Linkage between Leaf–Litter–Soil, Microbial Resource Limitation, and Carbon-Use Efficiency in Successive Chinese Fir (Cunninghamia lanceolata) Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Site
2.2. Sample Collection
2.3. Leaf, Litter, and Soil Chemical Assays
2.4. Phospholipid Fatty Acid (PLFA)
2.5. Calculation of Plant N and P Resorption Efficiency (%), Stoichiometric Imbalance, Microbial Resource Limitation, and CUE
2.6. Statistical Analysis
3. Results
3.1. Leaf and Litter Stoichiometry, and N and P Resorption Efficiency
3.2. Soil Physicochemical Properties and Microbial Stoichiometry
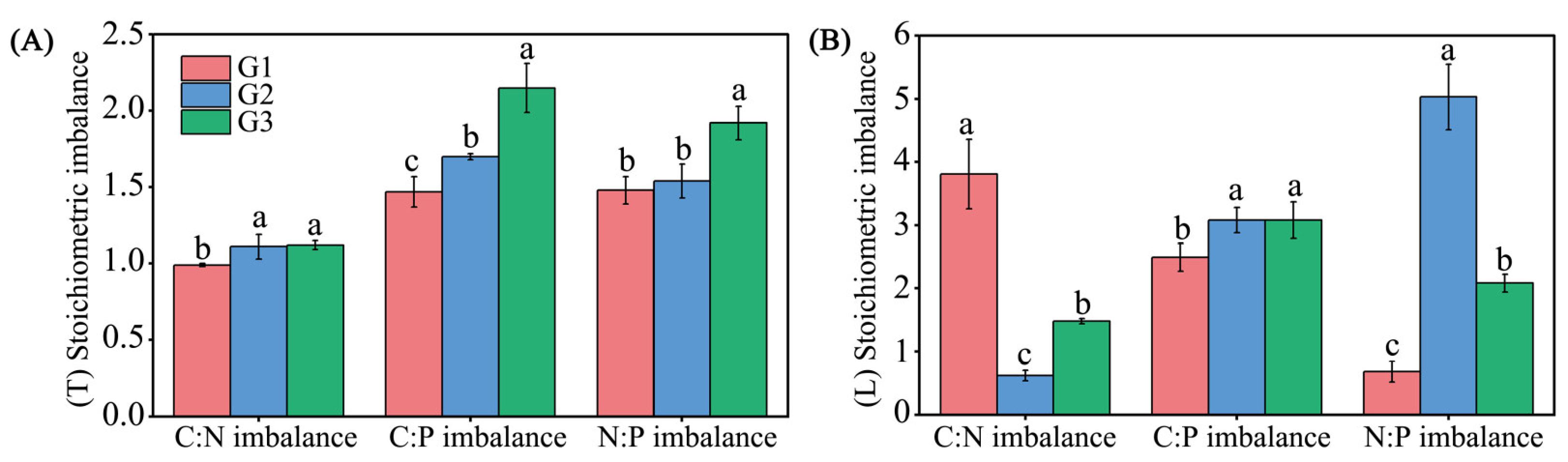
3.3. Changes in Stoichiometric Imbalance
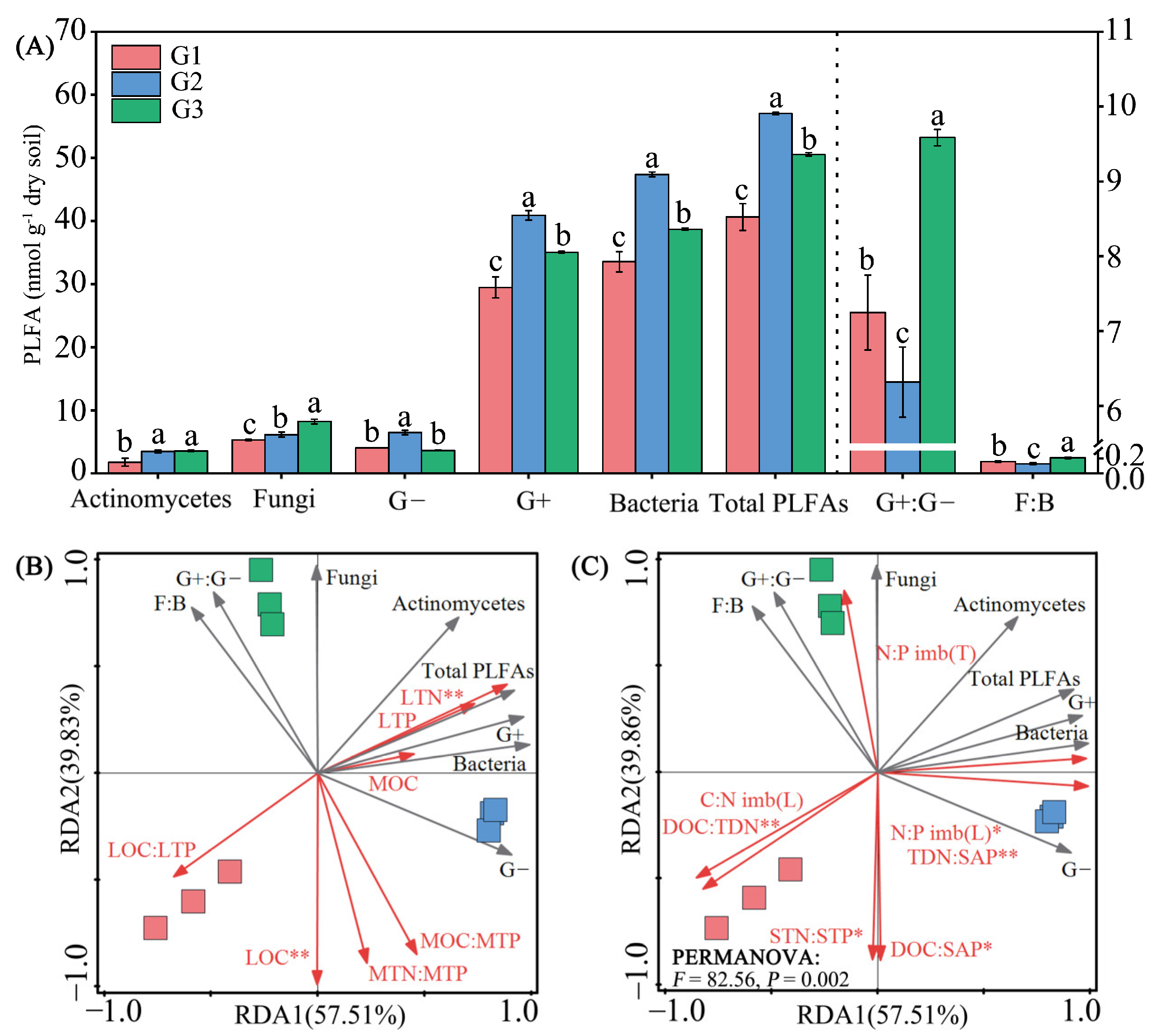
3.4. Microbial Community Structure
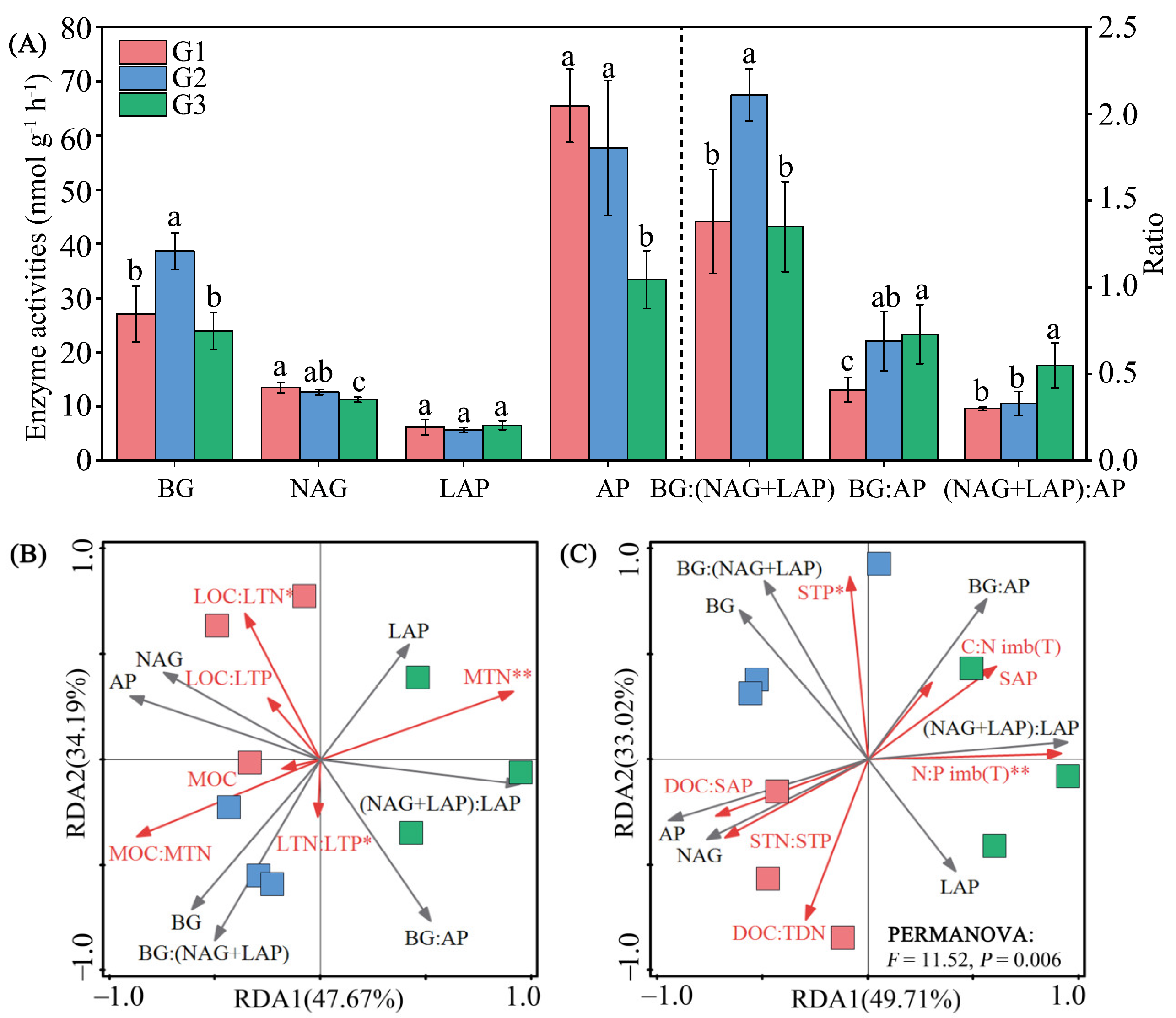
3.5. Extracellular Enzyme Activity
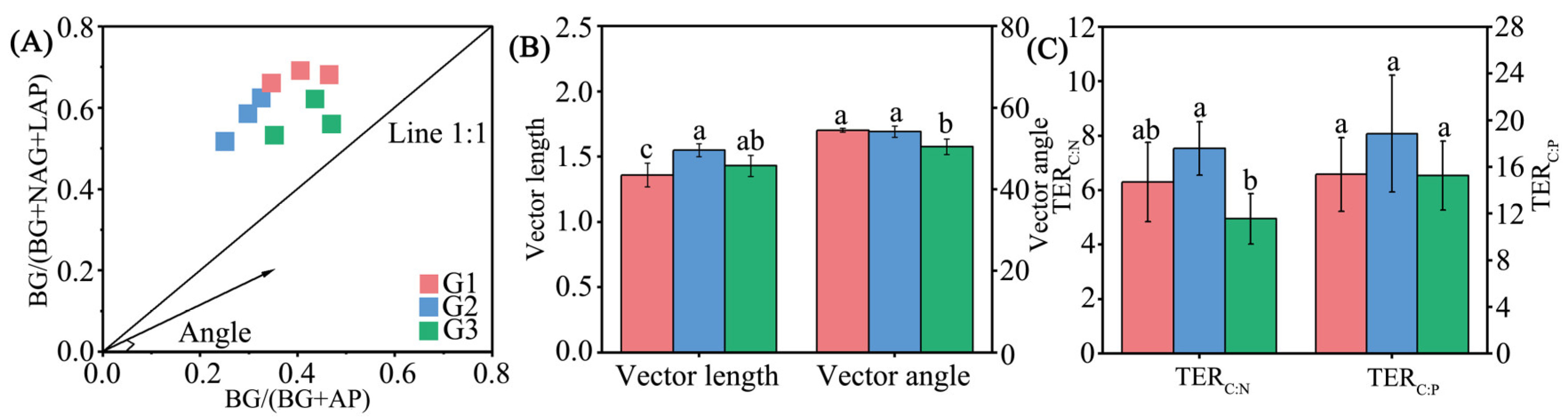
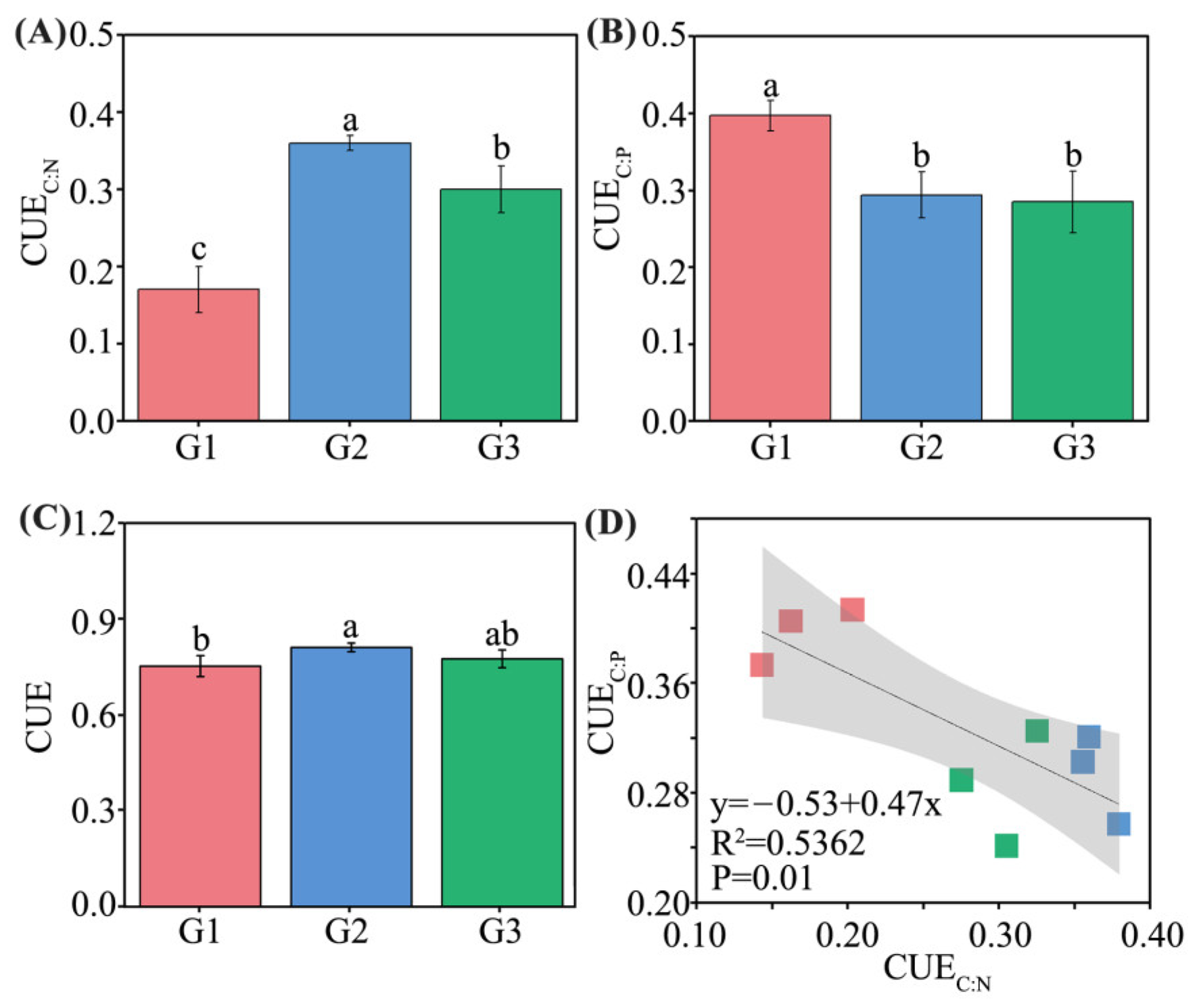
3.6. Indicators of Microbial Resource Limitation
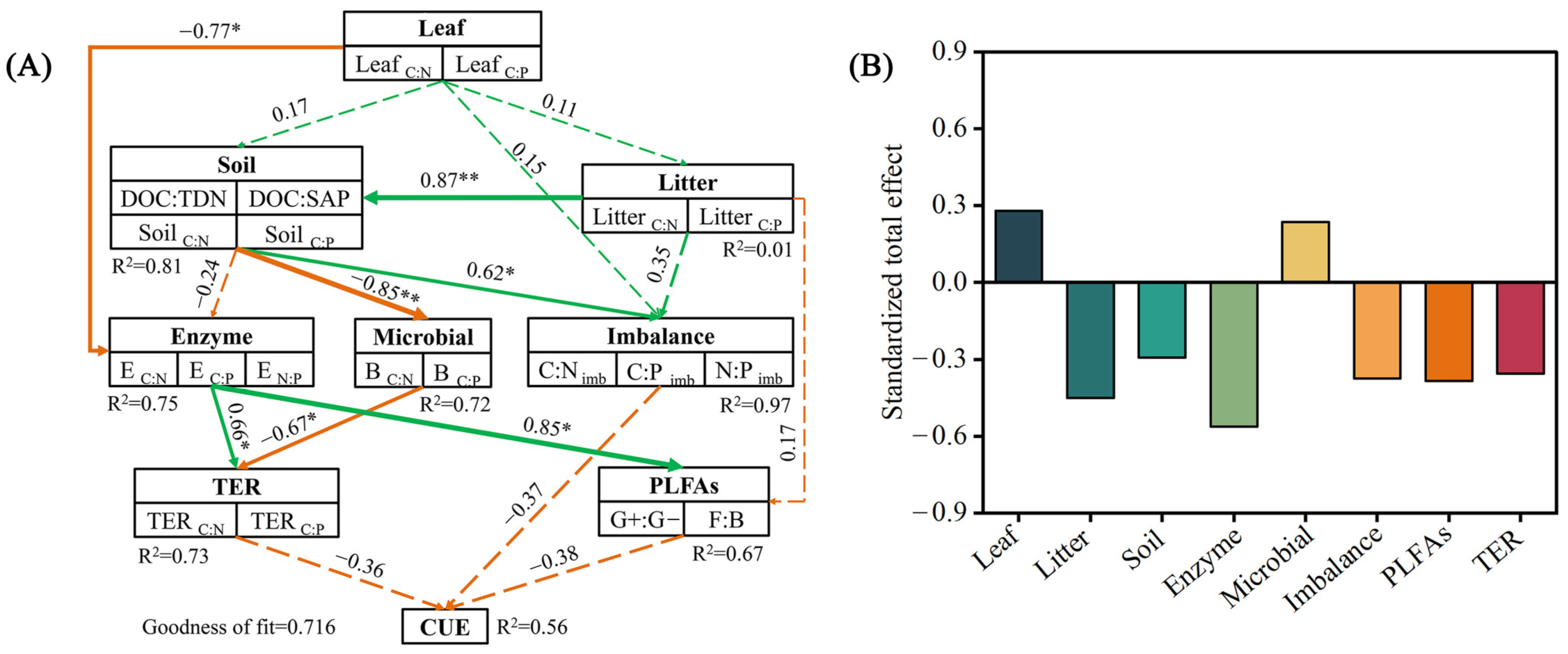
3.7. Linkages between Leaf, Soil, Microbial Resource Limitation, and Carbon-Use Efficiency
4. Discussion
4.1. Leaf, Litter and Soil Nutrient Reabsorption Efficiency and Soil Resource Limitation among Different Successive Planting Generations of Chinese Fir
4.2. Stoichiometric Imbalance and Microbial Resource Limitation among Different Successive Planting Generations of Chinese Fir
4.3. Soil Microbial Community Structure, Enzyme Activity, and CUE among Different Successive Planting Generations of Chinese Fir
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Yu, J.; Li, H.; Zhang, Y.; Pan, F.; Zhou, C.; Liu, A. Characteristics of soil phosphorus in Cunninghamia lanceolata plantations with different planting rotations. For. Res. 2021, 34, 10–18. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Z.; Ren, G.; Xie, J.; Yang, Y. Carbon storage in a chronosequence of Chinese fir plantations in southern China. For. Ecol. Manag. 2013, 300, 68–76. [Google Scholar] [CrossRef]
- Tian, D.; Xiang, W.; Chen, X.; Yan, W.; Fang, X.; Kang, W.; Dan, X.; Peng, C.; Peng, Y. A long-term evaluation of biomass production in first and second rotations of Chinese fir plantations at the same site. Forestry 2011, 84, 411–418. [Google Scholar] [CrossRef]
- Ma, X.; Fan, S.; Chen, S.; Lin, S. Study on biomass productivity of Chinese fir plantations after successive planting. Sci. Silvae Sin. 2003, 39, 78–83. [Google Scholar]
- Zhou, Y.; Kang, W.; Chen, R.; Tian, D.; Xiang, W. Nutrient uptake, accumulation, and utilization efficiency comparisons in plantations containing different generations of Chinese fir. Acta Ecol. Sin. 2018, 38, 3868–3878. [Google Scholar]
