Logging Affects Genetic Diversity Parameters in an Araucaria angustifolia Population: An Endangered Species in Southern Brazil
Abstract
1. Introduction
2. Material and Methods
2.1. Site and Sampling Design
2.2. DNA Extraction and Microsatellite Genotyping
2.3. Analysis of Genetic Diversity
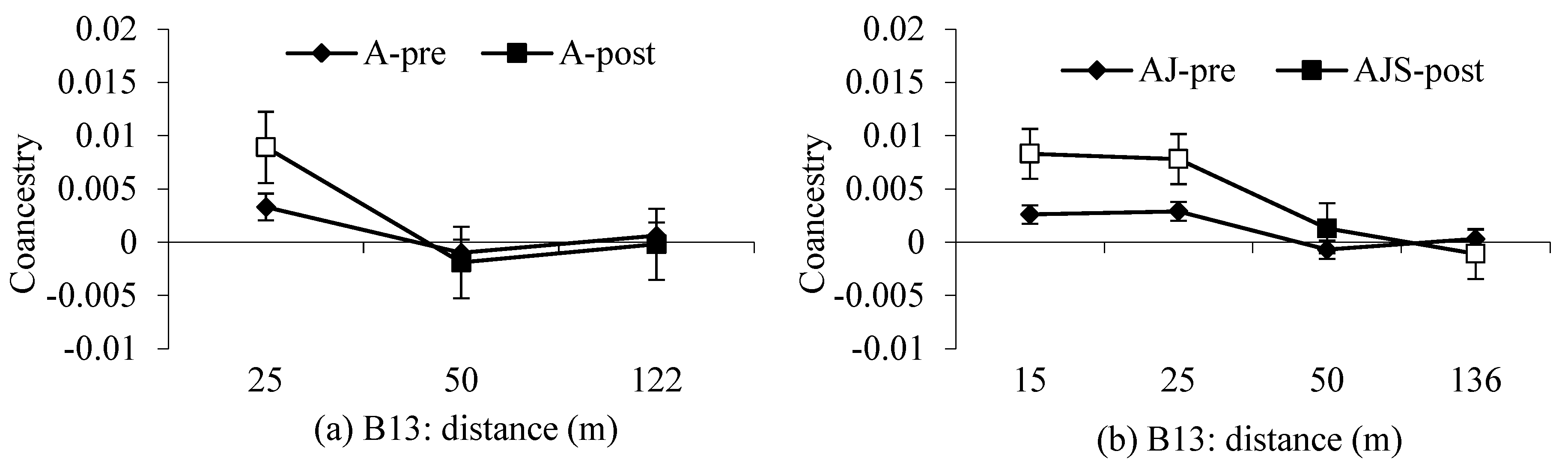
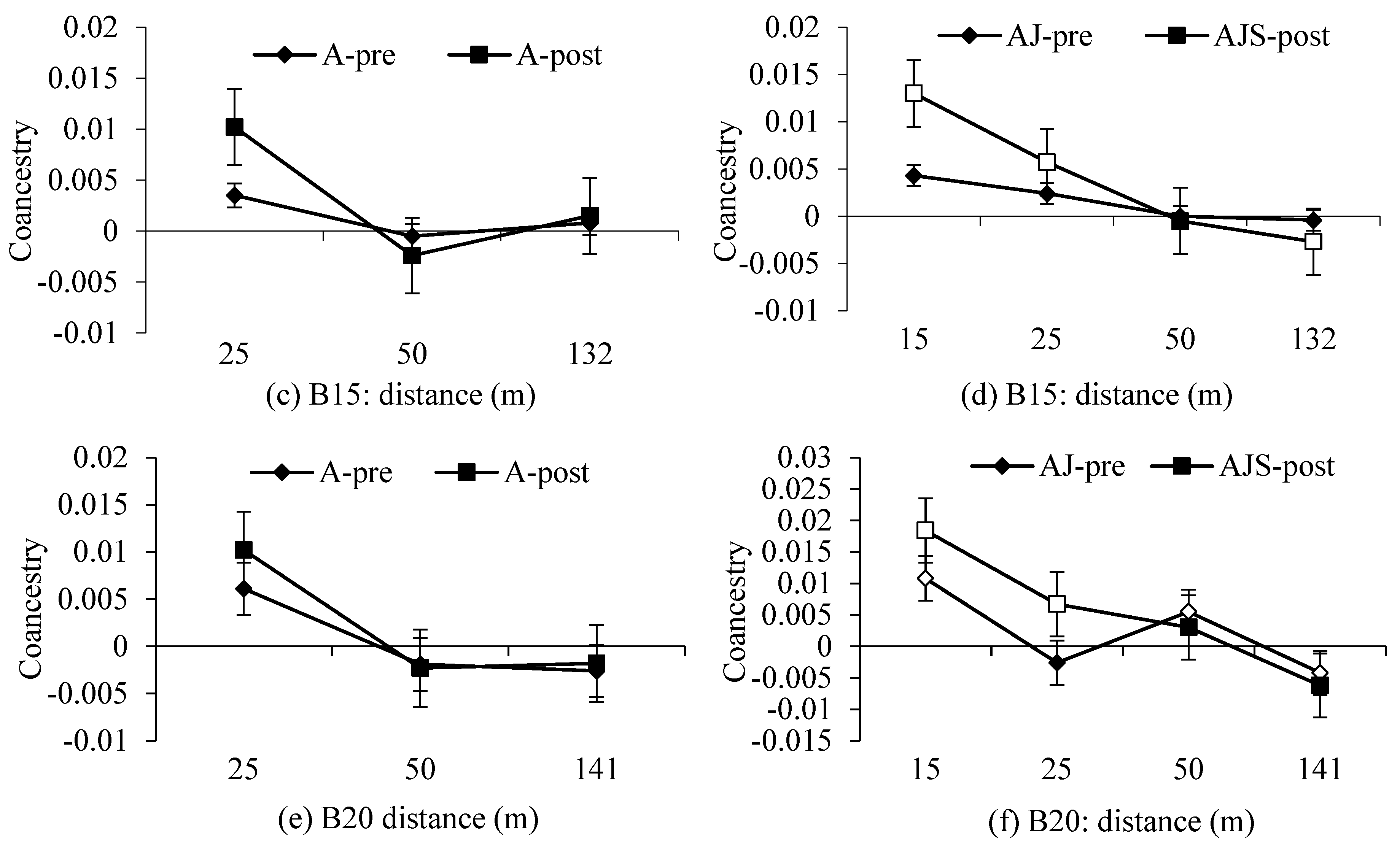
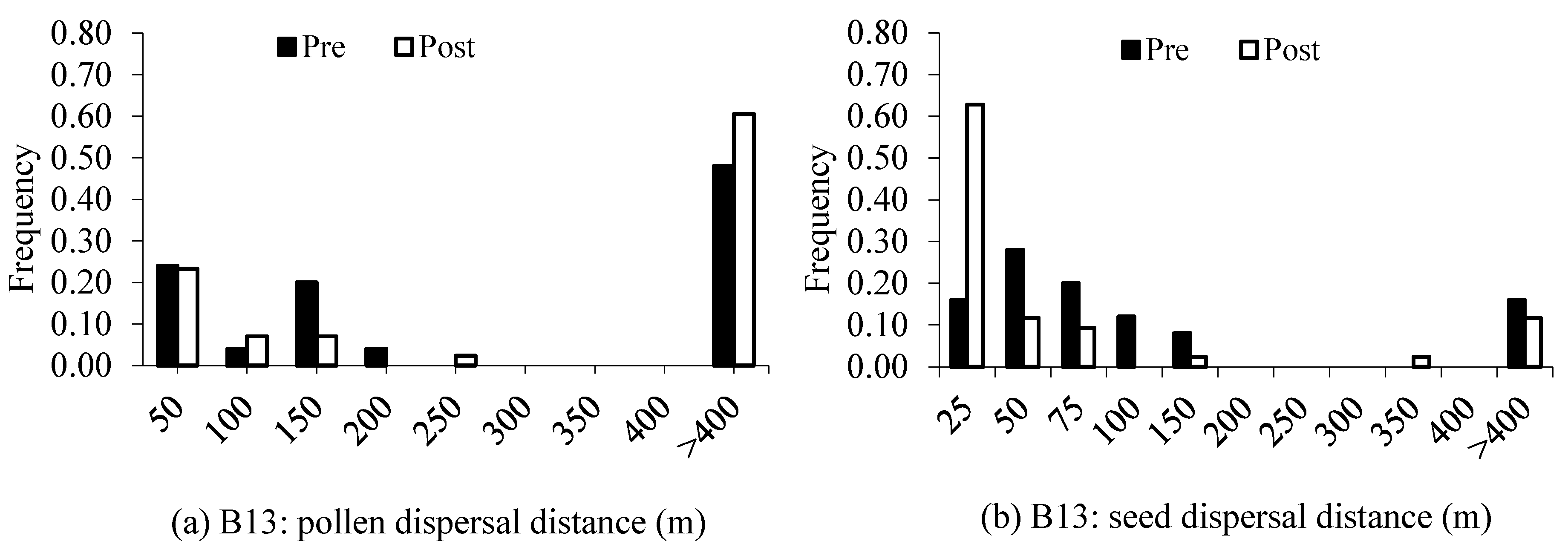
2.4. Spatial Genetic Structure and Effective Population Size
2.5. Parentage Analyses
3. Results
3.1. Genetic Diversity
3.2. Spatial Genetic Structure and
4. Discussion
4.1. Genetic Diversity and Effective Population Size
4.2. Gene Flow and Spatial Genetic Structure
4.3. Considerations for Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Degen, B.; Blanc, L.; Caron, H.; Maggia, L.; Kremer, A.; Gourlet-Fleury, S. Impact of selective logging on genetic composition and demographic structure of four tropical tree species. Biol. Conserv. 2006, 131, 386–401. [Google Scholar] [CrossRef]
- Sebbenn, A.M.; Degen, B.; Azevedo, V.C.R.; Silva, M.B.; Lacerda, A.E.B.; Ciampi, A.Y.; Kanashiro, M.; Carneiro, F.S.; Thompson, I.; Loveless, M.D. Modelling the long-term impacts for selective logging on genetic diversity and demographic structure of four tropical tree species in the Amazon forest. For. Ecol. Manag. 2008, 254, 335–349. [Google Scholar] [CrossRef]
- Lacerda, A.E.B.; Nimmo, E.R.; Sebbenn, A.M. Modeling the long-term impacts of logging on genetic diversity and demography of Hymenaea courbaril. For. Sci. 2013, 59, 15–26. [Google Scholar] [CrossRef]
- Dal Bem, E.A.; Bittencourt, J.V.M.; Moraes, M.L.T.; Sebbenn, A.M. Cenários de corte seletivo de árvores na diversidade genética e área basal de populações de Araucaria angustifolia com base em modelagem Ecogene. Sci. For. 2015, 43, 453–466. [Google Scholar]
- Vinson, C.C.; Kanashiro, M.; Sebbenn, A.M.; Williams, T.C.R.; Harris, S.A.; Boshier, D.H. Long-term impacts of selective logging on two Amazonian tree species with contrasting ecological and reproductive characteristics: Inferences from Ecogene model simulations. Heredity 2015, 115, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Sist, P.; Piponiot, C.; Kanashiro, M.; Pena-Claros, M.; Putz, F.E.; Schulze, M.; Verissimo, A.; Vidal, E. Sustainability of Brazilian forest concessions. For. Ecol. Manag. 2021, 496, 119440. [Google Scholar] [CrossRef]
- Lourmas, M.; Kjellberg, F.; Dessard, H.; Joly, H.I.; Chevallier, M.H. Reduced density due to logging and its consequence on mating system and pollen flow in the African mahogany Entandrophragma cylindricum. Heredity 2007, 99, 151–160. [Google Scholar] [CrossRef]
- Inza, M.V.; Aguirre, N.C.; Torales, S.L.; Pahr, N.M.; Fassola, H.E.; Fornes, L.F.; Zelener, N. Genetic variability of Araucaria angustifolia in the Argentinean Parana Forest and implications for management and conservation. Trees 2018, 32, 1135–1146. [Google Scholar] [CrossRef]
- Kavaliauskas, D.; Fussi, B.; Westergren, M.; Aravanopoulos, F.; Finzgar, D.; Baier, R.; Alizoti, P.; Bozic, G.; Avramidou, E.; Konnert, M.; et al. The interplay between forest management practices, genetic monitoring, and other long-term monitoring systems. Forests 2018, 9, 133. [Google Scholar] [CrossRef]
- Murawski, D.A.; Gunatilleke, I.A.U.N.; Bawa, K.S. The effects of selective logging on inbreeding in Shorea megistophylla (Dipterocarpaceae) from Siri Lanka. Conserv. Biol. 1994, 8, 997–1002. [Google Scholar] [CrossRef]
