Abstract
Seedlings are an important stage for plant populations, as the abundance and rigor of seedlings can indicate a changing forest structure in the future. Studying the different traits of the seedling can represent how the plant grows. Biomass is one of the traits that can represent the plant’s performance and many other growth processes of the seedling. Several allometric equations have been developed to estimate tree biomass. However, allometric equations for the biomass of seedlings remains poorly studied, especially those from the tropics. The objective of this research is to create and develop a model that can be used to predict the biomass of seedlings, including total biomass, aboveground biomass, and belowground biomass, from root collar diameter, shoot height, main stem length, and wood density from 205 two-year-old seedlings from twenty tree species found in dry evergreen forest in Huai Kha Khaeng Wildlife Sanctuary, Uthai Thani, Thailand. The results showed that the root collar diameter, shoot height, and wood density could be used to create a model to best predict the seedling biomass. This model should be tested with other seedlings in the wild and other datasets to evaluate the performance of the model. To our knowledge, this study is among the first to provide the first allometry for seedlings in tropical dry evergreen forest. The results from this study will allow ecologists to monitor and examine the growth of the seedlings at all stages of life in dynamic tropical environments in the future.
1. Introduction
Today, the global climate is changing, and the continuous increase in atmospheric carbon dioxide affects the global temperature and the environment in many areas. Plants are very important organisms to help reduce the rate of change because plants effectively absorb and store carbon from the atmosphere [1]. With photosynthesis, plants use atmospheric carbon and light energy to create organic carbon to be stored in their biomasses. Different life stages of plants have different capacities and rates of storing carbon. Several environmental and plant factors determine the carbon sequestration capacity of each plant species, such as water efficiency, growth rate, carbon absorption, and release rate [2]. Therefore, the study of plant biomass is important for ecological management as the global environment changes. An important step in the study of biomass is to create equations that show the relationships between plant traits. This can be used as a tool to assess the carbon content in the form of biomass, such as roots stems, leaves, and branches [3].
Carbon from the air is fixed in the leaf area through photosynthesis. Plants’ ability to fix and store carbon differs depending on the environment and the species of the plant [4]. The study of plant biomass can be analyzed to show the amount of carbon that plants accumulate in different parts in order to grow and survive. Plants are already helping to reduce the carbon in the atmosphere by fixing the carbon into mass. However, plants also release carbon into the atmosphere, through the process of respiration [5]. When photosynthesis can produce more energy than the respiration process, the energy is converted to structural carbohydrates in the form of biomass [6]. However, a direct measurement of biomass requires destructive sampling, which means interfering with the population dynamics and the rate of carbon sequestrations. Other indirect methods for measuring biomasses have been developed, but one of the most regularly used and practical methods involves the use of allometric relationships [7,8,9,10,11,12,13,14,15].
The allometric relationship is a relationship between plant functional traits that can be described as indicative, or proportional to another trait. This is often calculated by statistical methods in the form of the equation for the relation of both traits (allometric equation) [2,16]. Many studies have looked for traits that are easier to measure or collect and used to describe or predict some traits that are more difficult to measure or collect [17]. This relationship is used in forest ecology studies. Much research about using modeling methods for estimating the biomass of the tree has been published [18], starting with the determination of the correlation model of biomass, such as diameter or height, and then creating an equation that can be used to find biomass. Using appropriate statistics to calculate the equation depends on the type of forest, groups, categories, or species of plant studied [11,19,20].
While many equations exist for tree biomass, studies on seedling biomass are relatively rare. The seedling is the first stage of a tree after germinating from seed before reaching a mature stage with a standing stem. Generally, seedlings refer to plants with a height from the root collar diameter to the terminal bud level of less than 1.30 m [6]. Seedlings are critical in determining the fate of the plant population structure, including its composition, plant size, age, distribution pattern, and density [21]. The impact of seedlings on the population is often measured in terms of abundance, occurrence, or amount [22,23,24], while the ability to grow and accumulate biomass during this stage has rarely been studied.
This research aims to develop an allometric equation to predict seeding biomass from the morphological traits, including root collar diameter, shoot height, main stem length, and wood density of seedlings. The morphological traits of plant 20 species (Table 1) found in the 50-hectare long-term forest dynamics plot located in Huai Kha Khaeng Wildlife Sanctuary, Uthai Thani Province [25] (Figure 1) were used to fit candidate models to predict total biomass, aboveground biomass, and belowground biomass, separately. The candidate models were compared for their performance and errors, allowing for the selection of the most optimal model for predicting biomass. The obtained allometric equation will allow measurements of seedling biomass without disturbing the seedlings and can be used to assess plant performance for forest restoration planning.