- Wu, Z.; Li, J.; Zheng, J.; Liu, J.; Liu, S.; Lin, W.; Wu, C. Soil microbial community structure and catabolic activity are significantly degenerated in successive rotations of Chinese fir plantations. Sci. Rep. 2017, 7, 6691. [Google Scholar] [CrossRef]
- Farooq, T.H.; Yan, W.; Rashid, M.H.; Tigabu, M.; Gilani, M.M.; Zou, X.; Wu, P. Chinese fir (Cunninghamia lanceolata) a green gold of China with continues decline in its productivity over the successive rotations: A review. Appl. Ecol. Environ. Res. 2019, 17, 11055–11067. [Google Scholar] [CrossRef]
- Elser, J. Biological stoichiometry: A chemical bridge between ecosystem ecology and evolutionary biology. Am. Nat. 2006, 168, S25–S35. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Han, W.; Tang, L.; Chen, Y.; Fang, J. Relationship between the relative limitation and resorption efficiency of nitrogen vs. phosphorus in woody plants. PLoS ONE 2013, 8, e83366. [Google Scholar] [CrossRef]
- Deng, Q.; Hui, D.; Dennis, S.; Reddy, K.C. Responses of terrestrial ecosystem phosphorus cycling to nitrogen addition: A meta-analysis. Glob. Ecol. Biogeogr. 2017, 26, 713–728. [Google Scholar] [CrossRef]
- Hayes, P.; Turner, B.L.; Lambers, H.; Laliberte, E. Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J. Ecol. 2014, 102, 396–410. [Google Scholar] [CrossRef]
- Tong, R.; Zhou, B.; Jiang, L.; Ge, X.; Cao, Y. The growth of Chinese fir is limited by nitrogen: Evidences from N:P ratio, N or P variability and NuRE based on a regional investigation. For. Ecol. Manag. 2020, 460, 117905. [Google Scholar] [CrossRef]
- Zhang, T.; Li, F.; Shi, C.; Li, Y.; Tang, S.; Baoyin, T. Enhancement of nutrient resorption efficiency increases plant production and helps maintain soil nutrients under summer grazing in a semi-arid steppe. Agric. Ecosyst. Environ. 2020, 292, 8. [Google Scholar] [CrossRef]
- Zheng, Y.; Guan, F.; Fan, S.; Yan, X.; Huang, L. Dynamics of leaf-litter biomass, nutrient resorption efficiency and decomposition in a moso bamboo forest after strip clearcutting. Front. Plant Sci. 2022, 12, 3297. [Google Scholar] [CrossRef]
- Tully, K.L.; Wood, T.E.; Schwante, A.M.; Lawrence, D. Soil nutrient availability and reproductive effort drive patterns in nutrient resorption in Pentaclethra macroloba. Ecology 2013, 94, 930–940. [Google Scholar] [CrossRef]
- Wu, P.; Zeng, L.; Lei, P.; Hu, D.; Li, J.; Wang, M.; Zhong, Q. Content of Leaf Nutrients and Resorption Efficiency of Major Tree Species in Tsuga chinensis Forest in Wuyi Mountain, Jiangxi Province. Sci. Silvae Sin. 2022, 58, 13–21. [Google Scholar] [CrossRef]
- Tong, R.; Zhou, B.; Jiang, L.; Ge, X.; Cao, Y.; Shi, J. Leaf litter carbon, nitrogen and phosphorus stoichiometry of Chinese fir (Cunninghamia lanceolata) across China. Glob. Ecol. Conserv. 2021, 27, e01542. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Hai, X.; Shangguan, Z.; Deng, L. Dynamics of soil microbial C:N:P stoichiometry and its driving mechanisms following natural vegetation restoration after farmland abandonment. Sci. Total Environ. 2019, 693, 133613. [Google Scholar] [CrossRef]
