Abstract
Understanding the climatically suitable habitat of species plays a vital role in the sustainable use and management of target species. Calligonum mongolicum Turcz., a native shrub species found in desert areas of Central Asia, is generally considered as one of the top four tree species for desertification control. However, previous works on suitable habitat simulation had focused mainly on either the national or specific geographical scales rather than entire biota scales, which have underestimated the climatic tolerance of the species. Furthermore, the uncertainty outcomes of climate change were largely ignored. With these questions, the arid regions of Central Asia were selected as our research background area. Occurrence data of C. mongolicum were obtained from various sources, such as the Global Biodiversity Information Facility, the Chinese Virtual Herbarium, and the iPlant website. The maximum entropy model (MaxEnt) was used to simulate the suitable habitat change dynamics under various climate change scenarios [5 general circulation models (GCMs) × 3 shared socioeconomic pathways (SSPs)]. The uncertainty of climate change induced by GCMs and SSPs were decomposed by the two-way ANOVA method. Our results show that hydrological-related variables are more important for the species’ habitat suitability than thermal-related variables. The climatic threshold for the core suitable habitat was 1–30 mm for precipitation of the coldest quarter, 14–401 mm for annual precipitation, −16.01–12.42 °C for mean temperature of the driest quarter, 9.48–32.63 °C for mean temperature of the wettest quarter, and −25.01–−9.77 °C for the minimum temperature of the coldest month. The size of suitable habitat was about 287.4 × 104 km2 under the current climate condition, located in China and Mongolia. Climate change has less impact on the total area size, but it has bigger impacts on the gain area and loss area sizes. The loss area is mainly located in the southeast boundaries, whereas the gain area is mainly located in Mongolia and the Qinghai-Tibet Plateau. The decomposition uncertainty of climate change indicates that GCMs could explain 14.5%, 66.4%, and 97.0% of total variation, respectively, and SSPs could explain 85.5%, 33.6%, and 3.0% of the total variation for gain, loss, and total habitat sizes, respectively. Our work clearly demonstrates that while C. mongolicum has great planting potential in Central Asia under various climate change scenarios, the sensitive areas possess large uncertainties requiring long-term climate monitoring for afforestation projects.
1. Introduction
Desertification refers to the decline or loss of land productivity in arid, semi-arid, and sub-humid arid areas due to climate change or excessive human activities [1]. Serious desertification restricts the sustainable development of human society through productivity decline, sandstorms outbreaks, and natural disasters [2,3]. A series of biological, physical, and chemical measures have been used to solve the environmental problems caused by desertification [4,5]. Among them, biological measures are widely used in arid land, as it is the most economical and sustainable way. It refers to inhibiting degradation by planting trees or herbs on degraded land [6]. To achieve this goal, the selection of the right plant species, as well as exploring its planting potential, and the impact of climate change on its suitability, are all crucial for the sustainability of afforestation projects for desertification combating.
Calligonum mongolicum Turcz., also known as hairy grass, is a multi-branched shrub belonging to the family Polygonaceae, which is native in Central Asia. According to the report of Combating Desertification in Asia, Africa, and the Middle East, this species is listed as one of the four preferred and pioneer species for desertification control in contrast to Caragana korshinskii, Hippophae rhamnoides, and Haloxylon ammodendron [7,8,9]. This is mainly because C. mongolicum has a high ecological and economical value, such as wide tolerance range, drought resistance, and the sand fixation effect [10,11].
In past afforestation practice, C. mongolicum has received great attention. Based on 446 relevant references that reviewed this species, more than 90% of the research focused on the morphology and taxonomy of C. mongolicum [12,13], its genetics [14,15,16,17], physiological mechanisms of wind prevention, sand fixation effects, drought resistance abilities [18,19,20], and its response to environmental variables [7,21,22]. This provides valuable knowledge for us to use this species from the biological and ecological perspective. However, climate change will induce a range shift of species, challenging the sustainable afforestation and forest management for the species [23,24]. Currently, attention has been paid to the impact of climate change on the distribution area of C. mongolicum. For example, Hamit et al. [25] showed that climate change will either increase or decrease the suitable habitat of C. mongolicum in China depending on the CO2 emission scenarios. Xiao et al. [22], and Zhu [26], showed that climate change will increase the suitable habitat of C. mongolicum in the Junggar Basin of China. However, these studies only focused on either the national or specific geographical scales, rarely on the whole distribution scales, and did not provide the essential climatic thresholds, which limits our understanding of the climatic tolerance and climate change-induced range shift of the species at whole distribution scales.
Furthermore, future climate change is unpredictable. The outcomes of future climate data depend on CO2 emission scenarios and general circulation models (GCMs) [27]. CO2 emission scenarios are usually represented by representative concentration pathways (RCPs) or shared socioeconomic pathways (SSPs), which represent the optimistic and pessimistic estimates of global technological, economic, and social development [28]. The GCMs are mathematical models capable of representing the physical processes of the atmosphere and ocean to simulate responses of the global climate to the increasing CO2 emission [29]. Globally, there are dozens of GCMs that are continuously capable of producing future climate data. Previous studies [22,25,26,30,31,32] mainly reported the climate change uncertainty from the perspective of RCPs or SSPs, whereas the uncertainty of GCMs was ignored. However, Real et al. [32] suggested that the uncertainty of climate change mainly comes from GCMs rather than CO2 emission scenarios, which is mainly attributed to our insufficient knowledge concerning the effect of the atmosphere and oceans on the climate. Therefore, understanding the relative contribution of the two sources of variation (GCMs × RCPs/SSPs) is an important aspect of climate change studies.
