Differential Effects of Desiccation on Hornworts with Contrasting Life Histories in Tropical Montane Forests: A Functional Trait—Based Perspective
Abstract
1. Introduction
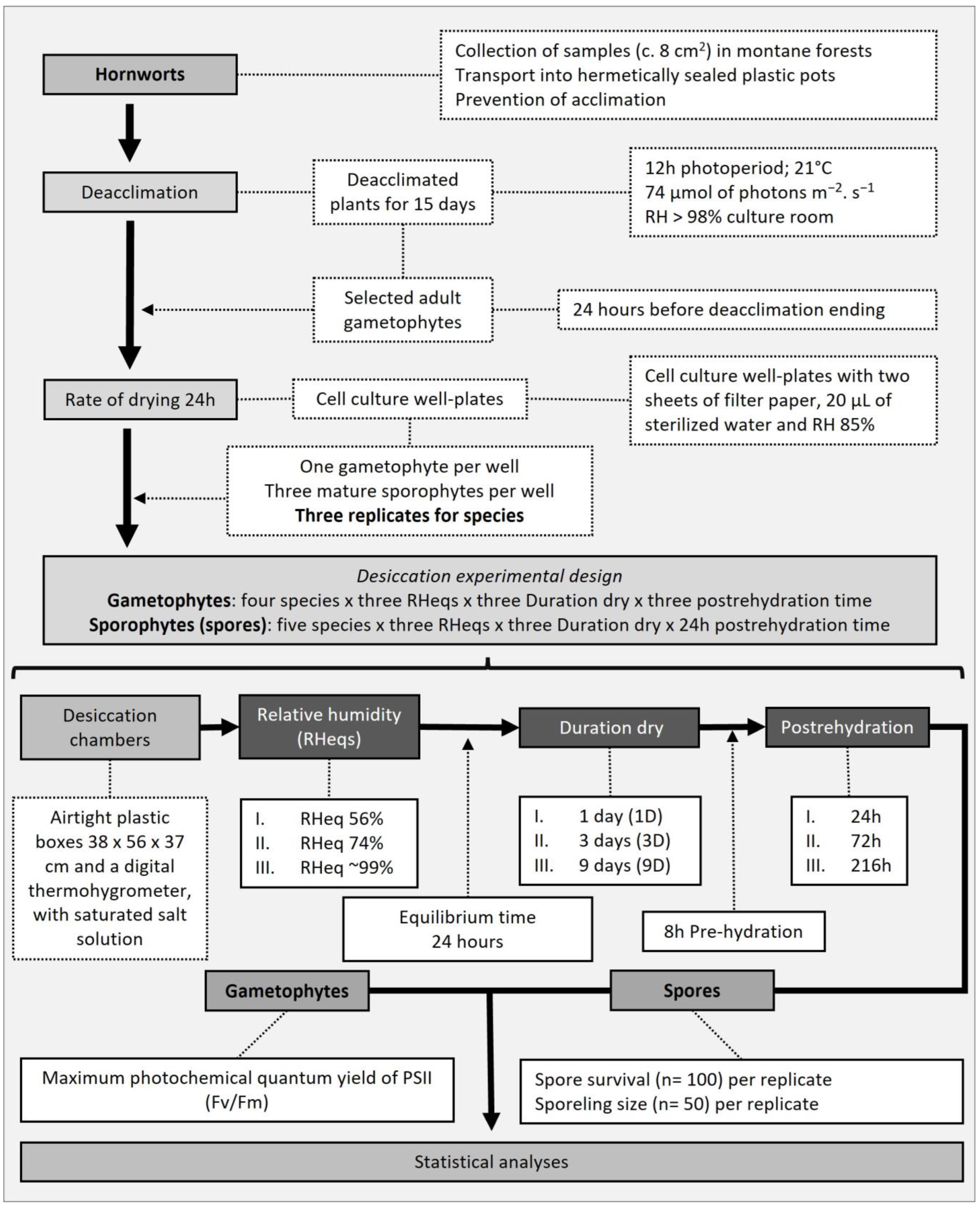
2. Materials and Methods
2.1. Species Descriptions
2.2. Field Sampling
2.3. Deacclimation
2.4. Experimental Design and Desiccation
2.5. Statistical Analyses
3. Results
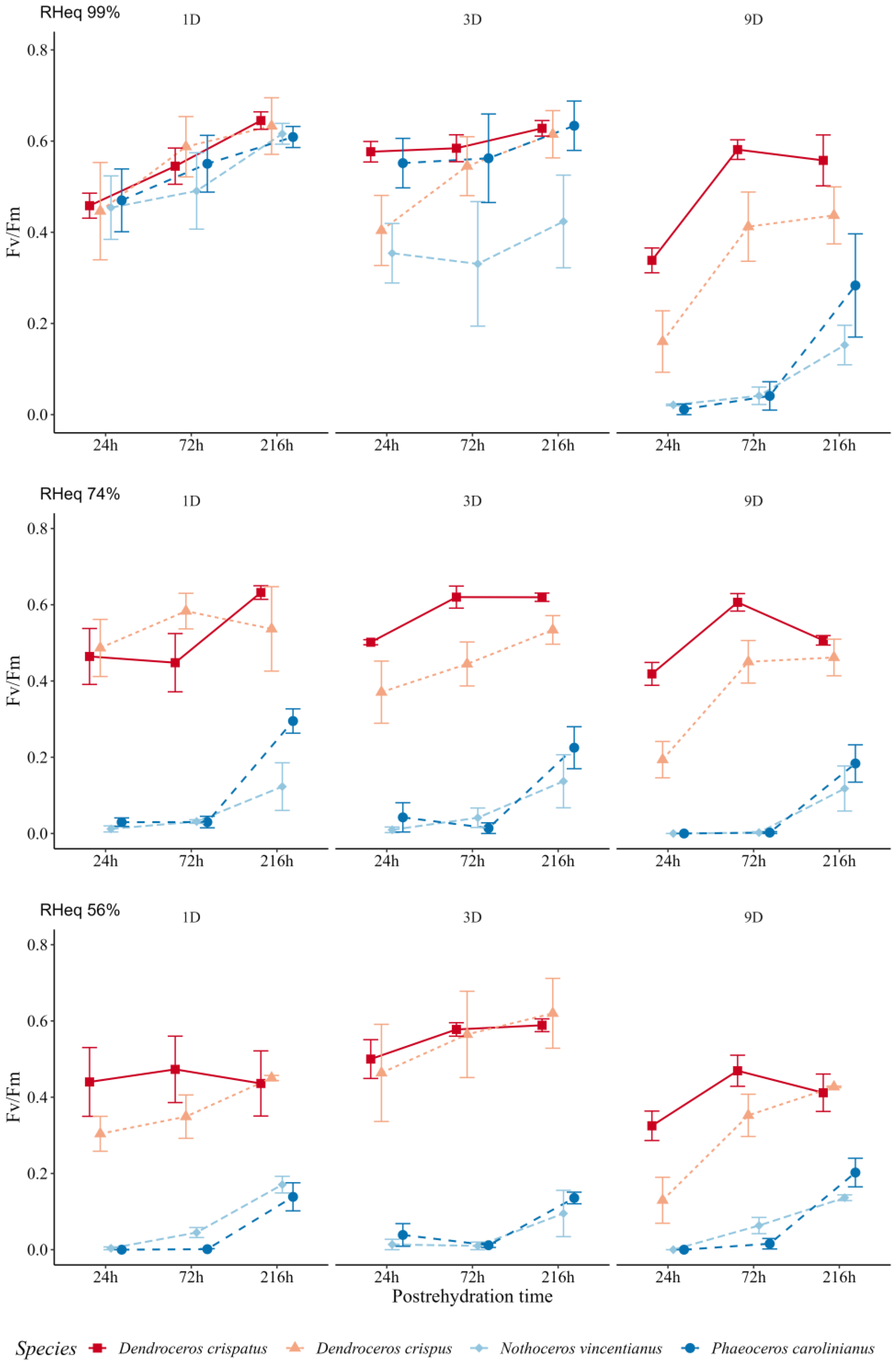
3.1. Desiccation Effects on Hornwort Gametophytes
3.2. Desiccation Effects on Hornwort Spores
4. Discussion
4.1. Desiccation Tolerance of Hornwort Gametophytes
4.2. Postrehydration Effects
4.3. Desiccation Tolerance of Hornwort Spores
4.4. Ecological Strategies of DT in Hornworts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stark, L.; Brinda, J. Developing sporophytes transition from an inducible to a constitutive ecological strategy of desiccation tolerance in the moss Aloina ambigua: Effects of desiccation on fitness. Ann. Bot. 2015, 115, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.J. The nature and distribution of vegetative desiccation-tolerance in hornworts, liverworts and mosses. Bryologist 2007, 110, 163–177. [Google Scholar] [CrossRef]
- Koster, K.; Balsamo, R.; Espinoza, C.; Oliver, M. Desiccation sensitivity and tolerance in the moss Physcomitrella patens: Assessing limits and damage. Plant Growth Regul. 2010, 62, 293–302. [Google Scholar] [CrossRef]
- Alpert, P. The discovery, scope, and puzzle of desiccation tolerance in plants. Plant Ecol. 2000, 151, 5–17. [Google Scholar] [CrossRef]
- Holzinger, A.; Karsten, U. Desiccation stress and tolerance in green algae: Consequences for ultrastructure, physiological and molecular mechanisms. Front. Plant Sci. 2013, 4, 327. [Google Scholar] [CrossRef]
- Kranner, I.; Beckett, R.; Hochman, A.; Nash, T. Desiccation-tolerance in Lichens: A review. Bryologist 2008, 111, 576–593. [Google Scholar] [CrossRef]
- Potts, M.; Slaughter, S.M.; Hunneke, F.U.; Garst, J.F.; Helm, R.F. Desiccation tolerance of prokaryotes: Application of principles to human cells. Integr. Comp. Biol. 2005, 45, 800–809. [Google Scholar] [CrossRef]
- Proctor, M.; Oliver, M.; Wood, A.; Alpert, P.; Stark, L.; Cleavitt, N.; Mishler, B. Desiccation-tolerance in bryophytes: A review. Bryologist 2007, 110, 595–621. [Google Scholar] [CrossRef]
