The Effect of Biochar Amendment, Microbiome Inoculation, Crop Mixture and Planting Density on Post-Mining Restoration
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar and Hydrogel Amendments
2.2. Plant-Microbial Organisms
2.3. Greenhouse Mesocosm Experiment
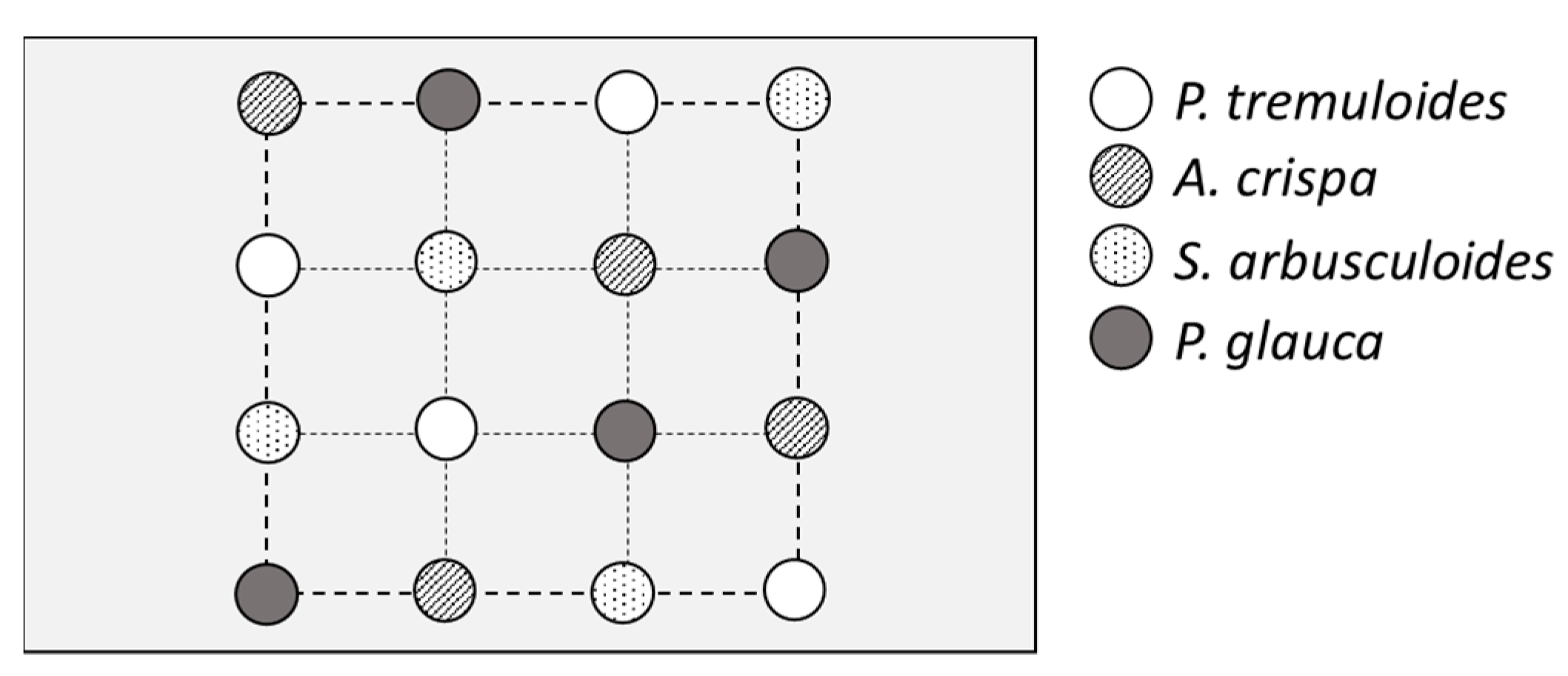
2.4. Field Trials
Set up of Experimental Design Field Trials
2.5. Statistical and Data Analyses
3. Results
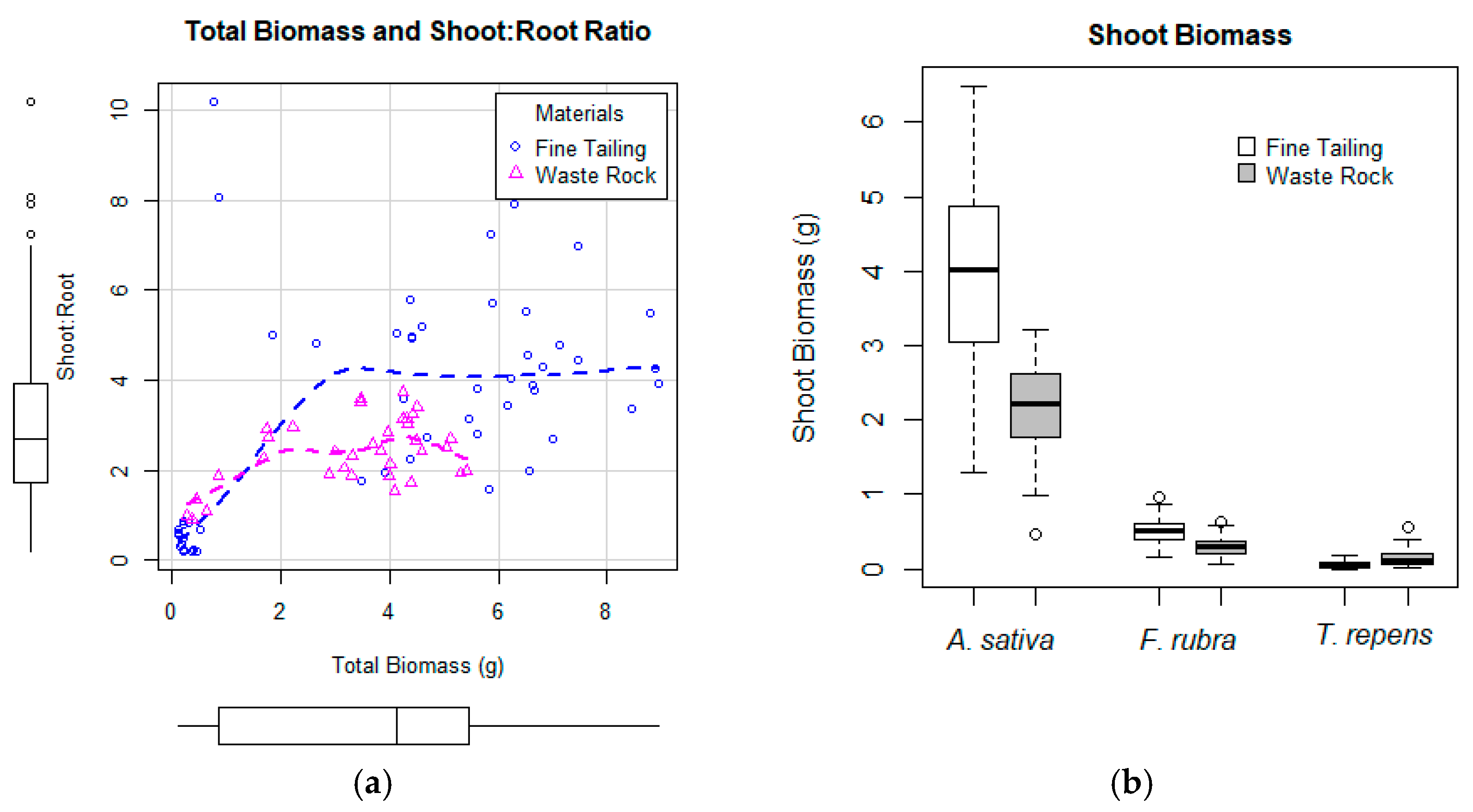
3.1. Greenhouse Experiment
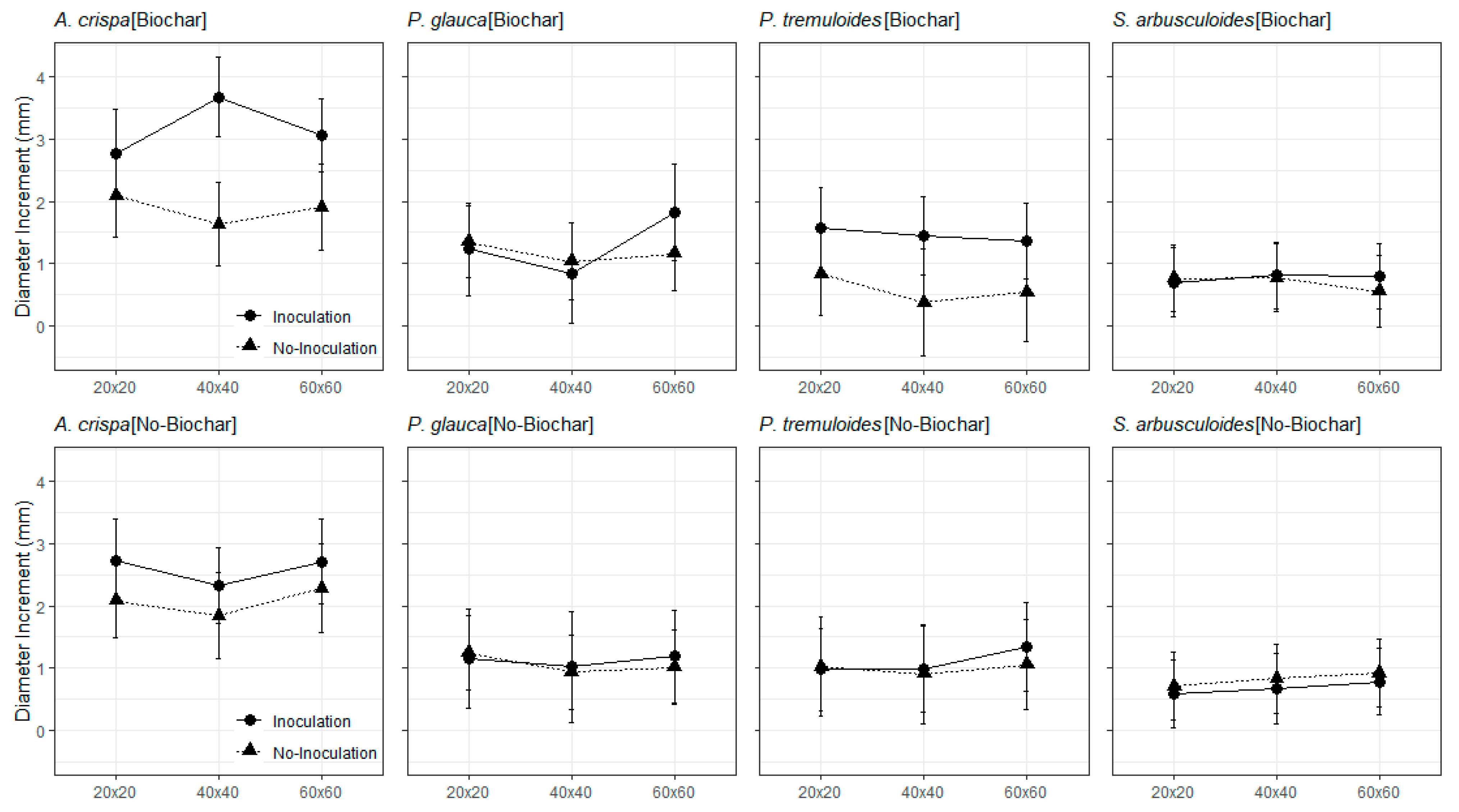
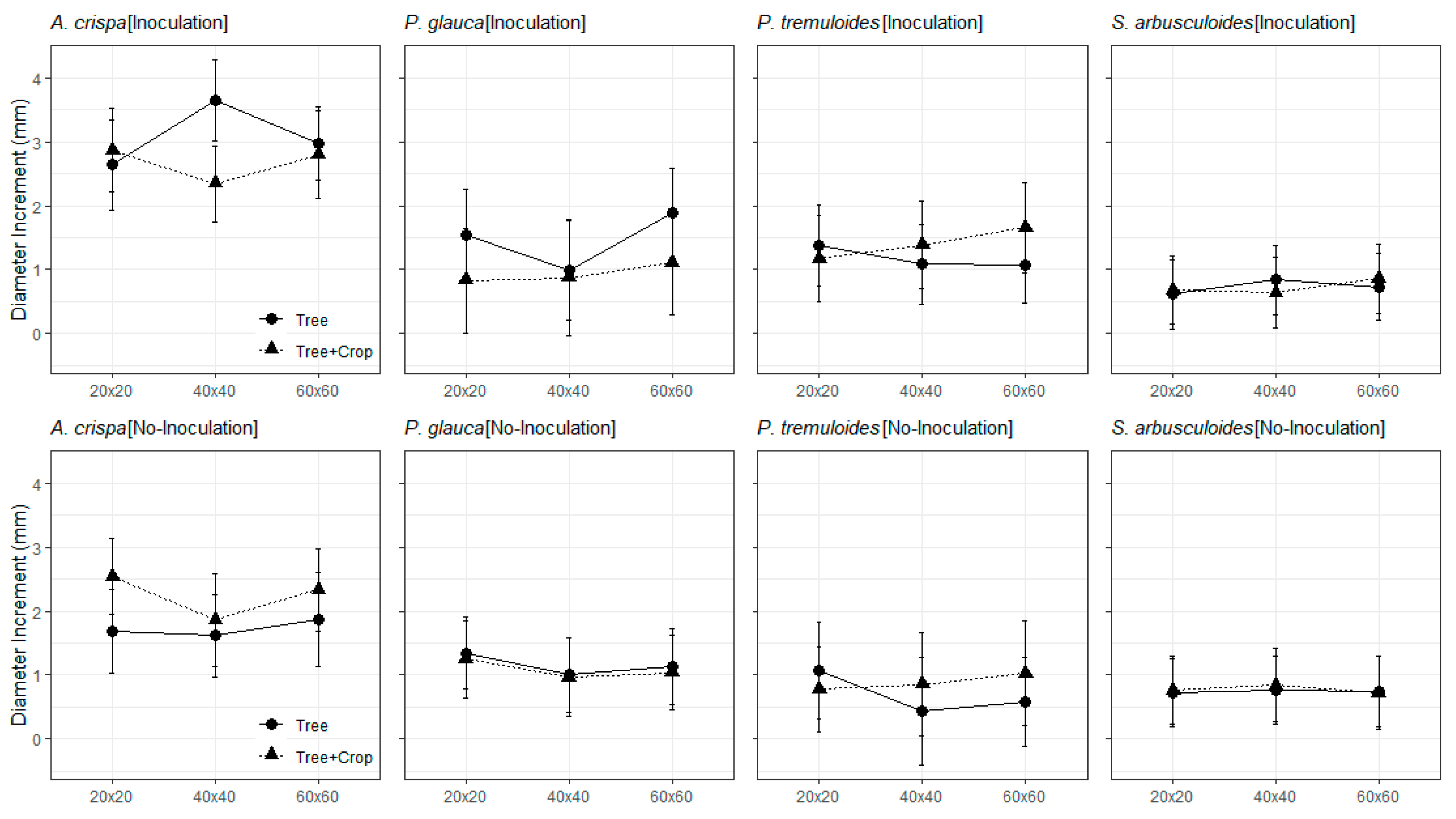
3.2. The Field Trials
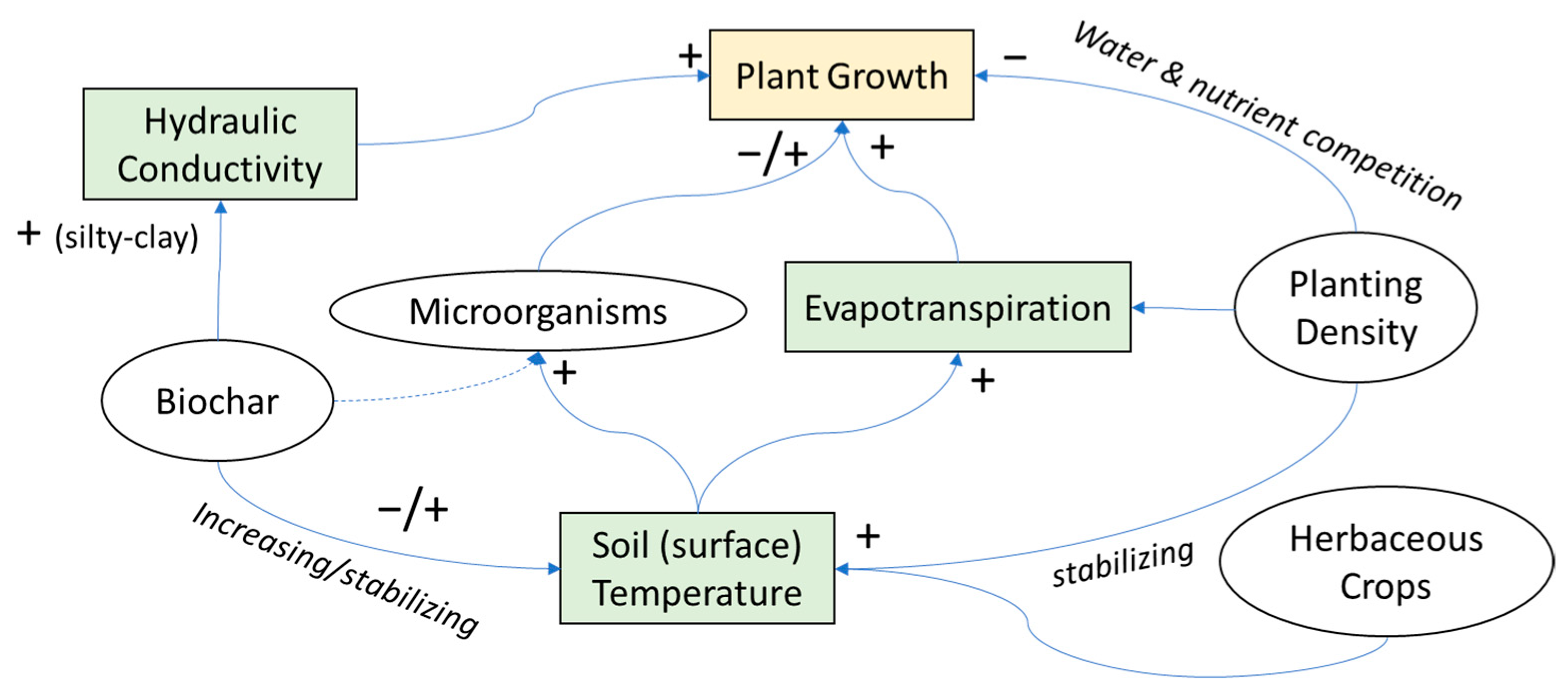
4. Discussion
4.1. Biochar Amendment
4.2. Microbial Inoculation
4.3. Mixed System Interactions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gann, G.D.; McDonald, T.; Walder, B.; Aronson, J.; Nelson, C.R.; Jonson, J.; Hallett, J.G.; Eisenberg, C.; Guariguata, M.R.; Liu, J.; et al. International Principles and Standards for the Practice of Ecological Restoration. Second Edition. Restor. Ecol. 2019, 27, S1–S46. [Google Scholar] [CrossRef]
- Choi, Y.D.; Temperton, V.M.; Allen, E.B.; Grootjans, A.P.; Halassy, M.; Hobbs, R.J.; Naeth, M.A.; Torok, K. Ecological Restoration for Future Sustainability in a Changing Environment. Ecoscience 2008, 15, 53–64. [Google Scholar] [CrossRef]
- Vieira, D.L.M.; Holl, K.D.; Peneireiro, F.M. Agro-Successional Restoration as a Strategy to Facilitate Tropical Forest Recovery. Restor. Ecol. 2009, 17, 451–459. [Google Scholar] [CrossRef]
- Jose, S.; Holzmueller, E.J.; Gillespie, A.R. Tree–Crop Interactions in Temperate Agroforestry. In North American Agroforestry: An Integrated Science and Practice; American Society of Agronomy, Inc.: Madison, WI, USA, 2009; pp. 57–74. [Google Scholar]
- Atangana, A.; Khasa, D.; Chang, S.; Degrande, A. Tropical Agroforestry; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-7722-4. [Google Scholar]
- Ong, C.K.; Black, C.R.; Wilson, J. (Eds.) Tree-Crop Interactions: Agroforestry in a Changing Climate, 2nd ed.; CAB International: Wallingford, UK; Boston, MA, USA, 2015; ISBN 978-1-78064-511-7. [Google Scholar]