To overcome the shortcomings of previous studies [22,25,26], we chose Central Asia as the research background region, which is a key area of the “the Belt and Road”, and an important lifeline of the Asian economy. This region is one of the regions with the fastest climate change in the world that is strongly threatened by climate change [33]. We collected the species occurrence data from multiple sources (detailed information can be seen in Section 2.2 species data) and used species distribution models to simulate the habitat suitability of the species across Central Asia. Species distribution models, also known as climate envelope models, are currently the mainstream method for studying the effects of climate change on species distribution. Our research objectives are as follows: (1) characterize the species’ climate thresholds, (2) predict the species’ suitable habitat in Central Asia, (3) assess the impact of climate change on the species’ habitats, and (4) decompose the sources of uncertainty regarding climate change. This study not only provides a case for understanding the risks of climate change on tree species in Central Asia, but also provides a scientific basis for the climate change adaptive management of C. mongolicum in practice.
2. Materials and Methods
2.1. Study Area
The study area is located in Central Asia (24°53′14″–56°16′24″ N, 43°44′43″–126°7′10″ E), covering an area of about 2.04 × 107 km2 (Figure 1). This was mainly due to the native range map of C. mongolicum recorded on the Plants of the World Online website (https://powo.science.kew.org/ (accessed on 3 June 2022)), which includes Kazakhstan, Mongolia, Uzbekistan, Turkmenistan, Iran, Tajikistan, Afghanistan, and China (represented by yellow polygons in Figure 1A). In this region, it possesses the characteristics of scarce rainfall, sufficient sunlight, dry climate, large evaporation, drastic temperature changes, and serious desertification. The annual mean temperature in this region is 7.41 °C, and the annual precipitation in this study area is 423 mm. Studies have shown that this region has one of the fastest climate changes in the world [33], which poses a serious threat to both the ecosystem and biodiversity [34,35].
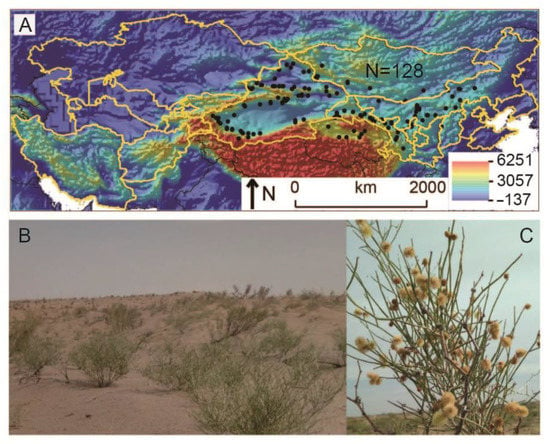
Figure 1.
Distribution records (black circles, (A)), as well as a landscape community photo (B) and flowering photo (C) of Calligonum mongolicum in the study area of Central Asia. The yellow polygons (A) represent the native range recorded on the Plants of the World Online website (https://powo.science.kew.org/ (accessed on 3 June 2022)).
2.2. Species Date
C. mongolicum usually grows on mobile sand dunes, semi-fixed sand dunes, fixed sand dunes, sandy desert, and gravel desert. The height of the C. mongolicum plant can reach 1.5 m. Figure 1 shows the landscape community photo (Figure 1B) and flowering photo of C. mongolicum (Figure 1C). Occurrence records of C. mongolicum were obtained from the following four sources: the Global Biodiversity Information Facility (https://www.gbif.org/ (accessed on 5 June 2022)), the Chinese Virtual Herbarium (https://www.cvh.ac.cn/ (accessed on 5 June 2022)), the iPlant Website (http://www.iplant.cn/ (accessed on 5 June 2022)), and other previous studies [36,37,38,39]. For records that only had the position information but with no coordinates, we obtained the relevant coordinate information through Google Earth positioning. Adjacent occurrences whose distance was less than a grid size (10 arc minutes (arc min)) were deleted, with only one of them being retained. Finally, 128 unique points were obtained (depicted by black circles in Figure 1A). These points are distributed in China and Mongolia, while they are almost invisible in the other native countries mentioned above. Interestingly, 7 occurrence points in the Qinghai province of China have been recorded, which expanded the native range map drawn by Plants of the World Online (yellow polygons in Figure 1A).
2.3. Climatic Variables and Climate Scenarios
To characterize the niche and to build the model, 19 climatic variables layers were downloaded from the WorldClim database (http://www.worldclim.org/ (accessed on 1 March 2022)): (1) Annual mean temperature (AMT); (2) Temperature seasonality (TS); (3) Annual range of temperature (ART); (4) Mean diurnal range (MDR); (5) Isothermality (Iso); (6) Maximum temperature of warmest month (MTWM); (7) Minimum temperature of coldest month (MTCM); (8) Mean temperature of wettest quarter (MTWQ); (9) Mean temperature of driest quarter (MTDQ); (10) Mean temperature of warmest quarter (MTWaQ); (11) Mean temperature of coldest quarter (MTCQ); (12) Annual precipitation (AP); (13) Precipitation seasonality (PS); (14) Precipitation of wettest month (PWM); (15) Precipitation of driest month (PDM); (16) Precipitation of wettest quarter (PWQ); (17) Precipitation of driest quarter (PDQ); (18) Precipitation of warmest quarter (PWaQ); (19) Precipitation of coldest quarter (PCQ). These 19 variables can accurately characterize the precipitation and temperature requirements of tree species around the world without further calculation [30,40,41,42]. These 19 current layers were based on thin plate smoothing splines, using the average latitude, longitude, altitude, monthly temperature, and precipitation data from 30 years of climate station records (1970–2000) [43].
