction effects were observed. Furthermore, correlation analysis indicated that litter mass loss was significantly positively correlated with the density of Prostigmata, Mesostigmata, and Oribatida, as well as the diversity of mesofauna. Overall, during the two-year decomposition process, our results suggest that N deposition and reduced precipitation interactively affected mesofaunal diversity and that N deposition adversely affected the mesofaunal community, while reduced precipitation increased the density of some groups but decreased mesofaunal diversity, consequently cascading on the decomposition of leaf litter.
1. Introduction
The world and its ecosystems face multiple global changes. These global changes, such as increasing anthropogenic nitrogen (N) deposition and alterations in precipitation patterns, are all predicted to become more frequent [1,2,3] and have affected a series of ecosystem processes. It is estimated that atmospheric N deposition will probably increase in regions with economic growth, such as Asia [3], and precipitation is projected to increase at high latitudes and decrease in most subtropical regions [4]. Moreover, these two global change factors usually occur simultaneously, and their concurrent action gives rise to a large uncertainty when predicting the effects of multiple global change factors on ecosystems [5].
Litter decomposition is a biogeochemical process fundamental to element cycling within ecosystems [6,7]. Soil faunal communities, by crushing, burrowing, feeding, and stimulating microbial activities directly or indirectly, play an important role in regulating litter decomposition [8,9,10]. Among the faunal communities, mesofauna such as Collembla and mites are believed to be the best-represented group in terms of abundance, richness, and diversity [11]; despite their lower biomass than that of macrofauna, they can efficiently affect litter decomposition by feeding, fragmentation, and stimulating microbial activity [8,12,13].
Nitrogen deposition and reduced precipitation may alter microhabitat and food resource availability, modifying and shifting the structure and function of the soil fauna community [14,15,16]. Numerous studies have reported the responses of soil mesofauna to N deposition; however, there are no consistent patterns of the effects of N deposition on mesofauna, and previous studies have shown dissimilar results, ranging from positive [17,18] to negative [16,19,20] to neutral [21,22]. The inconsistent results may be related to the difference in rates, form, duration of N addition, and ecosystem types among these studies [23,24]. Regarding how reduced precipitation affects mesofauna, in most arid to semiarid ecosystems, water availability is a major determinant of soil animal community composition and functioning [25,26]. Reduced precipitation led to less water availability and reduced the feeding activity of soil invertebrates [27], which may result in higher mortality and a reduction in mesofauna abundance [28]. Furthermore, reduced precipitation and N deposition often act in concert and interactively affect the soil fauna community [29], whereas due to the complexity of mesofaunal structure, understanding the interaction effect of reduced precipitation and N deposition on soil fauna is limited [27]. Together, N deposition and reduced precipitation profoundly affected the efficient decomposer soil mesofauna community [14,30], consequently with cascading effects on the litter decomposition process. However, although increasing attention has been given to the influence of N deposition and reduced precipitation on litter decomposition, the role of mesofauna in these processes has largely been ignored, and our knowledge is incomplete regarding the response of litter-dwelling mesofauna to reduced precipitation and N deposition and their relationships with litter decomposition.
To understand the effects of increasing atmospheric N deposition and reduced precipitation on litter-dwelling soil mesofauna and their relationships with litter decomposition, we carried out a two-year field experiment in a subtropical evergreen broad-leaved forest in southern China, studying the diversity and density of litter-dwelling mesofauna and litter decomposition. We hypothesized that (1) both N deposition and reduced precipitation would decrease the density and diversity of soil mesofauna. We expected negative impacts of N deposition on mesofauna as N enrichment induces declines in soil pH and microbial biomass based on our previous study [31], and soil acidification and shortage in living resources may negatively affect mesofauna density and diversity [14,19,32]. Negative effects of reduced precipitation were expected due to a reduction in water availability, which is the main regulating factor of the soil fauna community [33]. (2) N deposition and reduced precipitation would interactively affect the density and diversity of mesofauna. We expected interactive effects because changes in precipitation patterns would affect the deposition of atmospheric N [34]. (3) Given the vital role of mesofauna in litter decay [6,35,36], N deposition and reduced precipitation would affect litter decomposition due to changes in the mesofaunal community.
2. Materials and Methods
2.1. Study Site
The study was conducted in a natural evergreen broad-leaved forest in Ya’an city (102°59′ E, 30°03′ N, 1170 m a.s.l.), which is at the western edge of the Sichuan Basin, southwestern China. This region has a subtropical humid monsoon mountain climate. Atmospheric N deposition in this area is dominated by wet deposition because of climate and topography, and the average annual wet N deposition was 95 kg ha−1 yr−1 from 2008–2010 [37]. The mean annual precipitation is 1730 mm, and the mean annual temperature is 16.2 °C. Vegetation covers 80% of the study site, which is dominated by Schima superba Gardn. et Champ., Lithocarpus hancei (Benth.) Rehd., Pittosporum tobira (Thunb.) Ait., Machilus pingii W. C. Cheng ex Yen C. Yang and Acer davidii Franch.. The soil at this site is classified as lithic dystrudepts (USDA Soil Taxonomy) with a depth of more than 60 cm. More detailed descriptions and the location of the study site are provided by Zhou et al. [31,38,39].
