Responses of Fine Root Traits and Soil Nitrogen to Fertilization Methods and Nitrogen Application Amounts in a Poplar Plantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plant Material
2.3. Experimental Design
2.4. Sample Collection and Analysis
2.5. Data Analysis
3. Results
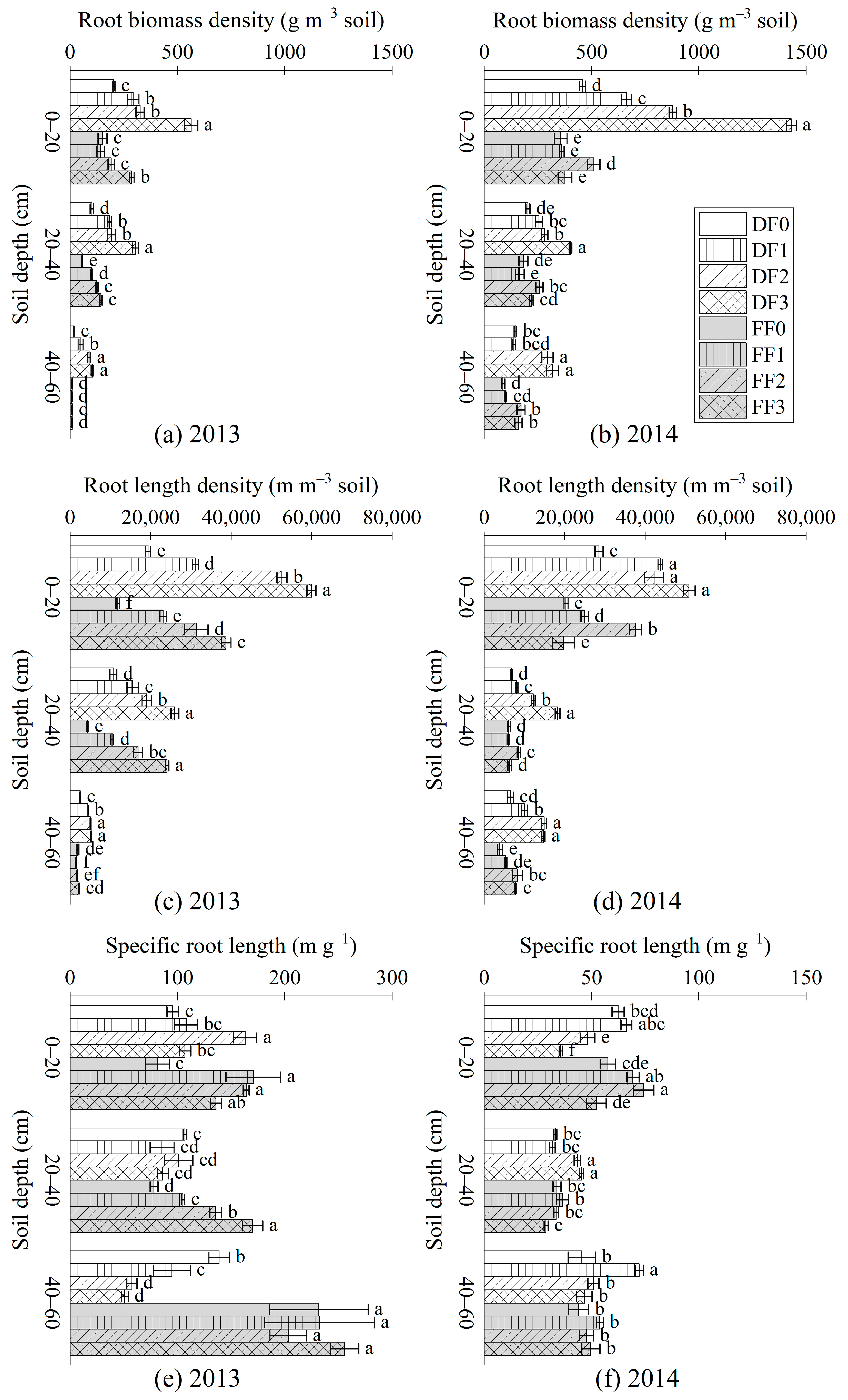
3.1. FRBD, FRLD and SRL
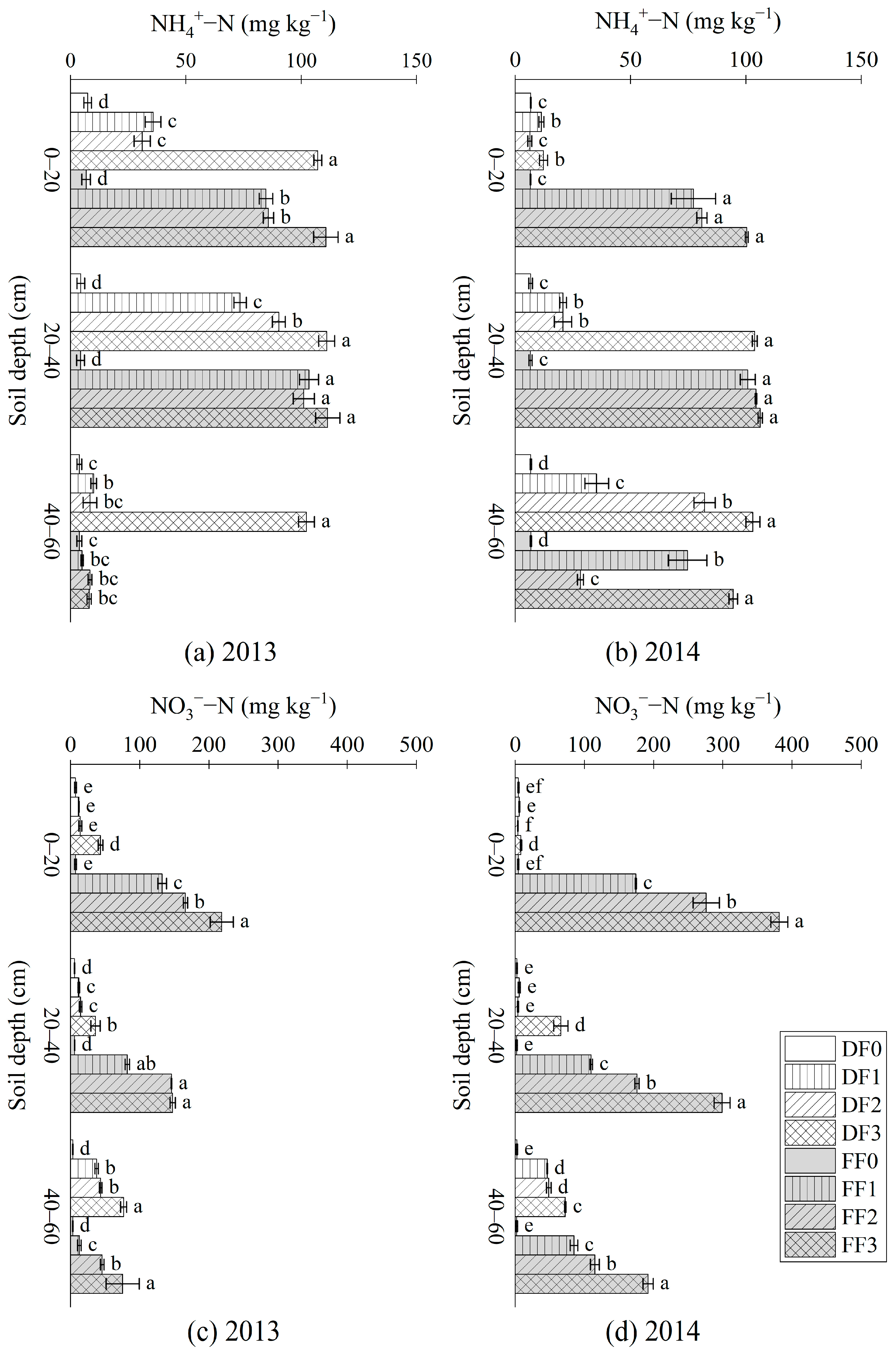
3.2. NH4+-N, NO3−-N, SIN and STN
3.3. Relationships between Fine Root Traits and Soil N
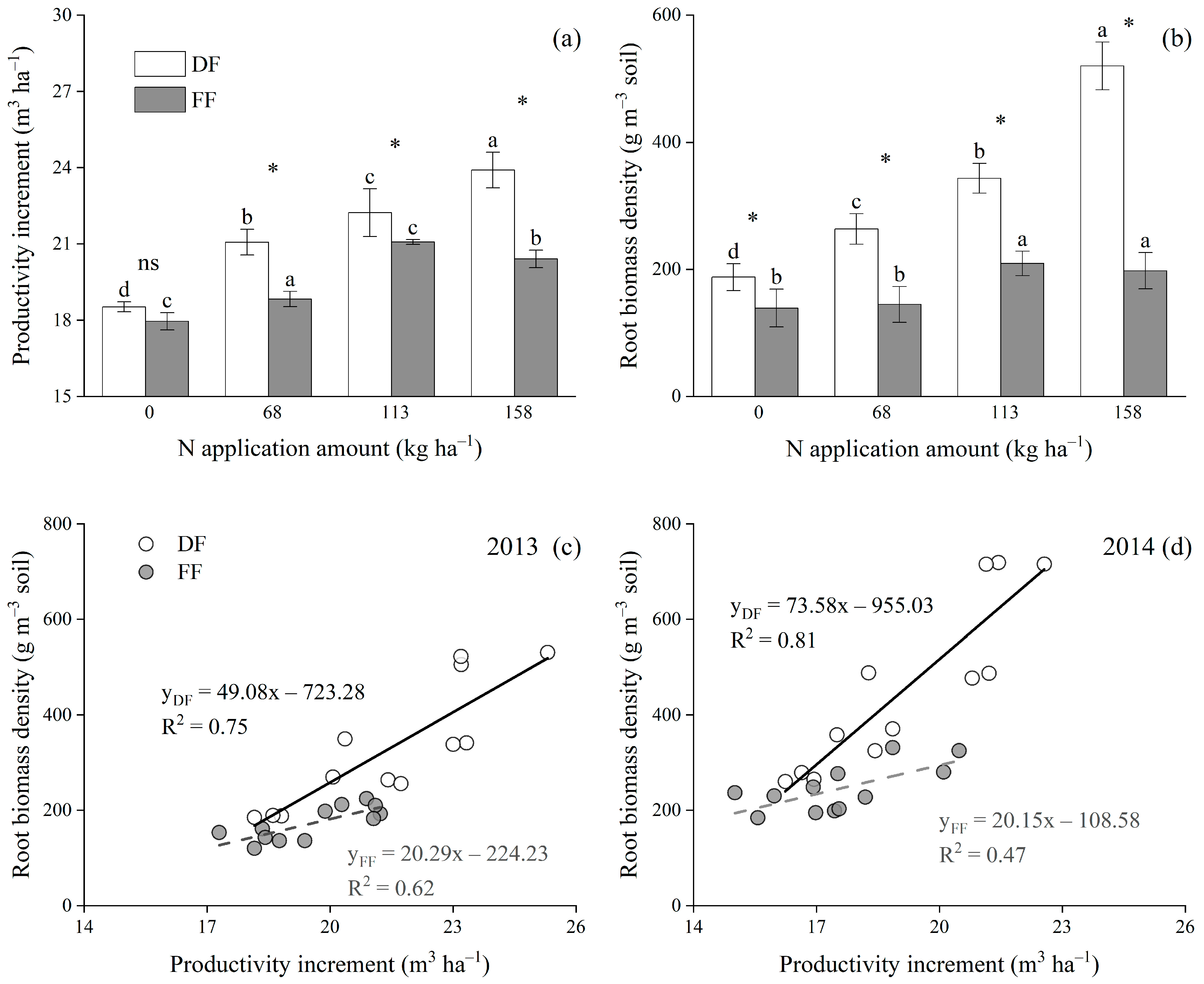
3.4. Relationships among N Application Amount, FRBD and Productivity Increment under Two Fertilization Methods
4. Discussion
4.1. Influence of Fertilization Methods and Application Rates on Fine Root Traits
4.2. Influence of Fertilization Methods and Application Rates on Soil N
4.3. Relationships among Soil N, Fine Root Traits and Productivity Increment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dickmann, D.I.; Nguyen, P.V.; Pregitzer, K.S. Effects of irrigation and coppicing on above-ground growth, physiology, and fine-root dynamics of two field-grown hybrid poplar clones. For. Ecol. Manag. 1996, 80, 163–174. [Google Scholar] [CrossRef]
- Wang, N.N.; Huang, J.; Ding, C.J.; Zhang, H.; Shen, Y.B.; Su, X.H. The relationship of shade tolerance of poplar and biomass production under different plantation density. For. Res. 2015, 28, 691–700. [Google Scholar]
- Yan, X.L.; Dai, T.F.; Jia, L.M. Evaluation of the cumulative effect of drip irrigation and fertigation on productivity in a poplar plantation. Ann. For. Sci. 2018, 75, 5. [Google Scholar] [CrossRef]
- Wang, Y.; Xi, B.Y.; Bloomberg, M.; Moltchanova, E.; Li, G.D.; Jia, L.M. Response of diameter growth, biomass allocation and n uptake to n fertigation in a triploid populus tomentosa plantation in the North China Plain: Ontogenetic shift does not exclude plasticity. Eur. J. For. Res. 2015, 134, 889–898. [Google Scholar] [CrossRef]
- Rennenberg, H.; Wildhagen, H.; Ehlting, B. Nitrogen nutrition of poplar trees. Plant Biol. 2010, 12, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.K. High Yield Cultivation of Poplar; Golden Shield: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Xi, B.Y.; Wang, Y.; Jia, L.M.; Bloomberg, M.; Li, G.D.; Di, N. Characteristics of fine root system and water uptake in a triploid Populus tomentosa plantation in the North China Plain: Implications for irrigation water management. Agric. Water Manag. 2013, 117, 83–92. [Google Scholar] [CrossRef]