Table 1.
Sample size and range of seedling measurements from the current study *.
Figure 1.
Location of the 50-hectare long-term forest dynamics plot in Huai Kha Khaeng Wildlife Sanctuary, Uthai Thani Province, Thailand.
2. Materials and Methods
2.1. Study Area
Huai Kha Khaeng Wildlife Sanctuary (Coordinates: 15°00′–15°47′ N, 99°00′–99°27′ E) a part of the Western Forest Complex of Thailand, covers approximately 3800 km2 and is mostly in Uthai Thani Province. The average precipitation is 1357 mm/year, and the average annual temperature is 23.5 °C. The area is a valley with a river flowing through the middle, consisting of dry evergreen forest, mixed deciduous forest, deciduous dipterocarp forest, and hill evergreen forest [25].
2.2. Species Selection and Preparation of Seedlings
Seeds of the twenty common tree species were collected (Table 1) from at least three mother trees surrounding the 50-ha long-term forest dynamics plot (Figure 1). Species were selected to represent three groups of mature trees by the canopy layers: understory (less than 10 m tall), sub-canopy (10–30 m tall), and top canopy (more than 30 m tall). This strategy was to ensure that a wide range of species of different ecology was chosen for data collection [25]. The exploratory data analyses showed no significant difference in seedling biomass among the mature tree layer group.
All collected seeds were planted in the same planting material, which was a mixture of soil, black husk, and manure at the ratio of 6:3:1. Seedlings were grown under the same environment in the nurseries at Kasetsart University, Bangkok, Thailand in 2017–2019. The average temperature during the time of planting was 29 °C, and the average light intensity was 6379 lux. The seedlings were measured at the age of two years when they had a diameter at breast height of less than 1 cm.
2.3. Seedling Measurements
Shoot height (SH) was measured from a side-view photo of a seedling with the program Fiji [26], an extension of the ImageJ program (version 1.53q) [27]. SH was defined as the height from the root collar to the highest point of the seedling in its natural orientation. The root collar diameter (Di) measurements were taken twice, perpendicular to each other, with vernier calipers, and the average value of the two measurements was used as the diameter of the seedlings. The main stem length (SL) was measured from the root collar to the tip along the stem with a measuring tape. The SL sometimes exceeded the SH as the stem kept elongating but started to droop and did not go straight up further. The wood density (WD) was measured using the water displacement method [18]. A piece of 2 cm long stem at 1 cm above the root collar was cut from five seedlings for each species for the measurement. The wood density was calculated from the dry weight proportional to the volume of the wood, and the average of five values was used for each species (Table 1). The biomass of seedlings was investigated by weighing separate parts of the seedling, including leaves, stems, branches, and roots, after drying at 80 °C for 3–5 days, or until the specimens were completely dry. Dry weight was measured in three different partitions: total biomass (TBM, from the plant), aboveground biomass (AGB, from leaves, stem, and branches), and belowground biomass (BGB, from root).
2.4. Statistical Analyses
Raw data were first transformed to a logarithmic scale to adjust for their distribution. Linear mixed models were constructed, using biomasses as dependent variables, stem traits (SH, Di, SL, and WD) as effects, and species of the seedling as a random effect. Specification of species as a random effect allowed the better estimation of coefficients, less effect from a small sample size in each species, and the ability to apply to other species beyond this study. Candidate models were constructed based on the following equation for the cylinder volume multiplied by its density (in this case, wood density is WD):
where H is the height of the cylinder, Di is the diameter of the cylinder, WD is the density of the cylinder.
In this study, all of the candidate models set the root collar diameter as the main variable, as it was chosen to be a single variable in previous allometric studies (e.g., [18]). Additional independent variables (either SH or SL for H and WD) were also added to each of the candidate models. Two main approaches were used in constructing candidate models. First, each trait was specified as a separate predictor with its coefficient (β) to be estimated. Second, similar to the equation for the cylinder mass, the chosen stem traits were first multiplied and then fit into the model as a single predictor with one coefficient to be estimated. We also varied the use of the diameter as a regular term (Di) and a square term (Di2) to mimic the formula for cylinder mass. The four main types of candidate models were as followed:
where Yest is estimated biomass, Di is root collar diameter, X1 is either SL or SH, and WD is wood density.
ln(Yest) = α + β1ln(Di) + β2ln(X1) + β3ln(WD)
ln(Yest) = α + β1ln(Di2) + β2ln(X1) + β3ln(WD)
ln(Yest) = α + βln(Di·X1·WD)
ln(Yest) = α + βln(Di2·X1·WD)
A total of 22 candidate models were constructed for each type of partition of biomass including total, aboveground, and belowground (Table 2).