For future climate scenarios, 15 climate change scenarios were selected, including five GCMs (ACCESS-CM2, BCC-CSM2-MR, GISS-E2-1-G, MIROC-ES2L, and HadGEM3-GC31-LL), and three SSPs (SSP1-2.6, SSP2-4.5, and SSP5-8.5), to estimate the suitable habitat change of C. mongolicum from 2000 to 2080. SSP1-2.6 adopts the sustainable development path and represents a low level of greenhouse gas (GHG) emissions. SSP2-4.5 represents a moderate and stable level of GHG emissions. SSP5-8.5 uses a fossil fuel-based development pathway and represents a high level of GHG emissions [28,44]. In these 15 climate change scenarios, the future temperature will be expected to increase by 1.96–6.39 °C, and precipitation will be predicted to increase by 17–69 mm. The summary of these 15 future climate change scenarios is shown in Table 1.

Table 1.
Summary of future climate change scenarios (2080s) from five general circulation models (GCMs).
2.4. Model Simulation and Statistical Analysis
MaxEnt software [45] was used to simulate the current ecological and future suitable habitat of C. mongolicum. MaxEnt 3.3.3k was downloaded from the website (https://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 3 March 2021)). Our simulation was divided into the following four steps:
Firstly, we trained and evaluated the current niche model. All 128 occurrence records, as well as the 19 current bioclimatic variables, were imported into the MaxEnt software. We used 10-fold cross validation to evaluate the performance of the model and used the area under the curve (AUC) to represent it. The criteria for AUC, as according to Li et al. [42], and Swets [46], were as follows: poor (0.5–0.6); fair (0.6–0.7); good (0.7–0.8); very good (0.8–0.9); and excellent (0.90–1.00).
Secondly, we projected these simulated niche models under current and future climatic scenarios. Thus, we obtained a current habitat map and 15 future maps (5 GCMs × 3 SSPs). These maps had values between 0 and 1, with 0 indicating lowest fitness and 1 indicating maximum fitness. A threshold was required to convert these suitability maps into range maps (binary maps). Here, the optimal threshold (TH) was calculated based on the mean value of maximum sensitivity and specificity. Accordingly, 0.15 was calculated as the optimal threshold (TH = 0.15). Then, we defined three classes of habitat: unsuitable area (TH < 0.15), moderately suitable area (0.15 < TH < 0.5), and core area (TH > 0.5).
Thirdly, we calculated the range shift maps under climate change scenarios. These range shift maps were calculated according to the following formula [9]:
where f represents future range shift maps; fmap represents future binary maps; cmap represents current binary map, and the subscript i represents the future climate change scenarios (5 GCMs × 3 SSPs). Accordingly, we obtained 15 range shift maps with codes of 0, 1, 2, and 3, respectively. These were used to visualize the range shift dynamics of C. mongolicum under different climate change scenarios, with 0 representing unsuitable habitat, 1 representing loss habitat, 2 representing gain habitat, and 3 representing a stable habitat.
Finally, we assessed the uncertainty of climate change. Here, the gain, loss, and total area of C. mongolicum were used as the dependent variable, while the GCMs and SSPs were used as the independent variable. Two-way ANOVA was performed to study the significance of the GCMs and SPPs range dynamics of C. mongolicum. Due to the 15 experiments (5 GCMs × 3 SSPs) being non-repetitive, we were unable to decompose the interaction between the GCMs and SSPs. The independent contribution of GCMs and SPPs to range change dynamics was decomposed by the variance decomposition method. The above analysis was implemented on R software version 4.2.0.
3. Results
3.1. Model Accuracy and Important Variables
Our simulated MaxEnt model was found to be significantly better than that of the random model and had excellent accuracy. The average value of the training AUC was 0.97, the average value of the test AUC was 0.939, while the standard deviation was 0.03. According to the criterion threshold of maximum sensitivity and specificity, the average of the optimal threshold was 0.15. The climatic variables PCQ, AP, MTDQ, MTWQ, and MTCM explained 69.9% of the total variance, whereas AMT, PS, MTCQ, MTWaQ, ART, TS, MDR, PWQ, and PDQ explained only 28% of the total variance (Table 2). The top five climatic variables could be divided into hydrological-related variables (45.7%), and thermal-related variables (24.2%), among which hydrological-related variables were found to have contributed more to the suitable habitat than thermal-related variables.

Table 2.
Percent contribution of the climate variables and climatic threshold of the suitable habitat for Calligonum mongolicum by the MaxEnt.
3.2. Climatic Threshold and Current Suitable Habitat
The climatic thresholds of the current suitable habitat are shown in Table 2. The results showed that the core area of C. mongolicum was mainly distributed in areas where the precipitation of coldest quarter (PCQ) was only 1–30 mm, the annual precipitation (AP) was 14–401 mm, the mean temperature of driest quarter (MTDQ) was −16.01–12.42 °C, the mean temperature of wettest quarter (MTWQ) was 9.48–32.63 °C, and the minimum temperature of coldest month (MTCM) was −25.01–−9.77 °C.