- Matsuo, N.; Mochizuki, T. Assessment of three water-saving cultivations and different growth responses among six rice cultivars. Plant Prod. Sci. 2009, 12, 514–525. [Google Scholar] [CrossRef]
- Tarkalson, D.D.; Van Donk, S.J.; Petersen, J.L. Effect of nitrogen application timing on corn production using subsurface drip irrigation. Soil Sci. 2009, 174, 174–179. [Google Scholar] [CrossRef]
- Dai, T.F.; Xi, B.Y.; Yan, X.L.; Jia, L.M. Effects of fertilization method and nitrogen application rate on soil nitrogen vertical migration in a Populus × euramericana cv. ‘Guariento’ plantation. Chin. J. Appl. Ecol. 2015, 26, 1641–1648, (In Chinese with English Abstract). [Google Scholar]
- Bhat, R.; Sujatha, S. Cost-benefit analysis of ferti-drip irrigation in arecanut (Areca catechu L.). J. Plant. Crops 2006, 34, 263. [Google Scholar]
- Bhat, R.; Sujatha, S. Soil fertility and nutrient uptake by arecanut (Areca catechu L.) as affected by level and frequency of fertigation in a laterite soil. Agr. Water Manag. 2009, 96, 445–456. [Google Scholar] [CrossRef]
- Valin, M.I.; Cameira, M.R.; Teodoro, P.R.; Pereira, L.S. Depivot: A model for center-pivot design and evaluation. Comput. Electron. Agric. 2012, 87, 159–170. [Google Scholar] [CrossRef]
- O’Neill, M.K.; Allen, S.C.; Heyduck, R.F.; Lombard, K.A.; Smeal, D.; Arnold, R.N. Hybrid poplar (Populus spp.) adaptation to a semi-arid region: Results from northwest new mexico (2002–2011). Agrofor. Syst. 2014, 88, 387–396. [Google Scholar] [CrossRef]
- Liang, X.S.; Gao, Y.A.; Zhang, X.Y.; Tian, Y.Q.; Zhang, Z.X.; Gao, L.H. Effect of optimal daily fertigation on migration of water and salt in soil, root growth and fruit yield of cucumber (Cucumis sativus L.) in solar-greenhouse. PLoS ONE 2014, 9, e86975. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Brown, K.M. New roots for agriculture: Exploiting the root phenome. Philos. T. R. Soc. B 2012, 367, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Ostonen, I.; Puttsepp, U.; Biel, C.; Alberton, O.; Bakker, M.R.; Lohmus, K.; Majdi, H.; Metcalfe, D.; Olsthoorn, A.F.M.; Pronk, A.; et al. Specific root length as an indicator of environmental change. Plant Biosyst. 2007, 141, 426–442. [Google Scholar] [CrossRef]