- Traore, O.Y.; Kiba, D.I.; Arnold, M.C.; Fliessbach, A.; Oberholzer, H.R.; Nacro, H.B.; Lompo, F.; Oberson, A.; Frossard, E.; Buenemann, E.K. Fertilization practices alter microbial nutrient limitations after alleviation of carbon limitation in a Ferric Acrisol. Biol. Fertil. Soils 2016, 52, 177–189. [Google Scholar] [CrossRef]
- Manzoni, S.; Trofymow, J.A.; Jackson, R.B.; Porporato, A. Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol. Monogr. 2010, 80, 89–106. [Google Scholar] [CrossRef]
- Wild, B.; Schnecker, J.; Knoltsch, A.; Takriti, M.; Mooshammer, M.; Gentsch, N.; Mikutta, R.; Alves, R.J.; Gittel, A.; Lashchinskiy, N.; et al. Microbial nitrogen dynamics in organic and mineral soil horizons along a latitudinal transect in western Siberia. Glob. Biogeochem. Cycles 2015, 29, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Liu, M.; Gan, B.; Li, Q.; Xiao, W.; Song, X. Effects of Nitrogen and Phosphorus Addition on Soil Extracellular Enzyme Activity and Stoichiometry in Chinese Fir (Cunninghamia lanceolata) Forests. Front. Plant Sci. 2022, 13, 834184. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, G.; Li, P.; Li, Q.; Xue, S. Ecoenzymatic stoichiometry and microbial nutrient limitation during secondary succession of natural grassland on the Loess Plateau, China—ScienceDirect. Soil Tillage Res. 2020, 20, 104605. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.; Zhang, W.; Zhu, P.; Xiao, Q.; Wang, C.; Wu, L.; Tian, Y.; Xu, M.; Gunina, A. Stoichiometric imbalance of soil carbon and nutrients drives microbial community structure under long-term fertilization. Appl. Soil Ecol. 2021, 168, 9. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z.; Zhou, J.; Xu, X.; Zhu, Y. Long-term straw mulching with nitrogen fertilization increases nutrient and microbial determinants of soil quality in a maize–wheat rotation on China’s Loess Plateau. Sci. Total Environ. 2021, 775, 12. [Google Scholar] [CrossRef]
- Selvaraj, S.; Duraisamy, V.; Huang, Z.; Guo, F.; Ma, X. Influence of long-term successive rotations and stand age of Chinese fir (Cunninghamia lanceolata) plantations on soil properties. Geoderma 2017, 306, 127–134. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Liu, Y.; Chen, H.; Hu, Y. Response of Bacterial and Fungal Soil Communities to Chinese Fir (Cunninghamia lanceolate) Long-Term Monoculture Plantations. Front. Microbiol. 2020, 11, 181. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795-U117. [Google Scholar] [CrossRef]