The simulated suitable habitat for C. mongolicum was mainly distributed in China and Mongolia, with a total area of 287.4 × 104 km2 (Figure 2). The core suitable habitat of C. mongolicum was mainly distributed in provinces of China, such as Xinjiang, Inner Mongolia, Gansu, Ningxia, and Qinghai. These distribution ranges were very consistent with the arid desert with cold climate (BWk), and arid steppe with cold climate (Bsk) climatic regions of the Köppen climate system (Figure 2). Surprisingly, using the established model, it was difficult to extrapolate the suitable habitat to similar climatic conditions observed in West Asian countries, such as Kazakhstan, Uzbekistan, Turkmenistan, Afghanistan, and Iran.
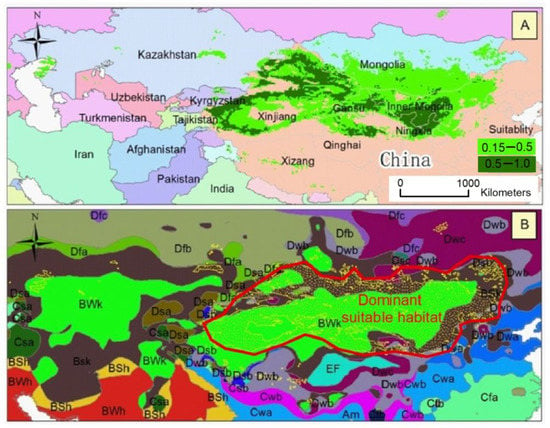
Figure 2.
Climatically suitable areas of Calligonum mongolicum (A) and its relationship with the Köppen climate system (B). The background colors represent the boundaries of countries (A), as well as the boundaries of the Köppen climate classification (B). Yellow polygons (in (B)) depict the total suitable habitat and the red polygons (in (B)) depict the dominant suitable habitat. The meanings of the background colors are described in abbreviations: BSk, arid steppe with cold climate; BWk, arid desert with cold climate; Dfb, cold region without dry season and warm summer; Dwc, cold region with dry winter and cold summer; BWh, arid desert with hot climate; Dwb, cold region with dry winter and warm summer; Cwa, temperate region with dry winter and hot summer; Cfa, temperate region without dry season and hot summer; Dwa, cold region with dry winter and hot summer; Dfa, cold region without dry season and hot summer; BSh, arid steppe with hot climate; Cwb, temperate region with dry winter and warm summer; Dfc, cold region without dry season and cold summer; Dsa, cold region with dry summer and hot summer; Csa, temperate region with dry summer and hot summer; EF, polar frost; Dsb, cold region with dry summer and warm summer; Cfb, temperate region without dry season and warm summer; Aw, tropical savannah; Am, tropical monsoon; Dsc, cold region with dry summer and cold summer; and Csb, temperate region with dry summer and warm summer.
3.3. Range Shift in the Future Climate Scenarios
Climate change will lead to a shift in the suitable habitat of C. mongolicum. Overall, the southeast boundary faces the risk of contraction, whereas expansion will occur in the northern boundary and the Qinghai-Tibet Plateau region (Figure 3). Nonetheless, a stable habitat will still account for a large proportion of the total suitable habitat, suggesting the species is either weak or moderately sensitive to climate change. The intensity shifts in the habitat caused by climate change depends on the GCMs and SSPs used. The loss habitat size under ACC.SSP585 was the largest, whereas it is smallest under the GISS.SSP245 scenario. The gain habitat size under BCC.SSP585, Had.SSP585, and MIR.SSP585 had the largest expansion area, while that under MIR.SSP126, Had.SSP126, and ACC.SSP245 had the smallest expansion (Table 3). These indicate that there is much simulation uncertainty using different climate change scenarios.
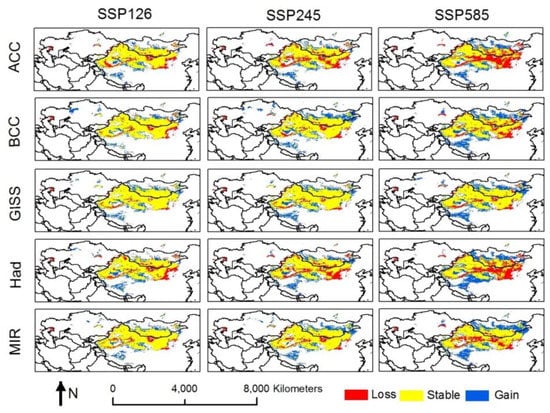
Figure 3.
Range shift of suitable habitat for Calligonum mongolicum under three shared socioeconomic pathways (SSPs) and five different general circulation models (GCMs). SSP126, SSP245, and SSP585 represent the three climate scenarios of SSP1-2.6, SSP2-4.5, and SSP5-8.5, respectively. ACC, BCC, GISS, Had, and MIR represent the five different general circulation models of ACCESS-CM2, BCC-CSM2-MR, GISS-E2-1-G, MIROC-ES2L, and HadGEM3-GC31-LL, respectively. Details of the five GCMs and SSPs can be seen in Table 1.

Table 3.
Loss, stable, and gain areas for Calligonum mongolicum under climate change. Loss + Stable = Current area, Stable + Gain = Future area, and Gain − Loss = Change area. Abbreviations of these climate scenarios can be seen in Table 1.
3.4. Uncertainty of Climate Change
The two-way ANOVA analysis showed that GCMs had a significant impact on the dynamics of gain area, loss area, and total area (Table 4), indicating that there is uncertainty for all aspects of range change in selecting these GCMs. For SSPs scenarios, they were found to only have a significant impact on gain area and loss area but had no significant impact on the total area. It indicates that the selection of SSPs had no impact on simulating the total area but had uncertainty in the estimation of the gain and loss areas.