- King, J.S.; Albaugh, T.J.; Allen, H.L.; Buford, M.; Strain, B.R.; Dougherty, P. Below-ground carbon input to soil is controlled by nutrient availability and fine root dynamics in loblolly pine. New Phytol. 2002, 154, 389–398. [Google Scholar] [CrossRef]
- Noguchi, K.; Nagakura, J.; Konopka, B.; Sakata, T.; Kaneko, S.; Takahashi, M. Fine-root dynamics in sugi (Cryptomeria japonica) under manipulated soil nitrogen conditions. Plant Soil 2013, 364, 159–169. [Google Scholar] [CrossRef]
- Artacho, P.; Bonomelli, C. Net primary productivity and allocation to fine-root production in field-grown sweet cherry trees under different soil nitrogen regimes. Sci. Hortic. 2017, 219, 207–215. [Google Scholar] [CrossRef]
- Phillips, D.L.; Johnson, M.G.; Tingey, D.T.; Storm, M.J.; Ball, J.T.; Johnson, D.W. CO2 and n-fertilization effects on fine-root length, production, and mortality: A 4-year ponderosa pine study. Oecologia 2006, 148, 517–525. [Google Scholar] [CrossRef]
- Jia, S.X.; Wang, Z.Q.; Li, X.P.; Sun, Y.; Zhang, X.P.; Liang, A.Z. N fertilization affects on soil respiration, microbial biomass and root respiration in Larix gmelinii and Fraxinus mandshurica plantations in China. Plant Soil 2010, 333, 325–336. [Google Scholar] [CrossRef]
- Xie, B.H.; Gu, J.X.; Yu, J.B.; Han, G.X.; Zheng, X.H.; Xu, Y.; Lin, H.T. Effects of n fertilizer application on soil N2O emissions and CH4 uptake: A two-year study in an apple orchard in eastern China. Atmosphere 2017, 8, 181. [Google Scholar] [CrossRef]
- Coleman, M. Spatial and temporal patterns of root distribution in developing stands of four woody crop species grown with drip irrigation and fertilization. Plant Soil 2007, 299, 195–213. [Google Scholar] [CrossRef]
- Xue, X.R.; Mai, W.X.; Zhao, Z.Y.; Zhang, K.; Tian, C.Y. Optimized nitrogen nitrogen fertilizer application enhances absorption of soil nitrogen and yield of castor with drip irrigation under mulch film. Ind. Crop. Prod. 2017, 95, 156–162. [Google Scholar] [CrossRef]
- Jia, L.M.; Liu, S.Q.; Zhu, L.H.; Hu, J.J.; Wang, X.P. Carbon storage and density of poplars in China. J. Nanjing For. Univ. 2013, 37, 1–7, (In Chinese with English Abstract). [Google Scholar]