- Solbrig, O.T. Plant Traits and Adaptive Strategies: Their Role in Ecosystem Function. In Biodiversity and Ecosystem Function; Schulze, E.-D., Mooney, H.A., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1994; ISBN 978-3-540-55804-0. [Google Scholar]
- Leakey, R. Environmental Resilience and Agroforestry. In Agroforestry-The Future of Global Land Use; Springer: Berlin/Heidelberg, Germany, 2012; pp. 11–12. [Google Scholar]
- Palmer, M.A.; Ambrose, R.F.; Poff, N.L. Ecological Theory and Community Restoration Ecology. Restor. Ecol. 1997, 5, 291–300. [Google Scholar] [CrossRef]
- Palmer, M.A.; Zedler, J.B.; Falk, D.A. Ecological Theory and Restoration Ecology. In Foundations of Restoration Ecology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 3–26. [Google Scholar]
- Martínez-Garza, C.; Howe, H.F. Restoring Tropical Diversity: Beating the Time Tax on Species Loss. J. Appl. Ecol. 2003, 40, 423–429. [Google Scholar] [CrossRef]
- Lamb, D. Regreening the Bare Hills; World Forests; Springer: Dordrecht, The Netherlands, 2011; Volume 8, ISBN 978-90-481-9869-6. [Google Scholar]
- Swift, M.J.; Izac, A.-M.N.; van Noordwijk, M. Biodiversity and Ecosystem Services in Agricultural Landscapes—Are We Asking the Right Questions? Agric. Ecosyst. Environ. 2004, 104, 113–134. [Google Scholar] [CrossRef]
- Paul, E.A. Soil Microbiology, Ecology and Biochemistry; Academic Press: Cambridge, MA, USA, 2014; ISBN 0-12-391411-6. [Google Scholar]
- van der Heijden, M.G.A.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal Fungal Diversity Determines Plant Biodiversity, Ecosystem Variability and Productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Vangronsveld, J. Phytoremediation: Plant–Endophyte Partnerships Take the Challenge. Curr. Opin. Biotechnol. 2009, 20, 248–254. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Bois, G.; Piché, Y.; Fung, M.Y.P.; Khasa, D.P. Mycorrhizal Inoculum Potentials of Pure Reclamation Materials and Revegetated Tailing Sands from the Canadian Oil Sand Industry. Mycorrhiza 2005, 15, 149–158. [Google Scholar] [CrossRef]
- Nadeau, M.B.; Laur, J.; Khasa, D.P. Mycorrhizae and Rhizobacteria on Precambrian Rocky Gold Mine Tailings: I. Mine-Adapted Symbionts Promote White Spruce Health and Growth. Front. Plant Sci. 2018, 9, 1267. [Google Scholar] [CrossRef]
