Abstract
Introduced willows have mostly been employed as a renewable feedstock for bioenergy in the northeastern USA. The question of whether introduced willows provide the same biodiversity value and attractiveness as native willows has not yet been explored. The objective of this study was to compare the attractiveness of native and introduced willows to different subfamilies of bees. The common garden experiment planted at Storrs, CT, USA, included three native (S. eriocephala, S. sericea, S. lucida) and three introduced (S. ‘SX64’, S. ‘Onondaga’, S. ‘S365’) willows. Various willow taxa flowered at different times through spring, allowing pollinators to be collected over a 6-week period using colored bowl traps. As a result, 2430 bees were collected and identified to the subfamily level. Andreninae was the most prevalent pollinator visiting all taxa of willows, followed by Hylaeinae and Colletinae. There were no significant differences in the number of pollinators collected from either native or introduced taxa during the progressive willow flowering during mid-March–May of 2021 and 2022, suggesting their equal attractiveness to pollinators. Similarly, during the concurrent flowering of the two native and two introduced taxa, there were no significant differences in the number of pollinators associated with either group suggesting that when pollinators had foraging choices, they were similarly attracted to both native and introduced willows. The overall finding of this study suggested that plantings of either native or introduced willows to meet bioenergy goals offer similar benefits of floral resources for pollinators.
1. Introduction
The development of sustainably produced biomass as feedstock for bioenergy is a critical national priority in the U.S. due to concerns about energy security, rural economic development, and the environment [1]. Bioenergy plantations serve many purposes, including contributing to energy security, promoting soil health, decreasing soil erosion, serving as wind, snow, and flood buffers, as well as maintaining and enhancing biodiversity that supports ecosystem functions [2,3]. Production of short-rotation woody crops, such as willows (Salix), is expected to play a significant role in future energy supplies [4,5]. Willows have been the recent research focus as a renewable feedstock for bioenergy in the northeastern United States [6,7]. Due to their rapid above-ground biomass growth, shallow diffuse root systems, and efficient nutrient use and uptake, willows are a viable source for bioenergy in agricultural systems [8,9]. They are also used as buffers and vegetative filters that remove waste nutrients [3]. Willows can grow on less than favorable land, which reduces the land-use competition between food and energy crops [10,11].
Willow plantations add structural diversity to landscapes as well as enrich biodiversity [12]. For example, willows support a high density of breeding bird communities by providing services such as nesting, food, and stopover sites [13]. Willows are considered an ecosystem “foundation species”, due to the high number of insects they bolster [14]. Furthermore, willow short rotation coppice (SRC) plantations reduce the negative consequences of habitat fragmentation with the provision of food and shelter for a high proportion of animals that move from adjacent habitats into the plantations [15]. Throughout several countries, multiple studies have shown that SRC plantations provide habitats for a wide array of animals and fungi and also support higher arthropod species richness and density when planted on established agricultural land [16,17,18,19]. Incorporating different willow taxa into bioenergy plantations has previously been suggested to result in more resilient plantings and reduction of herbivorous predation that ensure plant survival and, in turn, protect pollinator communities [20,21].
Since willows bloom in early spring, they offer the first floral resources for emerging bees [12]. Willow pollen provides essential nutrients for bees. Willow pollen has the following characteristics: a protein content ranging from 14.73%–23.19%, a moisture content ranging from 15.0%–22.40%, and an ash content from 1.75%–2.80% [22]. Additionally, willow pollen contains sugars, such as fructose (13.99%–20.05%), glucose (8.92%–15.87%), sucrose (5.87%–22.04%), maltose (1.56%–3.85%), melezitose (0.27%–1.54%), and raffinose (0.02%–0.23%). Overall, willow pollen is nutritionally balanced for bees, similar to many other flowering plant species.
Some willow species act as primary hosts to many oligolectic bees including the native family Andrenidae, representatives of which are some of the first bees to emerge after overwintering in New England [20]. Oligolectic bees only collect pollen from either a single plant species or a related plant species and rely heavily on the predictable timing of flower emergence [23]. Bees use floral scents to find flowers and to recognize and distinguish between plant species [24]. Salix utilizes olfactory and visual signaling to attract pollinators. Bees, such as Andrenids, detect aromatic compounds emitted by willows, even at minute traces, from far distances [25,26].
In addition to the specific differences in willow floral cues, the sex of willows also factors into pollinator attraction. There is an established connection between male willows and the attraction of a high number of bees [27]. Males, which provide both pollen and nectar, support insect diversity more readily and consistently compared to their female counterparts, which provide only nectar [21]. Male willows have been shown to support approximately 39% greater abundance, 20% more richness, and 9% greater diversity of bee visitors than female willows [12]. The use of male willows in plantations not only maximizes resources for bees but also minimizes unintentional risks of dispersal of non-native willows.
Native willows are known to provide food and habitat to a large range of wildlife species [20] with numerous insects interacting with native species. Research has found that native forbs and flowering shrubs, which have co-evolved with native arthropods, are able to provide superior nutrition for bees when compared to non-native flowering plants [28]. Local plant ecotypes have co-evolved with other wildlife populations as well, providing food, nesting sites, and shelter that they have become dependent upon. Non-local ecotypes frequently have different phenological cycles when compared to local ecotypes. This difference in phenological cycles can result in the misalignment of the presentation of foraging resources with the emergence of native bee populations [29]. In contrast, past research has suggested that if non-native plants are closely related to local natives, this could influence how insects are able to use these non-native species even though the native insects share no direct evolutionary history with the non-native plants [30,31]. Non-native plants that are related to native species share similar morphology, chemical composition, defenses, and other characteristics. Therefore, insects that are adapted to local plants can also use related introduced species for resources [32].
Introduced willows are most commonly used in SRC plantations in the northeastern United States [33,34]. It is imperative to study the possible consequences of increasing biomass production using introduced willows and its effect on biodiversity [35]. It has been documented that introduced willows can support a diverse range of bees that are in decline, such as Andrenidae and Halictidae [12]. However, the question of whether introduced cultivars of willows can provide the same biodiversity values as native willows has not yet been explored. We hypothesized that native and introduced willow characteristics can potentially influence their attractiveness to bees. Therefore, the objective of this study was to determine if introduced willows have a similar biodiversity value when compared to native willows. If non-native willows provide the same extent of resources to bees as native willow taxa, this then will support the utilization of introduced Salix taxa in biomass plantations in the northeastern regions of North America.