Table 2.
Model effectiveness results reported in the value of AIC, RMSE (root mean square error), and Avg%ER (average percent error) of all candidate models. The performances of models are reported separately for total, aboveground, and belowground biomasses. The models in bold are the best performing model for each type of biomass. Di = root collar diameter, SH = shoot height, SL = shoot length, and WD = wood density.
2.5. Models Comparison
The candidate models were compared using the Akaike information criterion (AIC), the root means square error (RMSE), and the relative error (%ER) to determine the effectiveness of the model. The AIC is a measure of the goodness-of-fit that penalizes parameter-rich models, as required by the principle of parsimony, using the following formula:
where L is the likelihood of the fitted model, and p is the number of parameters in the fitted model.
AIC = −2ln(L) + 2p
The model with a lower value of AIC indicates a better performance. RMSE and %ER directly compared the observed values with the predicted values from the model, using the following formulae:
where Yobs is the observed values, Yest is the predicted values, and n is the total number of observations.
All of the analyses were performed using R Version 4.1.2 [28] on RStudio version 2021.09.1 [29].
3. Results
3.1. Comparisons of Candidate Models
A total of 22 candidate models to predict the total, aboveground, and belowground biomass of seedlings were constructed using root collar diameter (Di), shoot height (SH), main stem length (SL), and wood density (WD). To compare the performances of the candidate models, the values of the Akaike information criterion (AIC), root mean square error (RMSE), and average relative error (Avg%ER) were calculated and reported for each model and each type of biomass (Table 2).
3.2. Model Selection
The best-fit models were the ones with the lowest AIC, RMSE, and %AvgER for each type of biomass. The results showed that the best model for each type of biomass was different. For total biomass (TBM) and aboveground biomass (AGB), the terms Di2, SH, and WD together were chosen to be the best predictor for modeling. However, for the belowground biomass (BGB), using Di, SH, and WD as separate terms yielded better predictions (Table 2).
The best-fit model in this study:
Total biomass (Figure 2a)
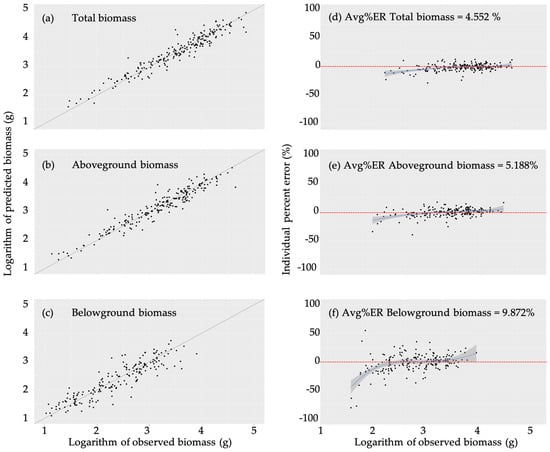
Figure 2.
Goodness-of-fit for the chosen models by plotting the predicted model against observed values of total (a), aboveground (b), and belowground (c) biomasses, where the black line represents the expectation of predicted values being equal to the observed values. Individual percent errors calculated using observed values of total (d) aboveground (e) and belowground (f) biomasses. The red dashed line represents the error at zero percent, while the solid blue line and the grey bar represent a spline (LOESS) regression of the data points and associated 95% confidence interval.
Aboveground biomass (Figure 2b)
Belowground biomass (Figure 2c)
The predicted values from the best models were plotted against the observed values and used to calculate the relative error for individual observation (Figure 2). The results showed the average errors ranged between 4.5% and 9.8%. The model for the belowground biomass had the highest error, as the model overestimated at the lower values and underestimated at the high values of biomasses (Figure 2f)
As wood density (WD) is not always available for seedlings, the following alternative models without wood density were also chosen (Table 2).
The alternative model:
Total biomass
Aboveground biomass
Belowground biomass
where TBMest is the estimated total biomass, AGBest is the estimated aboveground biomass, BGBest is the estimated belowground biomass, SH is a shoot height, Di is a root collar diameter, and WD is a wood density.
