Soil Organic Carbon and pH Dominate the Effects of Nitrogen Addition on Soil Microarthropods in a Poplar Plantation in Coastal Eastern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Experimental Setup
2.4. Sample Measurements
2.4.1. Soil Microarthropods Identification
2.4.2. Soil Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benedek, K.; Bálint, J.; Máthé, I.; Mara, G.; Felföldi, T.; Szabó, A.; Fazakas, C.; Albert, C.; Buchkowski, R.W.; Schmitz, O.J.; et al. Linking intraspecific variation in plant chemical defence with arthropod and soil bacterial community structure and N allocation. Plant Soil 2019, 444, 383–397. [Google Scholar] [CrossRef]
- Feng, X.; Wang, R.; Yu, Q.; Cao, Y.; Jiang, Y. Decoupling of plant and soil metal nutrients as affected by nitrogen addition in a meadow steppe. Plant Soil 2019, 443, 337–351. [Google Scholar] [CrossRef]
- Maisto, G.; Santorufo, L.; Milano, V.; Arena, C. Relationships between Quercus ilex L. litter characteristics and soil microarthropod community in an urban environment at different climatic conditions. Appl. Soil Ecol. 2016, 99, 98–109. [Google Scholar] [CrossRef]
- Braun, S.; Thomas, V.F.; Quiring, R.; Flückiger, W. Does nitrogen deposition increase forest production? The role of phosphorus. Environ. Pollut. 2010, 158, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Divito, G.A.; Rozas, H.R.S.; Echeverría, H.E.; Studdert, G.A.; Wyngaard, N. Long term nitrogen fertilization: Soil property changes in an Argentinean Pampas soil under no tillage. Soil Tillage Res. 2011, 114, 117–126. [Google Scholar] [CrossRef]
- Ge, Z.; Fang, S.; Chen, H.; Zhu, R.; Peng, S.; Ruan, H. Soil Aggregation and Organic Carbon Dynamics in Poplar Plantations. Forests 2018, 9, 508. [Google Scholar] [CrossRef]
- Carson, C.M.; Jumpponen, A.; Blair, J.M.; Zeglin, L.H. Soil fungal community changes in response to long-term fire cessation and N fertilization in tallgrass prairie. Funct. Ecol. 2019, 41, 45–55. [Google Scholar] [CrossRef]
- Knorr, M.; Frey, S.D.; Curtis, P.S. Nitrogen additions and litter decomposition: A meta-analysis. Ecology 2005, 86, 3252–3257. [Google Scholar] [CrossRef]
- Lu, X.; Gilliam, F.S.; Yu, G.; Li, L.; Mao, Q.; Chen, H.; Mo, J. Long-term nitrogen addition decreases carbon leaching in a nitrogen-rich forest ecosystem. Biogeosciences 2013, 10, 3931–3941. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, J.; Clark, C.M.; Naeem, S.; Pan, Q.; Huang, J.; Zhang, L.; Han, X. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: Evidence from inner Mongolia Grasslands. Glob. Chang. Biol. 2010, 16, 358–372. [Google Scholar] [CrossRef]
- Evans, C.D.; Goodale, C.L.; Caporn, S.J.M.; Dise, N.B.; Emmett, B.A.; Fernandez, I.J.; Field, C.D.; Findlay, S.E.G.; Lovett, G.M.; Meesenburg, H.; et al. Does elevated nitrogen deposition or ecosystem recovery from acidification drive increased dissolved organic carbon loss from upland soil? A review of evidence from field nitrogen addition experiments. Biogeochemistry 2008, 91, 13–35. [Google Scholar] [CrossRef]
- Treseder, K.K.; Allen, E.B.; Egerton-Warburton, L.M.; Hart, M.M.; Klironomos, J.N.; Maherali, H.; Tedersoo, L. Arbuscular mycorrhizal fungi as mediators of ecosystem responses to nitrogen deposition: A trait-based predictive framework. J. Ecol. 2018, 106, 480–489. [Google Scholar] [CrossRef]