2.2. Experimental Design
According to the amount of wet N deposition (95 kg ha−1 yr−1) and based on an increasing trend [37,40], we set two levels of N deposition (ambient N deposition and plus 150 kg ha−1 yr−1 N deposition). The decreasing trend of annual precipitation has decreased by more than 40 mm every 10 years in the past 50 years [38,41]. According to the trend of decreasing rainfall, two levels of precipitation (ambient throughfall and 20% throughfall reduction) were set. The treatments were as follows: control (CK, ambient N deposition with no throughfall reduction), N deposition (N, plus 150 kg ha−1 yr−1 with no throughfall reduction), reduced precipitation (RP, ambient N deposition and 20% throughfall reduction) and combined N deposition and reduced precipitation (N + RP, plus 150 kg ha−1 yr−1 and 10% throughfall reduction). Twelve 5 m × 5 m plots (3 replicates per treatment) with intervals of 5 m were randomly established. From March 2016, N deposition was simulated using ammonium nitrate (NH4NO3) every half month. Each time N was applied, 44.64 g NH4NO3 was weighed and dissolved in 2 L water and then applied to the N deposition plots, and other plots received equal amounts of water without NH4NO3. Reduced precipitation treatment was achieved by building a throughfall reduction device. A 6 m × 6 m roof with a rain gutter covered with 6 m × 0.05 m translucent V-shaped PVC sheets mounted to wood frames was installed above each throughfall reduction plot. The number of sheets for throughfall reduction treatments was 10, covering 10% of the plot area; more details of the throughfall reduction device are described in Zhou et al. [38,39].
In November 2015, freshly fallen leaf litter of dominant tree species in the natural evergreen broad-leaved forest was collected by suspended litter traps and transported to the laboratory. The litter was mixed evenly at an approximately 5:2:2 ratio of Schima superba, Lithocarpus hancei and Pittosporum tobira, resembling the ratio in the field. The litter was oven dried in the laboratory at 65 °C, and then 15.0 g of dry mixed leaf litter was placed in nylon-mesh litter bags (20 cm × 20 cm; the surface layer mesh size was 3.00 mm to allow the entrance of mesofauna [9,35], and the mesh size of the ground layer was 0.05 mm to prevent litter loss through the mesh net). In March 2016, litter bags were evenly arranged on the soil surface of the 12 plots, and at least 10 cm spacing was maintained between adjacent litter bags to avoid interaction. Seventy-two litter bags were placed in each plot (12 sampling times × 6 bags per sampling time), and a total of 864 litter bags were placed in the field. In each plot, an in situ thermocouple was placed in a litter bag to measure the temperature during the litter decomposition process. After litter bags settled in the plots, simulated N deposition and reduced precipitation were carried out. During N deposition and reduced precipitation (from May 2016 to March 2018), litter bags were collected every two months. When sampled, litter bags were placed in sealed and breathable black cloth bags at a low temperature and then transported to the laboratory.
2.3. Mesofauna Species Identification
At each sampling time, three out of six litterbags per plot were collected to separate and extract soil mesofauna. Soil mesofauna was extracted by Tullgren dry funnels for 96 h and stored in 70% ethyl alcohol. Then, soil mesofauna was counted under a microscope equipped with double-tube anatomical lenses. Soil mesofauna samples were identified at the family level according to the Pictorial Keys for the Soil Animals of China [42]. The Shannon-Weiner diversity index (H′) was calculated as followed [16,19]:
where pi represents the relative abundance of family i.
2.4. Litter Mass Loss and Moisture
The remaining three litterbags per plot were air-dried, and each leaf fragment was wiped clean individually before being weighed to prevent litter contamination by soil. Then, drying was conducted at 65 °C, followed by weighing, to calculate litter moisture content, litter cumulative mass loss, and litter mass loss. Litter moisture content was calculated as the ratio of dry mass to fresh mass, litter cumulative mass loss was calculated as the ratio of remaining dry mass to initial dry mass, and litter mass loss was evaluated by the difference in cumulative mass loss between two sampling periods.
2.5. Statistical Analyses
We used linear mixed-effects models (LMMs) employing restricted maximum likelihood to examine the main effects of N deposition (ambient and elevated), reduced precipitation (ambient and reduced), and their interactions on litter cumulative mass loss, moisture, temperature, density, and diversity of soil mesofauna; treatments (CK, N, RP, N + RP) and sampling period were fixed factors, and blocks (12 plots) were random factors. Then, we performed Spearman correlation analysis to assess the relationships among litter mass loss and density of dominant mesofauna (Prostigmata, Mesostigmata, Oribatida, and Collembola), diversity of mesofauna and microclimate conditions (litter temperature and moisture). Linear mixed-effects models (LMMs) were performed using SPSS 20.0 for Windows, and correlation analysis was performed with R software, version 3.6.3 [43].
3. Results
3.1. Microclimate
Nitrogen deposition had no significant effects on either litter moisture or temperature (Figure 1; Table 1). Reduced precipitation significantly decreased litter moisture but little affected litter temperature (Figure 1; Table 1). In addition, there was no significant interaction effect of reduced precipitation and N deposition on litter moisture and temperature (Table 1).
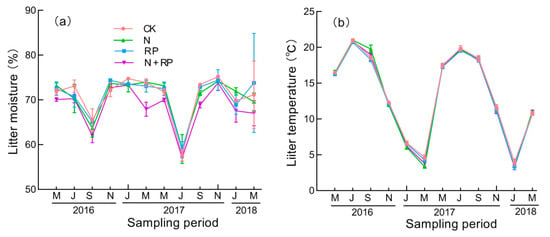
Figure 1.
