Complete Chloroplast Genome of Bamboo Species Pleioblastus ovatoauritus and Comparative Analysis of Pleioblastus from China and Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Sample and Data Collection
2.2. Chloroplast Genome Assembly and Annotation
2.3. Comparative Analysis of Complete Genomes
2.4. Phylogenic Analysis
2.5. Development of Primers for SSRs
3. Results
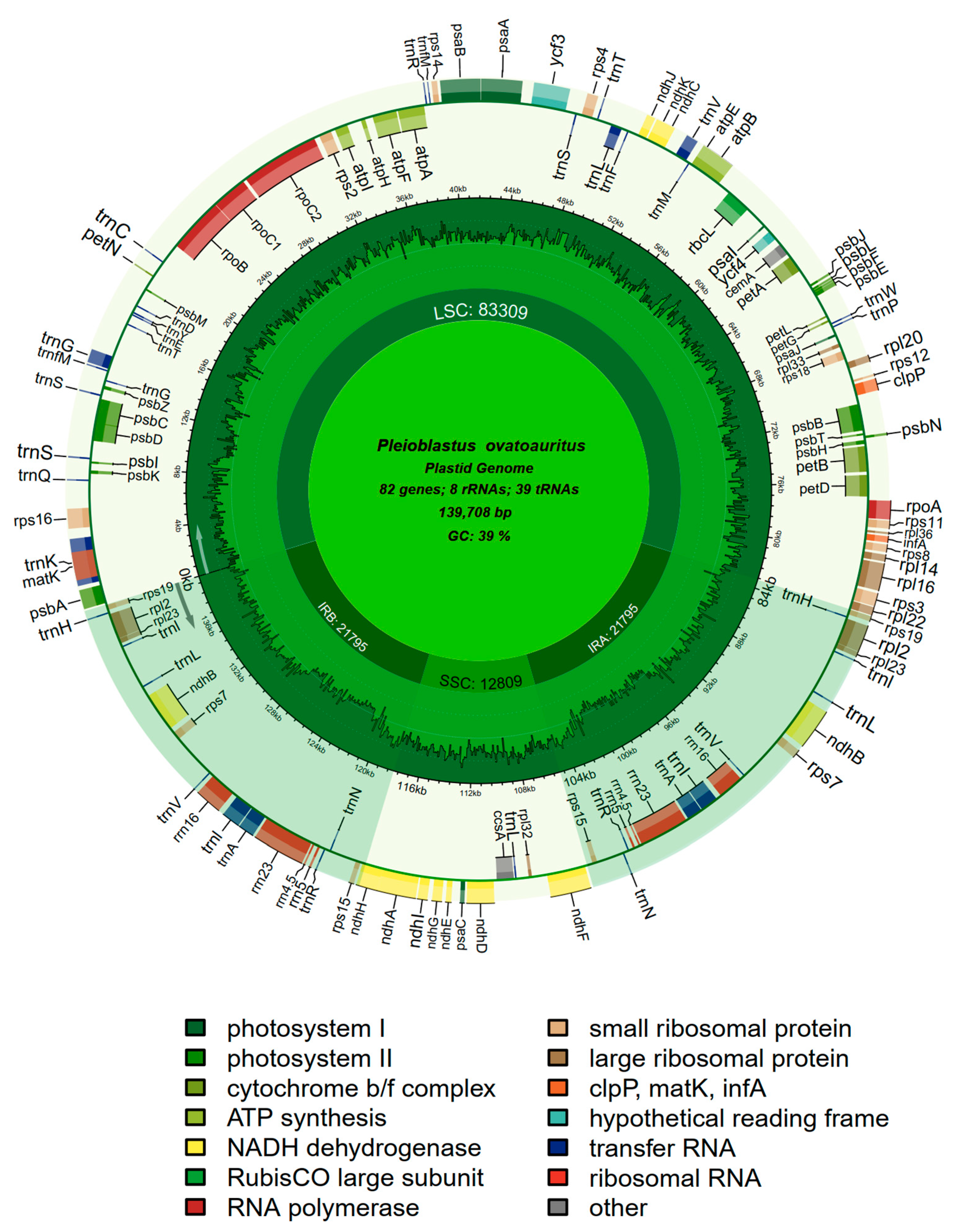
3.1. Chloroplast Genome of Pl. ovatoauritus
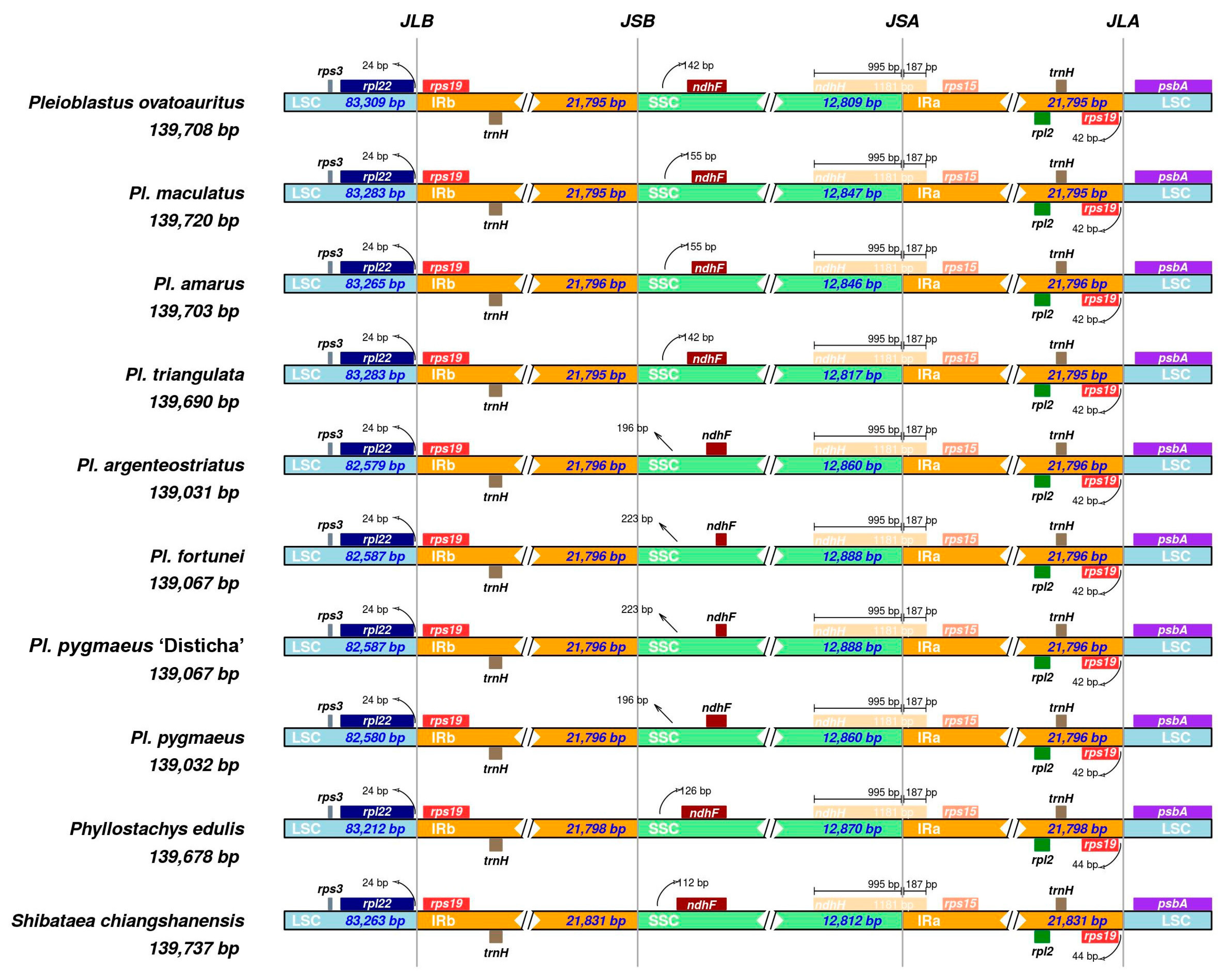
3.2. Chloroplast Genome Structure Analysis of Genus Pleioblastus
3.3. Codon Usage
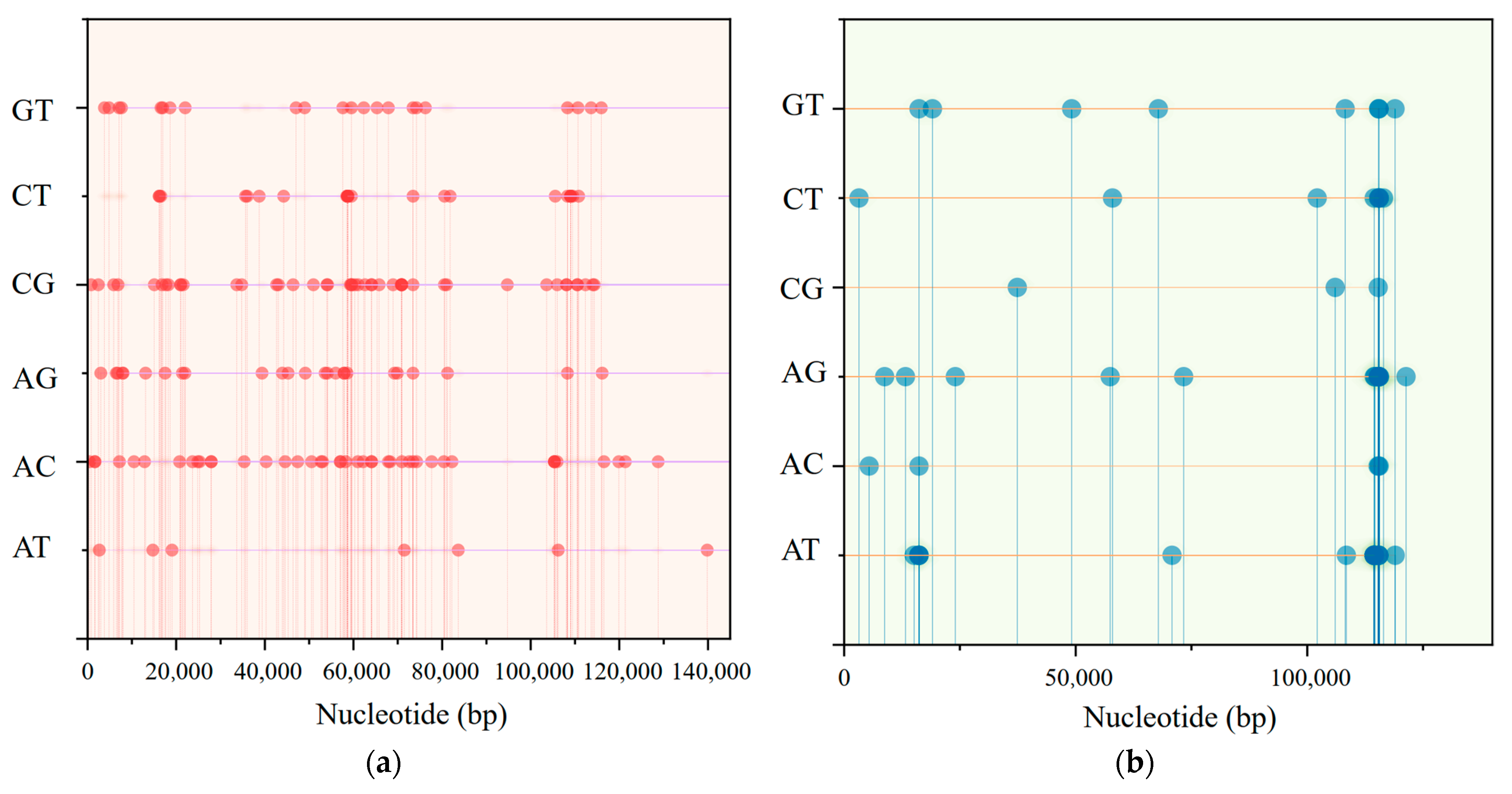
3.4. Sequence Divergence and Nucleotide Diversity
3.5. Phylogenetic Analysis of Genus Pleioblastus spp.
3.6. SSR Distribution and Primer Design of Genus Pleioblastus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primer | Unit | Forward 5′ → 3′ | Reverse 5′ → 3′ |
|---|---|---|---|
| PP1 | (T) 9 | ACCGGTCATGTTTCTTGGAT | AGTCTATTCTCTCTCCTACAACTCT |
| PP2 | (T) 9 | GGCGAACGAATAATCATTAAGTCCT | AGATCCGAACACTTGCCTCG |
| PP3 | (T) 9 | TTCTACGACTCTTTTCCACACT | ATCCAACTGATCCCCACGTC |
| PP4 | T | AAGAAATCGCAACTCCTTTCCG | TCCATGACTCCTATTTCAAAGCCT |
| PP5 | T | TCTCCCCAATAGAGCTTAGAAGT | TCTGGCTGTCTCGCAATACC |
| PP6 | A | CGTGGCTCTAGTATGAATCTAAGGT | TGGCTCATCTGTCTTTCTTTCTT |
| PP7 | AT | TGTGCGTAGAAGAGATTGTGGT | GCTCGAAATGGTTGTGCTCG |
| PP8 | TA | CCCGATCCGATAGTACCCGT | CGTCTTTTGTCATTCTTTGCTCCT |
| PP9 | TA | CGTCTTTTGTCATTCTTTGCTCCT | CCCGATCCGATAGTACCCGT |
References
- Zhong, Y.P.; Wu, C.M.; Hu, Y.P.; Mi, Y.Y.; Yu, Z.Y.; Cao, F.L. Updating for the World Checklist of Bamboos. Subtrop. Plant Sci. 2019, 48, 176–180. [Google Scholar]
- Vorontsova, M.S.; Clark, L.G.; Dransfield, J.; Govaerts, R.; Baker, W.J. World Checklist of Bamboos and Rattans; Royal Botanic Gardens: Kew, UK, 2016; ISBN 978-92-95098-99-2. [Google Scholar]