- Corredor, B.B.; Lang, B.; Russell, D. Effects of nitrogen fertilization on soil fauna—A global meta-analysis. In Proceedings of the EGU General Assembly Conference Abstracts, Online, 19 April 2021. [Google Scholar]
- Nijssen, M.E.; WallisDeVries, M.F.; Siepel, H. Pathways for the effects of increased nitrogen deposition on fauna. Biol. Conserv. 2017, 212, 423–431. [Google Scholar] [CrossRef]
- Zheng, C.; Ouyang, F.; Liu, X.; Ma, J.; Ge, F. Effect of coupled reduced irrigation and nitrogen fertilizer on soil mite community composition in a wheat field. Ecol. Evol. 2019, 9, 11367–11378. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Rocha, I.; Stevens, C.J.; Manrique, E.; Lucianez, M.J. Simulated nitrogen deposition affects soil fauna from a semiarid Mediterranean ecosystem in central Spain. Biol. Fert. Soils 2014, 50, 191–196. [Google Scholar] [CrossRef]
- Xu, C.; Xu, X.; Ju, C.; Chen, H.Y.; Wilsey, B.J.; Luo, Y.; Fan, W. Long-term, amplified responses of soil organic carbon to nitrogen addition worldwide. Glob. Chang. Biol. 2021, 27, 1170–1180. [Google Scholar] [CrossRef]
- Peguero, G.; Folch, E.; Liu, L.; Ogaya, R.; Penuelas, J. Divergent effects of drought and nitrogen deposition on microbial and arthropod soil communities in a Mediterranean forest. Eur. J. Soil Biol. 2021, 103, 103275. [Google Scholar] [CrossRef]
- Tan, X.; Machmuller, M.B.; Cotrufo, M.F.; Shen, W. Shifts in fungal biomass and activities of hydrolase and oxidative enzymes explain different responses of litter decomposition to nitrogen addition. Biol. Fertil. Soils 2020, 56, 423–438. [Google Scholar] [CrossRef]
- Berg, M.; Ruiter, P.D.; Didden, W.; Janssen, M.; Schouten, T.; Verhoef, H. Community food web, decomposition and nitrogen mineralisation in a stratified Scots pine forest soil. Oikos 2010, 94, 130–142. [Google Scholar] [CrossRef]
- Fang, Y.; Xun, F.; Bai, W.; Zhang, W.; Li, L. Long-term nitrogen addition leads to loss of species richness due to litter accumulation and soil acidification in a temperate steppe. PLoS ONE 2012, 7, e47369. [Google Scholar] [CrossRef]
- Eckert, M.; Gaigher, R.; Pryke, J.S.; Samways, M.J. Rapid recovery of soil arthropod assemblages after exotic plantation tree removal from hydromorphic soils in a grassland-timber production mosaic. Restor. Ecol. 2019, 27, 1357–1368. [Google Scholar] [CrossRef]
- Wang, S.; Tan, Y.; Fan, H.; Ruan, H.; Zheng, A. Responses of soil microarthropods to inorganic and organic fertilizers in a poplar plantation in a coastal area of eastern China. Appl. Soil Ecol. 2015, 89, 69–75. [Google Scholar] [CrossRef]
- Chi, L.; Yao, M.; Stegen, J.C.; Rui, J.; Li, J.; Li, X. Long-term nitrogen addition affects the phylogenetic turnover of soil microbial community responding to moisture pulse. Sci. Rep. 2017, 7, 17492. [Google Scholar]
- Xue, W.; Rui, C.; Wu, X.; Eisenhauer, N.; Sun, S. Effect of water table decline on the abundances of soil mites, springtails, and nematodes in the Zoige peatland of eastern Tibetan Plateau. Appl. Soil Ecol. 2018, 129, 77–83. [Google Scholar]
- Zhou, Y.; Liu, C.; Ai, N.; Tuo, X.; Zhang, Z.; Gao, R.; Qin, J.; Yuan, C. Characteristics of soil macrofauna and its coupling relationship with environmental factors in the loess area of Northern Shaanxi. Sustainability 2022, 14, 2484. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, X.; Wang, F. Composition and Spatial Distribution of Soil Mesofauna Along an Elevation Gradient on the North Slope of the Changbai Mountains, China. Pedosphere 2015, 25, 811–824. [Google Scholar] [CrossRef]
