The Identification and Expression Analysis of the Liriodendron chinense (Hemsl.) Sarg. SOD Gene Family
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification and Classification of LcSOD Gene Family
2.2. Phylogenetic Analysis of LcSOD Genes
2.3. Motifs, Gene Structure, Chromosomal Location, and Syntenic Analysis
2.4. Cis-Regulatory Element Analysis
2.5. Expression Analysis of LcSOD Genes
2.6. Plant Material and Cold Treatment
2.7. RNA Isolation and qRT-PCR
3. Results
3.1. Identification of SODs in L. chinense
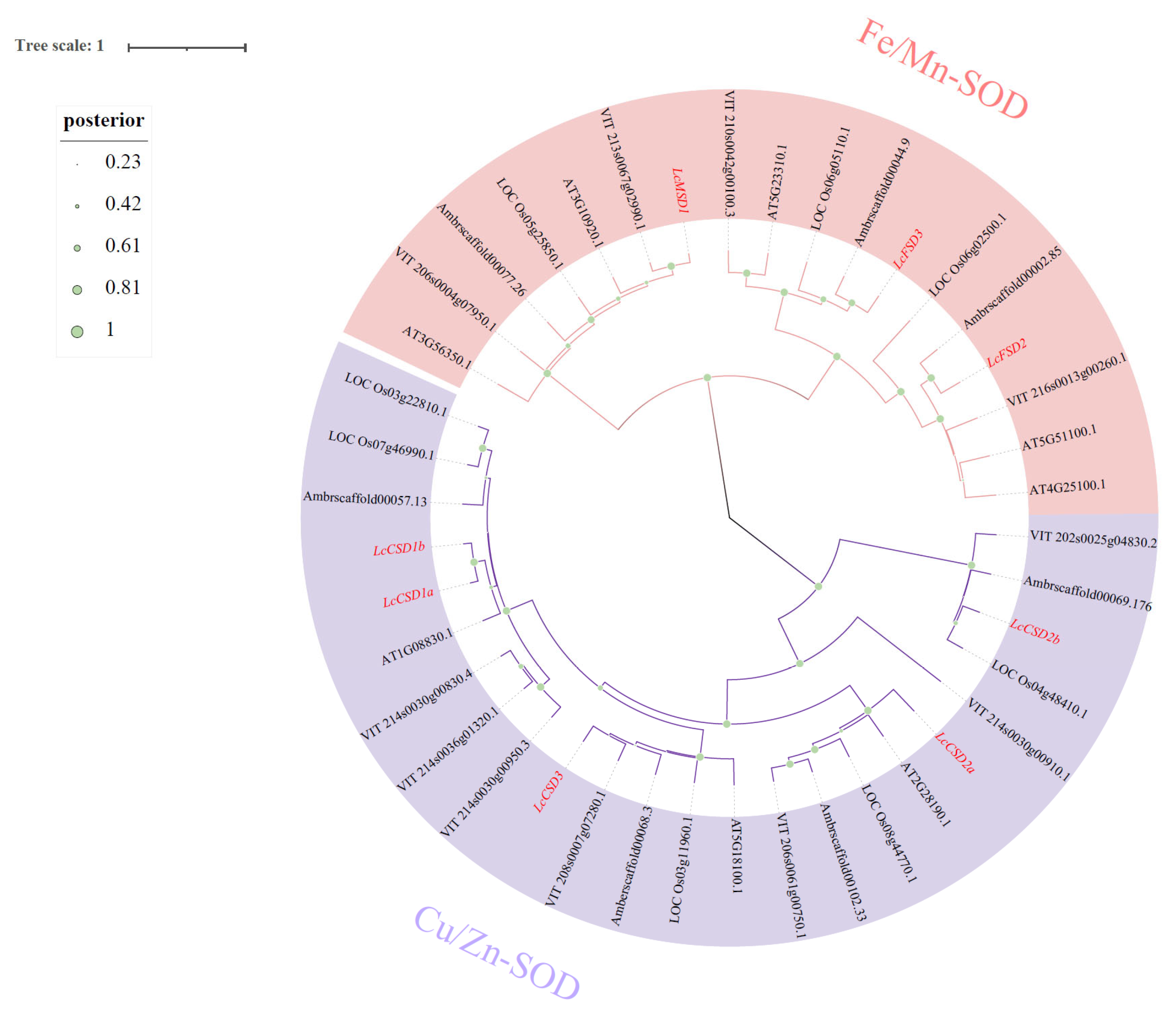
3.2. Phylogenetic Analysis of LcSODs in L. chinense
3.3. Chromosome Distribution and Syntenic Analysis of LcSODs
3.4. Analysis of the SOD Family Gene Structure in L. chinense
3.5. Cis-Regulatory Element Analysis
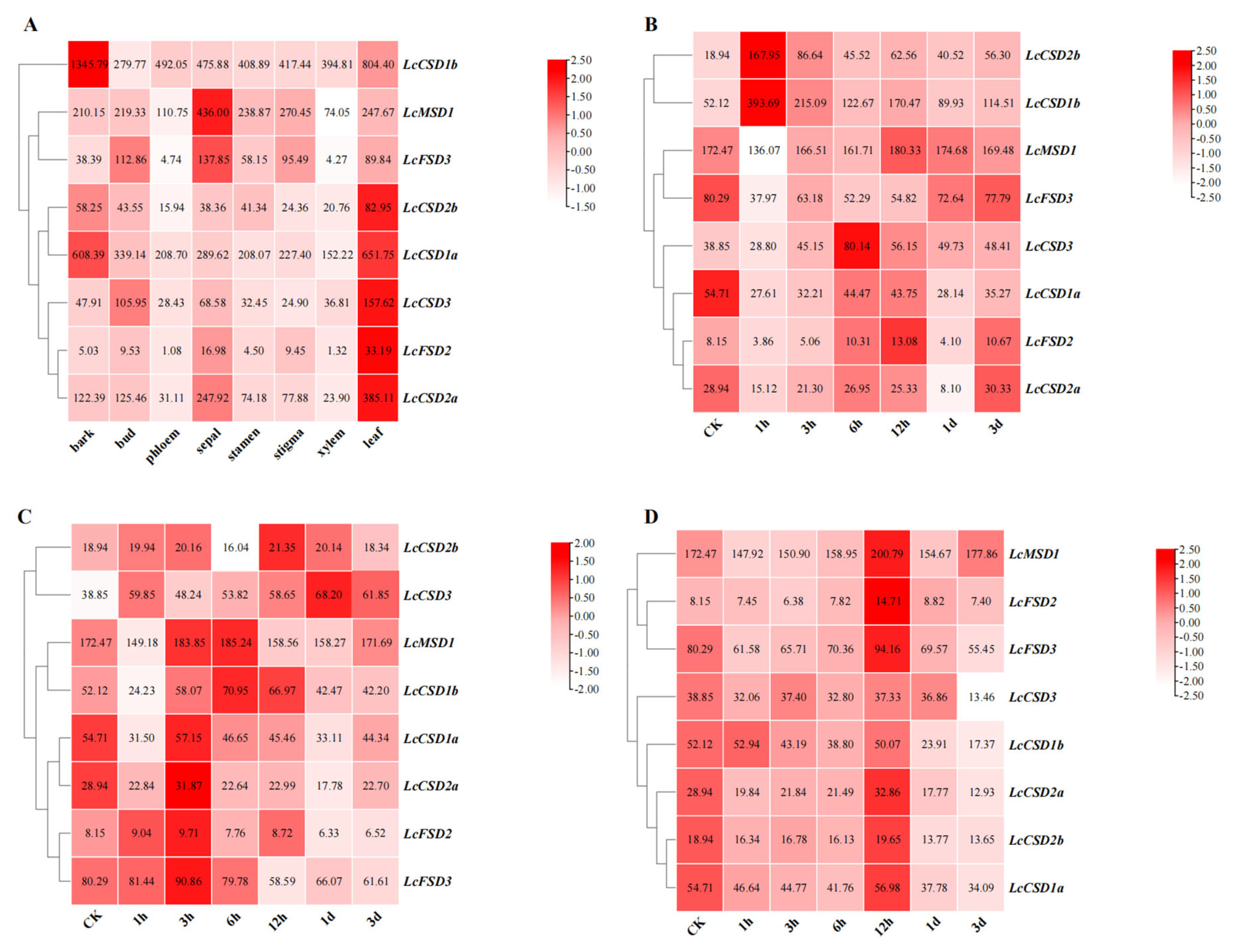
3.6. Expression Analysis of LcSOD Genes across Different Tissues and Abiotic Stresses
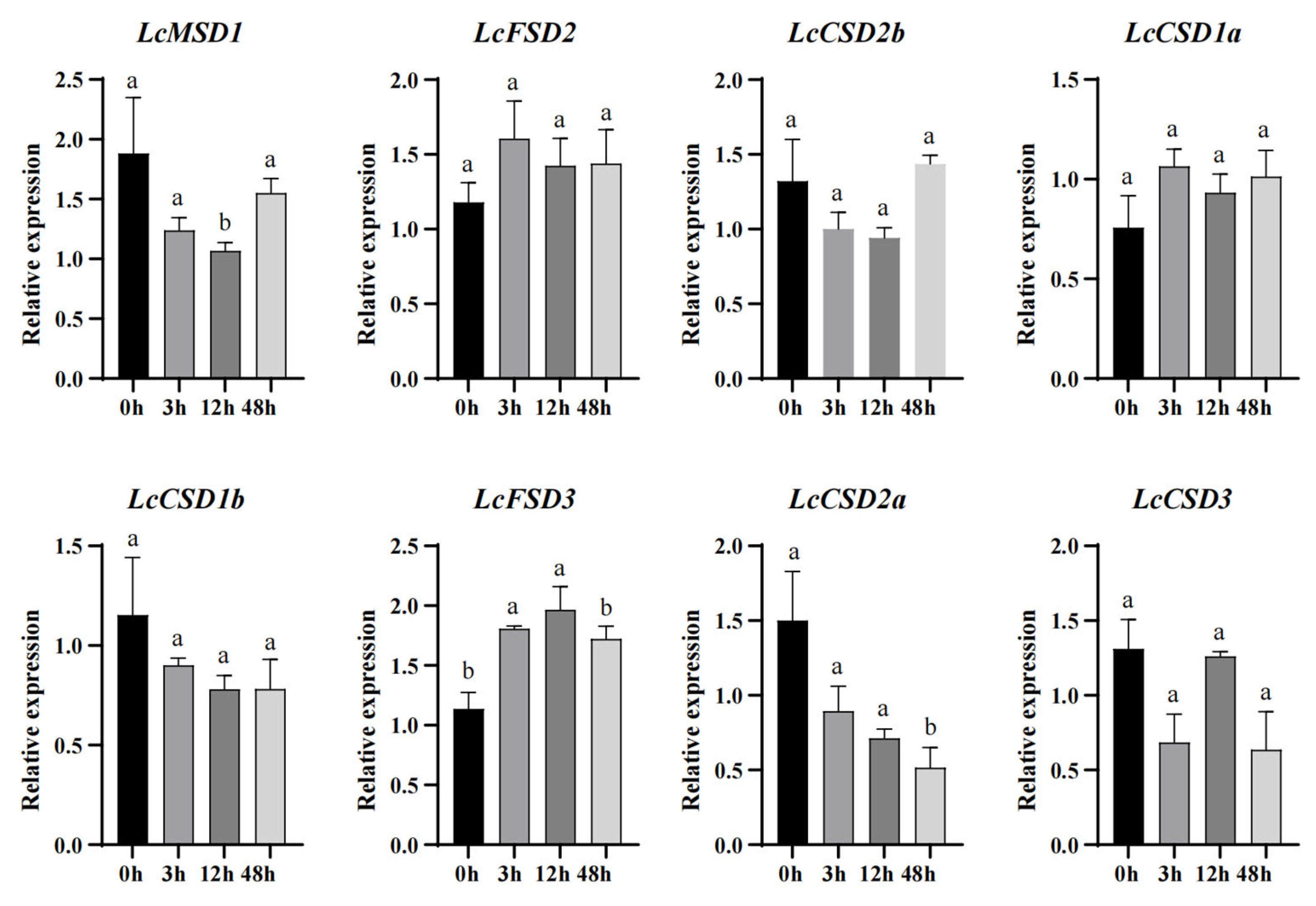
3.7. Expression Analysis of LcSOD Genes under Cold Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Guo, C. Cloning, characterization and expression analysis of two superoxide dismutase (SOD) genes in wheat (Triticum aestivum L.). Front. Agric. China 2008, 2, 141–149. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- Gill, S.S.; Peter Singh, L. Generation and scavenging of reactive oxygen species in plants under stress. In Improving Crop Resistance to Abiotic Stress; Wiley Online Library: Hoboken, NJ, USA, 2012; pp. 49–70. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Lai, Z. Genome-wide identification and characterization of the superoxide dismutase gene family in Musa acuminata cv. Tianbaojiao (AAA group). BMC Genom. 2015, 16, 823. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A. Superoxide dismutase—Mentor of abiotic stress tolerance in crop plants. Environ. Sci. Pollut. Res. 2015, 22, 10375–10394. [Google Scholar] [CrossRef]