- He, Y.; Lan, Z.P.; Sun, S.W.; Fu, J.P.; Liu, J.Q. Study on n, p, and k uptake and fertilization of young ‘107’ poplar clone with drip irrigation. For. Res. 2015, 25, 426–430, (In Chinese with English Abstract). [Google Scholar]
- Xi, B.; Wang, Y.; Jia, L. Effects of nitrogen application rate and frequency on biomass accumulation and nitrogen uptake of Populus tomentosa under drip fertigation. Sci. Silvae Sin. 2017, 53, 63–73, (In Chinese with English Abstract). [Google Scholar]
- Fang, C.H.; Zhou, K.Y.; Zhang, Y.W.; Li, B.Z.; Han, M.Y. Effect of root pruning and nitrogen fertilization on growth of young ‘fuji’ apple (Malus domestica borkh.) trees. J. Plant Nutr. 2017, 40, 1538–1546. [Google Scholar] [CrossRef]
- Yan, X.; Dai, T.; Xing, C.; Jia, L.; Zhang, L. Coupling effect of water and nitrogen on the morphology and distribution of fine root in surface soil layer of young Populus × euramericana plantation. Acta Ecol. Sin. 2015, 35, 3692–3701, (In Chinese with English Abstract). [Google Scholar]
- Chen, J.; Xiao, G.L.; Kuzyakov, Y.K.; Jenerette, G.D.; Ma, Y.; Liu, W.; Wang, Z.F.; Shen, W.J. Soil nitrogen transformation responses to seasonal precipitation changes are regulated by changes in functional microbial abundance in a subtropical forest. Biogeosciences 2017, 14, 2513–2525. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agro-Chemistrical Analysis; China Agriculture Press: Beijing, China, 2007. [Google Scholar]
- Shen, Y.F.; Wang, N.; Cheng, R.M.; Xiao, W.F.; Yang, S.; Guo, Y.; Lei, L.; Zeng, L.X.; Wang, X.R. Characteristics of fine roots of Pinus massoniana in the three gorges reservoir area, China. Forests 2017, 8, 183. [Google Scholar] [CrossRef]
- Bo, H.; Wen, C.; Song, L.; Yue, Y.; Nie, L. Fine-root responses of Populus tomentosa forests to stand density. Forests 2018, 9, 562. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. Mapping the global distribution of deep roots in relation to climate and soil characteristics. Geoderma 2005, 126, 129–140. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ke, Z.Q.; Shi, Q.I.; Yan, Z.; Mei, W.D. Root system distribution characteristics of plants on the terrace banks and their impact on soil moisture. Acta Ecol. Sin. 2005, 25, 500–506, (In Chinese with English Abstract). [Google Scholar]
- Singh, B.; Tripathi, K.P.; Jain, R.K.; Behl, H.M. Fine root biomass and tree species effects on potential n mineralization in afforested sodic soils. Plant Soil 2000, 219, 81–89. [Google Scholar] [CrossRef]