Litter moisture (a) and temperature (b) during the experimental period. Means indicate average values of litter moisture and temperature, and vertical error bars indicate standard deviations of means (n = 3). The sampling periods were 2016 May, July, September and November, 2017 January, March, May, July, September and November, 2018 January and March. CK: ambient nitrogen and ambient precipitation; N: N deposition and ambient precipitation; RP: ambient nitrogen and reduced precipitation; N + RP: N deposition and reduced precipitation.

Table 1.
Linear mixed-effects models; ANOVA table of F and p values on the effect of N deposition and reduced precipitation and their interactions on microclimate and cumulative litter mass loss. Significant p values are in boldface.
3.2. Density and Diversity of Mesofauna
We collected and identified 19,782 individuals of mesofauna in litterbags during the whole experiment (Table 2). Mites (Prostigmata, Mesostigmata, and Oribatida) and Collembola comprised almost 90% of the total number of individuals collected and dominated the soil mesofauna in our study (Table 2; Figure A1).

Table 2.
Total individual numbers of soil mesofauna in all treatments.
The linear mixed-effects model showed that N deposition significantly decreased the density of Oribatida mites, Collembola, and total mesofauna (Table 3; Figure 2a,c,e) but did not significantly affect the density of Prostigmata or Mesostigmata mites or the diversity of mesofauna (Table 3; Figure 2b,e and Figure 3). Reduced precipitation significantly increased the density of Collembola, Oribatida mites, and total mesofauna and marginally increased the density of Mesostigmata mites (Table 3; Figure 2a–c,e), while the density of Prostigmata mites was little affected by reduced precipitation (Table 3; Figure 2d). In addition, reduced precipitation significantly decreased the diversity of mesofauna (Table 3; Figure 3). Furthermore, although we did not detect significant interaction effects between N deposition and reduced precipitation on the total density of mesofauna, there were significant interaction effects on the density of Prostigmata mites, Oribatida mites, Collembola, and diversity of mesofauna and a marginally significant interaction effect on the density of Mesostigmata mites (Table 3).

Table 3.
Total individual numbers of soil mesofauna in all treatments.
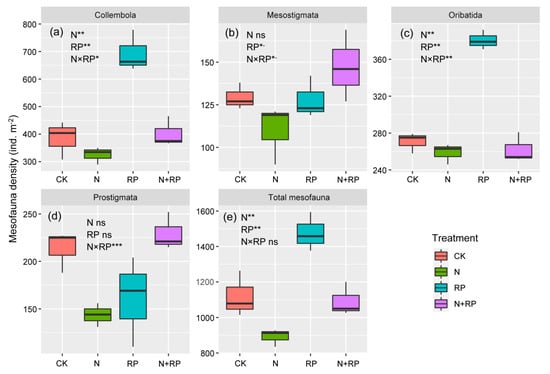
Figure 2.
Response of mesofauna abundance ((a): Collembola, (b): Mesostigmata, (c): Oribatida, (d): Prostigmata, (e): Total mesofauna) to N deposition and reduced precipitation. Boundaries of boxes indicate the first and third quartiles, and lines and squares within boxes represent the median and mean, respectively. Significance levels: *- 0.05 < p ≤ 0.10; * 0.01 < p ≤ 0.05; ** 0.001 < p ≤ 0.01; *** p ≤ 0.001. CK: ambient nitrogen and ambient precipitation; N: N deposition and ambient precipitation; RP: ambient nitrogen and reduced precipitation; N + RP: N deposition and reduced precipitation; “ns” means “no significant effects”.
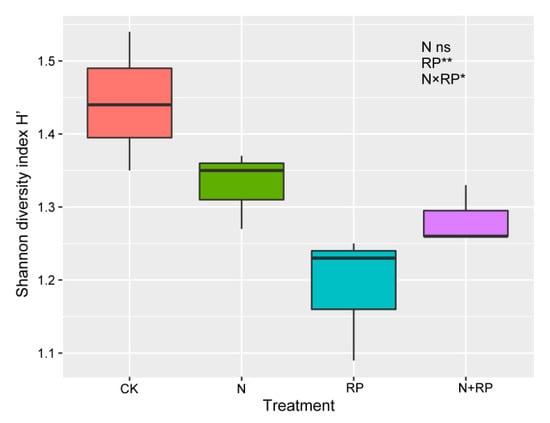
Figure 3.
Response of mesofauna diversity to N deposition and reduced precipitation. Boundaries of boxes indicate the first and third quartiles, and lines and squares within boxes represent the median and mean, respectively. Significance levels: * 0.01 < p ≤ 0.05; ** 0.001 < p ≤ 0.01: ambient nitrogen and ambient precipitation; N: N deposition and ambient precipitation; RP: ambient nitrogen and reduced precipitation; N + RP: N deposition and reduced precipitation; “ns” means “no significant effects”.
3.3. Litter Cumulative Mass Loss
During the whole experiment, litter cumulative mass loss increased continuously over 24 months (Figure 4). N deposition marginally significantly inhibited litter decomposition; likewise, reduced precipitation significantly retarded litter decomposition (Table 1; Figure 4). Additionally, there was no significant interaction effect of N deposition and reduced precipitation on litter cumulative mass loss (Table 1).