- Triplett, J.K. Phylogenetic Relationships among the Temperate Bamboos (Poaceae: Bambusoideae) with an Emphasis on Arundinaria and Allies. Ph.D. Thesis, Iowa State University, Iowa, IA, USA, 2008. [Google Scholar]
- Guo, C.; Ma, P.F.; Yang, G.Q.; Ye, X.Y.; Guo, Y.; Liu, J.X.; Liu, Y.L.; Eaton, D.A.R.; Guo, Z.H.; Li, D.Z. Parallel DdRAD and Genome Skimming Analyses Reveal a Radiative and Reticulate Evolutionary History of the Temperate Bamboos. Syst. Biol. 2021, 70, 756–773. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Chu, W.C.; Ayele, M.; Ho, J.; Bruggeman, E.; Yourstone, K.; Rafalski, A.; Smith, O.S.; McMullen, M.D.; Bezawada, C.; et al. Development of Single Nucleotide Polymorphism (SNP) Markers for Use in Commercial Maize (Zea Mays L.) Germplasm. Mol. Breed. 2009, 24, 165–176. [Google Scholar] [CrossRef]
- Li, D.Z.; Wang, Z.P.; Zhu, Z.D.; Xia, N.H.; Jia, L.Z.; Guo, Z.H.; Yang, G.Y.; Stapleton, C.M.A. Bambuseae (Poaceae). In Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China, 2006; Volume 22, p. 7. [Google Scholar]
- Keng, P.C.; Wang, Z.P. Gramineae-Subfam Bambusoideae. In Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1996; Volume 9, p. 1. [Google Scholar]
- Zeng, C.-X.; Zhang, Y.-X.; Triplett, J.K.; Yang, J.-B.; Li, D.-Z. Large Multi-Locus Plastid Phylogeny of the Tribe Arundinarieae (Poaceae: Bambusoideae) Reveals Ten Major Lineages and Low Rate of Molecular Divergence. Mol. Phylogenet. Evol. 2010, 56, 821–839. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.-J.; Zhang, W.-Y.; Yuan, J.-L. New Taxa and New Records of Bambusoideae (Poaceae) in Zhejiang Province, China. J. Bamboo Res. 2018, 37, 71–74. [Google Scholar]
- Sugiura, M. The Chloroplast Genome; Springer: Berlin/Heidelberg, Germany; Dordrecht, The Netherlands, 1992. [Google Scholar]