- Yuan, X.; Niu, D.; Gherardi, L.A.; Liu, Y.; Wang, Y.; Elser, J.J.; Fu, H. Linkages of stoichiometric imbalances to soil microbial respiration with increasing nitrogen addition: Evidence from a long-term grassland experiment. Soil Biol. Biochem. 2019, 138, 107580. [Google Scholar] [CrossRef]
- Hessen, D.O.; Agren, G.I.; Anderson, T.R.; Elser, J.J.; De Ruiter, P.C. Carbon, sequestration in ecosystems: The role of stoichiometry. Ecology 2004, 85, 1179–1192. [Google Scholar] [CrossRef]
- Cha, S.; Kim, Y.S.; Lee, A.L.; Lee, D.H.; Koo, N. Liming alters the soil microbial community and extracellular enzymatic activities in temperate coniferous forests. Forests 2021, 12, 190. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, M.; Zhang, W.; Ni, Z.; Hashidoko, Y.; Shen, W. Ammonium nitrogen content is a dominant predictor of bacterial community composition in an acidic forest soil with exogenous nitrogen enrichment. Sci. Total Environ. 2018, 624, 407–415. [Google Scholar] [CrossRef]
- Jones, D.L.; Kielland, K.; Sinclair, F.L.; Dahlgren, R.A.; Newsham, K.K.; Farrar, J.F.; Murphy, D.V. Soil organic nitrogen mineralization across a global latitudinal gradient. Glob. Biogeochem. Cycles 2009, 23, GB1016. [Google Scholar] [CrossRef]
- Geyer, K.M.; Kyker-Snowman, E.; Grandy, A.S.; Frey, S.D. Microbial carbon use efficiency: Accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochemistry 2016, 127, 173–188. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Turner, B.L.; Talbot, J.M.; Waring, B.G.; Powers, J.S.; Kuske, C.R.; Moorhead, D.L.; Follstad Shah, J.J. Stoichiometry of microbial carbon use efficiency in soils. Ecol. Monogr. 2016, 86, 18. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Agren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Wrb, I.W.G. World reference base for soil resources 2014: International soil classification system for naming soils and creating legends for soil maps. World Soil Resour. Rep. 2014, 106, 12–21. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil Agrochemistry; China Agricultural Science and Technology Publishing House: Beijing, China, 1999; pp. 18–99. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Verchot, L.V.; Borelli, T. Application of para-nitrophenol (pNP) enzyme assays in degraded tropical soils. Soil Biol. Biochem. 2005, 37, 625–633. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, G.; Zhang, X.; He, N.; Wang, Q.; Wang, S.; Wang, R.; Zhao, N.; Jia, Y.; Wang, C. Soil enzyme activity and stoichiometry in forest ecosystems along the North-South Transect in eastern China (NSTEC). Soil Biol. Biochem. 2017, 104, 152–163. [Google Scholar] [CrossRef]