- Miao, L.; Clair, D.K.S. Regulation of superoxide dismutase genes: Implications in disease. Free. Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, X. A wheat superoxide dismutase gene TaSOD2 enhances salt resistance through modulating redox homeostasis by promoting NADPH oxidase activity. Plant Mol. Biol. 2016, 91, 115–130. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free. Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Han, X.M.; Chen, Q.X. Genome-wide analysis of superoxide dismutase genes in Larix kaempferi. Gene 2019, 686, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.L.; Neupane, K. Diversity, function and evolution of genes coding for putative Ni-containing superoxide dismutases. Environ. Microbiol. 2008, 10, 1831–1843. [Google Scholar] [CrossRef]
- Sen, R.S.; Deng, X.W. The role of superoxide dismutase in combating oxidative stress in higher plants. Bot. Rev. 2000, 66, 89–98. [Google Scholar] [CrossRef]
- Molina-Rueda, J.J.; Tsai, C.J. The Populus superoxide dismutase gene family and its responses to drought stress in transgenic poplar overexpressing a pine cytosolic glutamine synthetase (GS1a). PLoS ONE 2013, 8, e56421. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Yu, J. The SOD gene family in tomato: Identification, phylogenetic relationships, and expression patterns. Front. Plant Sci. 2016, 7, 1279. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Lakhanpal, N.; Singh, K. Genome-wide identification and characterization of abiotic-stress responsive SOD (superoxide dismutase) gene family in Brassica juncea and B. rapa. BMC Genom. 2019, 20, 227. [Google Scholar] [CrossRef]
- Chen, J.; Hao, Z.; Guang, X.; Zhao, C.; Wang, P.; Xue, L.; Zhu, Q.; Yang, L.; Sheng, Y.; Zhou, Y.; et al. Liriodendron genome sheds light on angiosperm phylogeny and species–pair differentiation. Nat. Plants 2019, 5, 18–25. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, J. Alterations in Population Distribution of Liriodendron chinense (Hemsl.) Sarg. and Liriodendron tulipifera Linn. Caused by Climate Change. Forests 2022, 13, 488. [Google Scholar] [CrossRef]