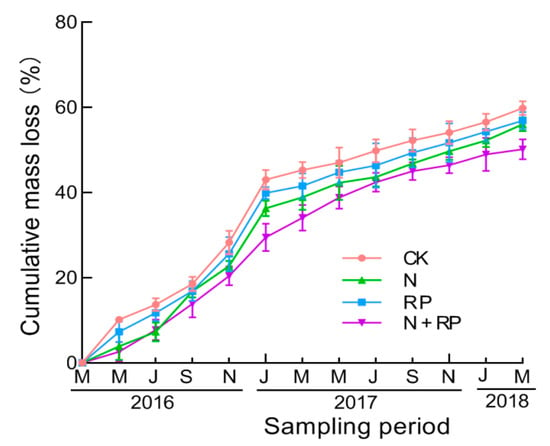
Figure 4.
Litter cumulative mass loss during the two-year period. Means indicate average values of litter cumulative mass loss, and vertical error bars indicate standard deviations of means (n = 3). CK: ambient nitrogen and ambient precipitation; N: N deposition and ambient precipitation; RP: ambient nitrogen and reduced precipitation; N + RP: N deposition and reduced precipitation; Sampling periods were May, July, September, and November 2016, January, March, May, July, September, and November 2017, January and March 2018.
3.4. The Relationship between Litter Mass Loss and the Mesofaunal Community as well as Microclimate
Spearman correlation showed that litter mass loss was significantly positively correlated with the density of Prostigmata (Figure 5a), Mesostigmata (Figure 5b), and Oribatida (Figure 5c) and the diversity of mesofauna (Figure 5d), whereas there was no significant correlation between litter mass loss and the density of Collembola, litter moisture, and temperature (Figure A2).
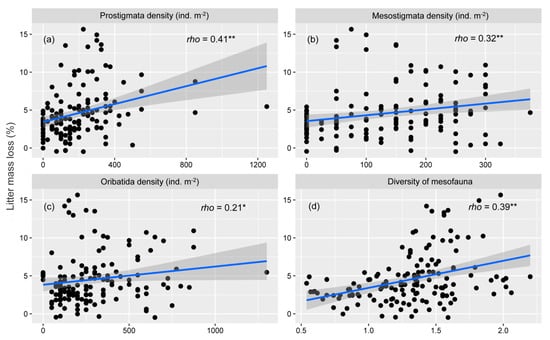
Figure 5.
Spearman correlation rho value for the correlations between litter mass loss and density of Prostigmata (a), Mesostigmata (b), and Oribatida (c) and diversity of mesofauna (d). We fit linear curves for litter mass loss and density of Prostigmata (a), Mesostigmata (b), and Oribatida (c) and diversity of mesofauna (d). In addition, the shaded areas denote 95% confidence intervals. Significance levels: * 0.01 < p ≤ 0.05; ** 0.001 < p ≤ 0.01.
4. Discussion
As hypothesized, our results showed that N deposition significantly decreased the total density of mesofauna, which was in line with some previous N addition experiments [20,29] and our first hypothesis. Two potential reasons accounted for the negative effects of N deposition on mesofauna density. First, the mesofaunal community is very sensitive to changes in soil pH, and N-induced soil acidification adversely affects mesofauna and then reduces its density [14,19]. Second, since microbes are the main living resources of mesofauna, N deposition reduces microbial biomass [44], which could induce resource limitation for mesofauna and thus decrease their biomass or abundance [45]. Our previous study also found that N deposition decreased soil pH and microbial biomass C and N [31]. According to the abovementioned two explanations, this N-induced decline in soil pH and living resources could help explain the observed decrease in the density of mesofauna in our study. In contrast to our results, some studies in temperate regions found that N addition increased soil mesofauna density [17,18]. The different responses of mesofaunal density presumably depend on the regional N content status. In N-poor ecosystems such as boreal forests, as a nutrient resource for soil biota, a certain amount of added N will increase the quality and quantity of food sources and increase mesofauna abundance [17,46]. As reported by Peng et al. [47], our studied forest is probably already N-saturated as a result of the high ambient atmospheric N deposition rate here (95 kg ha−1 yr−1). Excess N deposition would lead to a series of negative effects (e.g., decreasing microbial biomass [48]; eutrophication [49]; and acidification [50] and harm to mesofauna, hence adversely affecting mesofauna density. However, in contrast to our first hypothesis, the diversity of mesofauna was not significantly affected by N deposition. The nonsignificant effect of N deposition on the diversity of mesofauna could be attributed to three explanations. First, we did not investigate diversity at the species level but rather family diversity, which is more probably much less sensitive to harmful effects of N deposition: in other words, species diversity may decrease while family diversity remains constant. Second, increases in tolerant species compensate for decreases in other species so that the diversity of the mesofauna did not change significantly [32]. Particularly, in our study, N deposition treatments increased some mesofauna families (e.g., Parasitidae, Veigaiidae, and Ceratopogonidae; Table 3) but decreased other families (e.g., Segestriidae, Pholcidae, and Phoridae; Table 3), presumably resulting in the nonsignificant alteration of mesofauna diversity. Third, our study only lasted for 2 years, and the response of the diversity of mesofauna to N deposition was presumably realized after a longer period of time, which requires further research [51].