2. Materials and Methods
2.1. Study System and Design
The common garden was established in the spring of 2017 at the University of Connecticut’s Plant Science Research and Education Facility located at Storrs, CT (41°48′04″ N, 72°13′57″ W) (Figure 1 and Figure 2). The total size of the garden was 900 m² (30 m × 30 m) and included six willow taxa (20 plants of each taxon; with a total of 120 willows randomly placed within 20 rows). There were five replications per each taxon of willow. These willows were planted as 25 cm unrooted dormant hardwood cuttings. Plants were arranged in rows 3 m apart with 3 m between plants within each row. This was executed differently from the plantation experimental design where the spacing between the rows and between plants usually are 1.5 m and 0.7 m, respectively [36]. This spacious experimental design provided better feeding conditions and easier navigation for studied organisms, as dense plantation designs may impact the quality of the plants. Mechanical and chemical weed controls were applied as needed to maintain weed cover below 20% during the first year of establishment. At the end of the first year, following leaf drop, all willows were coppiced at 5 cm above the soil surface, to promote denser growth during the following years.

Figure 1.
The common garden in the spring of 2017 at the University of Connecticut’s Plant Science Research and Education Facility located at Storrs, CT (41°48′04″ N, 72°13′57″ W): the upper photo was taken in 2017, and the lower photo was taken in 2021.

Figure 2.
White and yellow bowl traps set out at catkin level on 1 m poles by the willow taxa in the common garden at Storrs, Connecticut.
2.2. Salix Taxa
This study included three native species—Salix eriocephala Muhl., S. sericea Marsh., and S. lucida Muhl and three introduced biomass cultivars—S. ‘Onondaga’ (S. purpurea L.), S. ‘SX64’ (S. miyabeana Seemen.), and S. ‘S365’ (S. caprea L. hybrid). Salix ‘Onondaga’ was developed through controlled willow breeding in 1999 at The State University of New York College of Environmental Science and Forestry as a high biomass-yielding and pest-resistant cultivar [37] (Table 1). Salix ‘SX64’ was selected from native habitats in Asia and introduced by the University of Toronto as a high-yield and pest-resistant willow cultivar [38]. Salix ‘S365’ is a high-yield, triploid hybrid variety obtained from the University of Toronto [39]. Each taxon was represented by one male clone.

Table 1.
Characteristics of the six native and introduced willows planted in 2017 in the common garden at Storrs, Connecticut.
2.3. Bee Collection
Bees were collected during March–May 2021 and 2022 using pan traps (Version Plasticpro B08CY7lPXK, CA). Pan traps offer a reduced-labor, unbiased, and cost-effective way to estimate the relative abundance and diversity of insects [40]. Active sampling, such as net sweeping, captures insects at a particular moment in time. In contrast, pan traps are placed out for a specific duration, usually 24 h, enabling the collection of a wider insect population [41,42]. Bowls of different colors allow for a larger array of sampling as insects are attracted to different reflective properties [12]. White and blue bowls were used in 2021. The blue bowls did not collect many specimens. Therefore, white and yellow colored bowls, which possess qualities of higher light reflectance, were used in 2022.
The bowls were filled halfway with water with two drops of Dawn dish soap (Version 97591965_RET_NG, Toronto, ON) to break the surface tension of the water. This made it less likely that the specimens could escape and enabled a higher-yielding collection of insects. The bowls were set at a height of 1 m above the ground on polyvinyl chloride (PVC)(Version Schedule 40 PVC pipe, Charlotte Pipe, Monroe, NC) poles to be close to the catkin level where active bee activity occurred (Figure 2). Each plant acted as one individual unit. The units for data collection were randomized using the PLAN procedure in SAS 9.2 (SAS Institute Inc., Cary, NC, USA) with five replications per willow taxon. Bee foraging among male willows was most prevalent during the morning hours [12], therefore, the bowls were set out between 9:00 and 10:00 a.m. and then retrieved 24 h later to maintain a consistent window of collection. The specimens were collected on sunny, calm days when ambient temperatures were above 12 °C for the adequate foraging activities performed by bees. Foraging activity performed by bees slows considerably when temperatures are below 12 °C [12,20] (Figure 3).
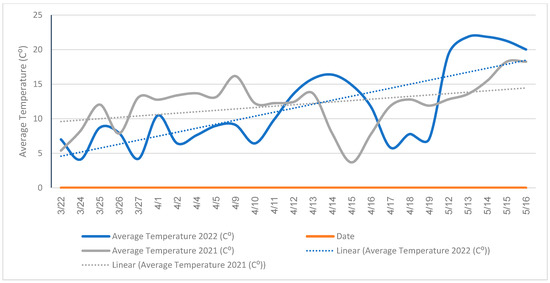
Figure 3.
The average air temperature (°C) during insect collection at Storrs, Connecticut, in the spring of 2021 and 2022.
The bee collection for 2021 occurred during the active flowering time of each species: S. ‘Onondaga’—March 20–31, S. ‘S365’—April 6–11, S. eriocephala—April 9–18, S. ‘SX64’—April 5–10, S. sericea—April 14–19 and S. lucida—May 9–14 (Figure 4).
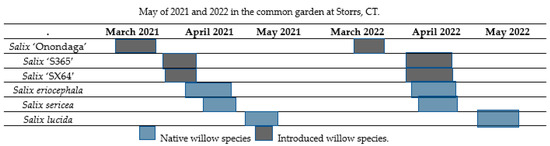
Figure 4.
The flowering periods of the native and introduced willow taxa during March, April, and May of 2021 and 2022 in the common garden at Storrs, CT.