Table 4.
Two-way ANOVA analysis of gain, loss, and total area for Calligonum mongolicum.
Variation partition showed that GCMs could explain 14.5%, 66.4%, and 97.0% variance for gain, loss, and total areas, respectively. Meanwhile, the SSP scenarios could explain 85.5%, 33.6%, and 3.0% variance for gain, loss, and total areas, respectively (Table 4). This indicates that GCMs and SSPs have different weight influences on the components of range dynamics. In total, the uncertainty of the total area and loss area was mainly from the GCMs, while that of the gain area was mainly from the SSPs.
4. Discussion
In this study, we are the first to assess the impact of climate change on the habitat suitability of C. mongolicum in the arid region of Central Asia. Our results show that the trained MaxEnt model has a high accuracy. There is a great area of suitable habitat for C. mongolicum in Central Asia. We observed that climate change is generally conducive to the expansion of the suitable range of this species, although there were significant differences found across the 15 climate change scenarios (5 GCMs × 3 SSPs). However, the size of the suitable habitat was generally stable under future climate change scenarios, and the species will not face the risk of extinction in the future according to the species–area curve theory [47]. On the contrary, a gain surface of C. mongolicum was observed in the SSP585 scenario for all the GCMs evaluated, with a tendency to increase their surface in northern Central Asia. In previous studies using species distribution models, some species showed certain resilience to global warming, increasing their distribution in suitable climate niches and potential distribution range expansion under certain climate scenarios [48,49].
The differences between our study and the past three articles are shown in Table 5. Overall, our study is similar to the proportion of area changes obtained by Zhu [26] and Xiao et al. [22], although they conducted the experiment in different background regions. Regarding this point, it is significantly different from the simulation result of Hamit et al. [25], and their results indicated a slight decrease in the total area of the species. This is likely related to the selection of various parameters and scenarios for the input model. In addition, reducing the range of study region will likely reduce the accuracy of SDM model fitting. Furthermore, there were differences found in the top two drive factors affecting C. mongolicum in different studies, and while our research emphasizes the importance of precipitation-related variables, other relevant studies also emphasized the importance of annual mean temperature or elevation. Our research extends from previous research in the following three topics.

Table 5.
Comparing our research with other climate change studies of Calligonum mongolicum in terms of input parameters and outcomes.
First, we expanded our research region. The study region was not limited to just in China or specific geographical units but was rather focused on the entire distribution area of C. mongolicum. This has many advantages, such as enabling us to fully understand the size of the habitat suitable area for this species, as well as the impact of climate change on the range shift of the species. Thus, the species shift ability should not be subjected to the physical constraints of national or geographical boundaries. Typically, a range of widely distributed species crosses national boundaries [50,51]. If the research region was limited within a country, this would violate the hypothesis of infinite shift capacity for species distribution models [52]. In addition, many studies have shown that the selection of background regions has a significant impact on species distribution models. They recommended selecting regions where species could spread as training background areas [53,54], and our research was found to conform to this view.
Afterward, our research characterized the climate thresholds and climate envelope of C. mongolicum. Revealing such data makes research much more transparent, repeatable, and referential [41,55]. According to the climatic threshold, the climatic suitability of C. mongolicum can be evaluated based on local climatic conditions. In addition, unlike Zhu [26], and Hamit et al. [25], in which variables such as terrain were introduced, we believe that introducing variables such as terrain have no direct impact on the species, resulting in confusion regarding the role of climate change. For example, elevation is simply a substitute variable for temperature [56,57]. The temperature climate layers were just spatial interpolation based on altitude, as well as latitude and longitude by thin plate smoothing splines technology [41]. Additionally, elevation is not a direct factor affecting the physiological and ecological mechanisms of plant geographical distribution, but rather, a substitute variable. This relationship is likely to change under different spatiotemporal conditions, which cannot meet the requirements of the SDM model for the assumption of plant ecological niche conservatism [58]. Thus, the elevation factor does not have the ability to transfer on a time scale. Therefore, this study mainly focused on bioclimatic variables, captures climatic niches, and depicts the spatial changes and dynamics of the ecological niche space of C. mongolicum.
Finally, we assessed the uncertainty of climate change. This uncertainty is not only the uncertainty from the SSPs, but also the uncertainty from the GCMs [30,48]. In the past, too much attention has been paid to the uncertainty of climate change on the SSPs or RCPs, ignoring the uncertainty of GCMs [30,31,32]. Many studies have used fusion GCMs to alleviate this uncertainty [22,23,59,60], but the variance has not been well considered, with only a few studies having focused on this issue [61,62]. Our results show that GCMs could explain 14.5%, 66.4%, and 97.0% of total variation, while SSPs could explain 85.5%, 33.6%, and 3.0% of total variation for gain, loss, and total habitat sizes, respectively. These proportions indicate that GCMs and SSPs have different weight influences on the components of range dynamics, meaning that there are still significant differences for understanding climate change in arid regions using social development paths and climate change mechanism models. Climate sensitive areas have large uncertainties, demonstrating that special attention and long-term climate monitoring are required for afforestation projects using C. mongolicum in Central Asia.