- Betancur-Corredor, B.; Lang, B.; Russell, D.J. Organic nitrogen fertilization benefits selected soil fauna in global agroecosystems. Biol. Fertil. Soils 2023, 59, 1–16. [Google Scholar] [CrossRef]
- Dickson, C.H.; Underhay, V.S.H.; Ross, V. Effect of season, soil fauna and water content on the decomposition of cattle dung pats. New Phytol. 1981, 88, 129–141. [Google Scholar] [CrossRef]
- Liu, S.; Yang, X.; Ives, A.R.; Feng, Z.; Sha, L. Effects of Seasonal and Perennial Grazing on Soil Fauna Community and Microbial Biomass Carbon in the Subalpine Meadows of Yunnan, Southwest China. Pedosphere 2017, 27, 371–379. [Google Scholar] [CrossRef]
- Marshall, V.G. Seasonal and vertical distribution of soil fauna in a thinned and urea-fertilized Douglas-fir forest. Can. J. Soil Sci. 1974, 54, 491–500. [Google Scholar] [CrossRef]
- Manning, P.; Saunders, M.; Bardgett, R.D.; Bonkowski, M.; Bradford, M.A.; Ellis, R.J.; Kandeler, E.; Marhan, S.; Tscherko, D. Direct and indirect effects of nitrogen deposition on litter decomposition. Soil Biol. Biochem. 2008, 40, 688–698. [Google Scholar] [CrossRef]
- Sayer, E.J. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. 2010, 81, 1–31. [Google Scholar] [CrossRef]
- Boxman, A.W.; Roelofs, J. Effects of liming, sod-cutting and fertilization at ambient and decreased nitrogen deposition on the soil solution chemistry in a scots pine forest in The Netherlands. For. Ecol. Manag. 2006, 237, 237–245. [Google Scholar] [CrossRef]
- Fang, S.; Xie, B.; Liu, J. Soil nutrient availability, poplar growth and biomass production on degraded agricultural soil under fresh grass mulch. For. Ecol. Manag. 2008, 255, 1802–1809. [Google Scholar] [CrossRef]
- Xie, T.; Zheng, A.B.; Wang, G.B.; Ruan, H.H.; Xu, Y.M.; Xu, C.B.; Ge, Z.W. Seasonal variation patterns of soil labile organic carbon in poplar plantations with different ages in northern Jiangsu. Chin. J. Ecol. 2012, 31, 1171–1178. [Google Scholar]
- Wang, G.B.; Deng, F.F.; Xu, W.H.; Chen, H.Y.H.; Ruan, H.H. Poplar plantations in coastal China: Towards the identification of the best rotation age for optimal soil carbon sequestration. Soil Use Manag. 2016, 32, 303–310. [Google Scholar] [CrossRef]
- Garcia-Franco, N.; Albaladejo, J.; Almagro, M.; Martínez-Mena, M. Beneficial effects of reduced tillage and green manure on soil aggregation and stabilization of organic carbon in a Mediterranean agroecosystem. Soil Tillage Res. 2015, 153, 66–75. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, S.; Ruan, H.; Fan, H.; Xu, K.; Xu, Y.; Xu, C.; Cao, G. Community structure of soil fauna in different age poplar plantations. J. Nanjing For. Univer. (Nat. Sci. Ed.) 2014, 38, 8–12. [Google Scholar]
- Yang, B.; Zhang, W.; Fan, H.; Wang, S.; Ruan, H.; Shen, C.; Cao, G. Community structure of soil fauna under different land use types in the coastal area of Northern Jiangsu Province. J. Nanjing For. Univer. (Nat. Sci. Ed.) 2017, 41, 120–126. [Google Scholar]
- National Meteorological Information Center. Annual Data Sets of Meteorological Observation in China. Available online: http://data.cma.cn/ (accessed on 20 December 2020).
