Abstract
Camellia oleifera is a major woody oilseed species in China, but it is typically cultivated in nutrient-poor soils and may be affected by various trace elements. This study examined how spraying selenium, boron, and zinc trace elements affected the traits and functional active compounds of C. oleifera under nutrient deficiency. The results revealed significant variations in the effects of different trace element combinations on C. oleifera. Optimal concentrations of zinc and selenium are critical for promoting the growth and development of C. oleifera fruit. The transverse diameter of the fruit, the single fruit weight, the number of seeds per fruit, the single fresh seed weight, the oil content in the fruit, and the oil yield per plant of other treatments can be increased by up to 3.07%, 10.57%, 23.66%, 30.23%, 7.94%, and 21.95%, respectively, at most, compared to the control group. Diluting zinc from 1000 to 1500 times and maintaining a selenium concentration from 100 to 200 mg/L has been found to be beneficial for fruit growth. While low concentrations of selenium may promote an increase in fruit transverse diameter, high concentrations of selenium, along with high dilutions of zinc, can have the opposite effect, leading to a reduction in fruit diameter. However, a high concentration of selenium can positively impact the number of seeds per fruit. The most effective combination was found to be a selenium concentration of 0 mg/L, a boron concentration of 4 mg/L, and a zinc dilution of 1500. Interestingly, lower concentrations of selenium and boron, as well as lower dilutions of zinc, were found to increase the oil yield per plant. This suggests that a careful balance of trace elements is required to promote both fruit growth and oil content. The total sterol, squalene, total flavonoid, and polyphenol content of other treatments can be increased by up to 28.81%, 32.07%, 188.04%, and 92.61%, respectively, at most, compared to the control group. Selenium fertilizer and boron fertilizer increased the total sterol content in Camellia oil and had a significant positive correlation at the 0.01 level, but zinc fertilizer had little influence on it. High concentration selenium fertilizer generally increased the squalene, total flavonoid, and polyphenol content in Camellia oil, but boron and zinc fertilizers had little effect on these components. The results suggested that choosing appropriate fertilizer combinations could improve nutrient deficiency in C. oleifera and enhance the functional active compounds of its oil, thereby enhancing its value.
1. Introduction
Camellia oleifera, a crucial oil crop in southern China, is often cultivated in arid and infertile soil [1]. The primary value of C. oleifera lies in its oil production. However, this production is often limited by soil nutrient deficiencies. Camellia oil contains antioxidant and functional active components that make it a valuable resource for the medical and cosmetic industries. These components are known to have balanced and healthy effects in reducing the risk of obesity, cancer, and heart disease [2,3]. Camellia oil is known for its nutritional value and health benefits, which are attributed to its high content of unsaturated fatty acids, antioxidants, and other bioactive compounds [4]. For example, the unsaturated fatty acids found in Camellia oil, such as oleic acid, have been shown to have cardioprotective effects and improve blood lipid profiles [5]. Tea polyphenols, and flavonoids, have potent antioxidant and anti-inflammatory properties, which may help protect against chronic diseases such as cancer and cardiovascular disease [6]. Squalene, a natural antioxidant found in Camellia oil, and saponins have been found to have antimicrobial and anti-inflammatory properties [7]. Overall, the combination of these beneficial compounds in Camellia oil makes it a valuable resource for the food, cosmetic, and pharmaceutical industries. Camellia flowers are abundant in phenolic compounds and serve as important natural sources of active ingredients for the food industry [8]. Previous studies have indicated that the levels of stigmasterol, tocopherol, beta-carotene, and lutein in Camellia oil are significantly higher than those found in olive oil [9]. The functional and active compounds found in Camellia oil play a crucial role in enhancing its quality. However, due to its tolerance in barren soil, C. oleifera is often planted in barren areas, which results in a reduction in the active compounds of Camellia oil and a decrease in nutritional value. Therefore, fertilization is needed to supplement nutrients.
The soil nutrient levels in C. oleifera forests in China exhibit substantial variability, and the growth of C. oleifera is restricted by different elements [10]. Stress induced by zinc and boron results in a significant decrease in the growth and relative water content of C. oleifera [11]. Selenium, on the other hand, can enhance the nutritional value of Camellia oil and reduce the potential for heavy metal contamination [12]. Trace elements, including zinc, boron, and selenium, play a crucial role in the growth and nutrient content of C. oleifera. Zinc is an essential micronutrient for plants, but an excess or deficiency of it can cause oxidative stress, which can damage plant cells. When Camellia plants are exposed to zinc stress, they experience an increase in reactive oxygen species, which can cause oxidative damage to cells. In response to this stress, Camellia plants increase the activity of antioxidant enzymes to prevent oxidative damage [13]. However, zinc can improve the water use efficiency of tea plants under drought stress and also reduce oxidative stress caused by drought by increasing the activity of antioxidant enzymes [14]. Studies have shown that the application of boron fertilizer can improve the yield and quality of C. oleifera by enhancing soil fertility, balancing nutrient availability, and preventing nutrient deficiencies [15]. The use of selenium fertilizer can significantly increase the selenium content in Camellia oil, which in turn can improve its antioxidant and other beneficial functional components [16]. Previous studies have demonstrated that both soil and foliar fertilizer applications can significantly increase the selenium content in C. oleifera oil. However, the enrichment effect of foliar applications is superior to that of root applications [17]. Additionally, applying an active selenium compound fertilizer to soil can increase the nutrient content in mature C. oleifera forests, and the foliar spraying of selenium compound fertilizer can improve both the quality and yield of C. oleifera [18]. Therefore, by understanding the role of trace elements and using fertilizer effectively, it may be possible to increase both the production and quality of C. oleifera in barren conditions. However, there are limited studies on the effects of trace elements on the functional active compounds of Camellia oil, such as total sterols, squalene, total flavonoids, and polyphenols.
The objective of this study was to investigate the effects of trace elements such as zinc, boron, and selenium on the indicators of C. oleifera forests grown in nutrient-poor conditions and on the functional and active components of Camellia oil. The results of this research will provide a theoretical basis for improving the level and quality of Camellia oil and its functional active compounds, which would improve economic benefits.