- Oliver, M. Desiccation tolerance in bryophytes: A reflection of the primitive strategy for plant survival in dehydrating habitats? Integr. Comp. Biol. 2005, 45, 788–799. [Google Scholar] [CrossRef]
- Stark, L.R. Ecology of desiccation tolerance in bryophytes: A conceptual framework and methodology. Bryologist 2017, 120, 130–165. [Google Scholar] [CrossRef]
- Oliver, M.; Farrant, J.; Hilhorst, H.W.M.; Mundree, S.; Williams, B.; Bewley, J.D. Desiccation tolerance: Avoiding cellular damage during drying and rehydration. Annu. Rev. Plant Biol. 2020, 71, 435–460. [Google Scholar] [CrossRef] [PubMed]
- Lughadha, E.N.; Govaerts, R.; Belyaeva, I.; Black, N.; Lindon, H.; Allkin, R.; Magill, R.E.; Nicolson, N. Counting counts: Revised estimates of numbers of accepted species of flowering plants, seed plants, vascular plants and land plants with a review of other recent estimates. Phytotaxa 2016, 272, 82–88. [Google Scholar] [CrossRef]
- Greenwood, J.L.; Stark, L.; Chiquoine, L. Effects of rate of drying, life history phase, and ecotype on the ability of the moss Bryum Argenteum to survive desiccation events and the influence on conservation and selection of material for restoration. Front. Ecol. Evol. 2019, 7, 388. [Google Scholar] [CrossRef]
- Porembski, S.; Barthlott, W. Granitic and gneissuc outcrops (inselbergs) as centers of diversity for desiccation tolerant vascular plants. Plant Ecol. 2000, 151, 19–28. [Google Scholar] [CrossRef]
- Rascio, N.; Rocca, N. La Resurrection plants: The puzzle of surviving extreme vegetative desiccation. CRC Crit. Rev. Plant Sci. 2005, 24, 209–225. [Google Scholar] [CrossRef]
- Bewley, J. Physiological aspects of desiccation tolerance. Annu. Rev. Plant Physiol. 1979, 30, 195–238. [Google Scholar] [CrossRef]
- Carvalho, R.C.; Branquinho, C.; da Silva, J.M. Physiological consequences of desiccation in the aquatic bryophyte Fontinalis antipyretica. Planta 2011, 234, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Green, T.G.A.; Sancho, L.G.; Pintado, A. Ecophysiology of Desiccation/Rehydration Cycles in Mosses and Lichens. In Plant Desiccation Tolerance. Ecological Studies (Analysis and Synthesis); Lüttge, U., Beck, E., Bartels, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 215, pp. 89–120. ISBN 978-3-642-19106-0. [Google Scholar]
- Brinda, J.; Stark, L.; Clark, T.; Greenwood, J. Embryos of a moss can be hardened to desiccation tolerance: Effects of rate of drying on the timeline of recovery and dehardening in Aloina ambigua (Pottiaceae). Ann. Bot. 2016, 117, 153–163. [Google Scholar] [CrossRef]
