Interspecies Association and Community Stability of Plants in the Core Distribution Area of Thuja sutchuenensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Location Overview of Study Area
2.2. Plots Setting and Data Collection
2.3. Data Analysis
2.3.1. Interspecific Association
2.3.2. Community Stability Index
2.4. Statistics and Analysis
3. Results
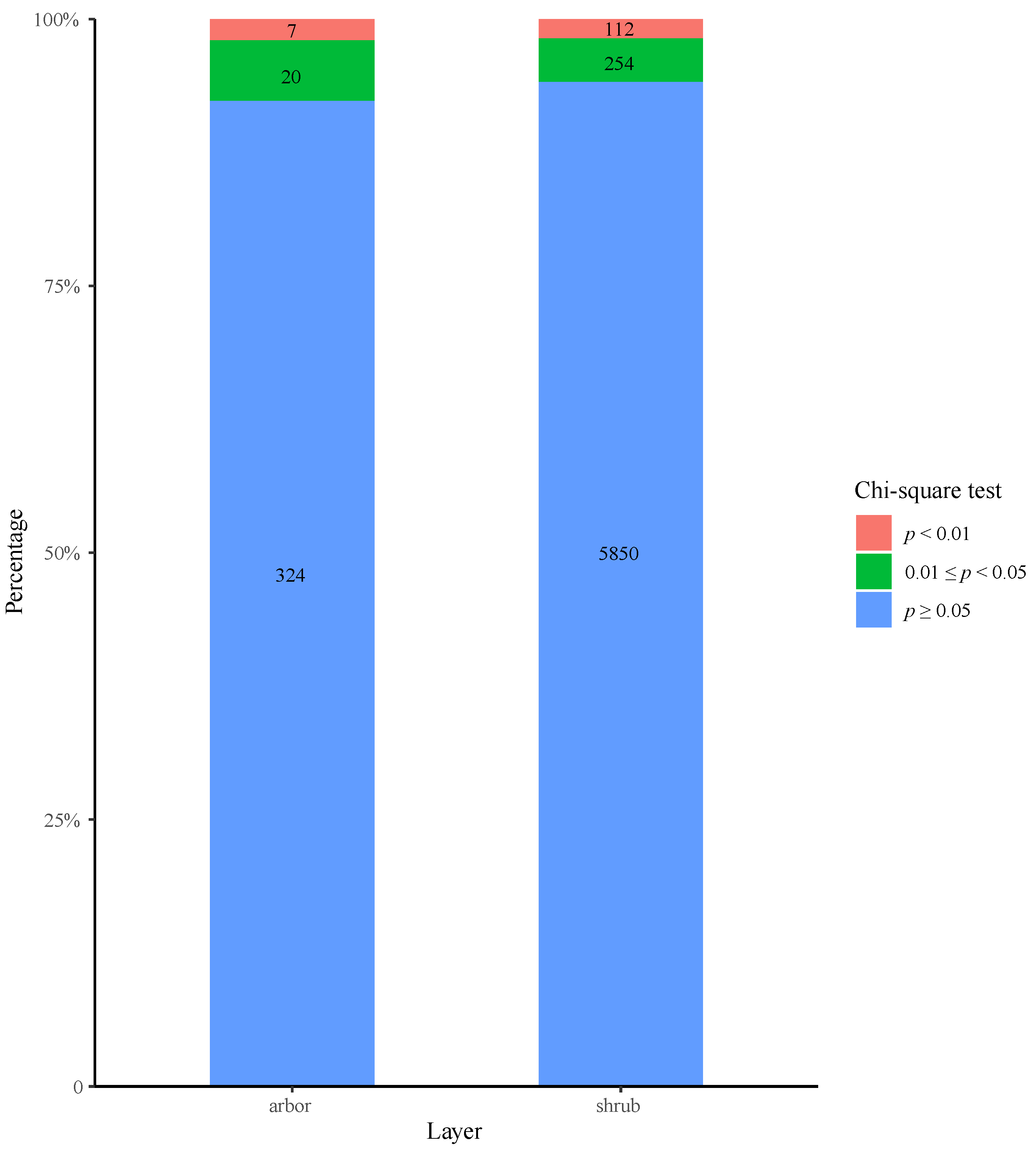
3.1. Overall Interspecies Association Analysis
3.2. Interspecific Association Analysis
3.3. Interspecies Association Coefficient Analysis
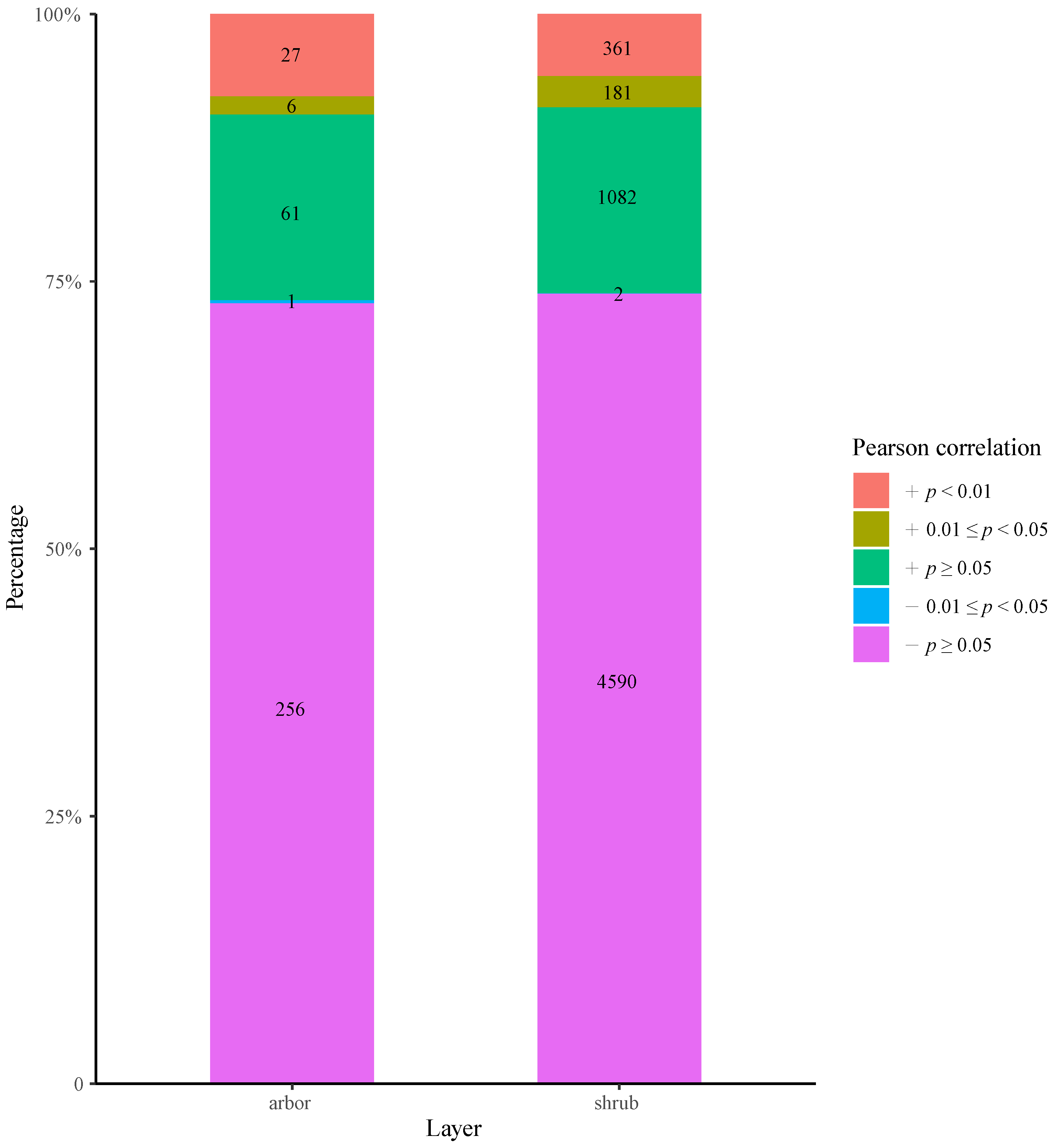
3.4. Interspecific Correlation Analysis
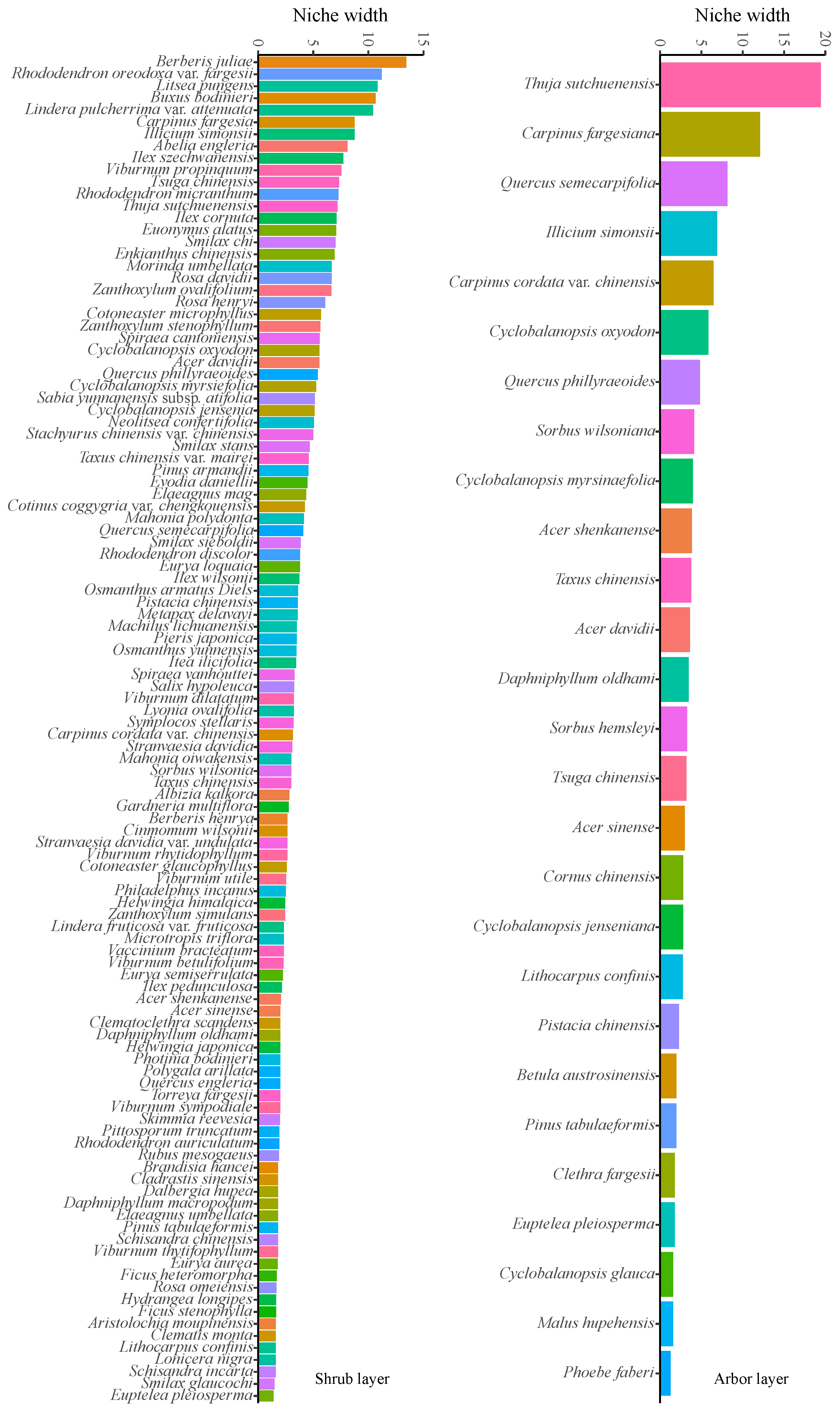
3.5. Niche Width Analysis
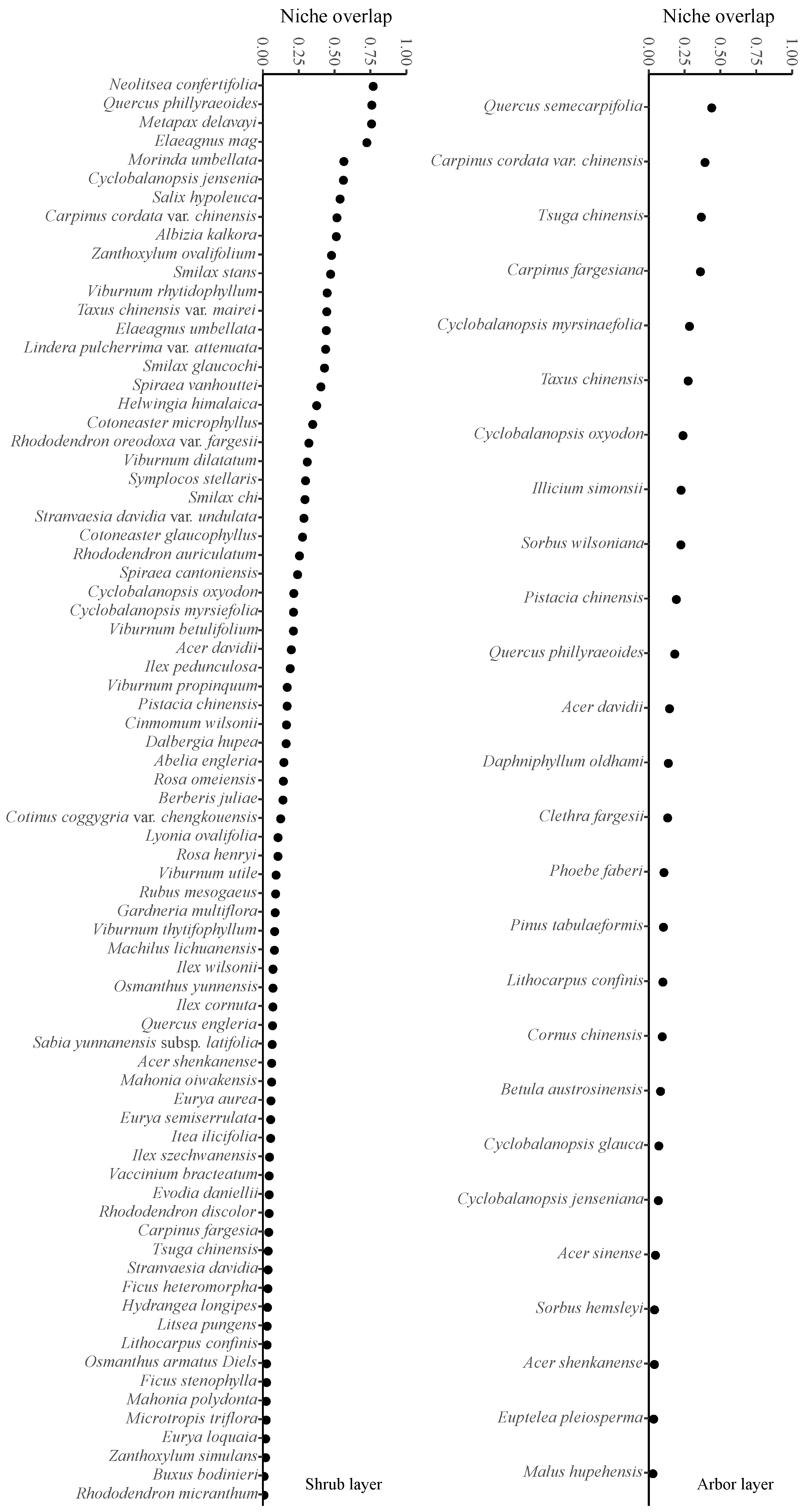
3.6. Niche Overlap Analysis
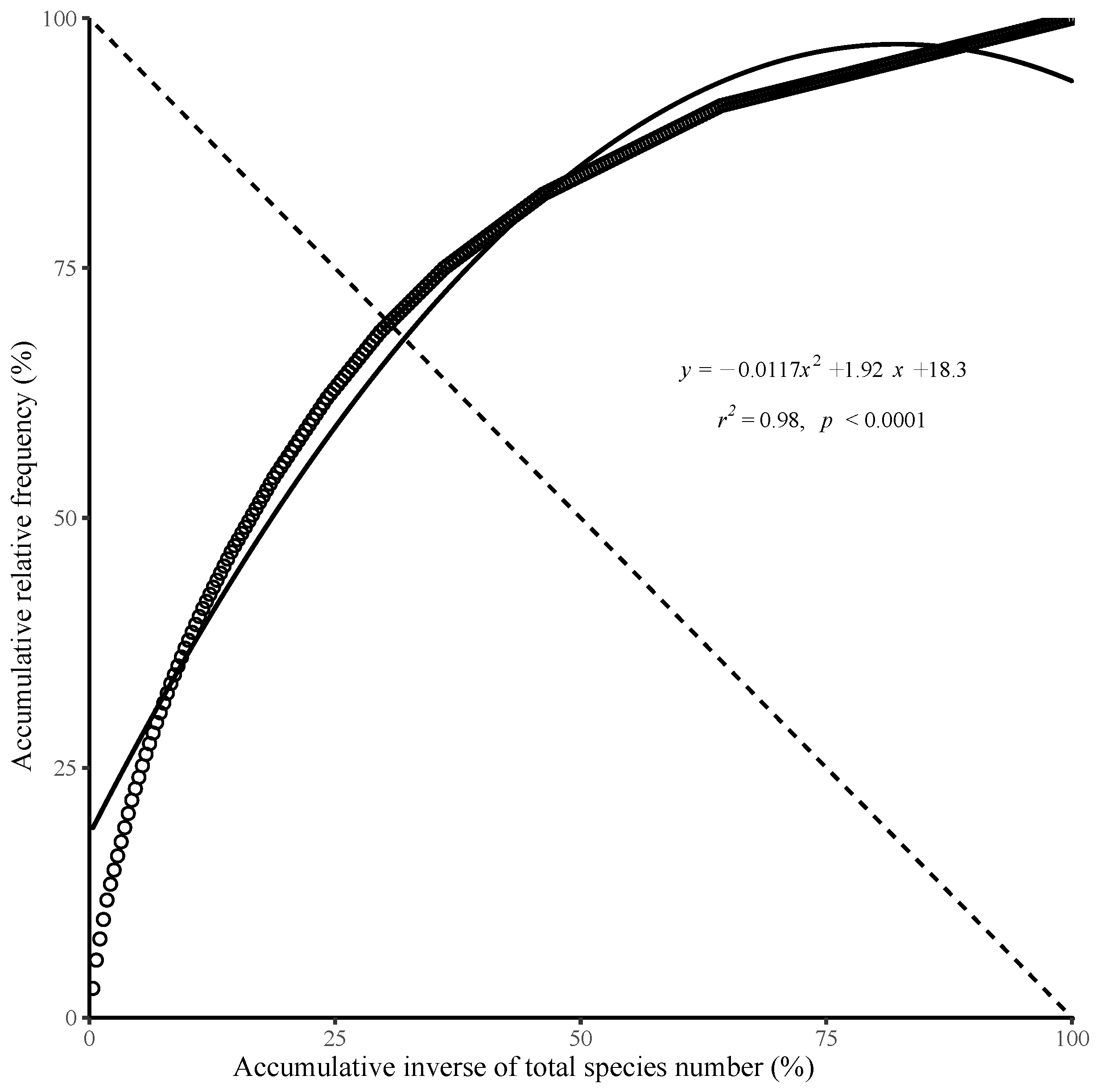
3.7. Community Stability Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; Zhao, C.; Deng, S.; Shen, G.; Xie, Z.; Xiong, G.; Li, J. Community structure and dynamics of a remnant forest dominated by Thuja sutchuenensis after deforestation. Biodivers. Sci. 2020, 28, 333. [Google Scholar]
- Qin, A.; Ding, Y.; Jian, Z.; Ma, F.; Worth, J.R.; Pei, S.; Xu, G.; Guo, Q.; Shi, Z. Low genetic diversity and population differentiation in Thuja sutchuenensis Franch., an extremely endangered rediscovered conifer species in southwestern China. Glob. Ecol. Conserv. 2021, 25, e01430. [Google Scholar] [CrossRef]
- Yang, Y.; Li, N.; Christian, T.; Luscombe, D. Thuja sutchuenensis. The IUCN Red List of Threatened Species 2013: e. T32378A2816862. Available online: https://www.iucnredlist.org/species/32378/2816862 (accessed on 4 April 2023).