4. Discussion
4.1. Comparisons of Candidate Models and Best Fit Model
The best model to predicts biomass used three stem variables: Di, SH, and WD. These variables are effective at predicting seedling biomass, as they are directly proportional to the biomass accumulated in the seedling stems. The best models included these three variables as the composite variable (Di2·SH·WD) with one coefficient, similar to the equation for the cylinder mass, suggesting that the use of cylinder mass equation as a candidate model was reasonable for seedling biomass.
To obtain the best estimates of the seedling biomass, it is necessary to use the WD variable, which requires a destructive sampling of the seedlings. Therefore, alternative models with similar performances without WD were also proposed as alternative models. These models with only Di and SH gave slightly higher AIC values, but almost identical in their RMSE and Avg%ER values, when compared to the best model with all three variables (Table 2). These slight difference between the best and alternative models suggested that wood density can be omitted in a larger scale study without the resource to measure seedlings wood density. Moreover, our analysis from this study showed that few differences were detected among species. Hence, the use of species as a random effect was reasonable in our study.
4.2. Errors of Models
The models for total and aboveground biomasses performed relatively well, with an average error rate of 4.5–5.18 percent, compared to the belowground biomass which had an average error rate of 9.8 percent (Figure 2). Measuring belowground biomass in the natural environment is challenging, especially for seedlings [30]. In our study, it was further complicated by the limited size of the pots in which the seedlings were grown, potentially affecting its proportions to other stem traits. The error rates were more pronounced at the low and high ends of the biomass spectrum, potentially due to a lower sample size at these ranges and/or the stunted or accelerated growth of some seedlings in response to the growing conditions. However, for normal-sized seedlings, the estimated biomass values produced by our models should be relatively accurate.
4.3. Comparisons with Previously Published Equations
Most of the previous biomass equations were generated for aboveground biomass of trees, where only diameter at breast height (DBH) can be measured reliably [12,18]. When available, tree heights (SH) and wood density (WD) were also included in the biomass equations, similar to our study with seedlings. When we compared the resulting equations for aboveground biomass to the other studies, our equation was the most similar to the equations for tropical trees [11] and rubberwood trees in plantation [10]. In both equations, the exponents of diameter were around 2 (1.97–2.05) and those of height and wood density were approximately 0.45 to 0.63. Another study estimated the aboveground biomass in tropical lowland Dipterocarp forests using only the diameter and found the exponent for the diameter at 2.1 [12]. Much less study was conducted for the biomass of seedlings. In the study of Jatropha curcas seedlings under different levels of drought stress [31], only the diameter was used in the equation with the exponent of 2.23. Despite using different sets of variables, most of the exponents and coefficients of the previous equations are similar in magnitude to our equation for aboveground biomass. The differences in these constants can be attributed to variations in geographical scopes, studied species, and planting conditions (Table 3).

Table 3.
Compared allometric models for predicting biomass with other studies *.
4.4. Potentials for Future Studies and Application in Restoration
In the current study, wood density data were important for a more precise prediction. However, these data were collected at the species level. While it would be ideal to have wood density for individual seedlings for biomass predictions, a destructive sampling of all seedlings is not feasible. A study on intraspecific variation of wood density among the studied species could be conducted to determine whether using species-level data for wood density is suitable. A previous study on tropical montane tree species found that the intraspecific variation of wood density was relatively low and should be able to represent the species as a whole [32].
Increasing the number of small and large seedling samples can also improve the model performance, as the errors were the highest at the end of biomass spectrum (Figure 2). Including smaller and larger seedlings will require a larger set of species and/or different stages of seedling growths, which were not set up for the current study [18,20,33]. Moreover, the belowground biomass from the pot-grown seedlings might be underestimated due to the space limitation. Planting seedlings in a natural soil can potentially yield a more accurate measurement of the belowground biomass.
Other methods are also available for biomass estimation [34]. Our current statistical method had an advantage in its simplicity and ease for applying with the seedling measurement. The additive biomass equation has a particular advantage in enhancing consistency when adding up multiple components of biomass, such as stem, bark, and leaf [35]. In the case of seedlings, we did not experience problems with additivity, probably because the leaf and bark components were relatively minor compared to the stem. Therefore, we chose the traditional allometric approach for our seedling data.
Along with the growth rate and functional trait data, biomass allometric equations make it possible to choose the tree species that are appropriate for different stages for restoration. Choosing tree seedlings that are fit to different successional stages will allow higher survival rate and can restore the forest more successfully [36]. After planting, we can use the seedling sizes to estimate how much carbon the forests store at different ages for planning for sustainable restoration and/or timber harvesting in the future.