We found that N deposition significantly decreased the density of Collembola and Oribatida mites (prey) but had no significant effects on Prostigmata mites or Mesostigmata mites (predators). This difference is probably due to the different adaptation strategies of predators and prey in response to environmental stress [52]. Predators (Mesostigmata mites and Prostigmata mites) have traits that most closely correspond to ‘‘K-selected’’ species and strategies that are preadapted to stress conditions [22]. When N deposition causes negative effects such as soil acidification or shortages in living resources [14], this increases the predation of prey to minimize negative impacts and the predator density may not be affected to some extent, the increased potential predation pressure will decrease prey (i.e., Collembola and Oribatida) abundance [53]. Moreover, our correlation analysis showed that the density of Prostigmata, Mesostigmata, and Oribatida mites as well as the diversity of mesofauna were positively related to litter mass loss (Figure 5), indicating their positive roles in promoting litter decomposition. Thus, the decrease in litter decomposition in the N deposition plots (Figure 4) may be related to decreasing the density of Oribatida mites to N deposition (Figure 2c), as the positive roles of Oribatida mites in promoting litter decomposition (Figure 5c) partly supported our third hypothesis.
In contrast to our first hypothesis, we found that reduced precipitation significantly increased the total density of mesofauna, presumably due to the reduced precipitation changing the air-filled pore space and anaerobic conditions. The sufficient mean annual precipitation in our region (almost 1700 mm [54]) may lead to the saturation of air-filled pore space and anaerobic conditions which adversely affect mesofauna [55]. Reduced precipitation decreased litter moisture (Figure 1a), which might have enhanced the porosity of litter and facilitated gas exchange and thus increased the mesofaunal population [56]. While other studies found that reduced precipitation decreased soil fauna abundance [57,58], which is inconsistent with our results, the dissimilar response may be associated with local precipitation conditions [25,33]. Our study has more sufficient mean annual precipitation (1700 mm) compared to the aforementioned reduced precipitation experiments (1150 mm [57]; 600 mm [59]; 600 mm [58]). Water availability may not be the predominant limiting factor in our region; hence, a 20% reduction in the quantity of precipitation would not have a significant adverse influence on soil fauna at least for a short period. Therefore, the effects of reduced precipitation on mesofauna were contingent on the water resource status; reduced precipitation would constrict mesofauna in water-limited regions and presumably not have adverse effects on mesofauna in water-rich areas in the short term [33]. In addition, given that the diversity of mesofauna was positively related to litter mass loss (Figure 5d), reduced precipitation decreased the diversity of mesofauna (Figure 3), indicating that reduced precipitation inhibited litter composition (Figure 4) may via reducing the diversity of mesofauna, which was in accord with our third hypothesis.
Although we did not detect significant interaction effects of N deposition and reduced precipitation on the total density of soil mesofauna, the diversity of mesofauna was significantly affected by interaction effects, partly in line with our second hypothesis, implying that reduced precipitation could affect the response of mesofauna diversity to N deposition. Moreover, our results showed that reduced precipitation and N deposition decreased the diversity more severely than N deposition alone (Figure 3), indicating that reduced precipitation would exacerbate the negative effects of N deposition on mesofauna diversity. Thus, under the N deposition and reduced precipitation scenarios in our studied region, the decline in mesofauna diversity should be considered.
5. Conclusions
Our study revealed the main effects of N deposition and reduced precipitation on the mesofaunal community. Specifically, N deposition adversely affected some faunal groups (e.g., decreased the density of Collembola and Oribatida mites and the total density of mesofauna), while reduced precipitation increased the density of some faunal groups (e.g., Collembola, Mesostigmata mites, Oribatida mites, and the total density of mesofauna) but decreased mesofaunal diversity, and N deposition and reduced precipitation interactively affected mesofaunal diversity. Reduced precipitation exacerbated the negative effects of N deposition on mesofaunal diversity. Correlation analysis showed the positive roles of the Prostigmata, Mesostigmata, and Oribatida mite densities as well as the diversity of mesofauna in promoting litter decomposition. Moreover, N deposition and reduced precipitation both inhibited litter decomposition. Taken together, N deposition and reduced precipitation affected the mesofaunal community and litter decay, and the shift in litter decay was presumably attributed to the altered mesofaunal community caused by N deposition and reduced precipitation. However, we did not investigate diversity at the species level but rather family diversity, which is probably much less sensitive to N deposition or reduced precipitation. To better understand the effects of N deposition and reduced precipitation on soil fauna community during litter decomposition, future studies can investigate diversity at the species level.
Author Contributions
Conceptualization, S.Z., J.H. and C.H.; methodology, S.Z., J.H. and C.H.; formal analysis, J.H.; Software, J.H., L.X., D.C. and X.C.; investigation, J.H., X.L., X.Z. and L.T.; data curation, J.H. and C.H.; writing-original draft preparation, S.Z. and J.H.; writing-review and editing, S.Z., J.H. and C.H.; visualization, J.H.; supervision, C.H.; project administration, C.H.; funding acquisition, S.Z. and C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Natural Science Foundation of Sichuan Province (2022NSFSC1134), Science and Technology Project of Sichuan Province (2021YFYZ0006) and the Forest Ecosystem Improvement in the Upper Reaches of Yangtze River Basin Program of World Bank (510201202038467). J.H. was financially supported by the China Scholarship Council.
Data Availability Statement
Data presented in this study are available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
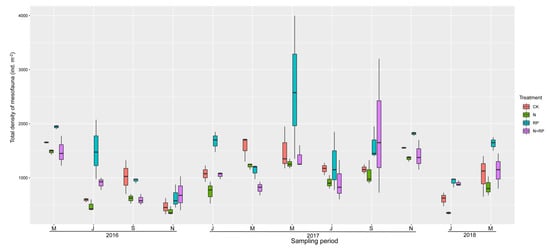
Figure A1.