- Tang, C.Q.; Yang, Y.; Ohsawa, M.; Momohara, A.; Yi, S.R.; Robertson, K.; Song, K.; Zhang, S.Q.; He, L.Y. Community structure and survival of tertiary relict Thuja sutchuenensis (Cupressaceae) in the subtropical Daba Mountains, southwestern China. PLoS ONE 2015, 10, e0125307. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Guo, Q.; Han, S.; Zhu, L.; Liu, Y.; Chen, Y. In vitro propagation of ‘Lazarus’ species Thuja sutchenensis Franch. Propag. Ornam. Plants 2018, 18, 77–86. [Google Scholar]
- Li, S.; Lang, X.; Huang, X.; Liu, W.; Su, J.; Xu, C.; Li, Z.; Xu, F. Interspecific association of woody plant species and community stability in the Eleutharrhena macrocarpa habitat. Biodivers. Sci. 2020, 28, 350. [Google Scholar]
- Jin, S.S.; Zhang, Y.Y.; Zhou, M.L.; Dong, X.M.; Chang, C.H.; Wang, T.; Yan, D.F. Interspecific association and community stability of tree species in natural secondary forests at different altitude aradients in the southern Taihang Mountains. Forests 2022, 13, 373. [Google Scholar] [CrossRef]
- Sanjerehei, M.M.; Rundel, P.W. A comparison of methods for detecting association between plant species. Ecol. Inform. 2020, 55, 101034. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Hu, G.; Zhu, J.D.; Luo, D.H.; Ni, J. Spatial patterns and interspecific associations of dominant tree species in two old-growth karst forests, SW China. Ecol. Res. 2010, 25, 1151–1160. [Google Scholar] [CrossRef]
- Wiegand, T.; Huth, A.; Getzin, S.; Wang, X.; Hao, Z.; Gunatilleke, C.V.S.; Gunatilleke, I.A.U.N. Testing the independent species’ arrangement assertion made by theories of stochastic geometry of biodiversity. Proc. Biol. Sci. 2012, 279, 3312–3320. [Google Scholar] [CrossRef]
- He, D.; Biswas, S.R. Negative relationship between interspecies spatial association and trait dissimilarity. Oikos 2019, 128, 659–667. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, X.; Wang, K.; Yu, W.; Deng, P.; Dong, J.; Li, Y.; Cui, K.; Liu, Y. Chloroplast and nuclear genetic diversity explain the limited distribution of endangered and endemic Thuja sutchuenensis in China. Front. Genet. 