- Obayashi, K.; Tsumura, Y.; Ihara-Ujino, T.; Niiyama, K.; Tanouchi, H.; Suyama, Y.; Washitani, I.; Lee, C.T.; Lee, S.L.; Muhammad, N. Genetic diversity and outcrossing rate between undisturbed and selectively logged forests of Shorea curtisii (Dipterocarpaceae) using microsattellite DNA analysis. Inter. J. Plant Sci. 2002, 163, 151–158. [Google Scholar] [CrossRef]
- Carneiro, F.S.; Lacerda, A.E.B.; Lemes, M.R.; Gribel, R.; Kanashito, M.; Wadt, L.H.O.; Sebbenn, A.M. Effects of selective logging on the mating system and pollen dispersal of Hymenaea courbaril L. (Leguminosae) in the Eastern Brazilian Amazon as revealed by microsatellite analysis. For. Ecol. Manag. 2011, 262, 1758–1765. [Google Scholar] [CrossRef]
- Tani, N.; Tsumura, Y.; Fukasawa, K.; Kado, T.; Taguchi, Y.; Lee, S.L.; Lee, C.T.; Muhammad, N.; Niiyama, K.; Otani, T.; et al. Mixed mating system are regulated by fecundity in Shorea curtisii (Dipterocarpaceae) as revealed by comparison under different pollen limited conditions. PLoS ONE 2015, 10, e0123445. [Google Scholar] [CrossRef] [PubMed]
- Arruda, C.C.B.; Silva, M.B.; Sebbenn, A.M.; Kanashiro, M.; Lemes, M.R.; Gribel, R. Mating system and genetic diversity of progenies before and after logging: A case study of Bagassa guianensis (Moraceae), a low-density dioecious tree of the Amazonian forest. Tree Genet. Genome 2015, 11, 3. [Google Scholar] [CrossRef]
- Lacerda, A.E.B.; Kanashiro, M.; Sebbenn, A.M. Long-pollen movement and deviation of random mating in a low-density continuous population of a tropical tree Hymenaea courbaril in the Brazilian Amazon. Biotropica 2008, 40, 462–470. [Google Scholar] [CrossRef]
- Silva, M.B.; Kanashiro, M.; Ciampi, A.Y.; Thompson, I.; Sebbenn, A.M. Genetic effects of selective logging and pollen gene flow in a low density population of the dioecious tropical tree Bagassa guianensis in the Brazilian Amazon. For. Ecol. Manag. 2008, 255, 1548–1558. [Google Scholar] [CrossRef]
- Rajora, O.P.; Rahman, M.H.; Buchert, G.P.; Dancik, B.P. Microsatellite DNA analysis of genetic effects of harvesting in old-growth eastern white pine (Pinus strobus) in Ontario, Canada. Mol. Ecol. 2000, 9, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Aravanopoulos, F.A. Do silviculture and forest management affect the genetic diversity and structure of long-impacted forest tree populations? Forests 2018, 9, 355. [Google Scholar] [CrossRef]
- Carvalho, P.E.R. Espécies Florestais Brasileiras: Recomendações Silviculturais, Potencialidades e Usos da Madeira; Embrapa: Brasília, Brazil, 1994. [Google Scholar]
- Hueck, K. Distribuição e habitat natural do Pinheiro do Paraná (Araucaria angustifolia). Boletim da Faculdade de Filosofia, Ciências e Letras, Universidade de São Paulo. Botânica 1953, 10, 3–24. [Google Scholar] [CrossRef]
- Reitz, R.; Klein, R.M. Araucariáceas; Herbário Barbosa: Itaja, Brazil, 1966; p. 62. [Google Scholar]
- Danner, M.A.; Ribeiro, J.Z.; Zanette, F.; Bittencourt, J.V.M.; Sebbenn, A.M. Impact of monoecy in the genetic structure of a predominately dioecious conifer species, Araucaria angustifolia (Bert.) O. Kuntze. Plant Syst. Evol. 2013, 299, 949–958. [Google Scholar] [CrossRef]