- Wallwork, J.A. The Distribution and Diversity of Soil Fauna; Academic Press: New York, NY, USA, 1976; p. 355. [Google Scholar]
- Yin, W. Pictorial Keys to Soil Animals of China; Science Press: Beijing, China, 2000. [Google Scholar]
- Chen, G.S.; Yang, Z.J.; Gao, R.; Xie, J.S.; Guo, J.F.; Huang, Z.Q.; Yang, Y.S. Carbon storage in a chronosequence of Chinese fir plantations in southern China. For. Ecol. Manag. 2013, 300, 68–76. [Google Scholar] [CrossRef]
- Marin, S.; Andrea, L.E.; Ramona, S.L. Assessment of metals bioavailability to vegetables under field conditions using DGT, single extractions and multivariate statistics. Chem. Cent. J. 2012, 6, 119. [Google Scholar]
- Vance, E.; Brookes, P.; Jenkinson, D. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Bates, D.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Grothendieck, G. lme4: Linear Mixed-Effects Models Using Eigen and S4. R Package Version 2017. Available online: https://cran.r-project.org/web/packages/lme4/ (accessed on 3 March 2020).
- Peterson, B.G.; Carl, P.; Boudt, K.; Bennett, R.; Ulrich, J.; Zivot, E.; Lestel, M.; Balkissoon, K.; Wuertz, D. Package ‘PerformanceAnalytics’. 2018. Available online: https://github.com/braverock/PerformanceAnalytics (accessed on 3 March 2020).
- R Development Core Team. R: A Language and Environment for for Statistical Computing. Version 3.6.3. 2020. Available online: https://cran.r-project.org/bin/windows/base/old/ (accessed on 3 March 2020).
- Cole, L.; Dromph, K.M.; Boaglio, V.; Bardgett, R.D. Effect of density and species richness of soil mesofauna on nutrient mineralisation and plant growth. Biol. Fertil. Soils 2004, 39, 337–343. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.Y.; Tan, Y.; Fan, H.; Ruan, H. Fertilizer regime impacts on abundance and diversity of soil fauna across a poplar plantation chronosequence in coastal Eastern China. Sci. Rep. 2016, 6, 20816. [Google Scholar] [CrossRef]