- Söderström, L.; Hagborg, A.; von Konrat, M.; Bartholomew-Began, S.; Bell, D.; Briscoe, L.; Brown, E.; Cargill, D.C.; da Costa, D.P.; Crandall-Stotler, B.J.; et al. World checklist of hornworts and liverworts. PhytoKeys 2016, 59, 1–828. [Google Scholar] [CrossRef]
- Desirò, A.; Duckett, J.; Pressel, S.; Villarreal, J.C.; Bidartondo, M. Fungal symbioses in hornworts: A chequered history. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130207. [Google Scholar] [CrossRef]
- Villarreal, J.C.; Renzaglia, K. Structure and development of Nostoc strands in Leiosporoceros dussii (Anthocerotophyta): A novel symbiosis in land plants. Am. J. Bot. 2006, 93, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Hauser, D.; Li, F.-W. Symbiotic cyanobacteria communities in hornworts across time, space, and host species. bioRxiv 2020. [Google Scholar] [CrossRef]
- Villarreal, J.C.; Renner, S. Hornwort pyrenoids, carbon-concentrating structures, evolved and were lost at least five times during the last 100 million years. Proc. Natl. Acad. Sci. USA 2012, 109, 18873–18878. [Google Scholar] [CrossRef]
- Li, F.-W.; Villarreal, J.C.; Szövényi, P. Hornworts: An overlooked window into carbon-concentrating mechanisms. Trends Plant Sci. 2017, 22, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, J.C.; Renzaglia, K. Sporophyte structure in the neotropical hornwort Phaeomegaceros fimbriatus: Implications for phylogeny, taxonomy, and character evolution. Int. J. Plant Sci. 2006, 167, 413–427. [Google Scholar] [CrossRef]
- Peñaloza-Bojacá, G.; Villarreal, J.C.; Maciel-Silva, A. Phylogenetic and morphological infrageneric classification of the genus Dendroceros (Dendrocerotaceae; Anthocerotophyta), with the addition of two new subgenera. Syst. Biodivers. 2019, 17, 712–727. [Google Scholar] [CrossRef]
- Renzaglia, K.; Villarreal, J.C.; Duff, R.J.; Goffinet, B. New Insights into Morphology, Anatomy, and Systematics of Hornworts. In Bryophyte Biology; Shaw, A.J., Ed.; Cambridge University Press: Cambridge, UK, 2009; pp. 139–172. [Google Scholar]
- Renzaglia, K.; Villarreal, J.C.; Garbary, D. Morphology supports the setaphyte hypothesis: Mosses plus liverworts form a natural group. Bryophyt. Divers. Evol. 2018, 40, 11–17. [Google Scholar] [CrossRef]