- Dobrogojski, J.; Adamiec, M.; Luciński, R. The chloroplast genome: A review. Acta Physiol. Plant. 2020, 42, 98. [Google Scholar] [CrossRef]
- Niu, Z.Y.; Cai, Z.Y.; Liao, C.L.; Xia, N.H. Chimonobambusa Sangzhiensis (Poaceae, Bambusoideae), a New Combination Supported by Morphological and Molecular Evidence. Phytokeys 2022, 195, 127–141. [Google Scholar] [CrossRef]
- Ma, P.F.; Zhang, Y.X.; Zeng, C.X.; Guo, Z.H.; Li, D.Z. Chloroplast Phylogenomic Analyses Resolve Deep-Level Relationships of an Intractable Bamboo Tribe Arundinarieae (Poaceae). Syst. Biol. 2014, 63, 933–950. [Google Scholar] [CrossRef]
- Ouyang, F.; Hu, J.; Wang, J.; Ling, J.; Wang, Z.; Wang, N.; Ma, J.; Zhang, H.; Mao, J.F.; Wang, J. Complete Plastome Sequences of Picea asperata and P. crassifolia and Comparative Analyses with P. abies and P. morrisonicola. Genome 2019, 62, 317–328. [Google Scholar] [CrossRef]
- Pei, J.; Wang, Y.; Zhuo, J.; Gao, H.; Vasupalli, N.; Hou, D.; Lin, X. Complete Chloroplast Genome Features of Dendrocalamusfarinosus and Its Comparison and Evolutionary Analysis with Other Bambusoideae Species. Genes 2022, 13, 1519. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Li, L.; Yue, J. The Complete Chloroplast Genome of Phyllostachys Edulis f. Tubiformis (Bambusoideae): A Highly Appreciated Type of Ornamental Bamboo in China. Mitochondrial DNA Part B 2022, 7, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.J.; Ma, J.X.; Yuan, J.L. Phyllostachys edulis ‘YuanBao’—A Bamboo Cultivar. J. Bamboo Res. 2022, 41, 1–4. [Google Scholar]
- Jin, J.J.; Yu, W.B.; Yang, J.B.; Song, Y.; de Pamphilis, C.W.; Yi, T.S.; Li, D.Z. GetOrganelle: A Fast and Versatile Toolkit for Accurate de Novo Assembly of Organelle Genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef]