2. Materials and Methods
2.1. Plant Material and Study Area
Camellia oleifera ‘Cenruan 3’, 8 years old, was used as the experimental species. The study area was located in Chini Town, Guangzhou City, China, and had a subtropical monsoon climate, warm and rainy, with long summers and short winters. The average monthly temperature was about 23.4 °C in October but, in exceptional months, was 13.1 °C. The average monthly precipitation was 98.5 mm in October [19]. The pH value of the soil was 5.6, the organic matter content was 21.26 mg/kg, the basic nitrogen content was 46.82 mg/kg, the available phosphorus content was 6.08 mg/kg, the available potassium content was 56.09 mg/kg, the available boron content was 0.22 mg/kg, the available zinc content was 1.76 mg/kg, and the selenium content was 0.159 mg/kg.
2.2. Experimental Design
An orthogonal experimental design was used to apply various levels of selenium (0, 100, 150, and 200 mg/L), boron (0, 2, 4, and 6 mg/L), and zinc (at dilutions of 0, 1000, 1500, and 2000) as a foliar fertilizer spray. The raw material for selenium is K3SeP3O10. The raw material for boron is Na2B4O7·10H2O. The raw material for zinc is C10H12N2O8ZnNa2, and the concentration of the original zinc solution before dilution was 145 mg/L. Mix the above raw materials with water and stir evenly. Each treatment (16 in total) equaled 30 L of fertilizer. The fertilizer was applied every two months from November 2020. Thirty trees were used for each fertilizer combination, representing a total of 480 trees. The fertilization plan is shown in Table 1.

Table 1.
Orthogonal design implementation.
2.3. Measurement of Camellia Fruit Characteristics and Economic Indicators
In October 2021, 15 trees were randomly selected for treatment and their yields were measured. Thirty fruits were randomly selected from thirty trees for each treatment, and the characteristics of the fruits were quantified. The longitudinal and transverse diameters of each fruit were measured with vernier calipers, and each fruit was weighed. Each fruit was then crushed with a hammer to release the peel and seeds. The peel thickness was measured with vernier calipers, and the number of seeds was counted. The fresh seeds from each fruit were also weighed. Seeds were dried to constant weight at 60 °C and stored in a refrigerator at 4 °C to measure the oil content.
Grinding the seeds into a powder. Two grams of dried seed powder per sample were wrapped in filter paper, baked to a constant weight at 105 °C, and then weighed on the filter paper and soaked overnight in ether. After 3 h at 65 °C in a crude fatty acid analyzer, the sample was dried again at 105 °C and weighed. The other fruit quality parameters were calculated using the following equations [20]: ratio of fresh seed per fruit (RFS) (%)= total mass of fresh seeds (g)/total mass of fresh fruit weight (g) × 100%; ratio of dry seed to fresh seed (RDS) (%) = total mass of dry seeds (g)/total mass of fresh seeds (g) × 100%; ratio of kernels in dry seed (RKS) (%) = total mass of fresh kernels (g)/total mass of dry seeds (g) × 100%; ratio of dry to fresh kernels (RDK) (%) = total mass of dry kernels (g)/total mass of fresh kernels (g) × 100%; oil content in dry kernels (OCK) (%) = total mass of oil (g)/total mass of dry kernels (g) × 100%; oil content in fruit (OCF) (%) = RFS × RDS × RKS × RDK × OCK.
2.4. Measurement of Functional Active Compounds of Camellia Oil
For each treatment, 5 kg of fruit was picked from 30 trees for oil extraction, and red or yellow peels were randomly selected. The fruit was dried indoors in the shade for a week and then left in the sun until it cracked, and then the peeled seeds baked at 105 °C to a constant weight. We then ground the seeds into powder and press oil and stored in a refrigerator at 4 °C.
Measurement of the total sterol content in Camellia oil according to standard GB/T 25223-2010 [21]. An amount of 250 mg of oil was placed in a 25 mL flask, and the unsaponifiable substances were extracted via an alumina column. Then, the unsaponifiable substances was separated on the thin layer chromatographic plate, and the total sterol content was obtained by gas chromatographic analysis.
Measurement of the squalene content in Camellia oil according to standard LS/T 6120-2017 [22]. An amount of 300 µL of internal standard squalene solution was accurately absorbed in a 250 mL round-bottomed flask and then dried with a nitrogen blower, and 0.2–2 g of the oil was added to the flask. An amount of 50mL of potassium hydroxide–ethanol solution was added for saponification and extraction, and the resulting concentrate was injected into a gas chromatograph for analysis of the squalene content.
The total flavonoid content of Camellia oil was determined using the following method [23]. An amount of 1 mL of oil from the solution to be tested was placed in a 10 mL centrifuge tube, and 0.4 mL 5% sodium nitrite was added. The solution was shaken well and left to stand for 6 min. Then, 0.4 mL 10% aluminum nitrate was added, shaken well, and left to stand for 6 min, followed by 4 mL 4% sodium hydroxide, which was again shaken well, and 4 mL 60% anhydrous ethanol. After 15 min, the absorbance was measured at 510 nm with a spectrophotometer after centrifugation at 3500 r/min for 10 min. The standard rutin, 10 mg, was added to a 10 mL volumetric bottle with 60% anhydrous ethanol to obtain a 1 mg/mL standard solution, and an absorbance of 0.025–0.4 mg/mL standard solution was determined. According to standard curve of rutin, the total flavonoid content was calculated.
The polyphenols content of Camellia oil was determined using the following method, according to standard LS/T 6119-2017 [24]. An amount of 1 mL of oil from the solution to be tested was put into a 10 mL colorimetric tube, and 5 mL 10% foline-phenol was added. It was then left to stand for 3–5 min, 4 mL 7.5% Na2CO3 was added, and it was again left to stand for 1 h. Absorbance was measured at 625 nm with a spectrophotometer, alongside a blank control. A standard curve was created using 30 mg gallic acid as a standard in a 100 mL volumetric bottle with distilled water added to obtain a 300 µg/mL solution. The absorbance value of 10–50 µg/mL of the standard solution was determined. According to standard curve of gallic acid, the polyphenols content was calculated.