- Leebens-Mack, J.; Barker, M.; Carpenter, E.; Deyholos, M.; Gitzendanner, M.; Graham, S.; Grosse, I.; Li, Z.; Melkonian, M.; Mirarab, S.; et al. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 2019, 574, 679–685. [Google Scholar] [CrossRef]
- Wickett, N.J.; Mirarab, S.; Nguyen, N.; Warnow, T.; Carpenter, E.; Matasci, N.; Ayyampalayam, S.; Barker, M.S.; Burleigh, J.G.; Gitzendanner, M.A.; et al. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc. Natl. Acad. Sci. USA 2014, 111, E4859–E4868. [Google Scholar] [CrossRef]
- Duff, J.; Villarreal, J.C.; Cargill, C.; Renzaglia, K. Progress and challenges toward developing a phylogeny and classification of the hornworts. Bryologist 2007, 110, 214–243. [Google Scholar] [CrossRef]
- Gradstein, S.R.; Churchill, S.P.; Salazar-Allen, N. Guide to the Bryophytes of Tropical America; The New York Botanical Garden Press: New York, NY, USA, 2001; Volume 86, ISBN 0-89327-435-6. [Google Scholar]
- Villarreal, J.C.; Renzaglia, K. The hornworts: Important advancements in early land plant evolution. J. Bryol. 2015, 37, 157–170. [Google Scholar] [CrossRef]
- Hosokawa, T.; Kubota, H. On the osmotic pressure and resistance to desiccation of epiphytic mosses from a beech forest, south-west Japan. J. Ecol. 1957, 45, 579. [Google Scholar] [CrossRef]
- Schuette, S.; Renzaglia, K. Development of multicellular spores in the hornwort genus Dendroceros (Dendrocerotaceae, Anthocerotophyta) and the occurrence of endospory in Bryophytes. Nov. Hedwigia 2010, 91, 301–316. [Google Scholar] [CrossRef]
- Leon-Vargas, Y.; Engwald, S.; Proctor, M. Microclimate, light adaptation and desiccation tolerance of epiphytic bryophytes in two Venezuelan cloud forests. J. Biogeogr. 2006, 33, 901–913. [Google Scholar] [CrossRef]
- Stark, L.; Greenwood, J.; Brinda, J.; Oliver, M. The desert moss Pterygoneurum lamellatum (Pottiaceae) exhibits an inducible ecological strategy of desiccation tolerance: Effects of rate of drying on shoot damage and regeneration. Am. J. Bot. 2013, 100, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- McLetchie, N.; Stark, L. Rate of drying influences tolerance of low water contents in the moss Funaria hygrometrica (Funariaceae). Bryologist 2019, 122, 271. [Google Scholar] [CrossRef]
- Lloyd, R.M.; Klekowski, E.J. Spore germination and viability in Pteridophyta: Evolutionary significance of chlorophyllous spores. Biotropica 1970, 2, 129. [Google Scholar] [CrossRef]