2021, 12, 801229. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Liu, B.; Guo, Q.; Bussmann, R.W.; Ma, F.; Jian, Z.; Xu, G.; Pei, S. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Wang, X. Community Ecology of Thuja sutchuenensis. Master’s Thesis, Chinese Academy of Forestry, Beijing, China, 2008. [Google Scholar]
- Ma, F.; Guo, Q.; Qin, A.; Jian, Z.; Huang, J.; Wang, Z.; Yang, Q.; Zhang, S. Survival and Growth of Reintroduced Thuja sutchuenensis Seedlings in Relation to Environmental Factors. Sci. Silvae Sin. 2021, 57, 1–12. [Google Scholar]
- Liu, J.; Shi, S.; Chang, E.; Yang, W.; Jiang, Z. Genetic diversity of the critically endangered Thuja sutchuenensis revealed by ISSR markers and the implications for conservation. Int. J. Mol. Sci. 2013, 14, 14860–14871. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Huang, B.H.; Zhang, Y.; Liao, P.C.; Li, J.Q. Chloroplast genome of an extremely endangered conifer Thuja sutchuenensis Franch.: Gene organization, comparative and phylogenetic analysis. Physiol. Mol. Biol. Plants 2020, 26, 409–418. [Google Scholar] [CrossRef]
- Zuo, Y.W.; Zhang, J.H.; Ning, D.H.; Zeng, Y.L.; Li, W.Q.; Xia, C.Y.; Zhang, H.; Deng, H.P. Comparative analyses of rhizosphere bacteria along an elevational gradient of Thuja sutchuenensis. Front. Microbiol. 2022, 13, 881921. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.W.; He, P.; Zhang, J.H.; Li, W.Q.; Ning, D.H.; Zeng, Y.L.; Yang, Y.; Xia, C.Y.; Zhang, H.; Deng, H.P. Contrasting responses of multispatial soil fungal communities of Thuja sutchuenensis Franch., an Extremely Endangered Conifer in Southwestern China. Microbiol. Spectr. 2022, 10, e0026022. [Google Scholar] [CrossRef]