2.5. Statistical Analysis
Microsoft Excel 2019 (Microsoft Corp, Redmond, Washington, DC, USA) was used for data processing and mapping. SPSS 24.0 (SPSS Inc., Chicago, IL, USA) was used for multiple comparisons, variance analyses, and correlation analyses.
3. Results
3.1. The Effect of Trace Elements on Fruit Characteristics
As shown in Table 2, there were slight differences between treatments in peel thickness, fruit transverse diameter, fruit longitudinal diameter, single fruit weight, the number of seeds per fruit, and single fresh seed weight. The transverse diameter of the fruit in treatment Y3 (0 mg/L selenium, 6 mg/L boron, and 2000 dilution of zinc) was significantly lower than that in the control group (p < 0.05), suggesting that high concentrations of selenium and high dilutions of zinc have the effect of reducing fruit transverse diameter, while the other treatments showed no significant difference (p > 0.05). The transverse diameter of fruit in treatment Y11 was the highest, and 9.1% higher than in treatment Y3. Both had received 6 mg/L boron, suggesting that boron had no direct effect on fruit transverse diameter. Treatment Y11 included 150 mg/L selenium, while treatment Y3 included 0 mg/L, and treatments Y4, Y5, Y7, and Y8 included 100, 100, 100, and 150 mg/L, respectively, indicating that low concentrations of selenium could perhaps promote an increase in fruit transverse diameter. However, there were no significant differences between the 15 treatments and the control group (p > 0.05). Treatment Y3 had the lowest fruit longitudinal diameter, while treatment Y2 had the highest fruit longitudinal diameter, which was 6.86% higher than that of treatment Y3. The transverse diameter of fruit of treatments Y2 can be increased by up to 3.07% compared to the control group. With a 1500 dilution of zinc, the fruit longitudinal diameter was higher, suggesting that this dilution had a positive effect on longitudinal diameter growth.

Table 2.
Multiple comparison of the effects of trace elements on Camellia oleifera fruit characteristics.
The peel thickness in treatment Y5 was significantly higher than that of the control group (p < 0.05), while the thickness in treatment Y10 was significantly lower than that of the control (p < 0.05) and 16.39% lower than that of treatment Y5. However, there were no significant differences in peel thickness between the other treatments and the control (p > 0.05). Both treatments Y5 and Y10 contained a 0 dilution of zinc, suggesting that zinc had no direct effect on peel thickness. Treatment Y10 (150 mg/L selenium, 4 mg/L boron, and 0 dilution of zinc) had the best effect on reducing peel thickness.
The single fruit weight of treatment 11 was the highest and 10.57% higher than in the control group, and that of treatment 3 was the lowest and 17.23% lower than in the control group. The single fruit weights of treatments 11, 7, 4, 8, and 13 were higher than in the control group. When the dilution of zinc is 1000–1500 times and the concentration of selenium is 100–200 mg/L, the growth of C. oleifera fruit is promoted.
Treatment Y11 yielded the most seeds per fruit, while treatment Y1 yielded the least grains per fruit. The single seed weight for treatment Y1 was the heaviest, while that of treatment Y14 was the lightest. Treatment Y11 had the highest fruit weight, 10.57% higher than the control group, while treatment Y3 had the lowest fruit weight, at 17.23% lower than the control group. The number of seeds per fruit in treatment 11, 14, 13, and 15 was higher than in control group. The amount of selenium applied was between 150–200 mg/L, suggesting that a high concentration of selenium can promote an increase in the number of seeds per fruit.
3.2. The Effect of Trace Elements on Economic Indicators
As shown in Table 3, different micronutrient treatments had variable effects on the oil yield of the fresh seeds. Treatment Y1 had the highest fresh seed ratio per fruit, 2.74% higher than the control, while treatment Y8 had the lowest fresh seed ratio. Treatment Y3 had the highest ratio of dry seed to fresh seed, while treatment Y4 had the lowest ratio of dry seed to fresh seed. Treatment Y2 had the highest ratio of kernels in the dried seeds, while treatment Y3 had the lowest ratio of kernels. Treatment Y6 had the most ratio of dried kernels to fresh kernels, while treatment Y9 had the least. Treatment Y2 had the highest yield of oil from both kernel and fruit, while treatment Y6 had the lowest yield. The oil content in fruit of treatments Y2 can be increased by up to 7.94% compared to the control group. Overall, the best effect was achieved with a selenium concentration of 0 mg/L, a boron concentration of 4 mg/L, and a zinc dilution of 1500, as demonstrated by the oil content of the fruits.

Table 3.
Effects of trace elements on economic indicators for Camellia oleifera.
Treatment Y5 had the highest fruit yield per plant at 17.05 kg, which was 44.41% higher than the control, followed by treatment Y13, while treatment Y7 had the lowest average yield. In combination with the oil content in fruit, the oil yield per plant for each treatment was ranked as Y1 > Y2 > Y5 > Y14 > Y13 > CK > Y15 > Y4 = Y9 > Y10 > Y11 > Y12 > Y8 > Y3 = Y6 = Y7. The oil yield per plant decreases to the minimum when the treatment is Y3, Y6, and Y7. The highest oil yield per plant for treatments Y1 (0 mg/L selenium, 2 mg/L boron, and 1000 dilution of zinc) and Y2 (0 mg/L selenium, 4 mg/L boron, and 1500 dilution of zinc) were 1.00 and 0.99 kg, respectively. The oil yield per plant of treatments Y1 can be increased by up to 21.95% compared to the control group. The results showed that a low concentration of selenium, a low concentration of boron, and a low dilution of zinc could increase the oil yield per plant.