Dynamic of total density of mesofauna during the two-year period. Boundaries of boxes indicate the first and third quartiles, lines and squares within boxes represent the median and mean, respectively. CK: ambient nitrogen and ambient precipitation; N: N deposition and ambient precipitation; RP: ambient nitrogen and reduced precipitation; N + RP: N deposition and reduced precipitation.
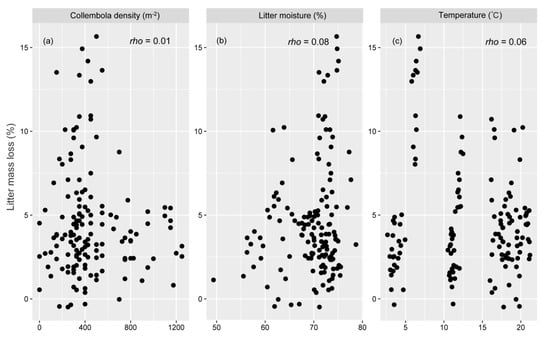
Figure A2.
Spearman correlation rho value for the correlations between litter mass loss and density of Collembola (a), litter moisture (b), temperature (c).
References
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Kanakidou, M.; Myriokefalitakis, S.; Daskalakis, N.; Fanourgakis, G.; Nenes, A.; Baker, A.R.; Tsigaridis, K.; Mihalopoulos, N. Past, Present, and Future Atmospheric Nitrogen Deposition. J. Atmos. Sci. 2016, 73, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Dore, M.H.I. Climate change and changes in global precipitation patterns: What do we know? Environ. Int. 2005, 31, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Ryo, M.; Lehmann, A.; Aguilar-Trigueros, C.A.; Buchert, S.; Wulf, A.; Iwasaki, A.; Roy, J.; Yang, G. The role of multiple global change factors in driving soil functions and microbial biodiversity. Science 2019, 366, 886–890. [Google Scholar] [CrossRef]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Handa, I.T.; Aerts, R.; Berendse, F.; Berg, M.P.; Bruder, A.; Butenschoen, O.; Chauvet, E.; Gessner, M.O.; Jabiol, J.; Makkonen, M.; et al. Consequences of biodiversity loss for litter decomposition across biomes. Nature 2014, 509, 218–221. [Google Scholar] [CrossRef]
- Frouz, J. Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 2018, 332, 161–172. [Google Scholar] [CrossRef]
- Peng, Y.; Vesterdal, L.; Peñuelas, J.; Peguero, G.; Wu, Q.; Heděnec, P.; Yue, K.; Wu, F. Soil fauna effects on litter decomposition are better predicted by fauna communities within litterbags than by ambient soil fauna communities. Plant Soil 2023, 1–11. [Google Scholar] [CrossRef]
- Fujii, S.; Berg, M.P.; Cornelissen, J.H.C. Living litter: Dynamic trait spectra predict fauna composition. Trends Ecol. Evol. 2020, 35, 886–896. [Google Scholar] [CrossRef]
- Ke, X.; Winter, K.; Filser, J. Effects of soil mesofauna and farming management on decomposition of clover litter: A microcosm experiment. Soil Biol. Biochem. 2005, 37, 731–738. [Google Scholar] [CrossRef]
- Wall, D.H.; Bradford, M.A.; St John, M.G.; Trofymow, J.A.; Behan-Pelletier, V.; Bignell, D.D.E.; Dangerfield, J.M.; Parton, W.J.; Rusek, J.; Voigt, W.; et al. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob. Change Biol. 2008, 14, 2661–2677. [Google Scholar] [CrossRef]
- Schaefer, M.; Migge-Kleian, S.; Scheu, S. The role of soil fauna for decomposition of plant residues. In Functioning and Management of European Beech Ecosystems; Springer: Berlin, Germany, 2009; pp. 207–230. [Google Scholar] [CrossRef]
- Nijssen, M.E.; WallisDeVries, M.F.; Siepel, H. Pathways for the effects of increased nitrogen deposition on fauna. Biol. Conserv. 2017, 212, 423–431. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, S.; Tie, L.; Liu, X.; Liu, X.; Zhao, A.; Lai, J.; Xiao, L.; You, C.; Huang, C. Effects of nitrogen addition on soil faunal abundance: A global meta-analysis. Glob. Ecol. Biogeogr. 2022, 31, 1655–1666. [Google Scholar] [CrossRef]
- Tie, L.; Wei, S.; Peñuelas, J.; Sardans, J.; Peguero, G.; Zhou, S.; Liu, X.; Hu, J.; Huang, C. Phosphorus addition reverses the negative effect of nitrogen addition on soil arthropods during litter decomposition in a subtropical forest. Sci. Total Environ. 2021, 781, 146786. [Google Scholar] [CrossRef]
- Van der Wal, A.; Geerts, R.; Korevaar, H.; Schouten, A.; op Akkerhuis, G.J.; Rutgers, M.; Mulder, C. Dissimilar response of plant and soil biota communities to long-term nutrient addition in grasslands. Biol. Fertil. Soils 2009, 45, 663–667. [Google Scholar] [CrossRef]
- Sjursen, H.; Michelsen, A.; Jonasson, S. Effects of long-term soil warming and fertilisation on microarthropod abundances in three sub-arctic ecosystems. Appl. Soil Ecol. 2005, 30, 148–161. [Google Scholar] [CrossRef]
- Xu, G.-L.; Schleppi, P.; Li, M.-H.; Fu, S.-L. Negative responses of Collembola in a forest soil (Alptal, Switzerland) under experimentally increased N deposition. Environ. Pollut. 2009, 157, 2030–2036. [Google Scholar] [CrossRef]
- Song, L.; Liu, J.; Yan, X.; Chang, L.; Wu, D. Euedaphic and hemiedaphic Collembola suffer larger damages than epedaphic species to nitrogen input. Environ. Pollut. 2016, 208, 413–415. [Google Scholar] [CrossRef]
- Trentini, C.P.; Villagra, M.; Gómez Pámies, D.; Laborde, V.B.; Bedano, J.C.; Campanello, P.I. Effect of nitrogen addition and litter removal on understory vegetation, soil mesofauna, and litter decomposition in loblolly pine plantations in subtropical Argentina. For. Ecol. Manag. 2018, 429, 133–142. [Google Scholar] [CrossRef]