- Nadeau, M.B.; Quoreshi, A.; Khasa, D.P. Ecological Restoration and Bioremediation of Canadian Mining Boreal Ecosystems. In Microbes for Restoration of Degraded Ecosystems; Bagyaraj, D.J., Jamaluddin, Eds.; New India Publishing Agency: Delhi, India, 2016; pp. 259–284. ISBN 978-93-85516-66-5. [Google Scholar]
- Joseph, S.; Lehmann, J. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009; ISBN 1-84407-658-X. [Google Scholar]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable Biochar to Mitigate Global Climate Change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef]
- Ogawa, M. Symbiosis of People and Nature in the Tropics. III. Trop. Agric. Using Charcoal. Farming Jpn. 1994, 28, 21–35. [Google Scholar]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological Nitrogen Fixation by Common Beans (Phaseolus vulgaris L.) Increases with Bio-Char Additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Steiner, C. Biochar Carbon Sequestration; University of Georgia, Biorefining and Carbon Cycling Program: Athens, Greece, 2008; p. 30602. [Google Scholar]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal Responses to Biochar in Soil–Concepts and Mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Barbour, M.G.; Billings, W.D. (Eds.) North American Terrestrial Vegetation, 2nd ed.; Cambridge University Press: Cambridge, UK, 2000; ISBN 978-0-521-55027-7. [Google Scholar]
- Abrahamson, I. Picea Glauca, White Spruce. In Fire Effects Information System; USDA, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Ft. Collins, CO, USA, 2015. [Google Scholar]
- Esser, L.L. Salix Arbusculoides. In Fire Effects Information System; USDA Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Ft. Collins, CO, USA, 1992. [Google Scholar]
- Howard, J.L. Populus Tremuloides. In Fire Effects Information System; USDA Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Ft. Collins, CO, USA, 1996. [Google Scholar]
- Matthews, R.F. Alnus Viridis Subsp. Crispa. In Fire Effects Information System; USDA Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Ft. Collins, CO, USA, 1992. [Google Scholar]