3.3. The Effects of Trace Elements on Functional and Active Components of Camellia Oil
3.3.1. Effects of Trace Elements on Total Sterol Content
As shown in Figure 1, applying foliar fertilizer could both significantly increase and significantly decrease the total sterol content of Camellia oil. The total sterol content under treatments Y11, Y6, Y15, Y9, and Y14 was significantly higher than that of the control group (p < 0.05), by 28.81%, 12.96%, 10.52%, and 7.62%, respectively. However, the total sterol content under treatment Y1 was significantly lower than that of the control group (p < 0.05). The total sterol content under the other treatments showed no significant difference with the control group (p > 0.05). The application rates of selenium were 150 mg/L, 100 mg/L, and 0 mg/L in treatments Y11, Y6, and Y1, respectively, the application rates of boron were 6 mg/L, 4 mg/L, and 2 mg/L, respectively, and the dilutions of zinc were 1000, 2000, and 1000, respectively, indicating that the high concentrations of selenium and boron could promote an increase in total sterol content, while zinc had no direct effect.
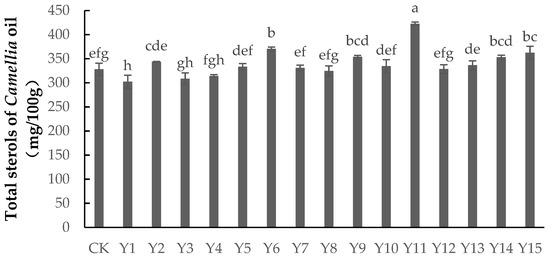
Figure 1.
The content of total sterols in Camellia oil under different treatments. Note: Data indicate mean ± SE (n = 3). Different letters indicate significant differences (p < 0.05) between different treatments.
3.3.2. Effects of Trace Elements on Squalene Content
As shown in Figure 2, applying foliar fertilizer could both significantly increase and significantly decrease the squalene content in Camellia oil. The squalene content of treatments Y12, Y13, Y14, Y11, Y15, Y2, Y6, Y8, Y9, and Y10 was significantly higher than that of the control group (p < 0.05). Of these, the squalene content was highest under Y12 and Y13, being 32.07% and 31.36% more, respectively, than in the control group. The squalene content under Y7 and Y4 was lower than the control group, but not significantly (p > 0.05), while the squalene content under Y1 and Y3 was significantly lower than in the control group (p < 0.05), by 4.66% and 5.68%, respectively. For treatments Y12, Y13, Y1, and Y3, the selenium dosage was 200 mg/L, 200 mg/L, 0 mg/L, and 0 mg/L, respectively, the boron dosage was 0 mg/L, 2 mg/L, 2 mg/L, and 6 mg/L, respectively, and the dilution of zinc was 2000, 1500, 1000, and 2000, respectively. This indicated that a high concentration of selenium could promote the squalene content of Camellia oil, while boron and zinc had no direct impact.
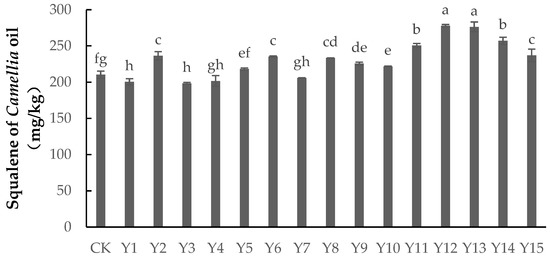
Figure 2.
The content of squalene in Camellia oil under different treatments. Note: Data indicate mean ± SE (n = 3). Different letters indicate significant differences (p < 0.05) between different treatments.
3.3.3. Effects of Trace Elements on Total Flavonoid Content
As shown in Figure 3, applying foliar fertilizer could both significantly increase and significantly decrease the total flavonoid content of Camellia oil. The total flavonoid content under treatments Y2, Y4, Y7, Y12, Y13, Y14, and Y15 was significantly higher than that of the control group (p < 0.05). Of these, Y4 had the highest total flavonoid content, being 188.04% higher than in the control group, followed by Y14 and Y13, which were 104.23% and 110.2% higher, respectively. The total flavonoid content under treatments Y1, Y3, Y5, Y6, Y8, Y9, and Y11 was significantly lower than in the control group (p < 0.05), with Y3 being 44.59% lower. The total flavonoid content under treatment Y10 was not significantly different from that of the control group (p > 0.05). Treatment Y4 received 100 mg/L of selenium, 0 mg/L of boron, and a 1000 dilution of zinc, indicating that this combination of elements maximized the total flavonoid content of Camellia oil. For treatments Y12, Y13, Y14, and Y15, the selenium application was 200 mg/L, indicating that 200 mg/L selenium could significantly increase the total flavonoid content of Camellia oil. The boron application was 0, 2, 4, and 6 mg/L, respectively, and the dilution of zinc 2000, 1500, 1000, and 0, respectively, indicating that boron and zinc had no direct effect on the total flavonoid content of Camellia oil.
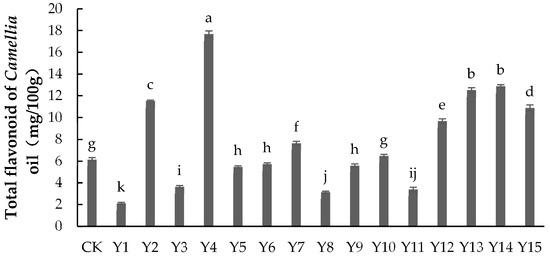
Figure 3.
The content of total flavonoid in Camellia oil under different treatments. Note: Data indicate mean ± SE (n = 3). Different letters indicate significant differences (p < 0.05) between different treatments.