- Figueiredo Filho, A.; Dias, A.N.; Stepka, T.F.; Sawczuk, A.R. Crescimento, mortalidade, ingresso e distribuição diamétrica em Floresta Ombrófila Mista. Floresta 2010, 40, 763–776. [Google Scholar] [CrossRef]
- Mantovani, A.; Morellato, L.P.C.; Reis, M.S.D. Fenologia reprodutiva e produção de sementes em Araucaria angustifolia (Bert.) O. Kuntze. Rev. Bras. Bot. 2004, 27, 787–796. [Google Scholar] [CrossRef]
- Anselmini, J.I.; Zanette, F. Development and growth curve of the pine cones of Araucaria angustifolia (Bert.) O. Ktze, in the region of Curitiba–PR. Braz. Arch. Biol. Technol. 2008, 51, 665–669. [Google Scholar] [CrossRef]
- Vieira, E.M.; Ribeiro, J.F.; Iob, G. Seed predation of Araucaria angustifolia (Araucariaceae) by small rodents in two areas with contrasting seed densities in the Brazilian Araucaria forest. J. Nat. Hist. 2011, 45, 843–854. [Google Scholar] [CrossRef]
- Wrege, M.S.; Fritzsons, E.; Soares, M.T.S.; Bognola, I.A.; Sousa, V.A.; Sousa, L.P.; Gomes, J.B.V.; Aguiar, A.V.; Gomes, G.C.; Matos, M.F.S.; et al. Distribuição natural e habitat da araucária frente às mudanças climáticas globais. Pesq. Flor. Bras. 2017, 37, 331–346. [Google Scholar] [CrossRef]
- Klein, R.M. O aspecto dinâmico do Pinheiro do Paraná. Sellowia 1960, 12, 17–44. [Google Scholar]
- Montagna, T.; Lauterjung, M.B.; Costa, N.C.F.; Bernardi, A.P.; Candido-Ribeiro, R.; Reis, M.S. Guidelines for seed collection of Araucaria angustifolia (Bertol.) Kuntze: A genetic, demographic and geographic approach. For. Ecol. Manag. 2019, 438, 10–17. [Google Scholar] [CrossRef]
- Bittencourt, J.V.M.; Sebbenn, A.M. Patterns of pollen and seed dispersal in a small, fragmented population of the wind-pollinated tree Araucaria angustifolia in southern Brazil. Heredity 2007, 99, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Paludo, G.F.; Mantovani, A.; Reis, M.S. Regeneração de uma população natural de Araucaria angustifolia (Araucariaceae). Rev. Árv. 2011, 35, 1107–1119. [Google Scholar] [CrossRef]
- Sant’Anna, C.S.; Sebbenn, A.M.; Klabunde, G.H.F.; Bittencourt, R.; Nodari, R.O.; Mantovani, A.; Reis, M.S. Realized pollen and seed dispersal within a continuous population of the dioecious coniferous Brazilian Pine (Araucaria angustifolia (Bertol.) Kuntze). Conserv. Genet. 2013, 14, 601–613. [Google Scholar] [CrossRef]
- Tagliari, M.M.; Vieilledent, G.; Alves, J.; Silveira, T.C.L.; Peroni, N. Relict populations of Araucaria angustifolia will be isolated, poorly protected, and unconnected under climate and land-use change in Brazil. Biodivers. Conserv. 2021, 30, 3665–3684. [Google Scholar] [CrossRef]
- Guerra, M.P.; Mantovani, N.S.A.; Nodari, R.O.; Reis, M.S.; Santos, K.L. Evolução, Ontogênese e Diversidade Genética em Araucaria angustifolia. In Origem e Evolução de Plantas Cultivadas; Barbieri, R.L., Stumpf, E.R.T., Eds.; Embrapa Informação Tecnológica: Brasília, Brazil, 2008; pp. 149–184. [Google Scholar]
- Reitz, R.; Klein, R.M.; Reis, A. Projeto Madeira de Santa Catarina; Sellowia: Itaja, Brazil, 1978; Volume 28/30, pp. 3–320. [Google Scholar]
- Stepka, T.F.; Mattos, P.P.; Figueiredo Filho, A.; Braz, E.M.; Machado, S.A. Growth of Araucaria angustifolia, Cedrela fissilis and Ocotea porosa in different locations within the Araucaria forest in the southern Brazil. For. Ecol. Manag. 2021, 486, 118941. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2020-2. 2020. Available online: https://www.iucnredlist.org. (accessed on 11 August 2021).