- Boxman, A.W.; Blanck, K.; Brandrud, T.E.; Emmett, B.A.; Gundersen, P.; Hogervorst, R.F.; Kjønaas, O.J.; Perssom, T.; Timmermann, V. Vegetation and soil biota response to experimentally-changed nitrogen inputs in coniferous forest ecosystems of the NITREX project. For. Ecol. Manag. 1998, 101, 65–79. [Google Scholar] [CrossRef]
- Meunier, C.L.; Gundale, M.J.; Sanchez, I.S.; Liess, A. Impact of nitrogen deposition on forest and lake food webs in nitrogen-limited environments. Glob. Chang. Biol. 2016, 22, 164–179. [Google Scholar] [CrossRef]
- Matson, P.; Lohse, K.A.; Hall, S.J. The Globalization of Nitrogen Deposition: Consequences for Terrestrial Ecosystems. Ambio 2002, 31, 113–119. [Google Scholar] [CrossRef]
- Wei, C.; Zheng, H.; Li, Q.; Lu, X.; Yu, Q.; Zhang, H.; Chen, Q.; He, N.; Kardol, P.; Liang, W.; et al. Nitrogen addition regulates soil nematode community composition through ammonium suppression. PLoS ONE 2012, 7, e43384. [Google Scholar] [CrossRef]
- Xin, X.L.; Yang, W.L.; Zhu, Q.G.; Zhang, X.F.; Zhu, A.N.; Zhang, J.B.; Goss, M. Abundance and depth stratification of soil arthropods as influenced by tillage regimes in a sandy loam soil. Soil Use Manag. 2018, 34, 286–296. [Google Scholar] [CrossRef]
- Whitford, W.G.; Freckman, D.W.; Elkins, N.Z.; Parker, L.W.; Parmalee, R.; Phillips, J.; Tucker, S. Diurnal migration and responses to simulated rainfall in desert soil microarthropods and nematodes. Soil Biol. Biochem. 1981, 13, 417–425. [Google Scholar] [CrossRef]
- Kuťáková, E.; Cesarz, S.; Münzbergová, Z.; Eisenhauer, N. Soil microarthropods alter the outcome of plant-soil feedback experiments. Sci. Rep. 2018, 8, 12139–12145. [Google Scholar] [CrossRef]