3.3.4. Effects of Trace Elements on Polyphenol Content
As shown in Figure 4, spraying foliar fertilizer could both significantly increase and significantly decrease the polyphenol content of Camellia oil. The polyphenol content under treatments Y2, Y13, Y14, Y12, Y15, and Y4 was significantly higher than that of the control group (p < 0.05), while the polyphenol content under treatments Y1, Y5, Y6, Y7, Y8, and Y11 was significantly lower (p < 0.05). There was no significant difference between treatments Y3, Y9, and Y10 and the control group (p > 0.05). Treatment Y2 had the highest polyphenol content, being 92.61% higher than in the control group, followed by treatments Y13 and Y14, which were 66.5% and 51.07% higher. The polyphenol content of treatment Y1 was the lowest, being 60.92% lower than in the control group, followed by Y6, which was 30.71% lower. Under treatment Y2, the selenium application was 0 mg/L, boron 4 mg/L, and the dilution of zinc 1500, indicating that this combination could maximize the polyphenol content of Camellia oil. For treatments Y12, Y13, Y14, and Y15, the selenium application was 200 mg/L, indicating that 200 mg/L selenium could significantly increase the polyphenol content of Camellia oil.
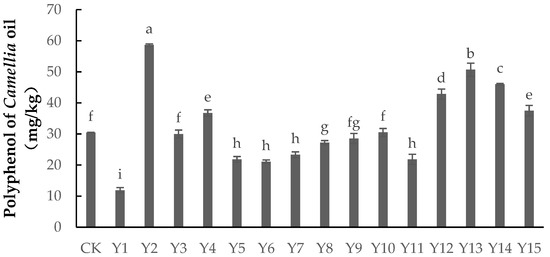
Figure 4.
The content of polyphenol in Camellia oil under different treatments. Note: Data indicate mean ± SE (n = 3). Different letters indicate significant differences (p < 0.05) between different treatments.
3.4. Correlation Analysis between Trace Elements and Components in Camellia Oil
As shown in Table 4, the total sterol content of Camellia oil had a very significant positive correlation with selenium and boron at the level of 0.01. The squalene content of Camellia oil had a very significant positive correlation with selenium at the level of 0.01, and there was a significant positive correlation between total flavonoids and selenium at the level of 0.05. There was no significant correlation between the polyphenol content of Camellia oil and the trace elements.

Table 4.
Correlation analysis between trace elements and components in Camellia oleifera.
As shown in Table 5, the variance analysis showed that selenium had a very significant effect on the squalene content of Camellia oil (p < 0.01) but no significant effect on the total sterol content. Boron and zinc had no significant effect on the squalene and total sterol content of Camellia oil. The range (R value) analysis showed that the relative weighting for the effect of the trace elements on the squalene content of Camellia oil was selenium > zinc > boron, and, for the total sterol content, selenium > boron > zinc. Selenium, boron, and zinc had no significant effect on the total flavonoid and polyphenol content of Camellia oil (p > 0.05), although selenium had a greater effect than zinc. The relative weighting for the effect of the trace elements on the polyphenol content of Camellia oil was selenium > boron > zinc.

Table 5.
Analysis of variance and extreme value analysis of trace elements and components in Camellia oil.
4. Discussion
Camellia plants that are in a nutrient-poor environment for an extended period of time will experience a significant reduction in yield, and the content of quality-related metabolites will be affected [25]. Previous research has shown that there are complex interactions between various elements in soil and C. oleifera, so the interaction between them should be fully considered to achieve precise fertilization and further improve fertilizer utilization [26]. In this study, 16 kinds of microelement combinations were designed to fertilize the C. oleifera forest under the environment of nutrient deficiency. The results showed that Y10 (150 mg/L selenium, 4 mg/L boron, and 0 diluted zinc) had the best effect on reducing the thickness of fruit peels. A low concentration of selenium might promote the increase in fruit transverse diameter. When the dilution of zinc was 1500, it had a positive effect on the growth of fruit longitudinal diameter, while boron concentration had little effect on fruit size. Xiang et al.’s research on tea plants aligns with the findings of this study, indicating that an appropriate concentration of selenium can result in a substantial increase in tea plant biomass and yield [27]. According to the research conducted by Hajiboland et al., the growth of tea plants was not found to be inhibited by a lack of boron, a critical micronutrient for many plants [28]. Recent studies, including one by Lu et al., have suggested that the application of a 0.05% zinc fertilizer concentration can significantly enhance the growth of C. oleifera [29]. Jiang et al. have shown that different fertilization methods, and the interaction of phosphorus and potassium fertilizers, have a significant impact on peel thickness. Nitrogen fertilizer applied at 50 g/plant, phosphorus fertilizer at 50 g/plant, and potassium fertilizer at 50 g/plant can all significantly reduce the peel thickness of C. oleifera [30]. The increase in pericarp thickness ends with the completion of lignin accumulation in the pericarp [31]. Cch NST1 and Cch BLH6 are lignin-regulated nuclear genes in C. oleifera fruit [32]. The change in the size of C. oleifera fruit after foliar spraying with selenium, boron and zinc may be due to the promotion or inhibition of CchNST1 and CchBLH6 gene expression.
The application of selenium, boron, and zinc had no significant effect on the fresh seed ratio or the number of seeds in C. oleifera fruit. The rate of seed inclusion during the accumulation of seed lignin and the number of ovules remaining in the fruit at harvest determine the fresh seed ratio and the number of seeds in C. oleifera fruit. C. oleifera seed inclusions accumulate from July to September, and fertilization during this period could increase the number of inclusions and improve the fresh seed ratio [33,34]. Therefore, applying appropriate fertilizer during this period could be beneficial to the number of seeds in C. oleifera fruit, but the physiological and molecular mechanisms that regulate ovule development have not yet been studied. In light of the selenium-poor soil conditions observed in the study area, the present research sheds new light on the crucial role that this trace element plays in promoting plant reproduction. Notably, the study’s results demonstrate that a concentration of selenium above a certain threshold can be a potent factor in boosting the number of seeds produced per fruit.