- Yu, W.; Kong, G.; Chao, J.; Yin, T.; Tian, H.; Ya, H.; He, L.; Zhang, H. Genome-wide identification of the rubber tree superoxide dismutase (SOD) gene family and analysis of its expression under abiotic stress. PeerJ 2022, 10, e14251. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bryant, S.H. CD-Search: Protein domain annotations on the fly. Nucleic Acids Res. 2004, 32 (Suppl. S2), W327–W331. [Google Scholar] [CrossRef]
- Garg, V.K.; Avashthi, H.; Tiwari, A.; Jain, P.A.; Ramkete, P.W.; Kayastha, A.M.; Singh, V.K. MFPPI–multi FASTA ProtParam interface. Bio. Inf. 2016, 12, 74–77. [Google Scholar] [CrossRef]
- Chou, K.C.; Shen, H.B. Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. Available online: https://pubmed.ncbi.nlm.nih.gov/11152613/ (accessed on 1 January 2023). [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; Maio, N.D.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37 (Suppl. S2), W202–W208. [Google Scholar] [CrossRef]
- Tang, H.; Bowers, J.E.; Wang, X.; Ming, R.; Alam, M.; Paterson, A.H. Synteny and collinearity in plant genomes. Science 2008, 320, 486–488. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, S.; Xu, L.; Zhu, L.; Wang, D.; Liu, Y.; Liu, S.; Hao, Z.; Lu, Y.; Yang, L.; et al. Genome-wide identification of the Liriodendron chinense WRKY gene family and its diverse roles in response to multiple abiotic stress. BMC Plant Biol. 2022, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; Sage Publications Ltd.: Thousand Oaks, CA, USA, 2013. [Google Scholar]
- Swift, M.L. GraphPad prism, data analysis, and scientific graphing. J. Chem. Inf. Comput. Sci. 1997, 37, 411–412. [Google Scholar] [CrossRef]