- Wang, M.; Huo, L.; Liu, H.; Zhao, L.; Xu, Z.; Tan, H.; Qiu, S.X. Thujasutchins N and O, two new compounds from the stems and roots of Thuja sutchuenensis. Nat. Prod. Res. 2022, 36, 2356–2362. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, X.; Bahaer, G.; Wan, X. Interspecific relationships of dominant tree species in Thuja sutchuenensis community. Chin. J. Ecol. 2007, 26, 1911–1917. [Google Scholar]
- Liu, L.; Wang, X.; Wen, Q.; Jia, Q.; Liu, Q. Interspecific associations of plant populations in rare earth mining wasteland in southern China. Int. Biodeterior. Biodegrad. 2017, 118, 82–88. [Google Scholar] [CrossRef]
- Schluter, D. A variance test for detecting species associations, with some example applications. Ecology 1984, 65, 998–1005. [Google Scholar] [CrossRef]
- Yates, F. Contingency tables involving small numbers and the χ2 test. Suppl. J. R. Stat. Soc. 1934, 1, 217–235. [Google Scholar] [CrossRef]
- Cole, L.C. The measurement of interspecific associaton. Ecology 1949, 4, 411–424. [Google Scholar] [CrossRef]
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations; Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Regmi, S.; Neupane, B.; Dhami, B.; Gautam, D.; Panthi, S.; Poudel, M. Niche breadth and overlap of spotted deer and domestic cattle with swamp deer in tropical region of Nepal. Ecol. Process. 2022, 11, 22. [Google Scholar] [CrossRef]
- Godron, M.; Daget, P.; Poissonet, P. Some aspects of heterogeneity in grasslands of Cantal. Stat. Ecol. 1971, 3, 397–415. [Google Scholar]
- Wu, S.; Wen, L.; Dong, S.; Gao, X.; Xu, Y.; Li, S.; Dong, Q.; Wessell, K. The plant interspecific association in the revegetated alpine grasslands determines the productivity stability of plant community across restoration time on Qinghai-Tibetan Plateau. Front. Plant Sci. 2022, 13, 850854. [Google Scholar] [CrossRef]