- Roy, S.; Khasa, D.P.; Greer, C.W. Combining Alders, Frankiae, and Mycorrhizae for the Revegetation and Remediation of Contaminated Ecosystems. Can. J. Bot. 2007, 85, 237–251. [Google Scholar] [CrossRef]
- Wong, M.H. Metal Cotolerance to Cooper, Lead, and Zinc in Festuca Rubra. Environ. Res. 1982, 29, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Rother, J.A.; Millbank, J.W.; Thornton, I. Nitrogen Fixation by White Clover (Trifolium repens) in Grasslands on Soils Contaminated with Cadmium, Lead and Zinc. J. Soil Sci. 1983, 34, 127–136. [Google Scholar] [CrossRef]
- Aubertin, M.; Bussiere, B.; Chapuis, R.P. Hydraulic Conductivity of Homogenized Tailings from Hard Rock Mines. Can. Geotech. J. 1996, 33, 470–482. [Google Scholar] [CrossRef]
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Macklin, M.G.; Hudson-Edwards, K.A. Mine Tailings Dams: Characteristics, Failure, Environmental Impacts, and Remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef]
- Nadeau, M.B. Development of a New Green Technology for the Revegetation of Abandoned Gold Mine Tailings Using Specific Symbionts Associated with Picea Glauca; Mémoire de Maîtrise, Département des Sciences du Bois et de la Forêt, Université Laval: Québec, QC, Canada, 2015. [Google Scholar]
- Gardner, C.M.; Laryea, K.B.; Unger, P.W. Soil Physical Constraints to Plant Growth and Crop Production; Land and Water Development Division, Food and Agriculture Organization: Rome, Italy, 1999. [Google Scholar]
- Lanini, W.T.; Orloff, S.B.; Vargas, R.N.; Orr, J.P.; Marble, V.L.; Grattan, S.R. Oat Companion Crop Seeding Rate Effect on Alfalfa Establishment, Yield, and Weed Control. Agron. J. 1991, 83, 330. [Google Scholar] [CrossRef]
- Dean, A.; Voss, D.; Draguljić, D. Split-Plot Designs. In Design and Analysis of Experiments; Springer International Publishing: Cham, Switzerland, 2017; pp. 703–764. ISBN 978-3-319-52250-0. [Google Scholar]
- Pardo, S. Mixed Models and Variance Components. In Statistical Analysis of Empirical Data: Methods for Applied Sciences; Springer International Publishing: Cham, Switzerland, 2020; pp. 107–119. ISBN 978-3-030-43328-4. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Blowes, D.W. The Environmental Effects of Mine Wastes. Proc. Explor. 1997, 97, 887–892. [Google Scholar]