- Wang, W.; Xia, M.X. Gene expression characteristics and regulation mechanisms of superoxide dismutase and its physiological roles in plants under stress. Biochemistry 2016, 81, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yang, L. Genome-wide identification and transcriptional expression analysis of superoxide dismutase (SOD) family in wheat (Triticum aestivum). PeerJ 2019, 7, e8062. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Monde, R.A. Superoxide dismutase in Arabidopsis: An eclectic enzyme family with disparate regulation and protein localization. Plant Physiol. 1998, 118, 637–650. [Google Scholar] [CrossRef]
- Filiz, E.; Tombuloğlu, H. Genome-wide distribution of superoxide dismutase (SOD) gene families in Sorghum bicolor. Turk. J. Biol. 2015, 39, 49–59. [Google Scholar] [CrossRef]
- Qureshi, A.M.I.; Sofi, M.U. Insilco identification and characterization of superoxide dismutase gene family in Brassica rapa. Saudi J. Biol. Sci. 2021, 28, 5526–5537. [Google Scholar] [CrossRef]
- Aleem, M.; Aleem, S. Characterization of SOD and GPX Gene Families in the Soybeans in Response to Drought and Salinity Stresses. Antioxidants 2022, 11, 460. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L. Genome-wide identification of the SOD gene family and expression analysis under drought and salt stress in barley. Plant Growth Regul. 2021, 94, 49–60. [Google Scholar] [CrossRef]
- Hu, X.; Hao, C. Genome-wide identification, characterization, and expression analysis of the grapevine superoxide dismutase (SOD) family. Int. J. Genom. 2019, 2019, 7350414. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, X. Genome-wide characterization and expression analyses of superoxide dismutase (SOD) genes in Gossypium hirsutum. BMC Genom. 2017, 18, 376. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Hua, W. Genome-wide identification and expression analysis of the superoxide dismutase (SOD) gene family in Salvia miltiorrhiza. Gene 2020, 742, 144603. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B. Genome-wide characterization and expression profiles of the superoxide dismutase gene family in Gossypium. Int. J. Genom. 2016, 2016, 8740901. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, L. Genome-wide identification and transcriptional expression analysis of cucumber superoxide dismutase (SOD) family in response to various abiotic stresses. Int. J. Genom. 2017, 2017, 7243973. [Google Scholar] [CrossRef]