Spraying with selenium, boron, and zinc can also increase the oil yield of C. oleifera. The results showed that Y1 and Y2 treatments could increase the oil content of fresh fruits and the oil yield per plant. Genetic quality, seed maturity, fruiting quantity, and the soil quality of C. oleifera forest land all affect the oil content of C. oleifera. For example, lime soil and calcium-rich purple sand soil are beneficial to the seed oil content of C. oleifera [33]. Fertilizer spraying and the application of amino acids can increase the oil content of C. oleifera [35]. Similarly, when selenium fertilizer is applied at 200–300 g/hm2, the oil content of sunflower kernels can be significantly improved [36]. In another study the application of 0.1% boron increased the oil content of C. oleifera to 8.25% more than that of the control [37]. The beneficial impact of selenium, boron, and zinc on the quality of Camellia oil could potentially be explained by their role in promoting the uptake and assimilation of other essential mineral elements by the plant. By facilitating nutrient absorption, these trace elements may improve the taste, aroma, and nutritional properties of the oil [8,12]. Lin et al. have discovered alleles of three enzymes that play important roles in enhancing the yield and quality of seed oil during C. oleifera domestication [38].
The trace elements can also significantly increase the content of functionally active components in Camellia oil. The study’s findings suggest that selenium and boron may have a synergistic effect on the total sterol content of Camellia oil, while zinc has no direct impact. Specifically, the application of selenium at a concentration of 150 mg/L, in conjunction with 6 mg/L of boron, can significantly enhance the total sterol content of Camellia oil. Song et al. have shown that applying selenium can significantly increase the total sterol content of Camellia oil [39]. Pinto et al. found that sterol-related enzymes were significantly altered only in the presence of a selenium deficiency [40]. Under the treatment of boron deficiency, the downregulation of two sterol methyltransferases decreased the sterol content [41]. This study shows that high concentrations of selenium can promote the squalene and total flavonoid content in Camellia oil, while boron and zinc have no direct impact. When the selenium concentration was 200 mg/L, the squalene and total flavonoid content was at its highest. Ma et al. have shown that the squalene content of Camellia oil is positively correlated with the selenium content, in accordance with the current study [42]. Spraying zinc fertilizer can significantly induce the expression of phenolic synthesis-related genes such as VvLDOX and VvMYBF1 during fruit development, further promoting phenolic synthesis [42]. The study’s results indicate that the application of selenium at a concentration of 200 mg/L can lead to a significant increase in the polyphenol content of Camellia oil, However, the effect of zinc fertilizer is not significant. This finding suggests that selenium may play a crucial role in the production of high-quality Camellia oil. The mechanism behind the influence of selenium, therefore, warrants further exploration. The polyphenol content of Camellia oil was significantly correlated with the total flavonoid and squalene content, suggesting that these three substances have the same response mechanism to selenium, boron, and zinc, and that they synergistically promote each other.
5. Conclusions
The present study is the first to apply zinc, boron, and selenium as foliar fertilizers to C. oleifera cultivated in nutrient-poor soil. The results show that the effects of micronutrient sprays on the phenotypic and economic traits of C. oleifera varied, with treatments Y4, 7, 8, and 11 all increasing the transverse, longitudinal diameter and fruit weight of C. oleifera fruit. Optimal concentrations of zinc and selenium are critical for promoting the growth and development of C. oleifera fruit. The transverse diameter of the fruit, the single fruit weight, the number of seeds per fruit, the single fresh seed weight, the oil content in the fruit, and the oil yield per plant of other treatments can be increased by up to 3.07%, 10.57%, 23.66%, 30.23%, 7.94%, and 21.95%, respectively, at most, compared to the control group. Diluting zinc from 1000 to 1500 times and maintaining a selenium concentration from 100 to 200 mg/L has been found to be beneficial for fruit growth. While low concentrations of selenium may promote an increase in fruit transverse diameter, high concentrations of selenium, along with high dilutions of zinc, can have the opposite effect, leading to a reduction in fruit diameter. However, a high concentration of selenium can positively impact the number of seeds per fruit. Treatments Y1 and Y2 were the most effective in increasing the oil yield of fresh fruits and the oil production per plant. The most effective combination was found to be a selenium concentration of 0 mg/L, a boron concentration of 4 mg/L, and a zinc dilution of 1500. Interestingly, lower concentrations of selenium and boron, as well as lower dilutions of zinc, were found to increase the oil yield per plant. This suggests that a careful balance of trace elements is required to promote both fruit growth and oil content.
This study’s findings suggest that high concentrations of selenium and boron can play a crucial role in promoting the content of functional active compounds in Camellia oil. The total sterol, squalene, total flavonoid, and polyphenol content of other treatments can be increased by up to 28.81%, 32.07%, 188.04%, and 92.61%, respectively, at most, compared to the control group. Specifically, the results reveal that treatments Y2, 6, 9, and 11 exhibited better performance in terms of increasing the total sterol content of the oil, with selenium and boron, showing a highly significant positive correlation with this compound. Moreover, the application of high selenium concentrations was found to have a significant Impact on the squalene and total flavonoid contents of Camellia oil, with treatments Y2, 11, 12, and 13 showing better performance in increasing the squalene content of the oil, while treatments Y2, 4, and 7 showed better performance in increasing the total flavonoid content. Furthermore, the study shows that spraying selenium at a concentration of 200 mg/L can lead to a significant increase in the polyphenol content of Camellia oil, with treatments Y2, 13, and 14 performing better in this regard.
Overall, the study provides a theoretical basis for improving the quality and yield of the C. oleifera industry by selecting suitable fertilizer combinations to increase the fresh kernel oil rate, the oil yield per plant, and the content of functional active components such as total sterols, squalene, total flavonoids, and polyphenols in Camellia oil. These findings emphasize the interrelationship between trace elements and the functional active components of Camellia oil and offer guidance for enhancing the growth of C. oleifera forests in nutrient-deficient soils.
Author Contributions
Conceptualization, Z.D. and Q.D.; methodology, Z.D. and Q.D.; software, Z.D. and Q.D.; validation, L.P., Y.Y. and Y.H.; formal analysis, Z.D. and Q.D.; investigation, L.P. and Q.D.; resources, L.P.; data curation, L.N. and Q.D.; writing—original draft preparation, Z.D. and Q.D.; writing—review and editing, Z.D.; visualization, L.N. and Q.D.; supervision, Y.H. and J.H.; project administration, Y.H. and J.H.; funding acquisition, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Forestry Science and Technology Innovation Project of Guangdong Province (2017KJCX005), and the Forestry Science and Technology Innovation Project of Guangdong Province (2023KJCX003).