Furthermore, the insect collection for 2022 took place during the following dates: S. ‘Onondaga’—March 22–27, S. ‘S365’—April 6–17, S. eriocephala—April 8–17, S. ‘SX64’—April 6–17, S. sericea—April 14–19 and S. lucida—May 12–19. Four taxa—S. eriocephala, S. sericea, S. ‘S365’, and S. ‘SX64’ had a period in which they flowered concurrently (Figure 4). The insects were collected from the bowls and placed into 75% EtOH for preservation. They were later identified to the subfamily level with a dissection microscope using previous knowledge of the species as well as a reference field guidebook [42,43,44].
Notably, the flowering time of S. ‘Onondaga’ coincided with a native species S. discolor (American pussy willow), which was observed in the natural habitats near the common garden in Storrs, in both years—around the last week of March. Salix discolor is not commonly used in biomass plantings because of its low biomass production compared to the introduced cultivars.
2.4. Statistical Analyses
We found a large discrepancy (0–50) between the number of specimens collected per bowl. This resulted in great variance and overdispersion in the data. Therefore, the number of specimens was transformed by a fitted model instead of being modeled linearly. The data were tested through different statistical models in R Studio 4.1—a Poisson model, a zero-inflated model, and a negative binomial model—to find a model of best fit. When the count of the specimens is less than the total variance of the summed count, a negative binomial model is the best to use when there is such over-dispersion in the variability of the data. The ANOVA indicated that the negative binomial was a more accurate depiction of the data. Under the negative binomial model, variances and deviations were much smaller than that of the Poisson model. There was no significant dispersion, outliers, or residual vs. predicted problems under the negative binomial model (p ≥ 0.05). A Type II Wald chi-square test was used as well to confirm whether the set of independent variables was collectively significant for the negative binomial model. Using this Type II Wald chi-square test revealed the willow taxa, as well as bowl color, were significant main effects in response to the number of bee specimens. The data were analyzed separately for each year using the fitted negative binomial model. The data were analyzed in log scale and then back transformed.
3. Results and Discussion
3.1. Bee Diversity
A total of 4970 specimens were collected during 2021 and 2022, including bees, flies, butterflies, moths, wasps, and planthoppers. Of those, 2510 were pollinators, which likely had specific insect–flower interactions with the willows. Overall, 2430 bees were collected and identified to the subfamily level as follows: Hymenoptera Andrenidae Andreninae, Hymenoptera Colletidae Hylaeinae, Hymenoptera Colletidae Colletinae, Hymenoptera Halictidae Halictinae, and Hymenoptera Apidae Bombus. Andreninae comprised the majority of the collected bee specimens (54.1%), followed by Hylaeinae (35.0%), Colletinae (9.8%), Halictinae (0.037%), and Bombus (0.025%) (Table 2).

Table 2.
The top bee subfamilies as well as genus (Bombus) and wasp families collected in 120 bowls during the spring of 2021 and 2022 from native and introduced willows grown in the common garden at Storrs, Connecticut.
The top three most abundant subfamilies of bees included Andreninae, Colletinae, and Hylaeinae. Tuminello et al. [12] also found that Andreninae was the most frequently occurring group associated with willows in the state of New York. Andreninae have been found to be in decline in New England due to the insufficient number of native shrubs, trees, or herbaceous plants that they need to survive [45]. As noted above, willows elicit an olfactory response in Andrenids that attract them specifically to their flowers. Andreninae pupate in late summer, overwinter as adults, and emerge in early spring. In spring, they use the willow’s pollen and nectar as resources to feed their young [12]. Andrenids rely on early blooming willows, such as S. ‘Onondaga’, for these resources. Most bees are unable to forage in temperatures below 12 °C; however, Andrenids forage in colder temperatures and have been seen in temperatures as low as 8 °C [12].
While representatives of Andreninae were the most frequently occurring bees associated with all willows, specimens from Hylaeinae were found to be the second most abundant subfamily of bees. Andrenid populations are known to be in decline, and it has been previously recorded in Connecticut state records of 2016 that the populations of Hylaeinae are increasing [45]. Hylaeinae are valuable pollinators of agricultural crops since they are generalists and pollinate many flowering plants that they may come across. Many species of Hylaeinae are bivoltine, meaning they have one brood that pollinates in the spring and another that is able to pollinate in the fall [46]. Colletinae was the third most abundant subfamily of bees. Like Andreninae, Colletinae are also considered to be in decline in the state of Connecticut [45]. Colletinae belongs to the same family as Hylaeinae; however its’ representatives are larger and more robust. Colletinae can be found foraging in New England during March and into late September. One reason that Colletinae was found in lower numbers is because they are more active pollinators in fall rather than spring. Additionally, while some are generalists, many Colletinae species are specialists that do not specifically pollinate willows and sometimes pollinate plants outside their specialty, such as willows, by accident [47].
Only nine specimens of Halictinae were collected in total. It was previously stated that they were rarely found pollinating willows [48]. Furthermore, only six Bombus specimens were collected. Bumble bees pollinate willows and have a stable population throughout New England; however, due to their large size, they are able to “swim” out of the bowl traps, dry off, and fly away [49]. Notably, Bombus representatives are more likely to be found in farm or field areas that contain willows rather than in farm or field areas that do not contain willows [50].
Besides bees, some wasps belonging to the Sphecid and Vespid families, which are also efficient pollinators, were collected during this study. The largest numbers of Sphecidae were associated with S. eriocephala and S. sericea, whereas Vespidae were found to be most frequently associated with S. sericea and S. lucida. There were no insects from Sphecid and Vespid families associated with the early-blooming S. ‘Onondaga’.
3.2. Bowl Color
In 2021, there was a significant difference in the number of specimens collected for the top three bee subfamilies in 2021 from white and blue bowls (Figure 5). These findings contrasted Droege’s findings [50], who found no significant difference in the number of bees collected from white, blue, or yellow bowls. Additionally, in 2021, there was no significant difference between the three subfamilies collected from either the white or blue bowls.