- Cole, L.; Buckland, S.M.; Bardgett, R.D. Influence of disturbance and nitrogen addition on plant and soil animal diversity in grassland. Soil Biol. Biochem. 2008, 40, 505–514. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, W.; Wang, K.; Song, T.; Du, H. Responses of the soil nematode community to management of hybrid napiergrass: The trade-off between positive and negative effects. Appl. Soil Ecol. 2014, 75, 134–144. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Hu, F.; Ran, W.; Shen, Q.; Li, H.; Whalen, J.K. Carbon-rich organic fertilizers to increase soil biodiversity: Evidence from a meta-analysis of nematode communities. Agric. Ecosyst. Environ. 2016, 232, 199–207. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Niklaus, P.A.; Hungate, B.A. A meta-analysis of responses of soil biota to global change. Oecologia 2011, 165, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, U.N.; Ball, B.A. Impacts of altered precipitation regimes on soil communities and biogeochemistry in arid and semi-arid ecosystems. Glob. Change Biol. 2015, 21, 1407–1421. [Google Scholar] [CrossRef]
- Thakur, M.P.; Del Real, I.M.; Cesarz, S.; Steinauer, K.; Reich, P.B.; Hobbie, S.; Ciobanu, M.; Rich, R.; Worm, K.; Eisenhauer, N. Soil microbial, nematode, and enzymatic responses to elevated CO2, N fertilization, warming, and reduced precipitation. Soil Biol. Biochem. 2019, 135, 184–193. [Google Scholar] [CrossRef]
- Santonja, M.; Rancon, A.; Fromin, N.; Baldy, V.; Hättenschwiler, S.; Fernandez, C.; Montès, N.; Mirleau, P. Plant litter diversity increases microbial abundance, fungal diversity, and carbon and nitrogen cycling in a Mediterranean shrubland. Soil Biol. Biochem. 2017, 111, 124–134. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Cesarz, S.; Koller, R.; Worm, K.; Reich, P.B. Global change belowground: Impacts of elevated CO2, nitrogen, and summer drought on soil food webs and biodiversity. Glob. Change Biol. 2012, 18, 435–447. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Zhou, S.; Xiang, Y.; Tie, L.; Han, B.; Huang, C. Simulated nitrogen deposition significantly reduces soil respiration in an evergreen broadleaf forest in western China. PLoS ONE 2018, 13, e0204661. [Google Scholar] [CrossRef]
- Lindberg, N.; Persson, T. Effects of long-term nutrient fertilisation and irrigation on the microarthropod community in a boreal Norway spruce stand. For. Ecol. Manag. 2004, 188, 125–135. [Google Scholar] [CrossRef]
- Sylvain, Z.A.; Wall, D.H.; Cherwin, K.L.; Peters, D.P.C.; Reichmann, L.G.; Sala, O.E. Soil animal responses to moisture availability are largely scale, not ecosystem dependent: Insight from a cross-site study. Glob. Change Biol. 2014, 20, 2631–2643. [Google Scholar] [CrossRef]
- Zhu, J.; He, N.; Wang, Q.; Yuan, G.; Wen, D.; Yu, G.; Jia, Y. The composition, spatial patterns, and influencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Sci. Total Environ. 2015, 511, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Butenschoen, O.; Barantal, S.; Handa, I.T.; Makkonen, M.; Vos, V.; Aerts, R.; Berg, M.P.; McKie, B.; Van Ruijven, J.; et al. Decomposition of leaf litter mixtures across biomes: The role of litter identity, diversity and soil fauna. J. Ecol. 2020, 108, 2283–2297. [Google Scholar] [CrossRef]
- Bradford, M.A.; Tordoff, G.M.; Eggers, T.; Jones, T.H.; Newington, J.E. Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos 2002, 99, 317–323. [Google Scholar] [CrossRef]
- Tu, L.H.; Chen, G.; Peng, Y.; Hu, H.L.; Hu, T.X.; Zhang, J.; Li, X.W.; Liu, L.; Tang, Y. Soil biochemical responses to nitrogen addition in a bamboo forest. PLoS ONE 2014, 9, e102315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huang, C.; Xiang, Y.; Tie, L.; Han, B.; Scheu, S. Effects of reduced precipitation on litter decomposition in an evergreen broad-leaved forest in western China. For. Ecol. Manag. 2018, 430, 219–227. [Google Scholar] [CrossRef]
- Zhou, S.; Yan, G.; Hu, J.; Liu, X.; Zou, X.; Tie, L.; Yuan, R.; Yang, Y.; Xiao, L.; Cui, X.; et al. The responses of leaf litter calcium, magnesium, and manganese dynamics to simulated nitrogen deposition and reduced precipitation vary with different decomposition stages. Forests 2021, 12, 1473. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, C.; Han, B.; Xiao, Y.; Tang, J.; Xiang, Y.; Luo, C. Simulated nitrogen deposition significantly suppresses the decomposition of forest litter in a natural evergreen broad-leaved forest in the Rainy Area of Western China. Plant Soil 2017, 420, 135–145. [Google Scholar] [CrossRef]
- Zhou, C.; Cen, S.; Li, Y.; Peng, G.; Yang, S.; Peng, J. Precipitation variation and its impacts in Sichuan in the last 50 years. Acta Geogr. Sin. 2011, 66, 619–630. [Google Scholar]
- Yin, W. Pictorical Keys to Soil Animals of China; Science Press: Beijing, China, 1998. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 24 April 2020).