- Darling, A.C.E.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple Alignment of Conserved Genomic Sequence with Rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Zheng, S.; Poczai, P.; Hyvönen, J.; Tang, J.; Amiryousefi, A. Chloroplot: An Online Program for the Versatile Plotting of Organelle Genomes. Front. Genet. 2020, 11, 576124. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. IRscope: An Online Program to Visualize the Junction Sites of Chloroplast Genomes. Bioinformatics 2018, 34, 3030–3031. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational Tools for Comparative Genomics. Nucleic Acids Res. 2004, 32, W273–W279. [Google Scholar] [CrossRef]
- Peden, J.F. Analysis of Codon Usage. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2000; pp. 73–74. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Peer, B. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-Web: A Web Server for Microsatellite Prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New Capabilities and Interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yao, W.; Guo, C.; Bian, L.; Ding, Y.; Lin, S. Chloroplast Genome Variation and Phylogenetic Analyses of Seven Dwarf Ornamental Bamboo Species. Forests 2022, 13, 1671. [Google Scholar] [CrossRef]
- Hu, Y.P.; Zhou, J.; Yu, Z.Y.; Li, J.J.; Xu, M.Y.; Guo, Q.R. The Complete Chloroplast Genome of Phyllostachys Heteroclada f. Solida (Poaceae). Mitochondrial DNA Part B 2021, 6, 566–567. [Google Scholar] [CrossRef]
- Triplett, J.K.; Clark, L.G. Phylogeny of the Temperate Bamboos (Poaceae: Bambusoideae: Bambuseae) with an Emphasis on Arundinaria and Allies. Syst. Bot. 2010, 35, 102–120. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zeng, C.X.; Li, D.Z. Complex Evolution in Arundinarieae (Poaceae: Bambusoideae): Incongruence between Plastid and Nuclear GBSSI Gene Phylogenies. Mol. Phylogenet. Evol. 2012, 63, 777–797. [Google Scholar] [CrossRef]