- Huo, C.; He, L. The Superoxide Dismutase Gene Family in Nicotiana tabacum, Genome-Wide Identification, Characterization, Expression Profiling and Functional Analysis in Response to Heavy Metal Stress. Front. Plant Sci. 2022, 13, 904105. [Google Scholar] [CrossRef]
- Fink, R.C.; Scandalios, J.G. Molecular evolution and structure–function relationships of the superoxide dismutase gene families in angiosperms and their relationship to other eukaryotic and prokaryotic superoxide dismutases. Arch. Biochem. Biophys. 2002, 399, 19–36. [Google Scholar] [CrossRef]
- Zhou, Y.; Ouang, L. Superoxide dismutase family genes in watermelon and their responses to different abiotic stresses. Front. Agric. Sci. Eng. 2021, 8, 645–658. [Google Scholar] [CrossRef]
- Bowler, C.; Van Camp, W. Superoxide dismutase in plants. Crit. Rev. Plant Sci. 1994, 13, 199–218. [Google Scholar] [CrossRef]
- Song, J.; Zeng, L. In silico identification and expression analysis of superoxide dismutase (SOD) gene family in Medicago truncatula. 3 Biotech 2018, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Meng, Z.; Zhang, G.; Qi, M.; Sun, Z.; Liu, Y.; Li, T. Sub-high temperature and high light intensity induced irreversible inhibition on photosynthesis system of tomato plant (Solanum lycopersicum L.). Front. Plant Sci. 2017, 8, 365. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | qRT-PCR Primers (5′-3′) | |
|---|---|---|
| LcMSD1 | F | ATCATGAAGCTCCACCACCA |
| R | GTGATTGACATGACCTCCGC | |
| LcFSD2 | F | CATGGGGCTGGAAAATCACC |
| R | GTGCCTTCCCCAGTGATACT | |
| LcFSD3 | F | TCCTGCCTAGTTCCTCCTCT |
| R | AAGTCCTTTGGGTCCGACAT | |
| LcCSD1a | F | ATGCTGGTGATCTCGGGAAT |
| R | TCCGCCCTTTCCAAGATCAT | |
| LcCSD1b | F | GGAGATGGCCCAACTACAGT |
| R | CCATGTTCTTTCCCAGCAGG | |