- Frostegard, A.; Baath, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Zak, D.R.; Pregitzer, K.S.; Curtis, P.S.; Holmes, W.E. Atmospheric CO2 and the composition and function of soil microbial communities. Ecol. Appl. 2000, 10, 47–59. [Google Scholar] [CrossRef]
- Zelles, L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 1997, 35, 275–294. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Zechmeister-Boltenstern, S.; Richter, A. Stoichiometric imbalances between terrestrial decomposer communities and their resources: Mechanisms and implications of microbial adaptations to their resources. Front. Microbiol. 2014, 5, 22. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: Informing enzyme-based decomposition models. Front. Microbiol. 2013, 4, 223. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A. Carbon use efficiency of microbial communities: Stoichiometry, methodology and modelling. Ecol. Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef]
- Hendricks, J.J.; Aber, J.D.; Nadelhoffer, K.J.; Hallett, R.D. Nitrogen controls on fine root substrate quality in temperate forest ecosystems. Ecosystems 2000, 3, 57–69. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Yang, X.; Chi, X.; Ji, C.; Liu, H.; Ma, W.; Mohhammat, A.; Shi, Z.; Wang, X.; Yu, S. Variations of leaf N and P concentrations in shrubland biomes across northern China: Phylogeny, climate, and soil. Biogeosciences 2016, 13, 4429–4438. [Google Scholar] [CrossRef]
- Deng, M.; Liu, L.; Sun, Z.; Piao, S.; Ma, Y.; Chen, Y.; Wang, J.; Qiao, C.; Wang, X.; Li, P. Increased phosphate uptake but not resorption alleviates phosphorus deficiency induced by nitrogen deposition in temperate Larix principis-rupprechtii plantations. New Phytol. 2016, 212, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.J.; Allen, R.B.; Doherty, J.E. Shifts in leaf N:P ratio during resorption reflect soil P in temperate rainforest. Funct. Ecol. 2008, 22, 738–745. [Google Scholar] [CrossRef]
- Haettenschwiler, S.; Aeschlimann, B.; Couteaux, M.M.; Roy, J.; Bonal, D. High variation in foliage and leaf litter chemistry among 45 tree species of a neotropical rainforest community. New Phytol. 2008, 179, 165–175. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, K.; Liu, X.; Zeng, F.; Song, T.; Peng, W.; Zhang, H.; Du, H. Stoichiometric characteristics of plants, litter and soils in karst plant communities of Northwest Guangxi. Chin. J. Plant Ecol. 2015, 39, 682–693. [Google Scholar]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Penuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Zotz, G. The resorption of phosphorus is greater than that of nitrogen in senescing leaves of vascular epiphytes from lowland Panama. J. Trop. Ecol. 2004, 20, 693–696. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; p. 1183. [Google Scholar]