Despite the significant advantages of this study, other uncertainties remain. First, we have only used one algorithm here without evaluating other algorithms, for example, the bioclimatic envelope (BIOCLIM) [63], the genetic algorithm for rule-set production (GARP) [64], and ecological-niche factor analysis (ENFA) [65]. Compared comprehensively, MaxEnt has been used very frequently worldwide, and has more explanatory power and application value than the other three models [66,67]. However, there is still a need to further evaluate the uncertainty of these algorithms [68]. Furthermore, non-systematic data collection may underestimate the climate tolerance of C. mongolicum. The occurrence data that we obtained only came from China and Mongolia without records from other countries such as Kazakhstan, Uzbekistan, Turkmenistan, Afghanistan, and Iran. This was mainly due to limited open access data from these countries. Our study shows that even if the Köppen climate is similar in these regions in contrast to climatically suitable habitats simulated in Figure 2, it is still difficult to extrapolate our established model to these regions. This indicates that the climate tolerance of C. mongolicum is likely to have been underestimated, which instructs that data collection efforts should be strengthened, and climate thresholds should be further improved and updated.
5. Conclusions
With this research, we simulated the habitat suitability and estimated climate thresholds for C. mongolicum in Central Asia. We also projected changes in the suitable habitat under various climate change scenarios. The obtained climatically suitable habitat map and habitat change maps could be guiding the introduction and afforestation of this species. The acquired climate thresholds could be used for inferring the habitat suitability of C. mongolicum on a site or plot scale using local weather station. Furthermore, the large uncertainty of climate change originating from the GCMs and SSPs over the habitat changes indicates the need for continuous climate monitoring in sensitive areas for afforestation projects in Central Asia.
Author Contributions
G.L. (Guan Liu), Y.Z., Q.L., K.A., Y.L., D.X., G.L. (Guoqing Li) and S.D. conceived and designed the experiments; G.L. (Guan Liu) and G.L. (Guoqing Li) analyzed the data and visualized it; G.L. (Guan Liu) and G.L. (Guoqing Li) wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China grant number [31971488] and the National Key Research and Development Program of China grant number [2017YFC0504601].
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyzes, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Geist, H.J.; Lambin, E.F. Dynamic causal patterns of desertification. Bioscience 2004, 54, 817–829. [Google Scholar] [CrossRef]
- LeHouérou, H.N. Climate change, drought and desertification. J. Arid. Environ. 1996, 34, 133–185. [Google Scholar] [CrossRef]
- Middleton, N. Rangeland management and climate hazards in drylands: Dust storms, desertification and the overgrazing debate. Nat. Hazards. 2018, 92, 57–70. [Google Scholar] [CrossRef]
- Glenn, E.; Smith, M.S.; Squires, V. On our failure to control desertification: Implications for global change issues, and a research agenda for the future. Environ. Sci. Policy 1998, 1, 71–78. [Google Scholar] [CrossRef]
- Li, C.J.; Fu, B.J.; Wang, S.; Stringer, L.C.; Wang, Y.P.; Li, Z.D.; Liu, Y.X.; Zhou, W.X. Drivers and impacts of changes in China’s drylands. Nat. Rev. Earth Environ. 2021, 2, 858–873. [Google Scholar] [CrossRef]
- Zhao, X.; Zuo, X.; Li, Y.; Liu, X.; Wang, S.; Chen, J.; Zhang, R. Biological Measures to Combat Aeolian Desertification. In Combating Aeolian Desertification in Northeast Asia; Springer Nature: Singapore, 2022; pp. 201–217. [Google Scholar]
- Jiang, X.; Ni, J. Species-Climate Relationships of 10 Desert Plant Species and their Estimated Potential Distribution Range in the Arid Lands of Northwestern China. Acta Pharmacol. Sin. 2005, 29, 98–107. [Google Scholar] [CrossRef]
- Zhang, X. Geographical Distribution and Climatic Suitability of Typical Ecoeconomical Tree Species in the Drwland of Northwest China. Ph.D. Thesis, University of Chinese Academy of Sciences (Research Center for Soil and Water Conservation and Ecological Environment, Ministry of Education, Chinese Academy of Sciences), Xianyang, China, 2018. [Google Scholar]
- Huang, J.; Li, G.; Li, J.; Zhang, X.; Yan, M.; Du, S. Projecting the Range Shifts in Climatically Suitable Habitat for Chinese Sea Buckthorn under Climate Change Scenarios. Forests 2018, 9, 9. [Google Scholar] [CrossRef]
- Shi, Y.; Du, T. Calligonum mongolicum. Chin. Vet. Med. Mag. 2003, Suppl, 142–143. [Google Scholar] [CrossRef]
- Fan, B. Natural Regeneration Strategies of Calligonum mongolicum under Different Aeolian Environments. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2017. [Google Scholar]
- Shi, W.; Wen, J.; Pan, B.R. A comparison of ITS sequence data and morphology for Calligonum pumilum and C. mongolicum (Polygonaceae) and its taxonomic implications. Phytotaxa 2016, 261, 157–167. [Google Scholar] [CrossRef]