5. Conclusions
In conclusion, this study aimed to develop a model to predict the biomass of seedlings in a dry evergreen forest in Huai Kha Khaeng Wildlife Sanctuary, Thailand. The results of the study showed that root collar diameter, shoot height, and wood density could be used to create a model that best predicted seedling biomass. The model with only shoot height and root collar diameter could also be an alternative when the wood density data is not available.
Our study provides a tool for researchers to measure and monitor seedling biomass without destructive sampling. Future studies on seedlings and their biomass can help predict future forest structure and growth patterns. This provides a foundation for potential applications in ecological monitoring and conservation efforts in tropical forests.
Author Contributions
Conceptualization, E.K. and N.P.; methodology, S.T., K.C., N.P. and E.K.; formal analysis, S.T. and E.K.; investigation, S.T. and K.C.; data curation, S.T.; writing—original draft preparation, S.T. and E.K.; writing—review and editing, S.T., K.C., N.P. and E.K.; visualization, S.T.; supervision, E.K. and N.P.; project administration, N.P.; funding acquisition, N.P. and E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Biodiversity-Based Economy Development Office (Public Organization), grant number 695296 in the fiscal year 2019.
Data Availability Statement
The raw datasets are available upon reasonable requests to the authors.
Acknowledgments
We are grateful for the permission to work in this protected area from the Department of National Parks, Wildlife, and Plants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tkemaladze, G.; Makhashvili, K. Climate changes and photosynthesis. Ann. Agrar. Sci. 2016, 14, 119–126. [Google Scholar] [CrossRef]
- Pallardy, S.G. Physiology of Woody Plants, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.J.; Crane, D.E. Carbon storage and sequestration by urban trees in the USA. Environ. Pollut. 2001, 116, 381–389. [Google Scholar] [CrossRef]
- Fernando, Y.L.; Adjie, F.F.; Jemi, R. Specific release of carbon dioxide of traditional medicinal plants used by the Dayak people of Palangka Raya. Acta Sci. Intellectus 2020, 6, 121–131. [Google Scholar]
- Viriyabuncha, C. Handbook of Stand Biomass Estimation; Department of National Parks, Wildlife and Plant Conservation: Bangkok, Thailand, 2003; p. 101.
- Chaiyo, U.; Garivait, S.; Wanthongchai, K. Carbon storage in above-ground biomass of tropical deciduous forest in Ratchaburi province, Thailand. World Acad. Sci. Eng. Technol. 2011, 58, 636–641. [Google Scholar] [CrossRef]
- Martínez Luna, J.E.; Carrillo Anzures, F.; Acosta Mireles, M.; Romero Sánchez, M.E.; Pérez Miranda, R. Allometric equations to estimate carbon in seedlings of Pinus hartwegii Lindl. Rev. Mex. Cienc. For. 2020, 11, 144–160. [Google Scholar]
- Pati, P.K.; Kaushik, P.; Khan, M.; Khare, P. Allometric equations for biomass and carbon stock estimation of small diameter woody species from tropical dry deciduous forests: Support to REDD+. Trees For. People 2022, 9, 100289. [Google Scholar] [CrossRef]
- Yang, X.; Blagodatsky, S.; Liu, F.; Beckschäfer, P.; Xu, J.; Cadisch, G. Rubber tree allometry, biomass partitioning and carbon stocks in mountainous landscapes of sub-tropical China. For. Ecol. Manag. 2017, 404, 84–99. [Google Scholar] [CrossRef]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Basuki, T.M.; van Laake, P.E.; Skidmore, A.K.; Hussin, Y.A. Allometric equations for estimating the above-ground biomass in tropical lowland Dipterocarp forests. For. Ecol. Manag. 2009, 257, 1684–1694. [Google Scholar] [CrossRef]
- Pothong, T.; Elliott, S.; Chairuangsri, S.; Chanthorn, W.; Shannon, D.P.; Wangpakapattanawong, P. New allometric equations for quantifying tree biomass and carbon sequestration in seasonally dry secondary forest in northern Thailand. New For. 2021, 53, 17–36. [Google Scholar] [CrossRef]