Data Availability Statement
All data relevant to the study are included in the article.
Conflicts of Interest
The authors declare no potential conflict of interest.
References
- Dong, B.; Wu, B.; Hong, W.; Li, X.; Li, Z.; Xue, L.; Huang, Y. Transcriptome analysis of the tea oil camellia (Camellia oleifera) reveals candidate drought stress genes. PLoS ONE 2017, 12, e0181835. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xie, Y.; Ren, H. Fatty acid composition and tocopherol, sitosterol, squalene components of Camellia reticulata oil. J. Consum. Prot. Food Saf. 2018, 13, 403–406. [Google Scholar] [CrossRef]
- Yu, J.; Yan, H.; Wu, Y.; Wang, Y.; Xia, P. Quality Evaluation of the Oil of Camellia spp. Foods 2022, 11, 2221. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.G.; Cao, Z.R.; Yao, H.; Li, C.; Zhao, Y.; Yuan, D.; Yang, G. The physicochemical properties and fatty acid composition of two new woody oil resources: Camellia hainanica seed oil and Camellia sinensis seed oil. CyTA J. Food 2021, 19, 208–211. [Google Scholar]
- Xiao, X.M.; He, L.; Chen, Y.Y.; Wu, L.; Wang, L.; Liu, Z.P. Anti-inflammatory and antioxidative effects of Camellia oleifera Abel components. Future Med. Chem. 2017, 9, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, G.Y.; Zhang, H.; Liu, J.A. Research progress on the health function of tea oil. J. Med. Plants Res. 2011, 5, 485–489. [Google Scholar]
- Xiang, Z.Y.; Xia, C.; Feng, S.; Chen, T.; Zhou, L.; Liu, L.; Kong, Q.; Yang, H.; Ding, C. Assessment of free and bound phenolics in the flowers and floral organs of two Camellia species flower and their antioxidant activities. Food Biosci. 2022, 49, 101905. [Google Scholar] [CrossRef]
- Zhang, L.X.; Wang, S.J.; Yang, R.; Mao, J.; Jiang, J.; Wang, X.; Zhang, W.; Zhang, Q.; Li, P.W. Simultaneous determination of tocopherols, carotenoids and phytosterols in edible vegetable oil by ultrasound-assisted saponification, LLE and LC-MS/MS. Food Chem. 2019, 289, 313–319. [Google Scholar] [CrossRef]
- Tang, G.X.; Tang, S.B.; Li, Y. Study on soil characters of Camellia oleifera land. Jiangxi For. Sci. Technol. 2002, 1, 20–24. [Google Scholar]
- Mukhopadhyay, M.; Mondal, T.K. Effect of Zinc and Boron on Growth and Water Relations of Camellia sinensis (L.) O. Kuntze cv. T-78. Natl. Acad. Sci. Lett.-India 2015, 38, 283–286. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, R.; Ye, H.; Wang, J.; Li, X.; Wang, Q.; Rui, Y. Determination of Trace Element Nutrition and Risk Assessment of Heavy Metals in Se-Enriched Camellia oleifera by ICP-MS. Asian J. Chem. 2013, 25, 8833–8834. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Das, A.; Subba, P.; Bantawa, P.; Sarkar, B.; Ghosh, P.; Mondal, T.K. Structural, physiological, and biochemical profiling of tea plantlets under zinc stress. Biol. Plant. 2013, 57, 474–480. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Dutta, B.K.; Panda, S.K. Zinc Modulates Drought-Induced Biochemical Damages in Tea Camellia sinensis (L) O Kuntze. J. Agric. Food Chem. 2013, 61, 6660–6670. [Google Scholar] [CrossRef]
- Mei, X.C.; Du, Y.X. Application Effect Test of Boron on Camellia oleifera’s Production. Jiangxi For. Sci. Technol. 2014, 42, 22–23. [Google Scholar]
- Wang, Y.H.; Liu, J.; Ma, X.L.; Jiang, J.M.; Wu, L.; Liu, F. Effects of different selenium–rich methods on quality characteristics and functional components of Camellia oleifera oil. Sci. Technol. Food Ind. 2017, 38, 54–59. [Google Scholar]
- Xu, W. Enrichment of selenium in Camellia oleifera and selenium fertilizer effect on the qualiy of Camellia oil. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2018. [Google Scholar]
- Zhao, J.P.; Wu, L.C.; Chen, Y.Z.; Li, F.C.; Qin, J. Effects of active selenium on soil chemical properties in Camellia oleifera forest. J. Cent. South Univ. For. Technol. 2011, 31, 75–81. [Google Scholar]
- National Meteorological Science Data Center of China. Available online: http://data.cma.cn/analysis/yearbooks.html (accessed on 20 March 2023).
- Wen, Y.; Zhang, Y.; Su, S.; Yang, S.; Ma, L.; Zhang, L.; Wang, X. Effects of Tree Shape on the Microclimate and Fruit Quality Parameters of Camellia oleifera Abel. Forests 2019, 10, 563. [Google Scholar] [CrossRef]
- GB/T 25223-2010; Animal and Vegetable Fats and Oils-Determination of Individual and Total Sterols Contents-Gas Chormatographic Method. ISO: Nanjing, China, 2010.
- LS/T 6120-2017; Inspection of Grain and Oil—Determination of Squalene in Vegetable Oil—Gas Chromatographic. ISO: Beijing, China, 2017.
- Yue, C.; Yu, N.; Li, H.; She, J.; Zhou, W.; Li, Z. Research on fatty acids and active components in Camellia oleifera Abel. seed oil of Hunan province. J. Food Saf. Qual. 2021, 12, 1972–1977. [Google Scholar]
- LS/T 6119-2017; Inspection of Grain and Oils—Determination of Polyphenols in Vegetable Oil—Spectrometric Method. ISO: Beijing, China, 2017.