- Saxton, K.E.; Rawls, W.J. Soil Water Characteristic Estimates by Texture and Organic Matter for Hydrologic Solutions. Soil Sci. Soc. Am. J. 2006, 70, 1569. [Google Scholar] [CrossRef]
- Nadeau, M.B.; Laur, J.; Khasa, D.P. Mycorrhizae and Rhizobacteria on Precambrian Rocky Gold Mine Tailings: II. Mine-Adapted Symbionts Alleviate Soil Element Imbalance for a Better Nutritional Status of White Spruce Seedlings. Front. Plant Sci. 2018, 9, 1268. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A Quantitative Review of the Effects of Biochar Application to Soils on Crop Productivity Using Meta-Analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A Review of Biochar and Its Use and Function in Soil. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2010; Volume 105, pp. 47–82. ISBN 978-0-12-381023-6. [Google Scholar]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota–A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Tarkka, M.T.; Frey-Klett, P. Mycorrhiza Helper Bacteria. In Mycorrhiza; Springer: Berlin/Heidelberg, Germany, 2008; pp. 113–132. [Google Scholar]
- Corbin, J.D.; Holl, K.D. Applied Nucleation as a Forest Restoration Strategy. For. Ecol. Manag. 2012, 265, 37–46. [Google Scholar] [CrossRef]
- Courchamp, F.; Berec, L.; Gascoigne, J. Allee Effects in Ecology and Conservation; Oxford Biology, Oxford University Press: Oxford, UK, 2008; ISBN 978-0-19-857030-1. [Google Scholar]
- Asmara, D.H.; Allaire, S.; van Noordwijk, M.; Khasa, D.P. Tree Establishment on Post-Mining Waste Soils: Species, Density, and Mixture Effects. Can. J. For. Res. 2021, 52, 79–89. [Google Scholar] [CrossRef]
- Verheijen, F.G.A.; Jeffery, S.; van der Velde, M.; Penížek, V.; Beland, M.; Bastos, A.C.; Keizer, J.J. Reductions in Soil Surface Albedo as a Function of Biochar Application Rate: Implications for Global Radiative Forcing. Environ. Res. Lett. 2013, 8, 044008. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Wu, Y.; Wang, X.; Du, Z.; Liu, X.; Song, J. Effects of Biochar Amendment on Soil Thermal Conductivity, Reflectance, and Temperature. Soil Sci. Soc. Am. J. 2013, 77, 1478. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Baldocchi, D.D.; Gershenson, A.; Goldstein, A.; Misson, L.; Wong, S. Microbial Soil Respiration and Its Dependency on Carbon Inputs, Soil Temperature and Moisture. Glob. Chang. Biol. 2007, 13, 2018–2035. [Google Scholar] [CrossRef]