- Ounban, W.; Puangchit, L.; Diloksumpun, S. Development of general biomass allometric equations for Tectona grandis Linn.f. and Eucalyptus camaldulensis Dehnh. plantations in Thailand. Agric. Nat. Resour. 2016, 50, 48–53. [Google Scholar] [CrossRef]
- Terakunpisut, J.; Gajaseni, N.; Ruankawe, N. Carbon sequestration potential in aboveground biomass of Thong Pha Phum national forest, Thailand. Appl. Ecol. Environ. Res. 2007, 5, 93–102. [Google Scholar] [CrossRef]
- Zianis, D.; Mencuccini, M. On simplifying allometric analyses of forest biomass. For. Ecol. Manag. 2004, 187, 311–332. [Google Scholar] [CrossRef]
- Enquist, B.J. Universal scaling in tree and vascular plant allometry: Toward a general quantitative theory linking plant form and function from cells to ecosystems. Tree Physiol. 2002, 22, 1045–1064. [Google Scholar] [CrossRef] [PubMed]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Annighöfer, P.; Ameztegui, A.; Ammer, C.; Balandier, P.; Bartsch, N.; Bolte, A.; Coll, L.; Collet, C.; Ewald, J.; Frischbier, N.; et al. Species-specific and generic biomass equations for seedlings and saplings of European tree species. Eur. J. For. Res. 2016, 135, 313–329. [Google Scholar] [CrossRef]
- Clough, B.F.; Dixon, P.; Dalhaus, O. Allometric Relationships for Estimating Biomass in Multi-stemmed Mangrove Trees. Aust. J. Bot. 1997, 45, 1023–1031. [Google Scholar] [CrossRef]
- Larsen, D.R.; Bliss, L.C. An analysis of structure of tree seedling populations on a Lahar. Landsc. Ecol. 1998, 13, 307–323. [Google Scholar] [CrossRef]
- Benitez-Malvido, J. Impact of forest fragmentation on seedling abundance in a tropical rain forest. Conserv. Biol. 1998, 12, 380–389. [Google Scholar] [CrossRef]
- Comita, L.S.; Aguilar, S.; Pérez, R.; Lao, S.; Hubbell, S.P. Patterns of woody plant species abundance and diversity in the seedling layer of a tropical forest. J. Veg. Sci. 2007, 18, 163. [Google Scholar] [CrossRef]
- Webb, C.O.; Peart, D.R. Seedling density dependence promotes coexistence of Bornean rain forest trees. Ecology 1999, 80, 2006–2017. [Google Scholar] [CrossRef]
- Bunyavejchewin, S.; Chamlongrach, Y.; Buasalee, R.; Rayangkul, P. Trees & Forest of Huai Kha Khaeng Wildlife Sanctuary; Thai Long-Term Forest Ecological Research & The Rabbit in the Moon Foundation: Bangkok, Thailand, 2016; p. 693. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2021. [Google Scholar]
- Tsakaldimi, M.; Zagas, T.; Tsitsoni, T.; Ganatsas, P. Root Morphology, Stem Growth and Field Performance of Seedlings of Two Mediterranean Evergreen Oak Species Raised in Different Container Types. Plant Soil 2005, 278, 85–93. [Google Scholar] [CrossRef]
- Achten, W.; Maes, W.H.; Reubens, B.; Mathijs, E.; Singh, V.; Verchot, L.; Muys, B. Biomass production and allocation in Jatropha curcas L. seedlings under different levels of drought stress. Biomass-Bioenergy 2010, 34, 667–676. [Google Scholar] [CrossRef]
- Sungpalee, W.; Itoh, A.; Kanzaki, M.; Sri-Ngernyuang, K.; Noguchi, H.; Mizuno, T.; Teejuntuk, S.; Hara, M.; Chai-Udom, K.; Ohkubo, T.; et al. Intra- and interspecific variation in wood density and fine-scale spatial distribution of stand-level wood density in a northern Thai tropical montane forest. J. Trop. Ecol. 2009, 25, 359–370. [Google Scholar] [CrossRef]
- Chen, X.; Li, B.-L. Testing the allometric scaling relationships with seedlings of two tree species. Acta Oecologica 2003, 24, 125–129. [Google Scholar] [CrossRef]
- Sanquetta, C.R.; Wojciechowski, J.; Dalla Corte, A.P.; Behling, A.; Netto, S.P.; Rodrigues, A.L.; Sanquetta, M.N.I. Comparison of data mining and allometric model in estimation of tree biomass. BMC Bioinform. 2015, 16, 185. [Google Scholar] [CrossRef]
- Bi, H.; Turner, J.; Lambert, M.J. Additive biomass equations for native eucalypt forest trees of temperate Australia. Trees 2004, 18, 467–479. [Google Scholar] [CrossRef]
- Khurana, E.; Singh, J. Ecology of seed and seedling growth for conservation and restoration of tropical dry forest: A review. Environ. Conserv. 2001, 28, 39–52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).