- Zhou, B.; Chen, Y.Y.; Zeng, L.T.; Cui, Y.Y.; Li, J.L.; Tang, H.; Liu, J.Y.; Tang, J.C. Soil nutrient deficiency decreases the postharvest quality-related metabolite contents of tea (Camellia sinensis (L.) Kuntze) leaves. Food Chem. 2022, 377, 132003. [Google Scholar] [CrossRef]
- Zhu, X.L.; Tang, J.M.; Qin, H.Z.; Bai, K.D.; Chen, Z.Y.; Zou, R.; Liu, S.Y.; Yang, Q.G.; Xiao, W.; Chai, S.F. Contrasting Adaptation Mechanisms of Golden Camellia Species to Different Soil Habitats Revealed by Nutrient Characteristics. Agronomy 2022, 12, 1511. [Google Scholar] [CrossRef]
- Xiang, J.; Rao, S.; Chen, Q.; Zhang, W.; Cheng, S.; Cong, X.; Zhang, Y.; Yang, X.; Xu, F. Research Progress on the Effects of Selenium on the Growth and Quality of Tea Plants. Plants 2022, 11, 2491. [Google Scholar] [CrossRef]
- Hajiboland, R.; Bahrami-Rad, S.; Bastani, S. Phenolics Metabolism in Boron-Deficient Tea Camellia sinensis (L.) O. Kuntze Plants. Acta Biol. Hung. 2013, 64, 196–206. [Google Scholar] [CrossRef]
- Huang, G.W.; Zhang, W.J.; Deng, S.G. Effects of Foliar Fertilizer on the Growth and Physiological Characteristics of Camellia oleifera Seedlings. J. West China For. Sci. 2021, 50, 56–63. [Google Scholar]
- Jiang, J.Y.; Yang, H.Q.; Ouyang, W.; An, M.; Yang, X.; Long, X.; Hu, Y. Effects of Combined Application of Organic Fertilizer and NPK on Growth and Economic Characters of Camellia oleifera. J. Sichuan Agric. Univ. 2022, 40, 73–82. [Google Scholar]
- Yan, C.; Yao, X.; Yin, H.; Wang, K.; Yin, R.; Teng, J. Fruit Development Dynamics and Lignin Accumulation Law of Oil Tea. Acta Agric. Univ. Jiangxiensis 2020, 42, 788–801. [Google Scholar]
- Yan, C. Molecular Regulation of Lignin Biosynthesis in Fruit Development of Oil-Camellia. Ph.D. Thesis, China Academy of Forestry Sciences, Beijing, China, 2020. [Google Scholar]
- Li, Z.J.; Hua, J.Q.; Zeng, Y.R. Oil content of Camellia oleifera fruit trees. J. Zhejiang A F Univ. 2010, 27, 935–940. [Google Scholar]
- Liang, W.J.; Xiao, P.; Cui, M.; Fu, Y.; Luo, L. The growth and development dynamics of Camellia oleifera Abel. fruits and seeds. J. Nanchang Univ. 2019, 43, 46–52. [Google Scholar]
- HU, Y.L.; Long, X.Y.; Yang, H.; Yang, X.; Yang, S.; Pan, Z. Effects of different foliar fertilization concentrations on the chlorophyll content and productivity of oil-tea Camellia. J. For. Environ. 2021, 41, 527–535. [Google Scholar]
- Zan, Y.L.; Wang, L. Effect of exogenous selenium on seed oil and fatty acid content in Helianthus annuus. Guizhou Agric. Sci. 2016, 44, 19–22. [Google Scholar]
- He, P.; Zhang, J.D.; Bai, X.Q. Effects of Some Trace Elements on the Flower and Fruit Drop and Oil Content of Camellia oleifera. Jiangxi For. Sci. Technol. 1984, 5, 5–8. [Google Scholar]
- Lin, P.; Wang, K.; Wang, Y.; Hu, Z.; Yan, C.; Huang, H.; Ma, X.; Cao, Y.; Long, W.; Liu, W.; et al. The genome of oil-Camellia and population genomics analysis provide insights into seed oil domestication. Genome Biol. 2022, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.R.; Jiang, X.G.; Peng, S.F.; Li, F.; Liu, J.; Chen, D.; Wu, L.; Liu, F. Effect of Selenium Content on the Quality and Functional Components of Selenium-riched Camellia oleifera Oil. J. Chin. Inst. Food Sci. Technol. 2015, 15, 142–149. [Google Scholar]
- Pinto, A.; Juniper, D.T.; Sanil, M.; Morgan, L.; Clark, L.; Sies, H.; Rayman, M.P.; Steinbrenner, H. Supranutritional selenium induces alterations in molecular targets related to energy metabolism in skeletal muscle and visceral adipose tissue of pigs. J. Inorg. Biochem. 2012, 114, 47–54. [Google Scholar] [CrossRef]
- Su, W.L.; Liu, N.; Mei, L.; Luo, J.; Zhu, Y.J.; Liang, Z. Global Transcriptomic Profile Analysis of Genes Involved in Lignin Biosynthesis and Accumulation Induced by Boron Deficiency in Poplar Roots. Biomolecules 2019, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Tang, W.L.; Liu, J.; Liu, J.; Wu, L.C.; Liu, F. Effects of selenium-enriched fertilizer on the main chemical characteristics and functional components of Camellia oil. Food Sci. Technol. 2015, 40, 148–154. [Google Scholar]
- Song, C.Z.; Liu, M.Y.; Meng, J.F.; Chi, M.; Xi, Z.M.; Zhang, Z.W. Promoting Effect of Foliage Sprayed Zinc Sulfate on Accumulation of Sugar and Phenolics in Berries of Vitis vinifera cv. Merlot Growing on Zinc Deficient Soil. Molecules 2015, 20, 2536–2554. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).