- Swift, M.J.; Anderson, J.M. Biodiversity and Ecosystem Function in Agricultural Systems. In Biodiversity and Ecosystem Function; Schulze, E.-D., Mooney, H.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 15–41. ISBN 978-3-642-58001-7. [Google Scholar]
- Fortin, J.A.; Plenchette, C.; Piché, Y. Les Mycorhizes. In La Nouvelle Révolution Verte; MultiMonde, Q., Ed.; Université Laval: Quebec City, QC, Canada, 2008. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Repr.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2009; ISBN 978-0-12-370526-6. [Google Scholar]
- Smith, F.A.; Grace, E.J.; Smith, S.E. More than a Carbon Economy: Nutrient Trade and Ecological Sustainability in Facultative Arbuscular Mycorrhizal Symbioses. Res. Review. New Phytol. 2009, 182, 347–358. [Google Scholar] [CrossRef]
- Franklin, O.; Näsholm, T.; Högberg, P.; Högberg, M.N. Forests Trapped in Nitrogen Limitation-an Ecological Market Perspective on Ectomycorrhizal Symbiosis. New Phytol. 2014, 203, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Khasa, P.D.; Chakravarty, P.; Robertson, A.; Thomas, B.R.; Dancik, B.P. The Mycorrhizal Status of Selected Poplar Clones Introduced in Alberta. Biomass Bioenergy 2002, 22, 99–104. [Google Scholar] [CrossRef]
- Walder, F.; Niemann, H.; Natarajan, M.; Lehmann, M.F.; Boller, T.; Wiemken, A. Mycorrhizal Networks: Common Goods of Plants Shared under Unequal Terms of Trade. Plant Physiol. 2012, 159, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Fellbaum, C.R.; Mensah, J.A.; Cloos, A.J.; Strahan, G.E.; Pfeffer, P.E.; Kiers, E.T.; Bücking, H. Fungal Nutrient Allocation in Common Mycorrhizal Networks Is Regulated by the Carbon Source Strength of Individual Host Plants. New Phytol. 2014, 203, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Bücking, H.; Mensah, J.A.; Fellbaum, C.R. Common Mycorrhizal Networks and Their Effect on the Bargaining Power of the Fungal Partner in the Arbuscular Mycorrhizal Symbiosis. Commun. Integr. Biol. 2016, 9, e1107684. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar Induced Soil Microbial Community Change: Implications for Biogeochemical Cycling of Carbon, Nitrogen and Phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Robertson, S.J.; Rutherford, P.M.; López-Gutiérrez, J.C.; Massicotte, H.B. Biochar Enhances Seedling Growth and Alters Root Symbioses and Properties of Sub-Boreal Forest Soils. Can. J. Soil Sci. 2012, 92, 329–340. [Google Scholar] [CrossRef]
- Graham, J.H.; Leonard, R.T.; Menge, J.A. Interaction of Light Intensity and Soil Temperature with Phosphorus Inhibition of Vesicular-Arbuscular Mycorrhiza Formation. New Phytol. 1982, 91, 683–690. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Hartley, I.P.; Ineson, P.; Fitter, A.H. Soil Temperature Affects Carbon Allocation within Arbuscular Mycorrhizal Networks and Carbon Transport from Plant to Fungus. Glob. Chang. Biol. 2008, 14, 1181–1190. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, A.; Jha, A.; Chaturvedi, O.P.; Prasad, R. Ajit Gupta Effects of Shade on Arbuscular Mycorrhizal Colonization and Growth of Crops and Tree Seedlings in Central India. Agrofor. Syst. 2009, 76, 95–109. [Google Scholar] [CrossRef]
- Hasselquist, N.J.; Metcalfe, D.B.; Inselsbacher, E.; Stangl, Z.; Oren, R.; Näsholm, T.; Högberg, P. Greater Carbon Allocation to Mycorrhizal Fungi Reduces Tree Nitrogen Uptake in a Boreal Forest. Ecology 2016, 97, 1012–1022. [Google Scholar] [CrossRef]
- Snapp, S.S.; Swinton, S.M.; Labarta, R.; Mutch, D.; Black, J.R.; Leep, R.; Nyiraneza, J.; O’neil, K. Evaluating Cover Crops for Benefits, Costs and Performance within Cropping System Niches. Agron. J. 2005, 97, 322–332. [Google Scholar] [CrossRef]
- Araujo, A.S.F.; Leite, L.F.C.; Iwata, B.d.F.; Lira, M.d.A.; Xavier, G.R.; Figueiredo, M.d.V.B. Microbiological Process in Agroforestry Systems. A Review. Agron. Sustain. Dev. 2012, 32, 215–226. [Google Scholar] [CrossRef]
- Holmgren, M.; Scheffer, M.; Huston, M.A. The Interplay of Facilitation and Competition in Plant Communities. Ecology 1997, 78, 1966–1975. [Google Scholar] [CrossRef]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; TielböRger, K.; Travis, J.M.J.; Anthelme, F.; et al. Facilitation in Plant Communities: The Past, the Present, and the Future. J. Ecol. 2007, 96, 18–34. [Google Scholar] [CrossRef]