- Guo, C. Phylogenomics of Arundinareae (Poaceae: Bambusoideae). Ph.D. Thesis, Unversity of Chinese Academy of Sciences, Kunming Institute of Botany, Kunming, China, 2019. [Google Scholar]


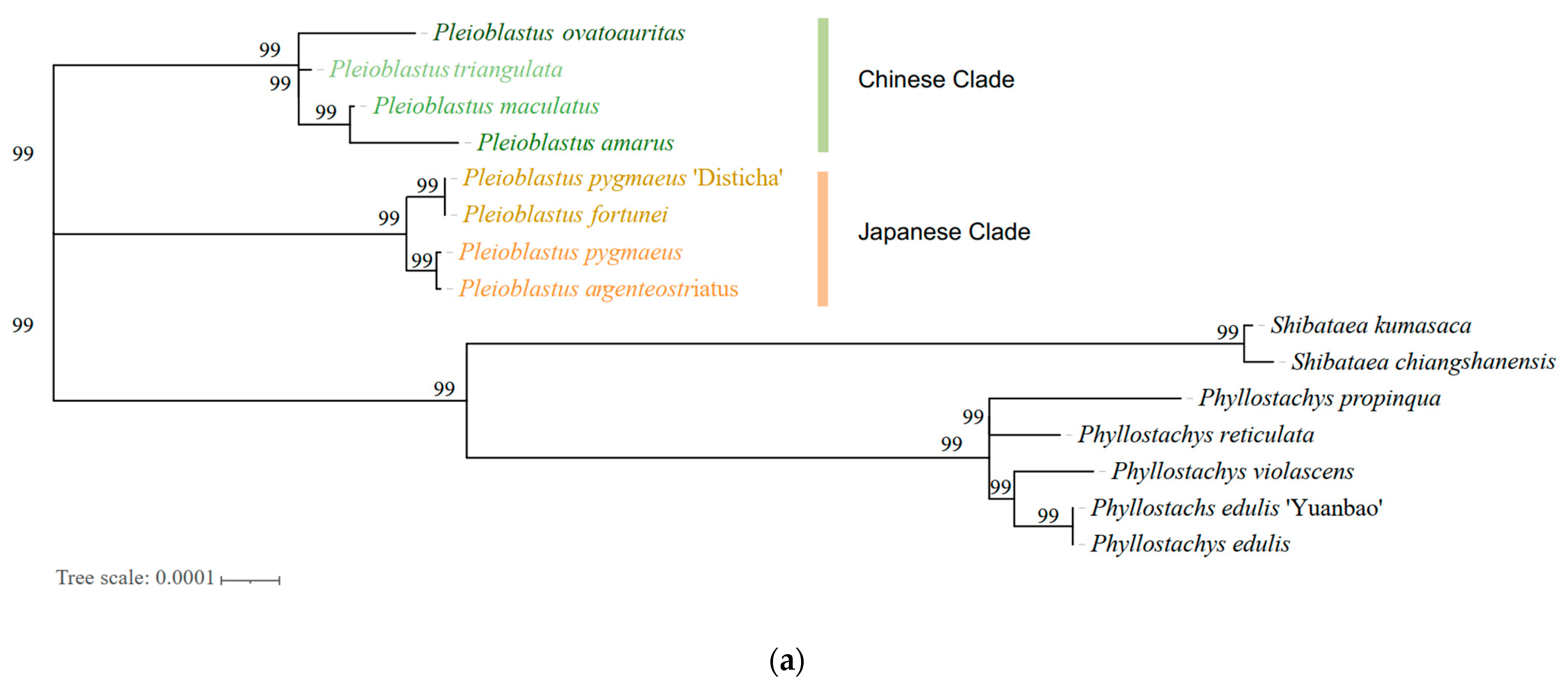
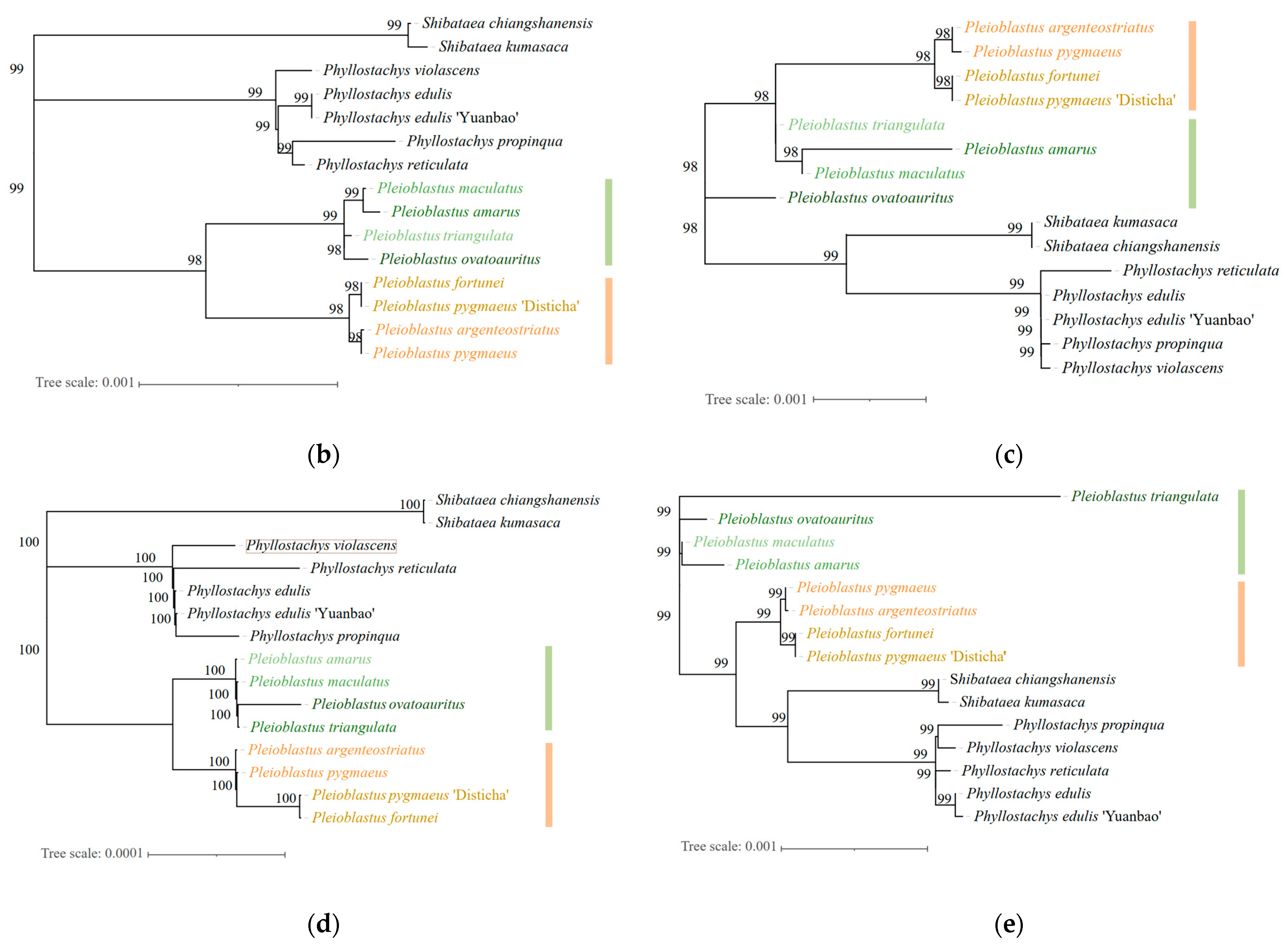
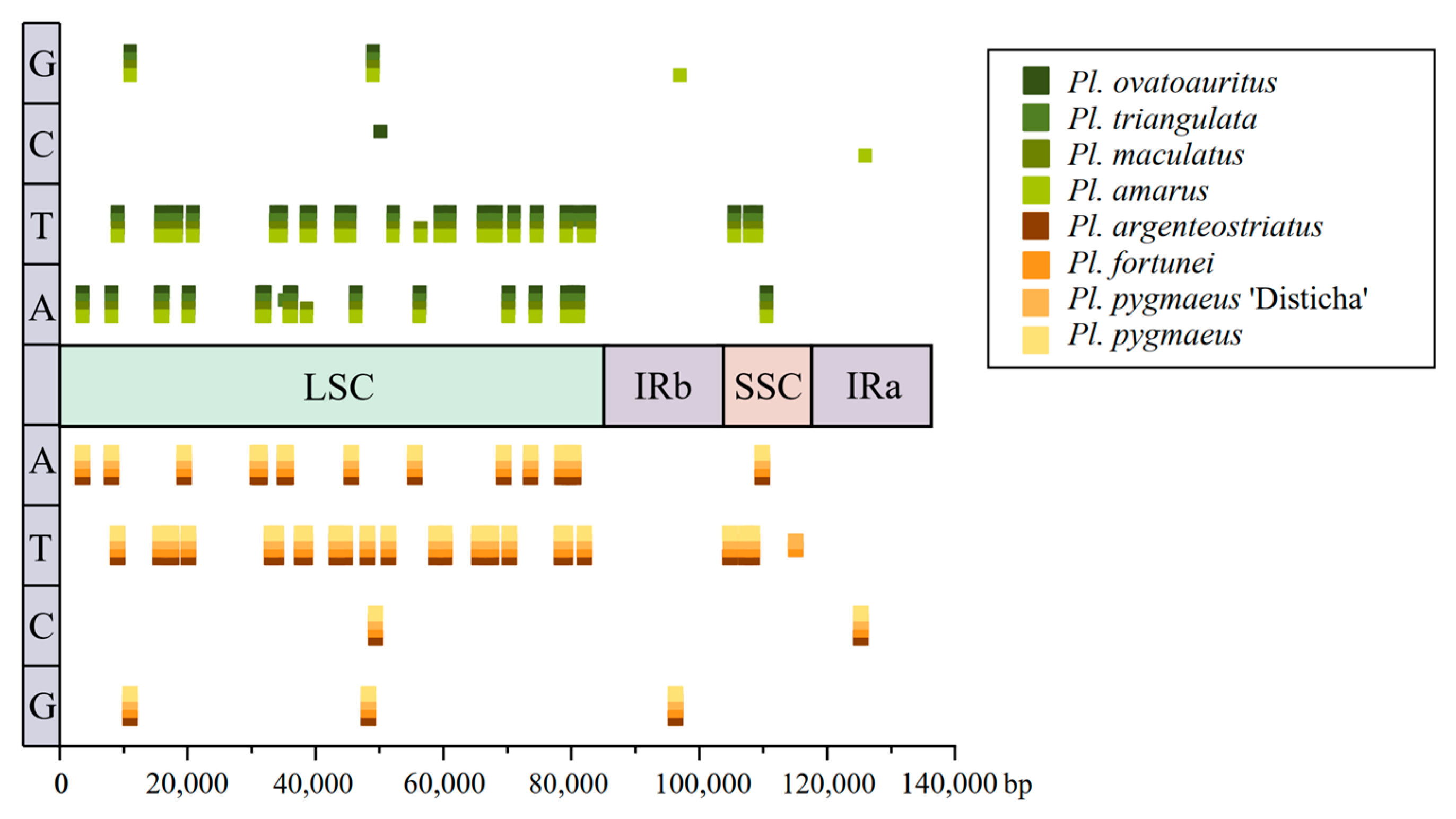
| Genus | Taxon | Accession |
|---|---|---|
| Ingroup | ||
| Pleioblastus | Pleioblastus ovatoauritus T.H.Wen | OP235916 |
| Pl. maculatus (McClure) C.D.Chu and C.S.Chao | JX513424 | |
| Pl. amarus (Keng) Keng f. | NC043892 | |
| Pl. ortuneate (Hsueh and T.P.Yi) N.H.Xia, Y.H.Tong and Z.Y.Niu | OK323193 | |
| Pl. argenteostriatus (Regel) Nakai | OP036432 | |
| Pl. ortune (Van Houtte) Nakai | OP036433 | |
| Pl. pygmaeus ‘Disticha’ | OP036434 | |
| Pl. pygmaeus (Miq.) Nakai | OP036435 | |
| Outgroup | ||
| Phyllostachys | Phyllostachys edulis (Carriere) J. Houzeau | MW007170 |
| Ph. Edulis ‘Yuanbao’ | MW874473 | |
| Ph. Reticulata (Ruprecht) K. Koch | MN537808 | |
| Ph. Propinqua McClure | JN415113 | |
| Ph. Violascens (Carrière) Riviere and C. Rivière | OP612331 | |
| Shibataea | Shibataea chiangshanensis T. W. Wen | NC036826 |
| S. kumasaca (Zoll. Ex Steud.) Makino ex Nakai | KU523578 |
| Taxon | Pl. ovatoauritus | Pl. maculata | Pl. amarus | Pl. triangulata | Pl. argenteostriatus | Pl. fortunei | Pl. pygmaeus ‘Disticha’ | Pl. pygmaeus |
|---|---|---|---|---|---|---|---|---|
| Accession No. | OP235916 | JX513424 | NC043892 | OK323193 | OP036432 | OP036433 | OP036434 | OP036435 |
| Plastome legnth | 139,708 | 139,720 | 139,703 | 139,690 | 139,031 | 139,067 | 139,067 | 139,032 |
| LSC | 83,309 | 83,283 | 83,265 | 83,283 | 82,579 | 82,587 | 82,587 | 82,580 |