- Soong, J.L.; Nielsen, U.N. The role of microarthropods in emerging models of soil organic matter. Soil Biol. Biochem. 2016, 102, 37–39. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Kallimanis, A.S.; Katana, E.; Stamou, G.P.; Sgardelis, S.P. Responses of soil microarthropods to experimental short-term manipulations of soil moisture. Appl. Soil Ecol. 2005, 29, 17–26. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, W.; Yang, H.; Yu, X.; Gutknecht, J.L.M.; Zhang, Z.; Wan, S.; Ma, K. Soil microbial responses to warming and increased precipitation and their implications for ecosystem C cycling. Oecologia 2013, 173, 1125–1142. [Google Scholar] [CrossRef]
- Qiu, L.; Yin, X.; Jiang, Y. Contributions of Soil Meso- and Microfauna to Nutrient Release During Broadleaved Tree Litter Decomposition in the Changbai Mountains. Environ. Entomol. 2019, 48, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Zhang, H.; Tang, Z. Responses of Litter Decomposition and Nutrient Dynamics to Nitrogen Addition in Temperate Shrublands of North China. Front. Plant Sci. 2021, 11, 2233. [Google Scholar] [CrossRef]
- Kleyer, M.; Trinogga, J.; Cebrián-Piqueras, M.A.; Trenkamp, A.; Fløjgaard, C.; Ejrnæs, R.; Bouma, T.J.; Minden, V.; Maier, M.; Mantilla-Contreras, J.; et al. Trait correlation network analysis identifies biomass allocation traits and stem specific length as hub traits in herbaceous perennial plants. J. Ecol. 2018, 107, 829–842. [Google Scholar] [CrossRef]
- Kinraide, T.B. Toxicity factors in acidic forest soils: Attempts to evaluate separately the toxic effects of excessive Al3+ and H+ and insufficient Ca2+ and Mg2+ upon root elongation. Eur. J. Soil Sci. 2003, 54, 232–333. [Google Scholar] [CrossRef]
- He, Z.; A.Tazisong, I.; Yin, X.; B.Watts, D.; Senwo, Z.N.; Torbert, H.A. Long-Term Cropping System, Tillage, and Poultry Litter Application Affect the Chemical Properties of an Alabama Ultisol. Pedosphere 2019, 29, 180–194. [Google Scholar] [CrossRef]