- Kaiser, C.; Franklin, O.; Dieckmann, U.; Richter, A. Microbial community dynamics alleviate stoichiometric constraints during litter decay. Ecol. Lett. 2014, 17, 680–690. [Google Scholar] [CrossRef]
- Niu, Y.; Chai, R.; Jin, G.; Wang, H.; Tang, C.; Zhang, Y. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Breuer, L.; Huisman, J.A.; Keller, T.; Frede, H.G. Impact of a conversion from cropland to grassland on C and N storage and related soil properties: Analysis of a 60-year chronosequence. Geoderma 2006, 133, 6–18. [Google Scholar] [CrossRef]
- Frost, P.C.; Benstead, J.P.; Cross, W.F.; Hillebrand, H.; Larson, J.H.; Xenopoulos, M.A.; Yoshida, T. Threshold elemental ratios of carbon and phosphorus in aquatic consumers. Ecol. Lett. 2006, 9, 774–779. [Google Scholar] [CrossRef]
- Averill, C. Divergence in plant and microbial allocation strategies explains continental patterns in microbial allocation and biogeochemical fluxes. Ecol. Lett. 2014, 17, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y. Study on the Characteristics Driving Mechanisms of Soil Microbial Nutrient Limitation. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2020. [Google Scholar]
- Zou, X.; Wu, P.; Chen, N.; Wang, P.; Ma, X. Chinese fir root response to spatial and temporal heterogeneity of phosphorus availability in the soil. Can. J. For. Resesrch 2015, 45, 402–410. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, C.; Shao, S.; Chen, J.; Qin, H.; Xu, Q. Linkages of litter and soil C:N:P stoichiometry with soil microbial resource limitation and community structure in a subtropical broadleaf forest invaded by Moso bamboo. Plant Soil 2021, 465, 473–490. [Google Scholar] [CrossRef]
- Tamura, M.; Tharayil, N. Plant litter chemistry and microbial priming regulate the accrual, composition and stability of soil carbon in invaded ecosystems. New Phytol. 2014, 203, 110–124. [Google Scholar] [CrossRef]
- Mi, C. Effect of Litter Decomposition and Nutrient Circulation on Soil Polarization in the Planted Pure Forests of Loess Plateau. Ph.D. Thesis, Northwest Agriculture and Forestry University, Xianyang, China, 2014. [Google Scholar]
- Briones, M.J.I.; Ineson, P. Decomposition of eucalyptus leaves in litter mixtures. Soil Biol. Biochem. 1996, 28, 1381–1388. [Google Scholar] [CrossRef]
- Li, J. Ecological Stoichiomrtry Characteristics and Seasonal Variation in Continuous Planted Eucalyptus Plantations. Master’s Thesis, Guangxi University, Nanning, China, 2017. [Google Scholar]