| LcCSD2a | F | TCCAGGGAAGTGCTGATCAA |
| R | CTGCTCCGACGAATATCCCT | |
| LcCSD2b | F | TCCCATGGATCATCGCCTAC |
| R | GTTGGTCGGGCAGTTGATTT | |
| LcCSD3 | F | AGGCTCTCTTCAGTTCGTCC |
| R | GAATTGCATCCGTTGGTGGT | |
| Gene ID | Number of Amino Acids (aa) | Molecular Weight (Da) | Aliphatic Index | GRAVY | pI | Subcellular Localization | Transmembrane Domain |
|---|---|---|---|---|---|---|---|
| LcCSD2b | 257 | 27,288.8 | 79.3 | −0.153 | 4.8 | Chloroplast | NO |
| LcCSD1b | 134 | 13,299.7 | 80.75 | −0.096 | 5.07 | Chloroplast | NO |
| LcFSD2 | 309 | 35,287.44 | 71.68 | −0.559 | 5.24 5.41 5.44 | Chloroplast | NO |
| LcCSD2a | 211 | 22,096.94 | 92.42 | −0.085 | Chloroplast | NO | |
| LcCSD1a | 178 | 18,166.16 | 78.88 | −0.166 | Chloroplast | NO | |
| LcMSD1 | 226 | 25,121.64 | 93.63 | −0.358 | 6.55 6.71 | Mitochondrion | NO |
| LcFSD3 | 271 | 31,106.47 | 75.9 | −0.409 | Mitochondrion | NO | |
| LcCSD3 | 150 | 15,125.9 | 87.2 | −0.173 | 7.16 | Chloroplast/Cytoplasm | NO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wu, H.; Hao, Z.; Zhu, L.; Lu, L.; Shi, J.; Chen, J. The Identification and Expression Analysis of the Liriodendron chinense (Hemsl.) Sarg. SOD Gene Family. Forests 2023, 14, 628. https://doi.org/10.3390/f14030628
Chen Y, Wu H, Hao Z, Zhu L, Lu L, Shi J, Chen J. The Identification and Expression Analysis of the Liriodendron chinense (Hemsl.) Sarg. SOD Gene Family. Forests. 2023; 14(3):628. https://doi.org/10.3390/f14030628
Chicago/Turabian StyleChen, Ya, Hua Wu, Zhaodong Hao, Liming Zhu, Lu Lu, Jisen Shi, and Jinhui Chen. 2023. "The Identification and Expression Analysis of the Liriodendron chinense (Hemsl.) Sarg. SOD Gene Family" Forests 14, no. 3: 628. https://doi.org/10.3390/f14030628
APA StyleChen, Y., Wu, H., Hao, Z., Zhu, L., Lu, L., Shi, J., & Chen, J. (2023). The Identification and Expression Analysis of the Liriodendron chinense (Hemsl.) Sarg. SOD Gene Family. Forests, 14(3), 628. https://doi.org/10.3390/f14030628