- Xu, G.L.; Schleppi, P.; Li, M.H.; Fu, S.L. Negative responses of Collembola in a forest soil (Alptal, Switzerland) under experimentally increased N deposition. Environ. Pollut. 2009, 157, 2030–2036. [Google Scholar] [CrossRef]
- Von Haden, A.C.; Dornbush, M.E. Patterns of root decomposition in response to soil moisture best explain high soil organic carbon heterogeneity within a mesic, restored prairie. Agric. Ecosyst. Environ. 2014, 185, 188–196. [Google Scholar] [CrossRef]
- Andriuzzi, W.S.; Adams, B.J.; Barrett, J.E.; Virginia, R.A.; Wall, D.H. Observed trends of soil fauna in the Antarctic Dry Valleys: Early signs of shifts predicted under climate change. Ecology 2018, 99, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Zárate-Valdez, J.L.; Zasoski, R.J.; Läuchli, A.E. Short-term effects of moisture content on soil solution pH and soil Eh. Soil Sci. 2006, 171, 423–431. [Google Scholar]
- Wang, H.; Liu, W.; Zhang, C.L. Dependence of the cyclization of branched tetraethers on soil moisture in alkaline soils from arid–subhumid China: Implications for palaeorainfall reconstructions on the Chinese Loess Plateau. Biogeosciences 2014, 11, 6755–6768. [Google Scholar] [CrossRef]
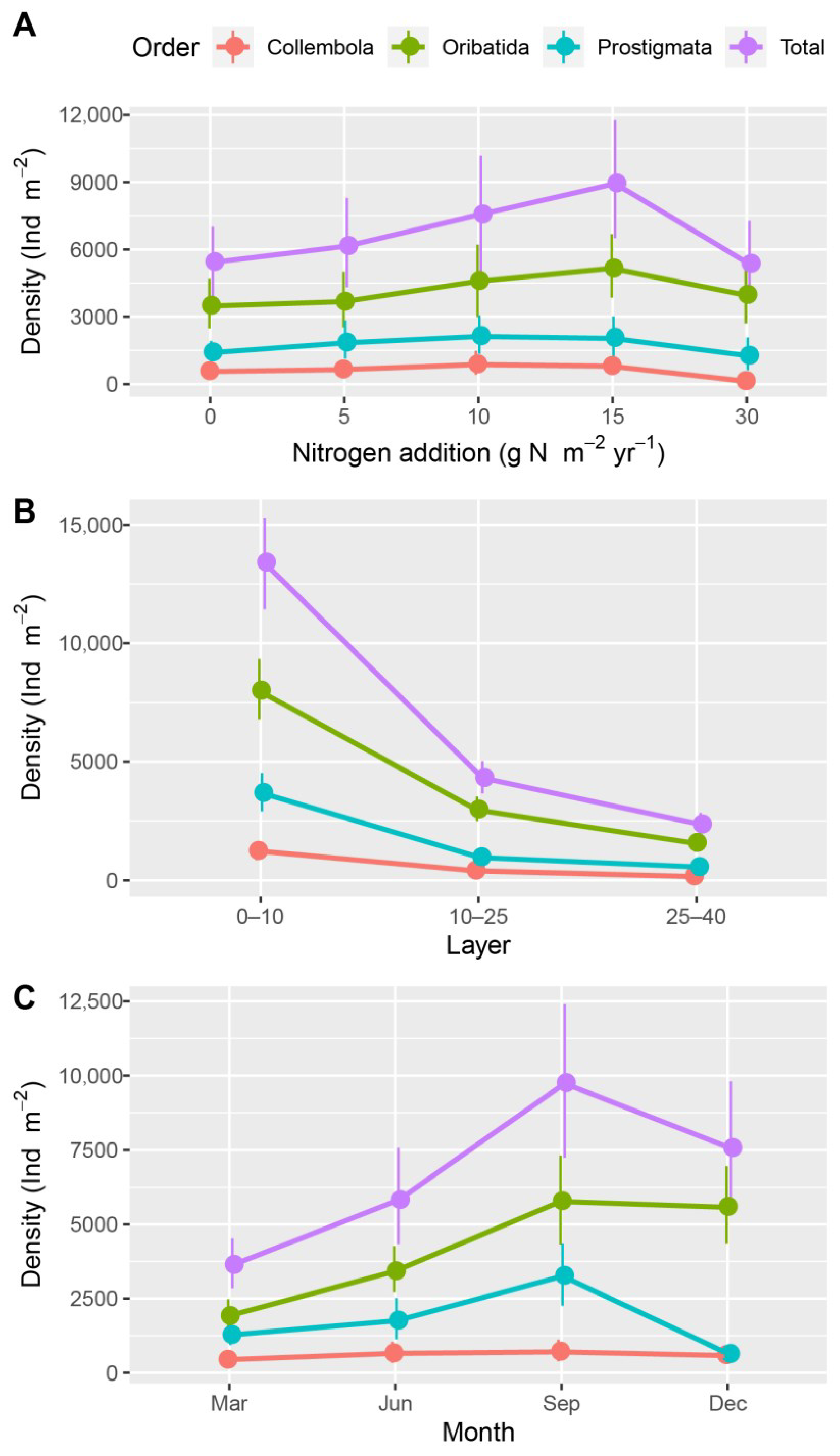
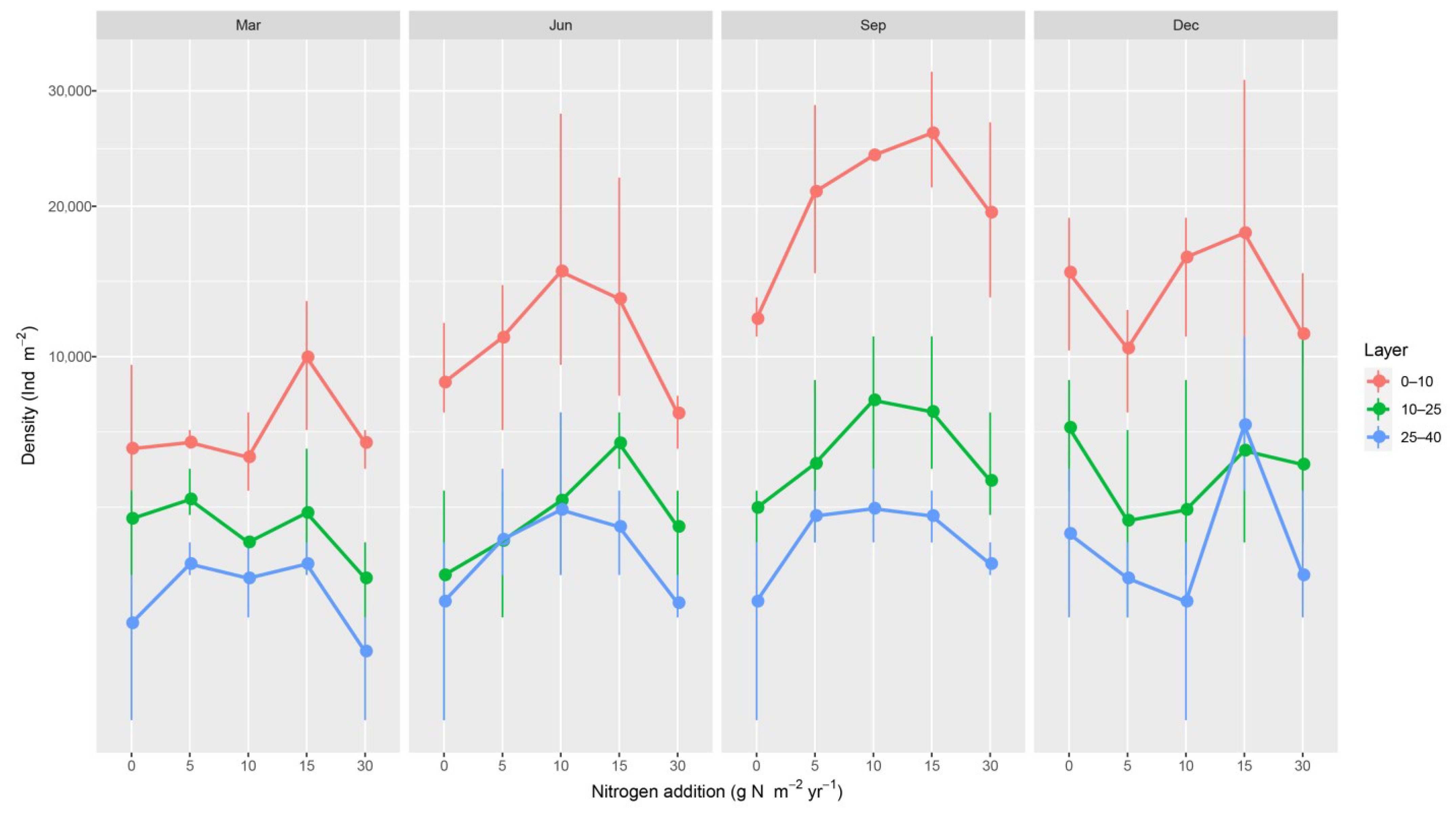


| Source | df | Sum Squares (×103) | F | p |
|---|---|---|---|---|
| N | 4, 8 | 12.4 | 8.3 | 0.006 |
| L | 2, 110 | 144.7 | 194.0 | <0.001 |
| D | 3, 110 | 26.8 | 24.0 | <0.001 |
| N × L | 8, 110 | 1.4 | 0.5 | 0.877 |
| N × D | 12, 110 | 8.5 | 1.9 | 0.042 |
| D × L | 6, 110 | 10.8 | 4.8 | <0.001 |
| N × D × L | 24, 110 | 4.8 | 0.5 | 0.958 |
| Effects | SOC | pH | Humidity | SMBC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | F | p | SS | F | p | SS | F | p | SS (×103) | F | p | |