- Souza, A.F.; Forgiarini, C.; Longhi, S.J.; Brena, D.A. Regeneration patterns of a long-lived dominant conifer and the effects of logging in southern South America. Acta Oecol. 2008, 34, 221–232. [Google Scholar] [CrossRef]
- Souza, A.F.; Cortez, L.S.R.; Longhi, S.J. Native forest management in subtropical South America: Long-term effects of logging and multiple-use on forest structure and diversity. Biodivers. Conserv. 2012, 21, 1953–1969. [Google Scholar] [CrossRef]
- Ebling, A.A.; Netto, S.P. Modelagem de ocorrência de coortes na estrutura diamétrica da Araucaria angustifolia (Bertol.) Kuntze. Cerne 2015, 21, 251–257. [Google Scholar] [CrossRef]
- Bittencourt, J.V.M.; Sebbenn, A.M. Pollen movement within a continuous forest of wind-pollinated Araucaria angustifolia, inferred from paternity and TwoGener analysis. Conserv. Genet. 2008, 9, 855–868. [Google Scholar] [CrossRef]
- Patreze, C.M.; Tsai, S.M. Intrapopulacional genetic diversity of Araucaria angustifolia (Bertol.) Kuntze is different when assessed on the basis of chloroplast or nuclear markers. Plant Syst. Evol. 2010, 284, 111–122. [Google Scholar] [CrossRef]
- Ferreira, D.K.; Nazareno, A.G.; Mantovani, A.; Bittencourt, R.; Sebbenn, A.M.; Reis, M.S. Genetic analysis of 50-year old Brazilian pine (Araucaria angustifolia) plantations: Implications for conservation planning. Conserv. Genet. 2012, 13, 435–442. [Google Scholar] [CrossRef]
- Costa, N.C.F.; Stedille, L.I.B.; Lauterjung, M.B.; Montagna, T.; Candido-Ribeiro, R.; Bernardi, A.P.; Mantovani, A.; Reis, M.S.; Nodari, R.O. Spatiotemporal variation in mating system and genetic diversity of Araucaria angustifolia: Implications for conservation and seed collection. For. Ecol. Manag. 2021, 481, 118716. [Google Scholar] [CrossRef]
- Medri, C.; Ruas, P.M.; Higa, A.R.; Murakami, M.; Ruas, C.F. Effects of forest management on the genetic diversity in a population of Araucaria angustifolia (Bert.) O. Kuntze. Silvae Genet. 2003, 52, 202–203. [Google Scholar]
- Zechini, A.A.; Lauterjung, M.B.; Candido-Ribeiro, R.; Montagna, T.; Bernardi, A.P.; Hoeltgebaum, M.P.; Mantovani, A.; Reis, M.S. Genetic conservation of Brazilian pine (Araucaria angustifolia) through traditional land use. Econ. Bot. 2018, 72, 166–179. [Google Scholar] [CrossRef]
- Silva, P.I.T.; Silva, O.B., Jr.; Resend, L.V.; Sousa, V.A.; Aguiar, A.V.; Grattapaglia, D. A 3K Axiom® SNP array from a transcriptome-wide SNP resource sheds new light on the genetic diversity and structure of the iconic subtropical conifer tree Araucaria angustifolia (Bert.) Kuntze. PLoS ONE 2020, 15, e0230404. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.A.; Reeves, P.A.; Reilley, A.; Aguiar, A.V.; Stefenon, V.M.; Richards, C.M. Genetic diversity and biogeographic determinants of population structure in Araucaria angustifolia (Bert.) O. Kuntze. Conserv. Genet. 2020, 21, 217–229. [Google Scholar] [CrossRef]
- Machado, J.A.R.; Aguiar, A.V.; Pontinha, A.A.S.; Souza, B.M.; Sebbenn, A.M.; Hallsworth, J.E.; Sousa, V.A.; Freitas, M.L.M. Enhancing genetic fitness while maintaining genetic variability in Araucaria angustifolia. Sci. For. 2021, 49, e3572. [Google Scholar] [CrossRef]
- Bittencourt, J.V.M.; Higa, M.C.; Mazza, M.C.; Ruas, P.M.; Ruas, C.F.; Caccavari, M.; Fassola, H. Conservation, Management and Sustainable use of Araucaria angustifolia Genetic Resources in Brazil. In Challenges in Managing Forest Genetic Resource for Livelihoods: Examples from Argentina and Brazil International Plant Genetic Resources; IPGRI: Rome, Italy, 2004; pp. 133–148. [Google Scholar]