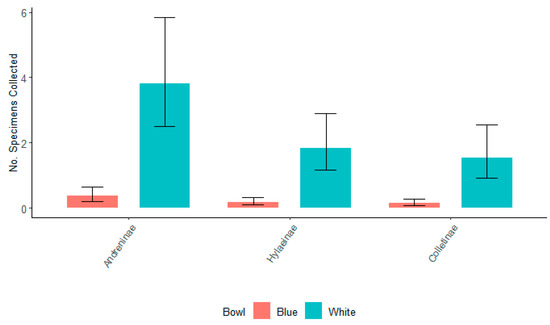
Figure 5.
The number of specimens of the top three subfamilies of bees, Andreninae, Colletinae, and Hylaeinae, for all willows collected in white and blue bowls in 2021. Non-overlapping confidence interval bars represent statistically different values within a 95% confidence interval.
After the switch from blue to yellow bowls during 2022, there was no significant difference in the number of specimens collected for the top three bee subfamilies for either white or yellow bowls (Figure 6). In 2022, Andreninae had a significantly higher number in both bowl colors. Colletinae counts were significantly smaller in both bowl colors compared to Andreninae and Hylaeinae. Although not significantly different, there was a slightly higher number of bees associated with the yellow-colored bowls.
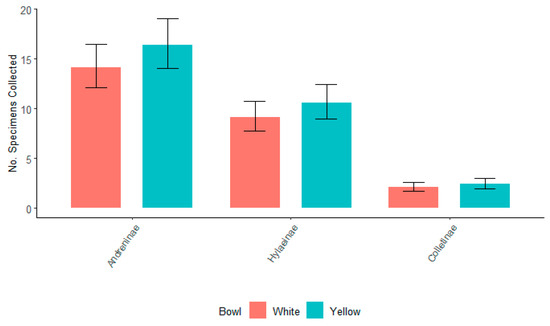
Figure 6.
The number of specimens of the top three subfamilies of bees, Andreninae, Colletinae, and Hylaeinae, for all willows collected in white and yellow bowls in 2022. Non-overlapping confidence interval bars represent statistically different values within a 95% confidence interval.
3.3. Total Number of Bees Associated with Native and Introduced Willows
When six willow taxa were grouped by origin, there were no significant differences in the total number of specimens for each bee subfamily associated with either native or introduced taxa for either 2021 or 2022 (p ≥ 0.05) (Figure 7 and Figure 8). For 2021, there was no significant difference between the number of specimens associated with any three subfamilies. However, for 2022, Andreninae and Hylaeinae had a significantly greater number of specimens associated with native and introduced willows compared to Colletinae. Colletinae had the smallest number of collected specimens.
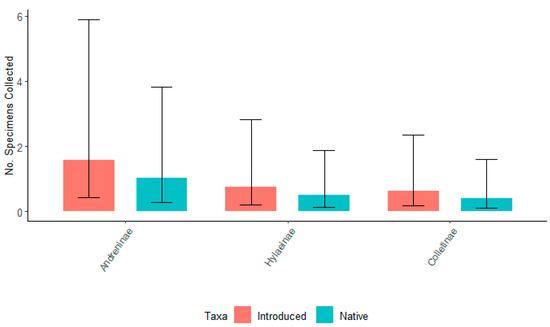
Figure 7.
The number of specimens collected for the top three subfamilies of bees, Andreninae, Hylaeinae, and Colletinae for native (S. eriocephala, S. sericea, S. lucida) and introduced (S. ‘Onondaga’, S. ‘SX64’, S. ‘S365’) willows in the spring of 2021. Non-overlapping confidence interval bars represent statistically different values within a 95% confidence interval.
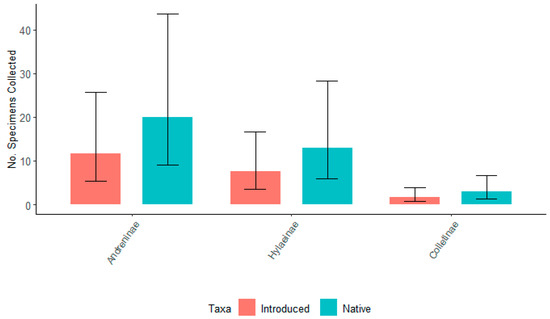
Figure 8.
The number of specimens collected for the top three subfamilies of bees, Andreninae, Hylaeinae, and Colletinae for native (S. eriocephala, S. sericea, S. lucida) and introduced (S. ‘Onondaga’, S. ‘SX64’, S. ‘S365’) willows in the spring of 2022. Non-overlapping confidence interval bars represent statistically different values within a 95% confidence interval.
3.4. Total Number of Bees Associated with Each Willow Taxa
For 2021, there were no significant differences between the top three subfamilies associated with each willow taxa (Figure 9). Andreninae representatives were the most frequently visiting bees associated with all willows but were not significantly different from Hylaeinae or Colletinae within each willow taxon. There was a significantly higher number of Andreninae, Hylaeus, and Colletinae associated with S. lucida, S. ‘Onondaga’, and S. ‘S365’ compared to other willow taxa. Meanwhile, there was a significantly lower number Andreninae, Colletinae, and Hylaeinae associated with S. eriocephala.
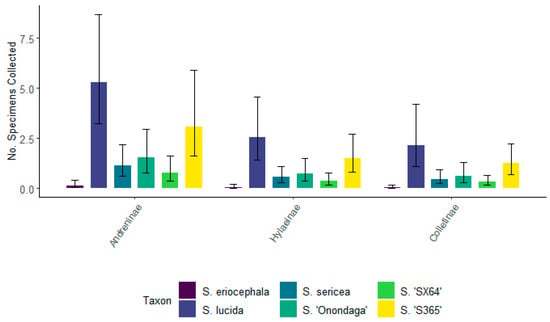
Figure 9.
The total number of specimens for the top three subfamilies of bees (Hylaeinae, Andreninae, and Colletinae) for six willows collected during the spring of 2021. Non-overlapping confidence interval bars represent statistically different values within a 95% confidence interval.