- Chang, R.Y.; Fu, B.J.; Liu, G.H.; Yao, X.L.; Wang, S. Effects of soil physicochemical properties and stand age on fine root biomass and vertical distribution of plantation forests in the loess plateau of China. Ecol. Res. 2012, 27, 827–836. [Google Scholar] [CrossRef]
- Yan, H.; Su, Y.Q.; Zhu, Y.Y.; Zhang, J.Q. Distribution characters of fine root of poplar plantation and its relation to properties of soil in the northern slope of Qinling Mountain. J. Nanjing For. Univ. 2009, 33, 85–89, (In Chinese with English Abstract). [Google Scholar]
- Verma, K.S.; Kohli, S.; Kaushal, R.; Chaturvedi, O.P. Root structure, distribution and biomass in five multipurpose tree species of Western Himalayas. J. Mt. Sci. Engl. 2014, 11, 519–525. [Google Scholar] [CrossRef]
- Chen, L.L.; Mu, X.M.; Yuan, Z.Y.; Deng, Q.; Chen, Y.L.; Yuan, L.Y.; Ryan, L.T.; Kallenbach, R.L. Soil nutrients and water affect the age-related fine root biomass but not production in two plantation forests on the loess plateau, China. J. Arid Environ. 2016, 135, 173–180. [Google Scholar] [CrossRef]
- Yan, X.L.; Dai, T.F.; Jia, L.M.; Dai, L.L.; Xin, F.M. Responses of the fine root morphology and vertical distribution of Populus × euramericana ‘guariento’ to the coupled effect of water and nitrogen. Chin. J. Plant Ecol. 2015, 39, 825–837, (In Chinese with English Abstract). [Google Scholar]
- Qi, D.; Hu, T.; Niu, X. Responses of root growth and distribution of maize to nitrogen application patterns under partial root-zone irrigation. Int. J. Plant Prod. 2017, 11, 209–224. [Google Scholar]
- Liu, X.J.A.; van Groenigen, K.J.; Dijkstra, P.; Hungate, B.A. Increased plant uptake of native soil nitrogen following fertilizer addition—Not a priming effect? Appl. Soil Ecol. 2017, 114, 105–110. [Google Scholar] [CrossRef]
- Block, R.M.A.; Rees, K.C.J.; Knight, J.D. A review of fine root dynamics in populus plantations. Agroforest Syst. 2006, 67, 73–84. [Google Scholar] [CrossRef]
- Price, J.S.; Hendrick, R.L. Fine root length production, mortality and standing root crop dynamics in an intensively managed sweetgum (Liquidambar styraciflua L.) coppice. Plant Soil 1998, 205, 193–201. [Google Scholar] [CrossRef]