- FUPEF. Inventário Florestal do Pinheiro no Sul do Brasil; FUPEF/IBDF: Curitiba, Brazil, 1978. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Feres, F.; Souza, A.P.; Amaral, M.C.E.; Bittrich, V. Avaliação de método de preservação de amostras de plantas de Savanas Neotropicais para a obtenção de DNA de alta qualidade para estudos moleculares. Rev. Bras. Bot. 2005, 28, 277–283. [Google Scholar] [CrossRef]
- Schmidt, A.B.; Ciampi, A.Y.; Guerra, M.P.; Nodari, R.O. Isolation and characterization of microsatellite markers for Araucaria angustifolia (Araucariaceae). Mol. Ecol. Notes 2007, 7, 340–342. [Google Scholar] [CrossRef]
- Salgueiro, F.; Caron, H.; Souza, M.I.F.; Kremer, A.; Margis, R. Characterization of nuclear microsatellite loci in South American Araucariaceae species. Mol. Ecol. Notes 2005, 5, 256–258. [Google Scholar] [CrossRef]
- Goudet, J. Fstat Version (1.2) A computer program to calculate F-Statistics. J. Hered. 2002, 86, 485–486. [Google Scholar] [CrossRef]
- Chybicki, I.J.; Burczyk, J. Simultaneous estimation of null alleles and inbreeding coefficients. J. Hered. 2009, 100, 106–113. [Google Scholar] [CrossRef]
- Loiselle, B.A.; Sork, V.L.; Nason, J.; Graham, C. Spatial genetic structure of a tropical understory shrub, Psychotria officinalis (Rubiaceae). Am. J. Bot. 1995, 82, 1420–1425. [Google Scholar] [CrossRef]
- Hardy, O.J.; Vekemans, X. Spagedi: A versatile computer program to analyses spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2002, 2, 618–620. [Google Scholar] [CrossRef]
- Lindgren, D.; Mullin, T.J. Relatedness and status number in seed orchard crops. Can. J. For. Res. 1998, 28, 276–283. [Google Scholar] [CrossRef]
- Cornuet, J.M.; Luikart, G. Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 1996, 144, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Dow, B.D.; Ashley, M.V. Microsatellite analysis of seed dispersal and parentage of saplings in bur oak, Quercus macrocarpa. Mol. Ecol. 1996, 5, 615–627. [Google Scholar] [CrossRef]
- Kalinowski, S.T. Counting alleles with rarefaction: Private alleles and hierarchical sampling designs. Conserv. Genet. 2004, 5, 539–543. [Google Scholar] [CrossRef]
- Carneiro, F.S.; Sebbenn, A.M.; Kanashiro, M.; Degen, B. Low interannual variation of mating system and gene flow of Symphonia globulifera in the Brazilian Amazon. Biotropica 2007, 39, 628–636. [Google Scholar] [CrossRef]
- Carneiro, F.S.; Degen, B.; Kanashiro, M.; Lacerda, A.E.B.; Sebbenn, A.M. High levels of pollen dispersal detected through paternity analysis from a continuous Symphonia globulifera population in the Brazilian Amazon. For. Ecol. Manag. 2009, 258, 1260–1266. [Google Scholar] [CrossRef]
- Sebbenn, A.M.; Seoane, C.E.S.; Kageyama, P.Y.; Vencovsky, R. Efeitos do manejo na estrutura genética de populações de caixeta (Tabebuia cassinoides). Sci. For. 2000, 58, 123–143. [Google Scholar]
- Sebbenn, A.M.; Seoane, C.E.S.; Kageyama, P.Y.; Lacerda, C.M.B. Estrutura genética em populações de Tabebuia cassinoides: Implicações para o manejo florestal e a conservação genética. Rev. Inst. Flor. 2001, 13, 99–113. [Google Scholar]
- Vinson, C.C.; Kanashiro, M.; Harris, S.A.; Boshier, D.H. Impacts of selective logging on inbreeding and gene flow in two Amazonian timber species with contrasting ecological and reproductive characteristics. Mol. Ecol. 2015, 24, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.M.M.; Wadt, L.H.O.; Mesquita, A.G.G.; Martins, K. Impacto da exploração madeireira na diversidade genética e área basal de jatobá na Amazônia sul-ocidental. Sci. For. 2016, 44, 545–555. [Google Scholar] [CrossRef][Green Version]
- Buchert, G.P.; Rajora, O.P.; Hood, J.V. Effects of harvesting on genetic diversity in old-growth eastern white pine in Ontario, Canada. Conserv. Biol. 1997, 11, 747–758. [Google Scholar] [CrossRef]