In 2022, Andreninae representatives were the most frequently visiting bees associated with all willow taxa, although they were not significantly different from Hylaeinae (Figure 10). The number of representatives for Andreninae was not significantly different when comparing S. eriocephala, S. sericea, S. ‘SX64’, and S. ‘S365’. Although, its number was significantly smaller for S. lucida when compared to S. eriocephala. Hylaeinae representatives were the second most frequently visiting subfamily of bees. The number of Hylaeinae specimens associated with S. eriocephala, S. sericea, S. ‘SX64’, and S. ‘S365’ was not significantly different. Like Andreninae, the number of Hylaeinae specimens associated with S. lucida was significantly smaller than the number associated with S. eriocephala. Colletinae representatives were the least frequently visiting bees of the top three subfamilies.
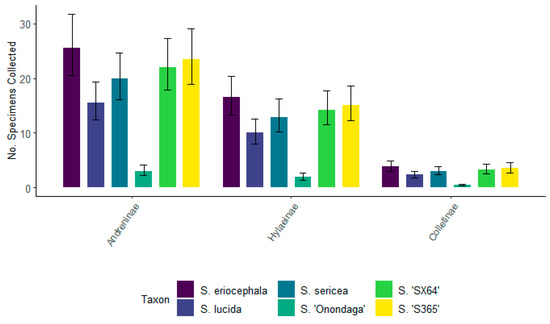
Figure 10.
The total number of specimens for the top three subfamilies of bees (Hylaeinae, Andreninae, and Colletinae) for six willows collected during the spring of 2022. Non-overlapping confidence interval bars represent statistically different values within a 95% confidence interval.
The following trends were recorded for the three bee subfamilies collected from the six willows.
For 2021, Andreninae and Hylaeinae had the greatest number of specimens associated with S. lucida, followed by S. ‘S365’ and S. ‘Onondaga’, although these numbers were not significantly different from one another within the respective subfamilies (Figure 11). Within the subfamilies, there was a significantly smaller number associated with S. ‘SX64’ and S. sericea. The least frequently visited willow for all subfamilies was S. eriocephala. This could be due to the large dip in temperature during its flowering from 13–17 April 2021. At this time the average temperature had plummeted to around 5 °C when bee foraging activity would not be occurring.
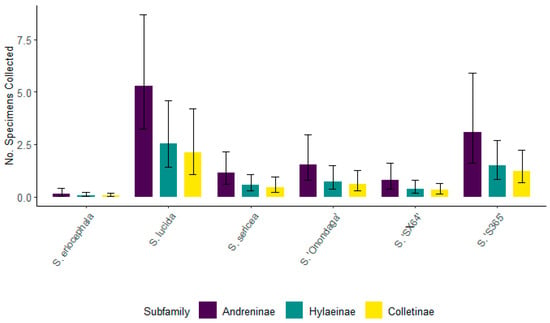
Figure 11.
The number of specimens for the top three bee subfamilies collected from six willows for 2021. Non-overlapping confidence interval bars represent statistically different values within a 95% confidence interval.
For 2022, the equally large numbers of specimens from Andreninae and Hylaeinae associated with S. eriocephala, S. sericea, S. ‘Onondaga’, and S. ‘S365’, were not significantly different across the respective subfamilies (Figure 12). Colletinae was the least frequently visiting group of bees to these willows. For S. lucida, there was a significant difference in the number of specimens collected for all three subfamilies of bees, with the greatest number associated with Andreninae, followed by Hylaeinae, then Colletinae. For S. sericea, Andreninae representatives were found to be the most prevalent visitors. Hylaeinae and Colletinae, which had significantly smaller numbers than Andreninae, were not different from one another. The most frequent visitors to S. ‘SX64’ were representatives of Andreninae followed by Hylaeinae, the number of which was significantly smaller. The least frequent subfamily of bees associated with S. ‘SX64’ was Colletinae, the number of which was significantly different.
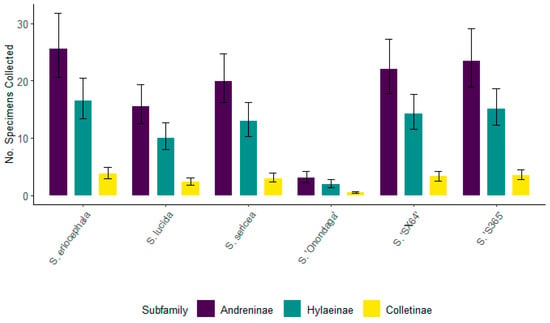
Figure 12.
The number of specimens for the top three bee subfamilies collected from six willows for 2022. Non-overlapping confidence interval bars represent statistically different values within a 95% confidence interval.
Additionally, for 2022, the number of Andreninae, Colletinae, and Hylaeinae associated with S. ‘Onondaga’ was significantly smaller than for other taxa of willows. Salix ‘Onondaga’ was the earliest willow to bloom during the last week of March and it had the fewest bees from all three subfamilies. This fact is most likely due to the cooler daily temperatures during S. ‘Onondaga’ flowering period which averaged from 3.3 °C to 8.9 °C during the day, and around 0 °C early in the morning (Figure 3). The number of bees continued to rise with rising temperatures.
3.5. Overlapping Blooming Periods
Four willows—two native (S. eriocephala and S. sericea) and two introduced (S. ‘S365’ and S. ‘SX64’) taxa—experienced concurrent flowering during 2022 (Figure 4), which allowed for direct comparisons of the bee preferences. The largest number of specimens collected for this study was during this time of simultaneous flowering.
When these four willows were grouped by native and introduced taxa, there were no significant differences in the number of specimens collected for the top three bee subfamilies Andreninae, Colletinae, and Hylaeinae (Figure 13). These findings suggest that when bees have foraging choices, they are similarly attracted to either native or introduced willows. Similar to the trends observed during the entirety of this study, the greatest number of bees were represented by Andreninae, followed by Hylaeinae, the number of which was just slightly smaller than Andreninae but not significantly different (Figure 14). There was a significantly smaller number of specimens associated with Colletinae for all willows flowering simultaneously. Again, this fact indicated that all willows were similarly attractive for these three subfamilies of bees.