| SSC | 12,809 | 12,847 | 12,846 | 12,817 | 12,860 | 12,888 | 12,888 | 12,860 |
| IR (single) | 21,795 | 21,795 | 21,796 | 21,795 | 21,796 | 21,796 | 21,796 | 21,796 |
| GC content % | 38.9 | 38.9 | 38.9 | 38.9 | 38.9 | 38.9 | 38.9 | 38.9 |
| No. of CDS | 82 | 82 | 82 | 82 | 82 | 82 | 82 | 82 |
| No. of tRNAs | 39 | 39 | 39 | 36 | 30 | 30 | 30 | 30 |
| No. of rRNAs | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| System | Group | Name |
|---|---|---|
| Photosynthesis | Subunits of ATP synthase | atpA, atpB, atpE, atpF, atpH, atpI |
| Subunits of NADH-dehydrogenase | ndhA, ndhBa, ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| Subunits of cytochrome b/f complex | petA, petB, petD, petG, petL, petN | |
| Subunits of photosystem I | psaA, psaB, psaC, psaI, psaJ | |
| Subunits of photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ | |
| Subunit of rubisco | rbcL | |
| Transcription and translation | Large subunit of ribosome | rpl2 a,rpl14, rpl16,rpl20, rpl22, rpl23 a, rpl32, rpl33, rpl36 |
| DNA dependent RNA polymerase | rpoA, rpoB, rpoC1, rpoC2 | |
| Small subunit of ribosomal proteins | rps2, rps3, rps4, rps7 a, rps8, rps11, rps12, rps14, rps15 a, rps16, rps18, rps19 a | |
| rRNA genes | rrn4.5 a, rrn5 a, rrn16 a, rrn23 a | |
| tRNA genes | trnA-UGC a, trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnfM-CAU a, trnG-GCC, trnG-UCC, trnH-GUG a,trnI-CAU a, trnIGAU a, trnK-UUU, trnL-CAA a, trnL-UAA, trnL-UAG, trnM-CAU, trnN-GUU a, trnP-GGG, trnQ-UUG,trnR-ACG a, trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC a, trnV-UAC, trnW-CCA, trnY-GUA | |
| Other genes | c-type cytochrome synthesis gene | ccsA |
| Envelope membrane protein | cemA | |
| ATP-dependent protease | clpP | |
| Maturase | matK | |
| Hypothetical chloroplast reading frames | ycf3, ycf4 | |
| Translational initiation factor | infA |