| N | 316.0 | 83.0 | <0.001 | 0.7 | 33.9 | <0.001 | 0.006 | 1.3 | 0.350 | 20.3 | 0.3 | 0.866 |
| L | 2656.7 | 1396.3 | <0.001 | 3.3 | 341.9 | <0.001 | 0.1 | 59.2 | <0.001 | 390.6 | 11.7 | <0.001 |
| D | 242.5 | 85.0 | <0.001 | 6.7 | 460.6 | <0.001 | 0.1 | 33.1 | <0.001 | 6936.6 | 139.0 | <0.001 |
| N × L | 38.6 | 5.1 | <0.001 | 0.1 | 1.3 | 0.236 | 0.007 | 0.8 | 0.645 | 78.2 | 0.6 | 0.786 |
| N × D | 42.0 | 3.7 | <0.001 | 0.4 | 6.2 | <0.001 | 0.01 | 1.1 | 0.403 | 214.1 | 1.1 | 0.390 |
| D × L | 38.8 | 6.8 | <0.001 | 0.1 | 4.8 | <0.001 | 0.03 | 4.5 | <0.001 | 244.1 | 2.4 | 0.029 |
| N × D × L | 81.5 | 3.6 | <0.001 | 0.3 | 2.4 | 0.001 | 0.03 | 1.0 | 0.425 | 334.9 | 0.8 | 0.680 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Z.; Xiao, H.; Pang, Y.; Peng, S.; Mao, L.; Ruan, H. Soil Organic Carbon and pH Dominate the Effects of Nitrogen Addition on Soil Microarthropods in a Poplar Plantation in Coastal Eastern China. Forests 2023, 14, 880. https://doi.org/10.3390/f14050880
Ge Z, Xiao H, Pang Y, Peng S, Mao L, Ruan H. Soil Organic Carbon and pH Dominate the Effects of Nitrogen Addition on Soil Microarthropods in a Poplar Plantation in Coastal Eastern China. Forests. 2023; 14(5):880. https://doi.org/10.3390/f14050880
Chicago/Turabian StyleGe, Zhiwei, Hanran Xiao, Yanbing Pang, Sili Peng, Lingfeng Mao, and Honghua Ruan. 2023. "Soil Organic Carbon and pH Dominate the Effects of Nitrogen Addition on Soil Microarthropods in a Poplar Plantation in Coastal Eastern China" Forests 14, no. 5: 880. https://doi.org/10.3390/f14050880
APA StyleGe, Z., Xiao, H., Pang, Y., Peng, S., Mao, L., & Ruan, H. (2023). Soil Organic Carbon and pH Dominate the Effects of Nitrogen Addition on Soil Microarthropods in a Poplar Plantation in Coastal Eastern China. Forests, 14(5), 880. https://doi.org/10.3390/f14050880