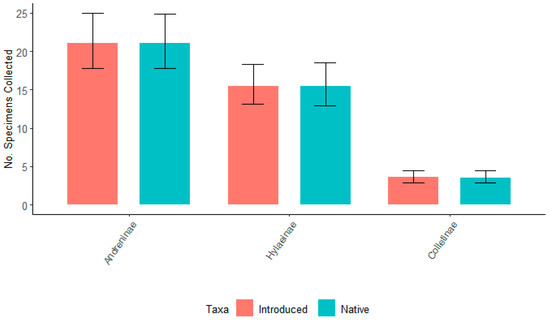
Figure 13.
The total number of specimens collected for the top three subfamilies bees—Andreninae, Colletinae, and Hylaeinae for native—(S. eriocephala and S. sericea) and introduced (S. ‘S365’ and S. ‘SX64’) taxa, which flowered concurrently during the spring of 2022 at Storrs, CT. Non-overlapping confidence interval bars represent statistically different values within a 95% confidence interval.
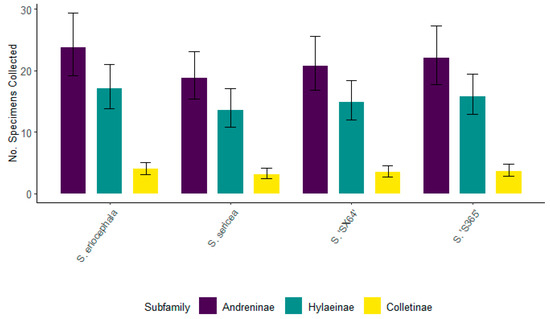
Figure 14.
The total number of specimens collected concurrently from two native (S. eriocephala and S. sericea) and two introduced (S. ‘S365’ and S. ‘SX64’) willows for the top three subfamilies of bees—Hylaeinae, Andreninae, and Colletinae—during the spring of 2022. Non-overlapping confidence interval bars represent statistically different values within a 95% confidence interval.
4. Conclusions
This study provided evidence about the bee attraction to both native and introduced willows and documented the bee groups associated with various willow taxa. The data obtained during this investigation suggested that bees did not have a preference for either native or introduced willows, and the origin of willows did not affect bee abundance or assemblages at the subfamily levels. The findings imply that the introduced willows employed in biomass plantations may offer similar resources for bees as native willows, and such plantings would provide valuable ecosystem services. Furthermore, the awareness of the number of bees associated with both native and introduced willows has management implications for the support of bees and enhancement of the overall biodiversity. In addition to the SRC plantations, both native and introduced willows can be integrated into various agricultural and managed landscapes to provide a wide range of ecosystem services. The phenological differences, particularly the various flowering time for different willow taxa recorded during this study, support the diversification of willows to provide a network of floral resources and a wide foraging window for pollinators throughout the season.
It has been previously suggested that planting willows near fruit and berry crops is an effective way to increase fruit production via increased pollination [14,51]. Both native and introduced willows may be strategically planted near agricultural fields along with pollinator-dependent crops to provide essential nutrient resources for bees prior to the mass flowering of agricultural crops. Also, it is important to note that while willows offer a nutrient-balanced pollen to bees and can stand alone, planting a mixture of local plant species can improve wild bee diets [52]. Various habitat configurations can be used for willow plantings to maximize overall benefit across landscapes, including windbreaks and snow fences, riparian buffers, and vegetation filters to create additional pollinator habitats. Either native or introduced willows may be used in these scenarios, offering additional opportunities to establish multifunctional landscapes, which support pollinators while providing other ecosystem services.
Author Contributions
Conceptualization and methodology, G.G., Y.A.K. and A.L.; data analysis, writing—original draft preparation, G.G. and Y.A.K.; writing—review and editing, G.G., Y.A.K. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this study was received from the U.S. Department of Agriculture National Institute of Food and Agriculture (HATCH grant number CONS00960).
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Langholtz, M.; Eaton, L.; Davis, M.; Shedden, M.; Brandt, C.; Volk, T.; Richard, T. Economic comparative advantage of willow biomass in the Northeast USA. Biofuels Bioprod. Biorefin. 2019, 13, 74–85. [Google Scholar] [CrossRef]
- Kuzovkina, Y.A.; Volk, T.A. The characterization of willow (Salix L.) varieties for use in ecological engineering applications: Co-ordination of structure, function, and autecology. Ecol. Eng. 2009, 35, 1178–1189. [Google Scholar] [CrossRef]
- Zumpf, C.; Quinn, J.; Cacho, J.; Grasse, N.; Negri, M.C.; Lee, D. Invertebrate and plant community diversity of an Illinois corn–soybean field with integrated shrub willow bioenergy buffers. Sustainability 2021, 13, 12280. [Google Scholar] [CrossRef]
- Rönnberg-Wästljung, A.C.; Dufour, L.; Gao, J.; Hansson, P.A.; Herrmann, A.; Jebrane, M.; Johansson, A.C.; Kalita, S.; Molinder, R.; Nordh, N.E.; et al. Optimized utilization of Salix—Perspectives for the genetic improvement toward sustainable biofuel value chains. GCB Bioenergy 2022, 14, 1128–1144. [Google Scholar] [CrossRef]
- Reddersen, J. SRC-willow (Salix viminalis) as a resource for flower-visiting insects. Biomass Bioenergy 2001, 20, 171–179. [Google Scholar] [CrossRef]
- Volk, T.A.; Berguson, B.; Daly, C.; Halbleib, M.; Miller, R.; Rials, T.; Abrahamson, L.; Buchman, D.; Buford, M.; Cunningham, M.; et al. Poplar and shrub willow energy crops in the United States: Field trial results from the multiyear regional feedstock partnership and yield potential maps based on the PRISM-ELM Model. GCB Bioenergy 2018, 10, 735–751. [Google Scholar] [CrossRef]
- Frank, J.; Therasme, O.; Volk, T.A.; Brown, T.; Malmsheimer, R.W.; Fortier, M.O.; Eisenbies, M.H.; Ha, H.; Heavey, J. Integrated stochastic life cycle assessment and techno-economic analysis for shrub willow production in the Northeastern United States. Sustainability 2022, 14, 9007. [Google Scholar] [CrossRef]
- Kuzovkina, Y.A.; Weih, M.; Abalos Romero, M.; Charles, J.; Hurst, S.; McIvor, I.; Karp, A.; Trybush, S.; Labrecque, M.; Teodorescu, T.; et al. Salix: Botany and global horticulture. Hortic. Rev. 2008, 34, 447–489. [Google Scholar]
- Rosa, D.; Clausen, J.; Kuzovkina, Y.A. Water quality changes in a short rotation woody crop riparian buffer. Bioenergy Biomass 2017, 107, 370–375. [Google Scholar] [CrossRef]
- Kuzovkina, Y.A.; Schulthess, C.; Zheng, D. Influence of soil chemical and physical characteristics on willow yield in Connecticut. Biomass Bioenergy 2018, 108, 297–306. [Google Scholar] [CrossRef]