- Adams, W.T.; Zuo, J.; Shimizu, J.Y.; Tappeiner, J.C. Impact of alternative regeneration methods on genetic diversity in coastal Douglas-fir. For. Sci. 1998, 44, 390–396. [Google Scholar] [CrossRef]
- Rajora, O.P. Genetic biodiversity impacts of silvicultural practices and phenotypic selection in white spruce. Theor. Appl. Genet. 1999, 99, 54–961. [Google Scholar] [CrossRef]
- El-Kassaby, Y.A.; Benowicz, A. Effects of commercial thinning on genetic, plant species and structural diversity in second-growth Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) stands. For. Genet. 2000, 7, 193–203. [Google Scholar]
- El-Kassaby, Y.A.; Dunsworth, B.G.; Krakowski, J. Genetic evaluation of alternative silvicultural systems in coastal montane forests: Western hemlock and amabilis fir. Theor. Appl. Genet. 2003, 107, 598–610. [Google Scholar] [CrossRef]
- Lee, K.S.; Wickneswari, R.; Choong, C.Y. Stand structure and the genetic diversity of Koompassia malaccensis and Dryobalanops aromatica in unlogged and logged-over stands. Sains Malays. 2007, 36, 233–242. [Google Scholar]
- Glaubitz, J.C.; Wu, H.X.; Moran, G.F. Impacts of silviculture on genetic diversity in the native forest species Eucalyptus sieberi. Conserv. Genet. 2003, 4, 275–287. [Google Scholar] [CrossRef]
- Hawley, G.J.; Schaberg, P.G.; Dehayes, D.H.; Brissette, J.C. Silviculture alters the genetic structure of an eastern hemlock forest in Maine, USA. Can. J. For. Res. 2005, 35, 143–150. [Google Scholar] [CrossRef]
- Valbuena-Carabana, M.; Gonzalez-Martınez, S.C.; Gil, L. Coppice forests and genetic diversity: A case study in Quercus pyrenaica Willd. from Central Spain. For. Ecol. Manag. 2008, 254, 225–232. [Google Scholar] [CrossRef]
- Ng, K.K.S.; Lee, S.L.; Ueno, S. Impact of selective logging on genetic diversity of two tropical tree species with contrasting breeding systems using direct comparison and simulation methods. For. Ecol. Manag. 2009, 257, 107–116. [Google Scholar] [CrossRef]
- Wickneswari, R. Threats to genetic viability of southeast Asian forest species. In Managing the Future of Southeast Asia’s Valuable Tropical Rainforests, a Practitioner’s Guide to Forest Genetics; Wickneswari, R., Cannon, C.H., Eds.; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; London, UK; New York, NY, USA, 2011; pp. 69–82. [Google Scholar]
- Fageria, M.S.; Rajora, O.P. Effects of silvicultural practices on genetic diversity and population structure of white spruce in Saskatchewan. Tree Genet. Genome 2014, 10, 287–296. [Google Scholar] [CrossRef]
- Ratnam, W.; Rajora, O.P.; Finkeldey, R.; Aravanopoulos, F.; Bouvet, J.M.; Vaillancourt, R.E.; Kanashiro, M.; Fady, B.; Tomita, M.; Vinson, C. Genetic effects of forest management practices: Global synthesis and perspectives. For. Ecol. Manag. 2014, 333, 52–65. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Thomas, B.R.; Cherniawsky, D.M.; Purdy, B.G. Managing genetic resources of lodgepole pine in west-central Alberta: Patterns of isozyme variation in natural populations and effects of forest management. For. Ecol. Manag. 2001, 152, 45–58. [Google Scholar] [CrossRef]
- Lee, C.T.; Wickneswari, R.; Mahani, M.C.; Zakri, A.H. Effect of selective logging on the genetic diversity of Scaphium macropodum. Biol. Conserv. 2002, 104, 107–118. [Google Scholar] [CrossRef]
- Rajora, O.P.; Pluhar, S.A. Genetic diversity impacts of forest fires, forest harvesting and alternative reforestation practices in black spruce (Picea mariana). Theor. Appl. Genet. 2003, 106, 1203–1212. [Google Scholar] [CrossRef]
- Buiteveld, J.; Vendramin, G.G.; Leonardi, S.; Kamer, K.; Geburek, T. Genetic diversity and differentiation in European beech (Fagus sylvatica L.) stands varying in management history. For. Ecol. Manag. 2007, 247, 98–106. [Google Scholar] [CrossRef]