- Holl, K.D.; Zahawi, R.A.; Cole, R.J.; Ostertag, R.; Cordell, S. Planting Seedlings in Tree Islands versus Plantations as a Large-scale Tropical Forest Restoration Strategy. Restor. Ecol. 2011, 19, 470–479. [Google Scholar] [CrossRef]
- Corbin, J.D.; Robinson, G.R.; Hafkemeyer, L.M.; Handel, S.N. A Long-Term Evaluation of Applied Nucleation as a Strategy to Facilitate Forest Restoration. Ecol. Appl. 2016, 26, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Bertoncello, R.; Oliveira, A.A.; Holl, K.D.; Pansonato, M.P.; Martini, A.M.Z. Cluster Planting Facilitates Survival but Not Growth in Early Development of Restored Tropical Forest. Basic Appl. Ecol. 2016, 17, 489–496. [Google Scholar] [CrossRef]
- Kammann, C.; Graber, E.R. Biochar Effects on Plant Ecophysiology. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK, 2015; pp. 391–420. [Google Scholar]
- Bodner, G.; Loiskandl, W.; Kaul, H.-P. Cover Crop Evapotranspiration under Semi-Arid Conditions Using FAO Dual Crop Coefficient Method with Water Stress Compensation. Agric. Water Manag. 2007, 93, 85–98. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asmara, D.H.; Allaire, S.; van Noordwijk, M.; Khasa, D.P. The Effect of Biochar Amendment, Microbiome Inoculation, Crop Mixture and Planting Density on Post-Mining Restoration. Forests 2023, 14, 856. https://doi.org/10.3390/f14040856
Asmara DH, Allaire S, van Noordwijk M, Khasa DP. The Effect of Biochar Amendment, Microbiome Inoculation, Crop Mixture and Planting Density on Post-Mining Restoration. Forests. 2023; 14(4):856. https://doi.org/10.3390/f14040856
Chicago/Turabian StyleAsmara, Degi Harja, Suzanne Allaire, Meine van Noordwijk, and Damase P. Khasa. 2023. "The Effect of Biochar Amendment, Microbiome Inoculation, Crop Mixture and Planting Density on Post-Mining Restoration" Forests 14, no. 4: 856. https://doi.org/10.3390/f14040856
APA StyleAsmara, D. H., Allaire, S., van Noordwijk, M., & Khasa, D. P. (2023). The Effect of Biochar Amendment, Microbiome Inoculation, Crop Mixture and Planting Density on Post-Mining Restoration. Forests, 14(4), 856. https://doi.org/10.3390/f14040856