- Yang, S.; Volk, T.A.; Fortier, M.O.P. Willow biomass crops are a carbon negative or low-carbon feedstock depending on prior land use and transportation distances to end users. Energies 2020, 13, 4251. [Google Scholar] [CrossRef]
- Tumminello, G.; Volk, T.A.; McArt, S.H.; Fierke, M.K. Maximizing pollinator diversity in willow biomass plantings: A comparison between willow sexes and among pedigrees. Biomass Bioenergy 2018, 117, 124–130. [Google Scholar] [CrossRef]
- Baril, L.M.; Hansen, A.J.; Renkin, R.; Lawrence, R. Songbird response to increased willow (Salix spp.) growth in Yellowstone’s Northern Range. Ecol. Appl. 2011, 21, 2283–2296. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.R.; DeBano, S.J.; Rowland, M.M.; Burrows, S. Feed the bees and shade the streams: Riparian shrubs planted for restoration provide forage for native bees. Restor. Ecol. 2022, 30, e13525. [Google Scholar] [CrossRef]
- Dauber, J.; Jones, M.B.; Stout, J.C. The impact of biomass crop cultivation on temperate biodiversity. GCB Bioenergy 2010, 2, 289–309. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Quine, C.P.; Sayer, J. Plantation forests and biodiversity: Oxymoron or opportunity? Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
- Verheyen, K.; Buggenhout, M.; Vangansbeke, P.; De Dobbelaere, A.; Verdonckt, P.; Bonte, D. Potential of short rotation coppice plantations to reinforce functional biodiversity in agricultural landscapes. Biomass Bioenergy 2014, 67, 435–442. [Google Scholar] [CrossRef]
- Rowe, R.L.; Goulson, D.; Doncaster, C.P.; Clarke, D.J.; Taylor, G.; Hanley, M.E. Evaluating ecosystem processes in willow short rotation coppice bioenergy plantations. GCB Bioenergy 2013, 5, 257–266. [Google Scholar] [CrossRef]
- Rowe, R.L.; Hanley, M.E.; Goulson, D.; Clarke, D.J.; Doncaster, C.P.; Taylor, G. Potential benefits of commercial willow Short Rotation Coppice (SRC) for farm-scale plant and invertebrate communities in the agri-environment. Biomass Bioenergy 2011, 35, 325–336. [Google Scholar] [CrossRef]
- Mosseler, A.; Major, J.; Ostaff, D.; Ascher, J. Bee foraging preferences on three willow (Salix) species: Effects of species, plant sex, sampling day and time of day. Ann. Appl. Biol. 2020, 177, 333–345. [Google Scholar] [CrossRef]
- Kollberg, I.; Weih, M.; Glynn, C. The effect of willow diversity on insect herbivory and predation. Agric. For. Entomol. 2022, 24, 27–39. [Google Scholar] [CrossRef]
- Prđun, S.; Svečnjak, L.; Valentić, M.; Marijanović, Z.; Jerković, I. Characterization of bee pollen: Physico-chemical properties, headspace composition and FTIR spectral profiles. Foods 2021, 10, 2103. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.J.; Keefover-Ring, K.; Park, Y.L.; Wimp, G.; Grady, J.; DiFazio, S.P. Characterization of Salix nigra floral insect community and activity of three native Andrena bees. Ecol. Evol. 2021, 11, 4688–4700. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.; Marquardt, M.; Babucke, K.; Heuel, K.C.; Ayasse, M.; Dötterl, S.; Galizia, C.G. Neural and behavioural responses of the pollen-specialist bee Andrena vaga to Salix odors. J. Exp. Biol. 2021, 224, jeb242166. [Google Scholar] [CrossRef] [PubMed]
- Dötterl, S.; Glück, U.; Jürgens, A.; Woodring, J.; Aas, G. Floral reward, advertisement, and attractiveness to honeybees in dioecious Salix caprea. PLoS ONE 2014, 9, e93421. [Google Scholar] [CrossRef]
- Füssel, U.; Dötterl, S.; Jürgens, A.; Aas, G. Inter-and intraspecific variation in floral scent in the genus Salix and its implication for pollination. J. Chem. Ecol. 2007, 33, 749–765. [Google Scholar] [CrossRef]
- Konatowska, M.; Rutkowski, P.; Wendzonka, J. The impact of willow flowering time on species composition and the number of Apoidea pollinators. J. Biosci. Med. 2021, 9, 89–100. [Google Scholar]
- Isaacs, R.; Tuell, J.K.; Fiedler, A.K.; Gardiner, M.; Landis, D.A. Maximizing arthropod-mediated ecosystem services in agricultural landscapes: The role of native plants. Front. Ecol. Environ. 2009, 7, 196–203. [Google Scholar] [CrossRef]
- Jones, A.T.; Hayes, M.J.; Sackville Hamilton, N.R. The effect of provenance on the performance of Crataegus monogyna in hedges. J. Appl. Ecol. 2001, 38, 952–962. [Google Scholar] [CrossRef]
- Baisden, E.C.; Tallamy, D.W.; Narango, D.L.; Boyle, E. Do cultivars of native plants support insect herbivores? HortTechnology 2018, 28, 596–606. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Lau, J.A.; Hambäck, P.A. Community heterogeneity and the evolution of interactions between plants and insect herbivores. Q. Rev. Biol. 2006, 81, 349–376. [Google Scholar] [CrossRef]
- Burghardt, K.T.; Tallamy, D.W. Not all non-natives are equally unequal: Reductions in herbivore β-diversity depend on phylogenetic similarity to native plant community. Ecol. Lett. 2015, 18, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Gouker, F.E.; Fabio, E.S.; Serapiglia, M.J.; Smart, L.B. Yield and biomass quality of shrub willow hybrids in differing rotation lengths and spacing designs. Biomass Bioenergy 2021, 146, 105977. [Google Scholar] [CrossRef]
- Montes, F.; Fabio, E.S.; Smart, L.B.; Kemanian, A.A. A semi-commercial case study of willow biomass production in the northeastern USA. Agron. J. 2021, 113, 1287–1302. [Google Scholar] [CrossRef]
- Fletcher, R.J., Jr.; Robertson, B.A.; Evans, J.; Doran, P.J.; Alavalapati, J.R.; Schemske, D.W. Biodiversity conservation in the era of biofuels: Risks and opportunities. Front. Ecol. Environ. 2011, 9, 161–168. [Google Scholar] [CrossRef]
- Fabio, E.S.; Volk, T.A.; Miller, R.O.; Serapiglia, M.J.; Gauch, H.G.; Van Rees, K.C.; Smart, L.B. Genotype× environment interaction analysis of North American shrub willow yield trials confirms superior performance of triploid hybrids. Bioenergy 2017, 9, 445–459. [Google Scholar] [CrossRef]
- Cameron, K.; Smart, L.; Ballard, B.; Volk, T.; Abrahamson, L. (Eds.) SUNY ESF. 2007a. Salix purpurea ‘Onondaga’. 2007. Available online: https://www.esf.edu/willow/documents/Onondaga.pdf (accessed on 25 March 2023).