- Zhang, M.t.; Kang, X.g.; Meng, J.h.; Zhang, L.x. Distribution patterns and associations of dominant tree species in a mixed coniferous-broadleaf forest in the Changbai Mountains. J. Mt. Sci. 2015, 12, 659–670. [Google Scholar] [CrossRef]
- Su, S.j.; Liu, J.f.; He, Z.s.; Zheng, S.q.; Hong, W.; Xu, D.w. Ecological species groups and interspecific association of dominant tree species in Daiyun Mountain National Nature Reserve. J. Mt. Sci. 2015, 12, 637–646. [Google Scholar] [CrossRef]
- Jian, M.; Liu, Q.; Zhu, D.; You, H. Inter-specific correlations among dominant populations of tree layer species in evergreen broad-leaved forest in Jiulianshan Mountain of subtropical China. J. Plant Ecol. 2009, 33, 672–680. (In Chinese) [Google Scholar]
- McCann, K.S. The diversity–stability debate. Nature 2000, 405, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Greig-Smith, P. Quantitative Plant Ecology, 3rd ed.; Blackwell Scientific Publications: Oxford, UK, 1983. [Google Scholar]
- Kershaw, K.A.; Looney, J.H.H. Quantitative and Dynamic Plant Ecology, 3rd ed.; Edward Arnold Limited: London, UK, 1985. [Google Scholar]
- Zhou, X.Y.; Wang, B.S.; Li, M.G.; Zan, Q.J. An analysis of interspecific associations in secondary succession forest communities in Heishiding Natural Reserve, Guangdong Province. Chin. J. Plant Ecol. 2000, 24, 332. [Google Scholar]
- Li, Y. Discussing on the Ecological Species Groups and Functional Groups Division based on the interspecific association—A case study on the arbor layer data in tropical lowland rain forest of Jianfenling, Hainan Island, China. Sci. Silvae Sin. 2007, 43, 9–16. [Google Scholar]
- Chen, Q.; Chen, J.; Zhong, J.J.; Ji, L.T.; Kang, B. Interspecific association and functional group classification of the dominant populations in shrub layer in secondary forest of Pinus tabuliformis in Qinling Mountain, China. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2018, 29, 1736–1744. [Google Scholar]