- Fageria, M.S.; Rajora, O.P. Effects of harvesting of increasing intensities on genetic diversity and population structure of white spruce. Evol. Appl. 2013, 6, 778–794. [Google Scholar] [CrossRef]
- Cristofoli, C. Dinâmica da Diversidade Genética de Araucaria angustifolia (Bertol.) Kuntze em Campo e Floresta no Sul do Brasil. Ph.D. Thesis, Federal University of Santa Catarina, Florianópolis, Brazil, 2017. [Google Scholar]
- Medina-Macedo, L.; Sebbenn, A.M.; Lacerda, A.E.B.; Ribeiro, J.Z.; Soccol, C.R.; Bittencourt, J.V.M. High levels of genetic diversity through pollen flow of the coniferous Araucaria angustifolia: A landscape level study in Southern Brazil. Tree Genet. Genome 2015, 11, 814. [Google Scholar] [CrossRef]
- Medina-Macedo, L.; Lacerda, A.E.B.; Sebbenn, A.M.; Ribeiro, J.Z.; Soccol, C.R.; Bittencourt, J.V.M. Using genetic diversity and mating system parameters estimated from genetic markers to determine strategies for the conservation of Araucaria angustifolia (Bert.) O. Kuntze (Araucariaceae). Conserv. Genet. 2016, 17, 413–423. [Google Scholar] [CrossRef]
- Sousa, V.A.; Hattemer, H.H. Pollen dispersal and gene flow by pollen in Araucaria angustifolia. Aust. J. Bot. 2003, 51, 309–317. [Google Scholar] [CrossRef]
- Mantovani, A.; Morellato, L.P.C.; Reis, M.S. Internal genetic structure and outcrossing rate in a natural population of Araucaria angustifolia (Bert.) O. Kuntze. J. Hered. 2006, 97, 466–472. [Google Scholar] [CrossRef]
- Stefenon, V.M.; Gailing, O.; Finkeldey, R. The role of gene flow in shaping genetic structures of the subtropical conifer species Araucaria angustifolia. Plant. Biol. 2008, 10, 356–364. [Google Scholar] [CrossRef]
- Degen, B.; Gregorius, H.R.; Scholz, F. ECO-GENE, a model for simulation studies on the spatial and temporal dynamics of genetic structures of tree populations. Silvae Genet. 1996, 45, 323–329. [Google Scholar]
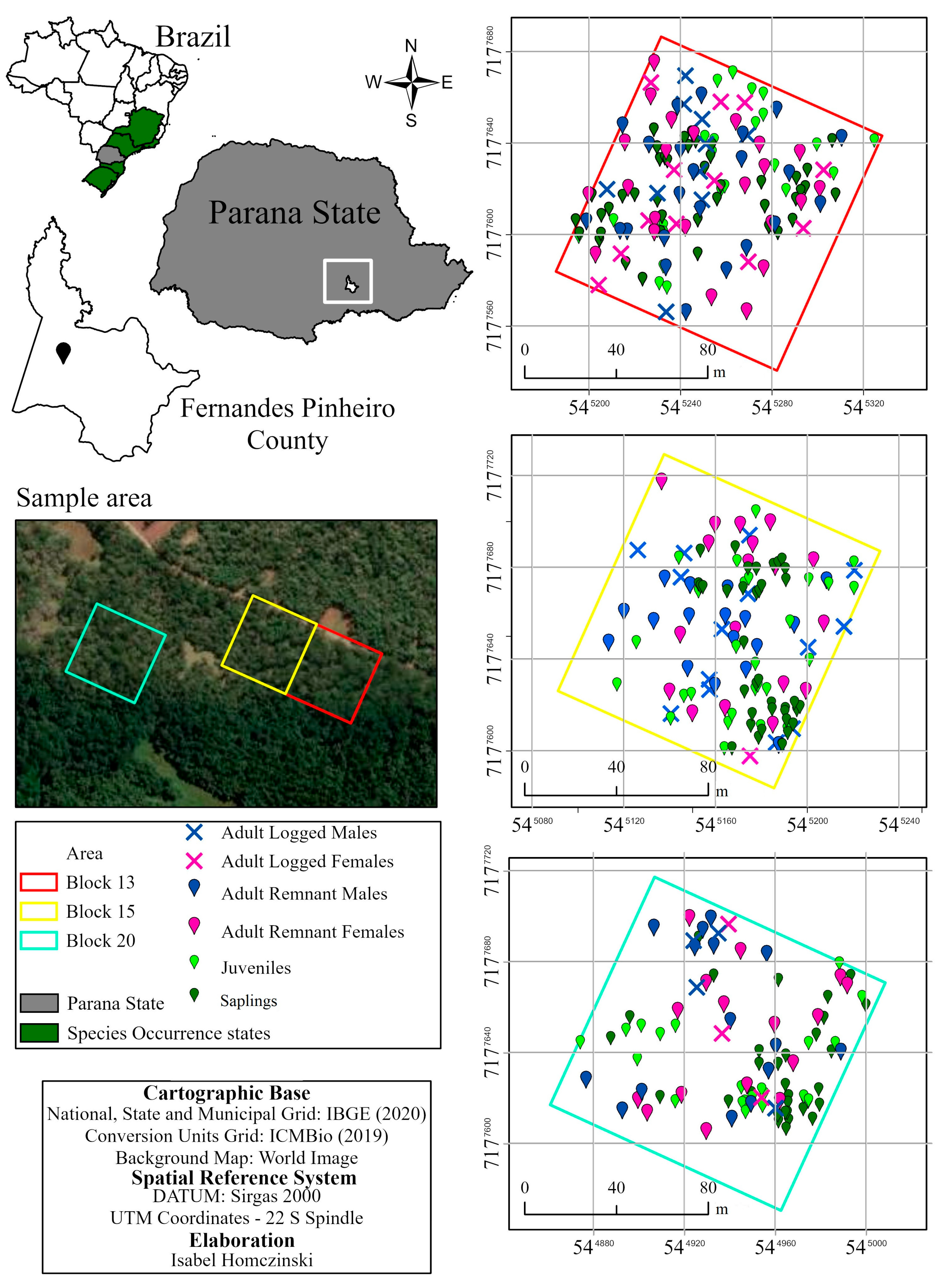
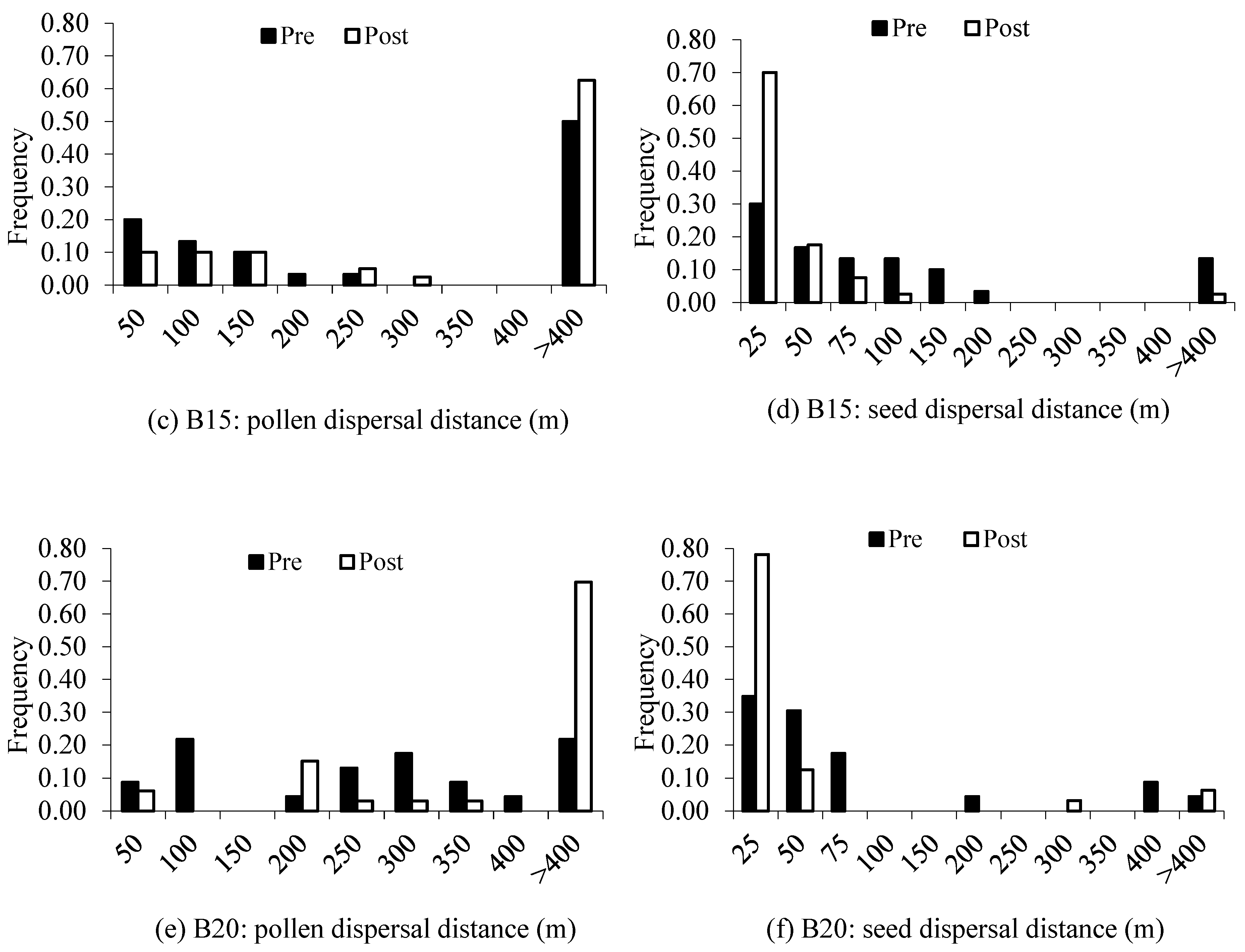
| B13 (LI = 32.3%) | |||||||
| Pre: adults (A) | 68 | 138 | 7 | 9.1 (6.4–11.9) | 0.821 (0.774–0.868) | 0.770 (0.686–0.854) | −0.066 * (−0.165–0.021) |
| Post: adults (A) | 46 | 128 | 0 | 9.0 (6.4–11.7) | 0.812 (0.762–0.861) | 0.769 (0.685–0.852) | −0.056 (−0.147–0.035) |
| Pre: juveniles (J) | 25 | 105 | 0 | 8.0 (5.7–10.3) | 0.830 (0.723–0.937) | 0.750 (0.650–0.850) | −0.107 (−0.167–0.047) |
| Pre: A + J | 93 | 136 | - | 9.0 (6.3–11.6) | 0.824 (0.764–0.885) | 0.769 (0.682–0.856) | −0.072 * (−0.151–0.007) |
| Post: saplings (S) | 43 | 131 | 2 | 9.0 (6.4–11.7) | 0.785 (0.671–0.898) | 0.753 (0.656–0.850) | −0.042 (−0.093–0.009) |
| Post: A + J + S | 114 | 136 | - | 8.8 (6.3–11.4) | 0.806 (0.724–0.887) | 0.762 (0.671–0.853) | −0.057 * (−0.102–0.012) |
| B15 (LI = 31.4%) | |||||||
| Pre: adults (A) | 51 | 130 | 9 | 9.2 (6.3–12.1) | 0.813 (0.745–0.881) | 0.769 (0.683–0.856) | −0.057 (−0.122–0.008) |
| Post: adults (A) | 35 | 123 | 0 | 9.2 (6.3–12.0) | 0.831 (0.773–0.890) | 0.772 (0.690–0.855) | −0.076 (−0.144–0.008) |
| Pre: juveniles (J) | 30 | 117 | 4 | 8.9 (6.3–11.5) | 0.783 (0.690–0.877) | 0.751 (0.661–0.841) | −0.043 (−0.079–0.007) |
| Pre: A + J | 81 | 137 | - | 9.3 (6.4–12.1) | 0.802 (0.726–0.877) | 0.765 (0.677–0.854) | −0.048 (−0.097–0.001) |
| Post: saplings (S) | 40 | 118 | 3 | 8.6 (6.0–11.4) | 0.799 (0.695–0.904) | 0.744 (0.652–0.837) | −0.074 (−0.129–0.019) |
| Post: A + J + S | 105 | 138 | - | 9.1 (6.3–11.9) | 0.805 (0.721–0.890) | 0.759 (0.670–0.848) | −0.061 * (−0.101–0.021) |
| B20 (LI = 18.4%) | |||||||
| Pre: adults (A) | 38 | 129 | 5 | 9.2 (6.6–11.7) | 0.761 (0.717–0.789) | 0.780 (0.682–0.878) | 0.025 (−0.051–0.101) |