- Rytter, R.M. The effect of limited availability of n or water on c allocation to fine roots and annual fine root turnover in Alnus incana and Salix viminalis. Tree Physiol. 2013, 33, 924–939. [Google Scholar] [CrossRef]
- Hu, J.L.; Lin, X.G.; Wang, J.H.; Dai, J.; Chen, R.R.; Zhang, J.B.; Wong, M.H. Microbial functional diversity, metabolic quotient, and invertase activity of a sandy loam soil as affected by long-term application of organic amendment and mineral fertilizer. J. Soil Sediment 2011, 11, 271–280. [Google Scholar] [CrossRef]
- Li, J.S.; Liu, Y.C. Water and nitrate distributions as affected by layered-textural soil and buried dripline depth under subsurface drip fertigation. Irrig. Sci. 2011, 29, 469–478. [Google Scholar] [CrossRef]
- Huang, J. Effects of bagging fertilization on plant growth and soil nutrient in cunninghamia lanceolata plantation. J. For. Environ. 2017, 37, 163–168, (In Chinese with English Abstract). [Google Scholar]
- Fan, Z.B.; Lin, S.; Zhang, X.M.; Jiang, Z.M.; Yang, K.C.; Jian, D.D.; Chen, Y.Z.; Li, J.L.; Chen, Q.; Wang, J.G. Conventional flooding irrigation causes an overuse of nitrogen fertilizer and low nitrogen use efficiency in intensively used solar greenhouse vegetable production. Agr. Water Manag. 2014, 144, 11–19. [Google Scholar] [CrossRef]
- Imada, S.; Taniguchi, T.; Acharya, K.; Yamanaka, N. Vertical distribution of fine roots of Tamarix ramosissima in an arid region of southern nevada. J. Arid Environ. 2013, 92, 46–52. [Google Scholar] [CrossRef]
- February, E.C.; Higgins, S.I. The distribution of tree and grass roots in savannas in relation to soil nitrogen and water. S. Afr. J. Bot. 2010, 76, 517–523. [Google Scholar] [CrossRef]
- Leuschner, C.; Hertel, D.; Schmid, I.; Koch, O.; Muhs, A.; Holscher, D. Stand fine root biomass and fine root morphology in old-growth beech forests as a function of precipitation and soil fertility. Plant Soil 2004, 258, 43–56. [Google Scholar] [CrossRef]
- Okada, K.; Aiba, S.; Kitayama, K. Influence of temperature and soil nitrogen and phosphorus availabilities on fine-root productivity in tropical rainforests on Mount Kinabalu, Borneo. Ecol. Res. 2017, 32, 145–156. [Google Scholar] [CrossRef]
- Hochmuth, G.; Hanlon, E. A Summary of N, P and K Research with Tomato in Florida; University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2020; Available online: http://edis.ifas.ufl.edu/cv236 (accessed on 8 December 2022).