| Genus | Pl. ovatoauritus | Pl. amarus | Pl. maculata | Pl. triangulata | Pl. argenteostriatus | Pl. fortunei | Pl. pygmaeus ‘Disticha’ | Pl. pygmaeus | |
|---|---|---|---|---|---|---|---|---|---|
| GC content of CDS (%) | 39.5 | 39.5 | 39.5 | 39.5 | 39.4 | 39.4 | 39.4 | 39.4 | |
| Codon preference at 3rd position | T (45.3%) | T (45.3%) | T (45.3%) | T (45.3%) | T (45.3%) | T (45.3%) | T (45.3%) | T (45.3%) | |
| Total AA | 19,695 | 19,695 | 19,695 | 19,695 | 19,705 | 19,705 | 19,705 | 19,705 | |
| Most preferred stop codon | UAA | UAA | UAA | UAA | UAA | UAA | UAA | UAA | |
| Most frequent AA | Leu | Leu | Leu | Leu | Leu | Leu | Leu | Leu | |
| Least frequent AA | Cys | Cys | Cys | Cys | Cys | Cys | Cys | Cys | |
| Parameter | 3rd Base | Number of Codon (TER excluded) | |||||||
| RSCU > 1 | A/U/C/G | 29 | 29 | 30 | 30 | 29 | 29 | 29 | 29 |
| A/U | 27 | 27 | 28 | 28 | 27 | 27 | 27 | 27 | |
| G/C | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| A | 11 | 11 | 12 | 12 | 11 | 11 | 11 | 11 | |
| U | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | |
| G | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| C | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Optimal | A/U/C/G | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| A/U | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | |
| G/C | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| A | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | |
| U | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | |
| G | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Motif Length | Base | Pl. ovatoauritus | Pl. triangulata | Pl. maculata | Pl. amarus | Pl. argenteostriatus | Pl. fortunei | Pl. pygmaeus ‘Disticha’ | Pl. pygmaeus |
|---|---|---|---|---|---|---|---|---|---|