- Maciel-Silva, A.; Da Silva, F.; Válio, I. All green, but equal? Morphological traits and ecological implications on spores of three species of mosses in the Brazilian Atlantic Forest. An. Acad. Bras. Cienc. 2014, 86, 1249–1262. [Google Scholar] [CrossRef]
- Pence, V. Survival of Chlorophyllous and Nonchlorophyllous fern spores through exposure to liquid nitrogen. Am. Fern J. 2000, 90, 119–126. [Google Scholar] [CrossRef]
- Sundue, M.; Vasco, A.; Moran, R. Cryptochlorophyllous spores in ferns: Nongreen spores that contain chlorophyll. Int. J. Plant Sci. 2011, 172, 1110–1119. [Google Scholar] [CrossRef]
- Bisang, I. Quantitative analysis of the diaspore banks of bryophytes and ferns in cultivated fields in Switzerland. Lindbergia 1996, 21, 9–20. [Google Scholar]
- Villarreal, J.C.; Cargill, D.; Hagborg, A.; Soderstrom, L.; Renzaglia, K. A synthesis of hornwort diversity: Patterns, causes and future work. Phytotaxa 2014, 9, 150. [Google Scholar] [CrossRef]
- Renzaglia, K.; Vaughn, K. Anatomy, development and classification of hornworts. In Bryophyte Biology; Shaw, A.J., Goffinet, B., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 1–35. [Google Scholar]
- Renzaglia, K. A comparative morphology and developmental anatomy of the Anthocerotophyta. J. Hattori Bot. Lab. 1978, 44, 31–90. [Google Scholar]
- Villarreal, J.C.; Campos, L.V.; Uribe-M., J.; Goffinet, B. Parallel evolution of endospory within hornworts: Nothoceros renzagliensis (Dendrocerotaceae), sp. nov. Syst. Bot. 2012, 37, 31–37. [Google Scholar] [CrossRef]
- Villarreal, J.C.; Renner, S. A review of molecular-clock calibrations and substitution rates in liverworts, mosses, and hornworts, and a timeframe for a taxonomically cleaned-up genus Nothoceros. Mol. Phylogenet. Evol. 2014, 78, 25–35. [Google Scholar] [CrossRef]
- Penjor, P.; Chantanaorrapint, S.; Meesawat, U. Morphological and anatomical features of cosmopolitan hornwort: Phaeoceros carolinianus (Michx.) Prosk. Walailak J. Sci. Technol. 2016, 13, 769–779. [Google Scholar]
- Proskauer, J. Studies on Anthocerotales V. Phytomorphology 1957, 7, 113–135. [Google Scholar]
- Ibarra-Morales, A.; Muñíz, M.E.; Valencia, S. The genus Anthoceros (Anthocerotaceae, Anthocerotophyta) in central Mexico. Phytotaxa 2015, 205, 215–228. [Google Scholar] [CrossRef]
- IBAMA Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis. Available online: https://uc.socioambiental.org/ (accessed on 16 March 2020).