- Yamashita, T.; Kasuya, N.; Nishimura, S.; Takeda, H. Comparison of two coniferous plantations in Central Japan with respect to forest productivity, growth phenology and soil nitrogen dynamics. For. Ecol. Manag. 2004, 200, 215–226. [Google Scholar] [CrossRef]
- Grissom, J.E.; Wu, R.; McKeand, S.E.; O’Malley, D.M. Phenotypic plasticity of fine root growth increases plant productivity in pine seedlings. Ann. Occup. Environ. Med. 2004, 4, 14. [Google Scholar]

| Depth cm | Sand % | Silt % | Clay % | Soil Texture (USDA Classification) | Bulk Density g·cm−3 | Organic Matter g·kg−1 | Total N g·kg−1 | NH4+-N mg·kg−1 | NO3−-N mg·kg−1 | Available P mg·kg−1 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0–20 | 69.96 ± 0.62 | 29.52 ± 0.64 | 0.52 ± 0.02 | Sandy loam | 1.68 ± 0.02 | 10.73 ± 0.20 | 0.58 ± 0.05 | 5.12 ± 0.05 | 3.88 ± 0.43 | 4.91 ± 0.97 |
| 20–40 | 67.19 ± 0.79 | 32.28 ± 0.77 | 0.53 ± 0.02 | Sandy loam | 1.64 ± 0.01 | 6.65 ± 0.29 | 0.49 ± 0.09 | 5.34 ± 0.44 | 2.56 ± 0.30 | 4.74 ± 0.69 |
| 40–60 | 63.52 ± 0.98 | 35.92 ± 0.96 | 0.56 ± 0.02 | Sandy loam | 1.62 ± 0.01 | 5.78 ± 0.23 | 0.44 ± 0.01 | 4.22 ± 0.39 | 6.63 ± 0.32 | 5.02 ± 0.68 |
| Treatment | Spring Irrigation (m3·ha−1·yr−1) | Irrigation Amount (m3·ha−1·yr−1) | Fertilizer Rate (kg N·ha−1·yr−1) | Fertilization Frequency (times yr−1) |
|---|---|---|---|---|
| DF1 | 3800 | 1395 | 68 | 6 |
| DF2 | 3800 | 1485 | 113 | 6 |
| DF3 | 3800 | 1580 | 158 | 6 |
| DF0 | 3800 | 1485 | 0 | 0 |
| FF1 | 3800 | 0 | 68 | 6 |
| FF2 | 3800 | 0 | 113 | 6 |
| FF3 | 3800 | 0 | 158 | 6 |
| FF0 | 3800 | 0 | 0 | 0 |
| Source of Variation | df | FRBD | FRLD | SRL |
|---|---|---|---|---|
| Year of experiment (Y) | 1 | <0.001 | <0.001 | <0.001 |
| Fertilization method (M) | 1 | <0.001 | <0.001 | <0.001 |
| Fertilizer amount (A) | 4 | <0.001 | <0.001 | 0.013 |
| Soil layers (S) | 2 | <0.001 | <0.001 | <0.001 |
| Y × M | 1 | 0.435 | 0.064 | <0.001 |
| Y × A | 1 | 0.135 | 0.545 | 0.719 |
| Y × S | 2 | <0.001 | <0.001 | 0.002 |
| M × S | 2 | <0.001 | <0.001 | <0.001 |
| A × S | 8 | <0.001 | <0.001 | 0.472 |
| M × A | 3 | <0.001 | <0.001 | 0.707 |
| Y × M × S | 2 | 0.012 | <0.001 | <0.001 |
| Y × M × A | 1 | 0.298 | 0.130 | 0.370 |
| M × S × A | 6 | <0.001 | <0.001 | 0.946 |
| Y × S × A | 2 | 0.180 | 0.025 | 0.419 |