- Treseder, K.K.; Berlemont, R.; Allison, S.D.; Martiny, A.C. Nitrogen enrichment shifts functional genes related to nitrogen and carbon acquisition in the fungal community. Soil Biol. Biochem. 2018, 123, 87–96. [Google Scholar] [CrossRef]
- Meunier, C.L.; Gundale, M.J.; Sánchez, I.S.; Liess, A. Impact of nitrogen deposition on forest and lake food webs in nitrogen-limited environments. Glob. Change Biol. 2016, 22, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Adair, K.L.; Lindgreen, S.; Poole, A.M.; Young, L.M.; Bernard-Verdier, M.; Wardle, D.A.; Tylianakis, J.M. Above and belowground community strategies respond to different global change drivers. Sci. Rep. 2019, 9, 2540. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Y.; Song, S.; Chen, Y.; Chen, G.; Tu, L. Nitrogen addition slows litter decomposition accompanied by accelerated manganese release: A five-year experiment in a subtropical evergreen broadleaf forest. Soil Biol. Biochem. 2022, 165, 108511. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Greaver, T.L.; Clark, C.M.; Compton, J.E.; Vallano, D.; Talhelm, A.F.; Weaver, C.P.; Band, L.E.; Baron, J.S.; Davidson, E.A.; Tague, C.L.; et al. Key ecological responses to nitrogen are altered by climate change. Nat. Clim. Change 2016, 6, 836–843. [Google Scholar] [CrossRef]
- Bowman, W.D.; Cleveland, C.C.; Halada, Ĺ.; Hreško, J.; Baron, J.S. Negative impact of nitrogen deposition on soil buffering capacity. Nat. Geosci. 2008, 1, 767–770. [Google Scholar] [CrossRef]
- Cole, L.; Buckland, S.M.; Bardgett, R.D. Relating microarthropod community structure and diversity to soil fertility manipulations in temperate grassland. Soil Biol. Biochem. 2005, 37, 1707–1717. [Google Scholar] [CrossRef]
- Fujii, S.; Takeda, H. Succession of soil microarthropod communities during the aboveground and belowground litter decomposition processes. Soil Biol. Biochem. 2017, 110, 95–102. [Google Scholar] [CrossRef]
- Melguizo-Ruiz, N.; Jimenez-Navarro, G.; De Mas, E.; Pato, J.; Scheu, S.; Austin, A.T.; Wise, D.H.; Moya-Larano, J. Field exclusion of large soil predators impacts lower trophic levels, and decreases leaf-litter decomposition in dry forests. J. Anim. Ecol. 2019, 89, 334–346. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Yang, S.; Liu, C.; Zhong, A.; Li, Y. Characteristics of the precipitation over the eastern edge of the Tibetan Plateau. Meteorol. Atmos. Phys. 2010, 106, 49–56. [Google Scholar] [CrossRef]
- Turnbull, M.S.; Lindo, Z. Combined effects of abiotic factors on Collembola communities reveal precipitation may act as a disturbance. Soil Biol. Biochem. 2015, 82, 36–43. [Google Scholar] [CrossRef]
- Wei, X.; Cao, R.; Wu, X.; Eisenhauer, N.; Sun, S. Effect of water table decline on the abundances of soil mites, springtails, and nematodes in the Zoige peatland of eastern Tibetan Plateau. Appl. Soil Ecol. 2018, 129, 77–83. [Google Scholar] [CrossRef]
- Lindberg, N.; Engtsson, J.B.; Persson, T. Effects of experimental irrigation and drought on the composition and diversity of soil fauna in a coniferous stand. J. Appl. Ecol. 2002, 39, 924–936. [Google Scholar] [CrossRef]
- Makkonen, M.; Berg, M.P.; van Hal, J.R.; Callaghan, T.V.; Press, M.C.; Aerts, R. Traits explain the responses of a sub-arctic Collembola community to climate manipulation. Soil Biol. Biochem. 2011, 43, 377–384. [Google Scholar] [CrossRef]
- Maraldo, K.; Krogh, P.H.; van der Linden, L.; Christensen, B.; Mikkelsen, T.N.; Beier, C.; Holmstrup, M. The counteracting effects of elevated atmospheric CO2 concentrations and drought episodes: Studies of enchytraeid communities in a dry heathland. Soil Biol. Biochem. 2010, 42, 1958–1966. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).