- Zhou, Q.L.; Liu, Z.M.; Xin, Z.M.; Daryanto, S.; Wang, L.X.; Qian, J.Q.; Wang, Y.C.; Liang, W.; Qin, X.P.; Zhao, Y.M.; et al. Relationship between seed morphological traits and wind dispersal trajectory. Funct. Plant Biol. 2019, 46, 1063–1071. [Google Scholar] [CrossRef]
- Shi, W.; Wen, J.; Zhao, Y.F.; Johnson, G.; Pan, B.R. Reproductive biology and variation of nuclear ribosomal ITS and ETS sequences in the Calligonum mongolicum complex (Polygonaceae). Phytokeys 2017, 16, 71–88. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, X.T. Microsatellite DNA loci from the drought desert plant Calligonum mongolicum Turcz. (Polygonaceae). Conserv. Genet. 2009, 10, 1891–1893. [Google Scholar] [CrossRef]
- Dong, B.C.; Yu, F.H.; Roiloa, S.R. Editorial: Ecoepigenetics in Clonal and Inbreeding Plants: Transgenerational Adaptation and Environmental Variation. Front. Plant Sci. 2019, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Pan, B.R. Karyotype analysis of the Calligonum mongolicum complex (Polygonaceae) from Northwest China. Caryologia 2015, 68, 125–131. [Google Scholar] [CrossRef]
- Su, P.X.; Liu, X.M.; Zhang, L.X.; Zhao, A.F.; Li, W.R.; Chen, H.S. Comparison of delta C-13 values and gas exchange of assimilating shoots of desert plants Haloxylon ammodendron and Calligonum mongolicum with other plants. Isr. J. Plant Sci. 2004, 52, 87–97. [Google Scholar] [CrossRef]
- Luo, W.C.; Zhao, W.Z. Adventitious roots are key to the development of nebkhas in extremely arid regions. Plant Soil. 2019, 442, 471–482. [Google Scholar] [CrossRef]
- Ji, X.B.; Zhao, W.Z.; Jin, B.W.; Liu, J.; Xu, F.N.; Zhou, H. Seasonal variations in energy exchange and evapotranspiration of an oasis-desert ecotone in an arid region. Hydrol. Process. 2021, 35, e14364. [Google Scholar] [CrossRef]
- Hamit, S.; Abdushalih, N.; Xu, Z.; Jiesisi, A. Analysis of Potential Distribution and Suitable Area of Calligonum mongolicum in Xinjiang Based on MaxEnt Model. J. Northwest For. U. 2018, 33, 71–77. [Google Scholar] [CrossRef]
- Xiao, J.; Eziz, A.; Zhang, H.; Wang, Z.; Tang, Z.; Fang, J. Responses of four dominant dryland plant species to climate change in the Junggar Basin, Northwest China. Ecol. Evol. 2019, 9, 13596–13607. [Google Scholar] [CrossRef]
- Li, G.; Huang, J. Multi-Directional Rather Than Unidirectional Northward-Dominant Range Shifts Predicted under Climate Change for 99 Chinese Tree Species. Forests 2022, 13, 1619. [Google Scholar] [CrossRef]
- Li, G.; Huang, J.; Guo, H.; Du, S. Projecting species loss and turnover under climate change for 111 Chinese tree species. For. Ecol. Manag. 2020, 477, 118488. [Google Scholar] [CrossRef]
- Hamit, S.; Abdushalih, N.; Li, X.; Shao, H.; Jiesisi, A.; Guli, A. Effects of Climate Change and Human Activities on the Distribution Pattern of Calligonum mongolicum Turcz. Arid. Zone Res. 2018, 35, 1450–1458. [Google Scholar] [CrossRef]
- Zhu, N. Predicting the Geographic Distribution of Calligonum mongolicum under Climate Change. J. Des. Res. 2019, 39, 136–144. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Eds.; Cambridge University Press: Cambridge, UK, 2021; pp. 1–41. [Google Scholar]
- Riahi, K.; VanVuuren, D.P.; Kriegler, E.; Edmonds, J.; O’neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Change 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Weart, S. The development of general circulation models of climate. Stud. Hist. Philos. Part B 2010, 41, 208–217. [Google Scholar] [CrossRef]
- Jiménez-García, D.; Peterson, A.T. Climate change impact on endangered cloud forest tree species in Mexico. Rev. Mex. Biodivers. 2019, 90, e902781. [Google Scholar] [CrossRef]
- Xie, G.Y.; Olson, D.H.; Blaustein, A.R. Projecting the Global Distribution of the Emerging Amphibian Fungal Pathogen, Batrachochytrium dendrobatidis, Based on IPCC Climate Futures. PLoS ONE 2016, 11, e0160746. [Google Scholar] [CrossRef]
- Real, R.; Marquez, A.L.; Olivero, J.; Estrada, A. Species distribution models in climate change scenarios are still not useful for informing policy planning: An uncertainty assessement using fuzzy logic. Ecography 2010, 33, 304–314. [Google Scholar] [CrossRef]
- He, H.; Luo, G.; Cai, P.; Hamdi, R.; Termonia, P.; De Maeyer, P.; Kurban, A.; Li, J. Assessment of Climate Change in Central Asia from 1980 to 2100 Using the Köppen-Geiger Climate Classification. Atmosphere 2021, 12, 123. [Google Scholar] [CrossRef]
- Dou, X.; Ma, X.; Zhao, C.; Li, J.; Yan, Y.; Zhu, J. Risk assessment of soil erosion in Central Asia under global warming. Catena 2022, 212, 106056. [Google Scholar] [CrossRef]
- Vakulchuk, R.; Daloz, A.S.; Overland, I.; Sagbakken, H.F.; Standal, K. A void in Central Asia research: Climate change. Cent. Asian Surv. 2022, 42, 1–20. [Google Scholar] [CrossRef]
- Liu, N.; Feng, Y.; Guan, K.; Fan, Y.; Chen, J. Geographic Distribution of Calligonum mongolicum. Arid. Zone Res. 2015, 32, 753–759. [Google Scholar] [CrossRef]
- Mao, Z.; Pan, B. The classification and distribution of the genus Calligonum L. in China. Acta Pharm. Sin. 1988, 24, 98–107. [Google Scholar]
- Mao, Z. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1998; pp. 120–133. [Google Scholar]