- Cameron, K.; Smart, L.; Volk, T.; Abrahamson, L. (Eds.) SUNY ESF. 2007b. Salix miyabeana ‘SX64’. 2007. SUNY ESF. Available online: https://www.esf.edu/willow/documents/SX64.pdf (accessed on 25 March 2023).
- Cameron, K.; Smart, L.; Ballard, B.; Volk, T.; Abrahamson, L. (Eds.) SUNY ESF. 2007c. Salix caprea Hybrid ‘S365’. 2007. SUNY ESF. Available online: https://www.esf.edu/willow/documents/S365.pdf (accessed on 25 March 2023).
- St. Clair, A.L.; Dolezal, A.G.; O’Neal, M.E.; Toth, A.L. Pan traps for tracking honeybee activity-density: A case study in soybeans. Insects 2020, 11, 366. [Google Scholar] [CrossRef]
- Templ, B.; Mózes, E.; Templ, M.; Földesi, R.; Szirák, Á.; Báldi, A.; Kovács-Hostyánszki, A. Habitat-dependency of transect walk and pan trap methods for bee sampling in farmlands. J. Apic. Sci. 2019, 63, 93–115. [Google Scholar] [CrossRef]
- Buffington, M.L.; Garretson, A.; Kula, R.R.; Gates, M.W.; Carpenter, R.; Smith, D.R.; Kula, A.A. Pan trap color preference across Hymenoptera in a forest clearing. Entomol. Exp. Appl. 2021, 169, 298–311. [Google Scholar] [CrossRef]
- Borror, D.J.; White, R.E. A Field Guide to Insects: America North of Mexico; Houghton Mifflin Harcourt: Boston, MA, USA, 1998; Volume 19. [Google Scholar]
- Cadotte, M.W.; Cadotte, I.J.; MacIvor, J.S. The Bees in Your Backyard: A Guide to North America’s Bees: Andrenidae; Princeton University Press: Princeton, NJ, USA, 2017. [Google Scholar]
- Dibble, A.C.; Drummond, F.A.; Averill, A.L.; Bickerman-Martens, K.; Bosworth, S.C.; Bushman, S.L.; Hoshide, A.K.; Leach, M.E.; Skyrm, K.; Venturini, E.; et al. Bees and Their Habitats in Four New England States. MR448; Maine Agricultural and Forest Experiment Station: Orono, ME, USA, 2018. [Google Scholar]
- Zarrillo, T.A.; Ascher, J.S.; Gibbs, J.; Stoner, K.A. New and noteworthy records of bees (Hymenoptera: Apoidea: Anthophila) for Connecticut. J. Kans. Entomol. Soc. 2016, 89, 138–157. [Google Scholar] [CrossRef]
- Wilson, J.S.; Carril, O.M. The Bees in Your Backyard. A Guide to North America’s Bees: Colletidae; Princeton University Press: Princeton, NJ, USA, 2015. [Google Scholar]
- Suni, S.S.; Scott, Z.; Averill, A.; Whiteley, A. Population genetics of wild and managed pollinators: Implications for crop pollination and the genetic integrity of wild bees. Conserv. Genet. 2018, 18, 667–677. [Google Scholar] [CrossRef]
- Berkley, N.A.; Hanley, M.E.; Boden, R.; Owen, R.S.; Holmes, J.H.; Critchley, R.D.; Carroll, K.; Sawyer, D.G.M.; Parmesan, C. Influence of bioenergy crops on pollinator activity varies with crop type and distance. GCB Bioenergy 2018, 10, 960–971. [Google Scholar] [CrossRef]
- Droege, S.; Tepedino, V.J.; LeBuhn, G.; Link, W.; Minckley, R.L.; Chen, Q.; Conrad, C. Spatial patterns of bee captures in North American bowl trapping surveys. Insect Conserv. Divers. 2010, 3, 15–23. [Google Scholar] [CrossRef]
- Ostaff, D.P.; Mosseler, A.; Johns, R.C.; Javorek, S.; Klymko, J.; Ascher, J.S. Willows (Salix spp.) as pollen and nectar sources for sustaining fruit and berry pollinating insects. Can. J. Plant Sci. 2015, 95, 505–516. [Google Scholar] [CrossRef]
- Filipiak, Z.M.; Denisow, B.; Stawiarz, E.; Filipiak, M. Unravelling the dependence of a wild bee on floral diversity and composition using a feeding experiment. Sci. Total Environ. 2022, 820, 153326. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).