| Y × M × S × A | 2 | 0.203 | 0.004 | 0.824 |
| Source of Variation | df | NH4+-N | NO3−-N | SIN | STN |
|---|---|---|---|---|---|
| Year of experiment (Y) | 1 | <0.001 | 0.105 | <0.001 | 0.090 |
| Fertilization method (M) | 1 | <0.001 | <0.001 | <0.001 | <0.001 |
| Fertilizer amount (A) | 4 | <0.001 | <0.001 | <0.001 | <0.001 |
| Soil layers (S) | 2 | <0.001 | <0.001 | <0.001 | <0.001 |
| Y × M | 1 | <0.001 | <0.001 | <0.001 | 0.002 |
| Y × A | 1 | <0.001 | 0.069 | <0.001 | 0.289 |
| Y × S | 2 | <0.001 | <0.001 | <0.001 | <0.001 |
| M × S | 2 | <0.001 | <0.001 | <0.001 | <0.001 |
| A × S | 8 | <0.001 | <0.001 | <0.001 | <0.001 |
| M × A | 3 | <0.001 | <0.001 | <0.001 | <0.001 |
| Y × M × S | 2 | <0.001 | <0.001 | 0.626 | <0.001 |
| Y × M × A | 1 | <0.001 | <0.001 | <0.001 | <0.001 |
| M × S × A | 6 | <0.001 | <0.001 | <0.001 | <0.001 |
| Y × S × A | 2 | <0.001 | 0.202 | <0.001 | 0.264 |
| Y × M × S × A | 2 | <0.001 | 0.465 | 0.011 | 0.002 |
| Methods | Traits | NH4+-N | NO3−-N | SIN | STN |
|---|---|---|---|---|---|
| FRBD | 0.15 | −0.09 | 0.06 | 0.86 ** | |
| DF | FRLD | 0.03 | −0.19 | −0.07 | 0.82 ** |
| SRL | −0.59 ** | −0.55 ** | −0.61 ** | 0.06 | |
| FRBD | 0.50 ** | 0.66 ** | 0.63 ** | 0.88 ** | |
| FF | FRLD | 0.55 ** | 0.74 ** | 0.71 ** | 0.93 ** |
| SRL | −0.37 * | −0.24 | −0.29 | −0.47 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Dai, T.; Gao, Y.; Di, N.; Jia, L. Responses of Fine Root Traits and Soil Nitrogen to Fertilization Methods and Nitrogen Application Amounts in a Poplar Plantation. Forests 2023, 14, 282. https://doi.org/10.3390/f14020282
Yan X, Dai T, Gao Y, Di N, Jia L. Responses of Fine Root Traits and Soil Nitrogen to Fertilization Methods and Nitrogen Application Amounts in a Poplar Plantation. Forests. 2023; 14(2):282. https://doi.org/10.3390/f14020282
Chicago/Turabian StyleYan, Xiaoli, Tengfei Dai, Yuan Gao, Nan Di, and Liming Jia. 2023. "Responses of Fine Root Traits and Soil Nitrogen to Fertilization Methods and Nitrogen Application Amounts in a Poplar Plantation" Forests 14, no. 2: 282. https://doi.org/10.3390/f14020282
APA StyleYan, X., Dai, T., Gao, Y., Di, N., & Jia, L. (2023). Responses of Fine Root Traits and Soil Nitrogen to Fertilization Methods and Nitrogen Application Amounts in a Poplar Plantation. Forests, 14(2), 282. https://doi.org/10.3390/f14020282