- Zhang, X.; Li, G.; Du, S. Simulating the potential distribution of Elaeagnus angustifolia L. based on climatic constraints in China. Ecol. Eng. 2018, 113, 27–34. [Google Scholar] [CrossRef]
- Booth, T.H. Why understanding the pioneering and continuing contributions of BIOCLIM to species distribution modelling is important. Austral Ecol. 2018, 43, 852–860. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Lu, Q.; Xiong, D.; Li, G.; Du, S. Understanding the Limiting Climatic Factors on the Suitable Habitat of Chinese Alfalfa. Forests 2022, 13, 482. [Google Scholar] [CrossRef]
- Li, G.; Liu, C.; Liu, Y.; Yang, J.; Zhang, X.; Guo, K. Effects of climate, disturbance and soil factors on the potential distribution of Liaotung oak (Quercus wutaishanica Mayr) in China. Ecol. Res. 2012, 27, 427–436. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Li, S.Y.; Miao, L.J.; Jiang, Z.H.; Wang, G.J.; Gnyawali, K.R.; Zhang, J.; Zhang, H.; Fang, K.; He, Y.; Li, C. Projected drought conditions in Northwest China with CMIP6 models under combined SSPs and RCPs for 2015-2099. Adv. Clim. Change Res. 2020, 11, 210–217. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Lomolino, M.V. Ecology’s most general, yet protean pattern: The species-area relationship. J. Biogeogr. 2000, 27, 17–26. [Google Scholar] [CrossRef]
- Aguado-Bautista, O.; Escalante, T. Changes on patterns of endemism of the Mexican land mammals by global warming. Rev. Mex. Biodivers. 2015, 86, 99–110. [Google Scholar] [CrossRef]
- Lara-Reséndiz, R.A.; Galina-Tesaro, P.; Pérez-Delgadillo, A.G.; Valdez-Villavicencio, J.H.; Méndez-de la Cruz, F.R. Effects of climate change on a widely distributed thermophilic lizard (Dipsosaurus dorsalis): An ecophysiological approach. Rev. Mex. Biodivers. 2019, 90, e902888. [Google Scholar] [CrossRef]
- Rands, M.R.W.; Adams, W.M.; Bennun, L.; Butchart, S.H.M.; Clements, A.; Coomes, D.; Entwistle, A.; Hodge, I.; Kapos, V.; Scharlemann, J.P.W.; et al. Biodiversity conservation: Challenges beyond 2010. Science 2010, 329, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Gaines, S.; Gonzalez, L.; Kaufman, D.M.; Kingsolver, J.; Townsend Peterson, A.; Sagarin, R. Empirical perspectives on species borders: From traditional biogeography to global change. Oikos 2005, 108, 58–75. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Booth, T.H. Assessing species climatic requirements beyond the realized niche: Some lessons mainly from tree species distribution modelling. Clim. Chang. 2017, 145, 259–271. [Google Scholar] [CrossRef]
- Montgomery, K. Variation in temperature with altitude and latitude. J. Geogr. 2006, 105, 133–135. [Google Scholar] [CrossRef]
- Kane, R.P.; Buriti, R.A. Latitude and altitude dependence of the interannual variability and trends of atmospheric temperatures. Pure Appl. Geophys. 1997, 149, 775–792. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Huang, J.; Wen, Z.; Du, S. Afforestation and climatic niche dynamics of black locust (Robinia pseudoacacia). For. Ecol. Manag. 2018, 407, 184–190. [Google Scholar] [CrossRef]
- Qin, Y.J.; Wang, C.; Zhao, Z.H.; Pan, X.B.; Li, Z.H. Climate change impacts on the global potential geographical distribution of the agricultural invasive pest, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Clim. Chang. 2019, 155, 145–156. [Google Scholar] [CrossRef]
- Li, G.; An, K.; Li, Y. Climate change induced range shifts in Chinese ash (Fraxinus chinensis) along three geographical dimensions in China at the late 21st century. Appl. Ecol. Environ. Res. 2022, 20, 4499–4513. [Google Scholar] [CrossRef]
- Koo, K.A.; Park, S.U.; Kong, W.-S.; Hong, S.; Jang, I.; Seo, C. Potential climate change effects on tree distributions in the Korean Peninsula: Understanding model & climate uncertainties. Ecol. Model. 2017, 353, 17–27. [Google Scholar] [CrossRef]
- Wang, T.; Wang, G.; Innes, J.; Nitschke, C.; Kang, H. Climatic niche models and their consensus projections for future climates for four major forest tree species in the Asia–Pacific region. For. Ecol. Manag. 2016, 360, 357–366. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. BIOCLIM: The first species distribution modelling package, its early applications and relevance to most current MAXENT studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Stockwell, D. The GARP modelling system: Problems and solutions to automated spatial prediction. Int. J. Geogr. Inf. Sci. 1999, 13, 143–158. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Hausser, J.; Chessel, D.; Perrin, N. Ecological-niche factor analysis: How to compute habitat-suitability maps without absence data? Ecology 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papes, M.; Eaton, M. Transferability and model evaluation in ecological niche modeling: A comparison of GARP and Maxent. Ecography 2007, 30, 550–560. [Google Scholar] [CrossRef]
- Morin, X.; Thuiller, W. Comparing niche- and process-based models to reduce prediction uncertainty in species range shifts under climate change. Ecology 2009, 90, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).