- Leimu, R.; Fischer, M. A meta-analysis of local adaptation in plants. PLoS ONE 2008, 3, e4010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.J.; Qing, H.; Zhao, L.Q.; Zhang, L.; Yang, J.; Huang, J.H. Spatial pattern and association of shrub species in gravel hilly and rocky low mountain desert dominated by relict Helianthemum songaricum in China. Glob. Ecol. Conserv. 2021, 32, e01914. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, G.; Sun, J.; Fornara, D.; Zhang, L.; Zhang, F.; Li, L. Temporal dynamics of nutrient uptake by neighbouring plant species: Evidence from intercropping. Funct. Ecol. 2017, 31, 469–479. [Google Scholar] [CrossRef]
- Silvertown, J.; Dodd, M.E.; Gowing, D.J.; Mountford, J.O. Hydrologically defined niches reveal a basis for species richness in plant communities. Nature 1999, 400, 61–63. [Google Scholar] [CrossRef]
- Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Gu, L.; Gong, Z.w.; Li, W.z. Niches and interspecific associations of dominant populations in three changed stages of natural secondary forests on Loess Plateau, P.R. China. Sci. Rep. 2017, 7, 6604. [Google Scholar] [CrossRef]
- Sun, C.; Li, L.; Dong, X.; Qin, F.; Yang, Z. Variations and factors characterizing ecological niches of understory herbaceous species in plantation forests. Sustainability 2022, 14, 10719. [Google Scholar] [CrossRef]
- Chu, C.; Kleinhesselink, A.R.; Havstad, K.M.; McClaran, M.P.; Peters, D.P.; Vermeire, L.T.; Wei, H.; Adler, P.B. Direct effects dominate responses to climate perturbations in grassland plant communities. Nat. Commun. 2016, 7, 11766. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.I.; Barabás, G.; Bimler, M.D.; Mayfield, M.M.; Miller, T.E. The evolution of niche overlap and competitive differences. Nat. Ecol. Evol. 2021, 5, 330–337. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, J.; Liu, Q.; Yang, W.; Wu, F.; Feng, M. Niche characteristics of main herbage populations in Eucalyptus grandis plantation. Acta Prataculturae Sin. 2010, 19, 16–21. [Google Scholar]