| Post: adults (A) | 31 | 125 | 0 | 9.1 (6.5–11.7) | 0.779 (0.686–0.872) | 0.778 (0.674–0.882) | −0.001 (−0.080–0.078) |
| Pre: juveniles (J) | 23 | 108 | 0 | 8.3 (6.0–10.6) | 0.796 (0.685–0.906) | 0.744 (0.640–0.849) | −0.069 (−0.130–-0.006) |
| Pre: A + J | 61 | 129 | - | 8.8 (6.4–11.3) | 0.774 (0.684–0.864) | 0.766 (0.666–0.866) | −0.011 (−0.069–0.047) |
| Post: saplings (S) | 32 | 120 | 5 | 8.5 (5.9–11.1) | 0.772 (0.642–0.902) | 0.749 (0.634–0.863) | −0.031 (−0.098–0.036) |
| Post: A + J + S | 86 | 133 | - | 8.7 (6.2–11.2) | 0.781 (0.674–0.888) | 0.759 (0.653–0.865) | −0.029 (−0.078–0.020) |
| Block 13 | Block 15 | Block 20 | |
|---|---|---|---|
| Logging intensity (%) | 32.3 | 31.4 | 18.4 |
| Total number of alleles in adults pre-logging | 138 | 130 | 129 |
| Total number of alleles in adults post-logging | 128 | 123 | 125 |
| Number of loss allele in adults post-logging (%) | 10 (7.2) | 7 (5.4) | 4 (3.1) |
| Maximum allele frequency | 0.022 | 0.029 | 0.028 |
| Number of loss alleles in adults present in juveniles | 3 | 4 | 2 |
| Number of loss alleles in adults present in saplings | 5 | 4 | 2 |
| Number of loss alleles in adults present in A + J + S | 6 | 5 | 3 |
| Number of alleles not found within blocks | 4 | 2 | 1 |
| Maximum allele frequency | 0.015 | 0.029 | 0.027 |
| M:F | Decrease | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B13 | 39.5 | |||||||||
| Pre | 33:35 | 68 | 1:0.94 | 0.0038 | 0.0097 | −0.0016 | 0.0135 | 37 | 0.54 | |
| Post | 23:23 | 46 | 1:1 | 0.0087 | 0.0114 | 0.0022 | 0.0224 | 22.4 | 0.49 | |
| B15 | 27.7 | |||||||||
| Pre | 31:20 | 51 | 1.55:1 | 0.0064 | 0.0113 | −0.0049 | 0.0128 | 39 | 0.76 | |
| Post | 17:18 | 35 | 1:0.94 | 0.0113 | 0.0119 | −0.0085 | 0.0177 | 28.2 | 0.81 | |
| B20 | 28.8 | |||||||||
| Pre | 19:19 | 38 | 1:1 | 0.0066 | 0.0066 | 0.00004 | 0.0132 | 37.9 | 0.99 | |
| Post | 15:16 | 31 | 1:1.07 | 0.0093 | 0.0092 | −0.0023 | 0.0185 | 27 | 0.87 |
| Pollen Dispersal (m) | Seed Dispersal (m) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Min/max | Mean | Median | Min/max | |||
| Block13 | ||||||||
| Pre: juveniles | 76 | 84 ± 29 | 89 | 9/162 | 28 | 55 ± 13 | 50 | 6/112 |
| Post: saplings | 74.4 | 68 ± 31 | 44 | 2/243 | 18.6 | 32 ± 18 | 18 | 2/313 |
| Block15 | ||||||||
| Pre: juveniles | 53.3 | 85 ± 31 | 59 | 9/233 | 30 | 56 ± 38 | 45 | 4/155 |
| Post: saplings | 85 | 112 ± 38 | 97 | 21/270 | 5 | 25 ± 6 | 18 | 2/97 |
| Block20 | ||||||||
| Pre: juveniles | 78.3 | 187 ± 51 | 219 | 41/368 | 13 | 68 ± 43 | 29 | 2/366 |
| Post: saplings | 96.9 | 178 ± 56 | 187 | 4/349 | 9.4 | 23 ± 17 | 12 | 3/268 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roque, R.H.; Sebbenn, A.M.; Boshier, D.H.; Filho, A.F.; Tambarussi, E.V. Logging Affects Genetic Diversity Parameters in an Araucaria angustifolia Population: An Endangered Species in Southern Brazil. Forests 2023, 14, 1046. https://doi.org/10.3390/f14051046
Roque RH, Sebbenn AM, Boshier DH, Filho AF, Tambarussi EV. Logging Affects Genetic Diversity Parameters in an Araucaria angustifolia Population: An Endangered Species in Southern Brazil. Forests. 2023; 14(5):1046. https://doi.org/10.3390/f14051046
Chicago/Turabian StyleRoque, Rafael H., Alexandre M. Sebbenn, David H. Boshier, Afonso F. Filho, and Evandro V. Tambarussi. 2023. "Logging Affects Genetic Diversity Parameters in an Araucaria angustifolia Population: An Endangered Species in Southern Brazil" Forests 14, no. 5: 1046. https://doi.org/10.3390/f14051046
APA StyleRoque, R. H., Sebbenn, A. M., Boshier, D. H., Filho, A. F., & Tambarussi, E. V. (2023). Logging Affects Genetic Diversity Parameters in an Araucaria angustifolia Population: An Endangered Species in Southern Brazil. Forests, 14(5), 1046. https://doi.org/10.3390/f14051046