- Zhou, L.; Li, J.; Luo, Y.; Liu, S.; Chen, J.; Wang, J.; Bai, Y.; Lin, W.; Wu, Z. Variation in soil fungal community structure during successive rotations of Casuarina equisetifolia plantations as determined by high-throughput sequencing analysis. Plant Growth Regul. 2019, 87, 445–453. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setala, H.; Vander Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Corneo, P.E.; Pellegrini, A.; Cappellin, L.; Roncador, M.; Chierici, M.; Gessler, C.; Pertot, I. Microbial community structure in vineyard soils across altitudinal gradients and in different seasons. Fems Microbiol. Ecol. 2013, 84, 588–602. [Google Scholar] [CrossRef]
- Inselsbacher, E.; Hinko-Najera Umana, N.; Stange, F.C.; Gorfer, M.; Schueller, E.; Ripka, K.; Zechmeister-Boltenstern, S.; Hood-Novotny, R.; Strauss, J.; Wanek, W. Short-term competition between crop plants and soil microbes for inorganic N fertilizer. Soil Biol. Biochem. 2010, 42, 360–372. [Google Scholar] [CrossRef]
- Dong, H.; Ge, J.; Sun, K.; Wang, B.; Xue, J.; Wakelin, S.A.; Wu, J.; Sheng, W.; Liang, C.; Xu, Q.; et al. Change in root-associated fungal communities affects soil enzymatic activities during Pinus massoniana forest development in subtropical China. For. Ecol. Manag. 2021, 482, 118817. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef]
- Corrales, A.; Turner, B.L.; Tedersoo, L.; Anslan, S.; Dalling, J.W. Nitrogen addition alters ectomycorrhizal fungal communities and soil enzyme activities in a tropical montane forest. Fungal Ecol. 2017, 27, 14–23. [Google Scholar] [CrossRef]
- Li, C.T. Relationship between Evolution of Understory Plant Functional Groups and Soil Nutrient in Eucalyptus Plantations; Guangxi University: Nanning, China, 2019. [Google Scholar]
- Tapia-Torres, Y.; Elser, J.J.; Souza, V.; Garcia-Oliva, F. Ecoenzymatic stoichiometry at the extremes: How microbes cope in an ultra-oligotrophic desert soil. Soil Biol. Biochem. 2015, 87, 34–42. [Google Scholar] [CrossRef]
- Wang, M.; Bhardwaj, G.; Webster, T.J. Antibacterial properties of PEKK for orthopedic applications. Int. J. Nanomed. 2017, 12, 6471–6476. [Google Scholar] [CrossRef]
| G1 | G2 | G3 | ||
|---|---|---|---|---|
| Leaf | MOC(g·kg−1) | 519.73 ± 2.33 a | 523.33 ± 5.12 a | 521.30 ± 1.90 a |
| MTN(g·kg−1) | 16.12 ± 0.59 b | 14.26 ± 0.33 c | 19.11 ± 0.84 a | |
| MTP(g·kg−1) | 1.56 ± 0.14 b | 1.39 ± 0.02 b | 2.64 ± 0.21 a | |
| MOC:MTN | 32.27 ± 1.23 b | 36.72 ± 1.19 a | 27.32 ± 1.26 c | |
| MOC:MTP | 334.99 ± 30.35 b | 376.56 ± 7.36 a | 198.24 ± 14.71 c | |
| MTN:MTP | 10.39 ± 1.00 a | 10.26 ± 0.19 a | 7.26 ± 0.54 b | |
| Litter | LOC(g·kg−1) | 577.82 ± 0.42 a | 573.27 ± 1.03 b | 562.04 ± 0.92 c |
| LTN(g·kg−1) | 12.43 ± 0.07 c | 13.62 ± 0.16 a | 13.20 ± 0.03 b | |
| LTP(g·kg−1) | 0.57 ± 0.03 c | 0.64 ± 0.02 a | 0.62 ± 0.03 ab | |
| LOC:LTN | 46.49 ± 0.23 a | 42.09 ± 0.55 b | 42.58 ± 0.09 b | |
| LOC:LTP | 1016.16 ± 59.98 a | 891.89 ± 29.95 b | 911.85 ± 45.13 b | |
| LTN:LTP | 21.86 ± 1.18 a | 21.19 ± 0.48 a | 21.42 ± 1.10 a | |