- Pulla, S.; Suresh, H.S.; Dattaraja, H.S.; Sukumar, R. Multidimensional tree niches in a tropical dry forest. Ecology 2017, 98, 1334–1348. [Google Scholar] [CrossRef]
- Guo, Q.; Qin, A.; Ma, F.; Jian, Z.; Pei, S. Research progress on Thuja sutchuenensis: A critically endangered species in the world. World For. Res. 2015, 28, 18–22. [Google Scholar]
- Jin, J.; Ren, F.; Xia, Y.; Liu, Z.; Chen, Y.; Zhang, J. Research on reproductive phenology, pollination, and embryonic development of Thuja sutchuenensis Franch., a plant species with extremely small populations. Plant Sci. J. 2020, 38, 696–706. [Google Scholar]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Liu, C.; Piao, S.; Sack, L.; Xu, L.; Luo, Y.; He, J.; Han, X.; Zhou, G.; Zhou, X. Ecosystem traits linking functional traits to macroecology. Trends Ecol. Evol. 2019, 34, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Yan, P.; He, N. How to improve the predictions of plant functional traits on ecosystem functioning? Front. Plant Sci. 2021, 12, 622260. [Google Scholar] [CrossRef]

| Plots | Location | Longitude | Latitude | Altitude (m) | Slope (°) | Aspect | Slope Position | Mean DBH (cm) | Mean H (m) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | TPM | 980 | 30 | Northwest | Lower | 7.16 | 5.34 | ||
| 2 | 1085 | 52 | West | Upper | 8.14 | 6.02 | |||
| 3 | 1580 | 49 | Northeast | Upper middle | 8.24 | 5.62 | |||
| 4 | 1802 | 42 | North | Middle | 8.59 | 7.80 | |||
| 5 | 1730 | 48 | Northwest | Middle | 7.82 | 6.48 | |||
| 6 | XBM | 2153 | 46 | North | Upper | 9.05 | 4.65 | ||
| 7 | 2120 | 25 | South | Upper middle | 11.68 | 6.34 | |||