| Mono-repeats | A | 20 | 20 | 20 | 20 | 18 | 18 | 18 | 18 |
| T | 29 | 29 | 29 | 29 | 28 | 29 | 29 | 28 | |
| G | 3 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | |
| C | 1 | 0 | 0 | 1 | 2 | 2 | 2 | 2 | |
| Total | 53 | 51 | 51 | 53 | 51 | 52 | 52 | 51 | |
| Di-repeats | 5 TA | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 2 |
| 5 AT | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | |
| 5 TC | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | |
| Total | 4 | 4 | 0 | 0 | 4 | 4 | 4 | 4 | |
| Tri-repeats | 4 AAT | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4 TAT | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 4 TCT | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Total | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Tetra-repeats | 4 GTAG | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Wang, B.; Shen, Z.; Guo, Q. Complete Chloroplast Genome of Bamboo Species Pleioblastus ovatoauritus and Comparative Analysis of Pleioblastus from China and Japan. Forests 2023, 14, 1051. https://doi.org/10.3390/f14051051
Peng W, Wang B, Shen Z, Guo Q. Complete Chloroplast Genome of Bamboo Species Pleioblastus ovatoauritus and Comparative Analysis of Pleioblastus from China and Japan. Forests. 2023; 14(5):1051. https://doi.org/10.3390/f14051051
Chicago/Turabian StylePeng, Weihan, Beibei Wang, Zhuolong Shen, and Qirong Guo. 2023. "Complete Chloroplast Genome of Bamboo Species Pleioblastus ovatoauritus and Comparative Analysis of Pleioblastus from China and Japan" Forests 14, no. 5: 1051. https://doi.org/10.3390/f14051051
APA StylePeng, W., Wang, B., Shen, Z., & Guo, Q. (2023). Complete Chloroplast Genome of Bamboo Species Pleioblastus ovatoauritus and Comparative Analysis of Pleioblastus from China and Japan. Forests, 14(5), 1051. https://doi.org/10.3390/f14051051