| Leaf Nutrient Resorption Efficiency | NRE (%) | 22.83 ± 2.73 b | 4.43 ± 3.30 c | 30.84 ± 3.04 a |
| PRE (%) | 63.38 ± 1.45 b | 53.72 ± 1.63 c | 76.58 ± 0.69 a | |
| NRE:PRE | 0.36 ± 0.05 a | 0.08 ± 0.05 b | 0.40 ± 0.04 a |
| G1 | G2 | G3 | ||
|---|---|---|---|---|
| Soil | pH | 4.02 ± 0.03 c | 4.10 ± 0.03 b | 4.19 ± 0.01 a |
| Moisture (%) | 15.15 ± 1.42 a | 17.75 ± 3.09 a | 17.30 ± 1.95 a | |
| SOC(g·kg−1) | 27.07 ± 0.13 b | 27.94 ± 0.39 a | 24.41 ± 0.39 c | |
| STN(g·kg−1) | 1.71 ± 0.04 b | 2.02 ± 0.03 a | 1.70 ± 0.03 b | |
| STP(g·kg−1) | 0.23 ± 0.01 b | 0.28 ± 0.01 a | 0.25 ± 0.02 b | |
| SOC: STN | 15.80 ± 0.33 a | 13.82 ± 0.25 b | 14.36 ± 0.47 b | |
| SOC: STP | 118.85 ± 5.00 a | 101.15 ± 1.86 b | 98.48 ± 7.26 b | |
| STN: STP | 7.52 ± 0.19 a | 7.32 ± 0.15 a | 6.85 ± 0.32 b | |
| DOC(mg·kg−1) | 348.05 ± 2.42 b | 373.31 ± 5.70 a | 328.41 ± 5.13 c | |
| TDN(mg·kg−1) | 5.79 ± 0.26 c | 48.91 ± 1.01 a | 17.29 ± 0.79 b | |
| SAP(mg·kg−1) | 1.74 ± 0.19 b | 2.05 ± 0.09 a | 2.33 ± 0.16 a | |
| DOC: TDN | 60.17 ± 3.00 a | 7.64 ± 0.28 c | 19.01 ± 0.76 b | |
| DOC: SAP | 202.02 ± 19.97 a | 182.58 ± 8.36 a | 141.23 ± 10.24 b | |
| TDN: SAP | 3.37 ± 0.45 c | 23.92 ± 1.11 a | 7.43 ± 0.39 b | |
| Microbe | MBC(mg·kg−1) | 338.04 ± 8.58 b | 362.20 ± 12.07 a | 331.93 ± 12.00 b |
| MBN(mg·kg−1) | 21.21 ± 0.48 c | 29.08 ± 1.50 a | 25.84 ± 0.24 b | |
| MBP(mg·kg−1) | 4.17 ± 0.05 c | 6.10 ± 0.16 b | 7.23 ± 0.13 a | |
| MBC: MBN | 15.94 ± 0.35 a | 12.49 ± 1.02 b | 12.85 ± 0.57 b | |
| MBC: MBP | 81.08 ± 2.75 a | 59.38 ± 1.54 b | 45.94 ± 2.45 c | |
| MBN: MBP | 5.09 ± 0.18 a | 4.77 ± 0.28 a | 3.57 ± 0.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Ye, S.; Liu, H.; Deng, X.; He, P.; Cheng, F. Linkage between Leaf–Litter–Soil, Microbial Resource Limitation, and Carbon-Use Efficiency in Successive Chinese Fir (Cunninghamia lanceolata) Plantations. Forests 2023, 14, 357. https://doi.org/10.3390/f14020357
Shen L, Ye S, Liu H, Deng X, He P, Cheng F. Linkage between Leaf–Litter–Soil, Microbial Resource Limitation, and Carbon-Use Efficiency in Successive Chinese Fir (Cunninghamia lanceolata) Plantations. Forests. 2023; 14(2):357. https://doi.org/10.3390/f14020357
Chicago/Turabian StyleShen, Lu, Shaoming Ye, Haiyu Liu, Xiangsheng Deng, Peng He, and Fei Cheng. 2023. "Linkage between Leaf–Litter–Soil, Microbial Resource Limitation, and Carbon-Use Efficiency in Successive Chinese Fir (Cunninghamia lanceolata) Plantations" Forests 14, no. 2: 357. https://doi.org/10.3390/f14020357
APA StyleShen, L., Ye, S., Liu, H., Deng, X., He, P., & Cheng, F. (2023). Linkage between Leaf–Litter–Soil, Microbial Resource Limitation, and Carbon-Use Efficiency in Successive Chinese Fir (Cunninghamia lanceolata) Plantations. Forests, 14(2), 357. https://doi.org/10.3390/f14020357