| 8 | 2169 | 45 | West | Upper | 11.11 | 6.71 | |||
| 9 | 2169 | 45 | Northwest | Upper | 9.13 | 6.10 |
| Species B | Sum | |||
|---|---|---|---|---|
| Present | Absent | |||
| Species A | Present | a | b | a + b |
| Absent | c | d | c + d | |
| sum | a + c | b + d | N = a + b + c + d | |
| Layer | Variance Ratio (VR) | Test Statistic (W) | , | Result |
|---|---|---|---|---|
| Arbor | 0.90 | 32.29 | 23.27, 51.00 | Non-significant negative association |
| Shrub | 1.18 | 42.55 | 23.27, 51.00 | Non-significant positive association |
| Niche Overlap Index | Arbor | Shrub | ||
|---|---|---|---|---|
| Species Pairs Number | % | Species Pairs Number | % | |
| 0 | 0.00 | 2 | 0.03 | |
| < 0.98 | 2 | 0.57 | 54 | 0.87 |
| < 0.78 | 8 | 2.28 | 124 | 1.99 |
| < 0.59 | 19 | 5.41 | 319 | 5.13 |
| < 0.40 | 41 | 11.68 | 597 | 9.60 |
| 281 | 80.06 | 5120 | 82.37 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, Y.; Li, Y.; Jin, J.; Guo, Q.; Pei, S. Interspecies Association and Community Stability of Plants in the Core Distribution Area of Thuja sutchuenensis. Forests 2023, 14, 762. https://doi.org/10.3390/f14040762
Wang X, Liu Y, Li Y, Jin J, Guo Q, Pei S. Interspecies Association and Community Stability of Plants in the Core Distribution Area of Thuja sutchuenensis. Forests. 2023; 14(4):762. https://doi.org/10.3390/f14040762
Chicago/Turabian StyleWang, Xiangfu, Yong Liu, Yuanhui Li, Jiangqun Jin, Quanshui Guo, and Shunxiang Pei. 2023. "Interspecies Association and Community Stability of Plants in the Core Distribution Area of Thuja sutchuenensis" Forests 14, no. 4: 762. https://doi.org/10.3390/f14040762
APA StyleWang, X., Liu, Y., Li, Y., Jin, J., Guo, Q., & Pei, S. (2023). Interspecies Association and Community Stability of Plants in the Core Distribution Area of Thuja sutchuenensis. Forests, 14(4), 762. https://doi.org/10.3390/f14040762