- INEA Plano de Manejo—Parque Estadual Dos Três Picos (PETP). Available online: http://www.inea.rj.gov.br/ (accessed on 23 December 2022).
- Hájek, T.; Vicherová, E. Desiccation tolerance of Sphagnum revisited: A puzzle resolved. Plant Biol. 2014, 16, 765–773. [Google Scholar] [CrossRef]
- Hellwege, E.M.; Dietz, K.-J.; Volk, O.H.; Hartung, W. Abscisic acid and the induction of desiccation tolerance in the extremely xerophilic liverwort Exormotheca holstii. Planta 1994, 194, 525–531. [Google Scholar] [CrossRef]
- Beckett, R. Partial dehydration and ABA induce tolerance to desiccation-induced ion leakage in the moss Atrichum androgynum. S. Afr. J. Bot. 1999, 65, 212–217. [Google Scholar] [CrossRef]
- Butler, W.L.; Kitajima, M. Fluorescence quenching in Photosystem II of chloroplasts. Biochim. Biophys. Acta Bioenerg. 1975, 376, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.-M.; Baker, N. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Cruz de Carvalho, R.; Maurício, A.; Pereira, M.F.; Marques da Silva, J.; Branquinho, C. All for one: The role of colony morphology in Bryophyte desiccation tolerance. Front. Plant Sci. 2019, 10, 1360. [Google Scholar] [CrossRef]
- Marks, R.A.; Burton, J.F.; McLetchie, D.N. Sex differences and plasticity in dehydration tolerance: Insight from a tropical liverwort. Ann. Bot. 2016, 118, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, T.; Kadota, A.; Wada, M. Photoinduction of spore germination in Marchantia polymorpha L. is mediated by photosynthesis. Plant Cell Physiol. 1999, 40, 1014–1020. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 15 October 2018).
- RStudio Team. RStudio: Integrated Development Environment for R. Available online: http://www.rstudio.com/ (accessed on 20 February 2019).
- Wickham, H.; Chang, W. Ggplot2: An Implementation of the Grammar of Graphics. R Package Version 0.8.3. Available online: http://CRAN.R-project.org/package=ggplot2,3 (accessed on 4 December 2019).
- Kemp, F. Modern Applied Statistics with S. J. R. Stat. Soc. Ser. D Stat. 2003, 52, 704–705. [Google Scholar] [CrossRef]
- Renzaglia, K.; Schuette, S.; Duff, J.; Ligrone, R.; Shaw, A.J.; Mishler, B.D.; Duckett, J.G. Bryophyte phylogeny: Advancing the molecular and morphological frontiers. Bryologist 2007, 110, 179–213. [Google Scholar] [CrossRef]
- Watkins, J.; Mack, M.; Sinclair, T.; Mulkey, S. Ecological and evolutionary consequences of desiccation tolerance in tropical fern gametophytes. N. Phytol. 2007, 176, 708–717. [Google Scholar] [CrossRef]
- Abel, W. Die Austrocknungsresistenz der Laubmoose. Sitzungsberichte der Akad. der Wissenschaften Math. Klasse 1956, 165, 619–707. [Google Scholar]
- Lakatos, M. Lichens and Bryophytes: Habitats and Species. In Plant Desiccation Tolerance. Ecological Studies (Analysis and Synthesis); Lüttge, U., Beck, E., Bartels, D., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2011; Volume 215, pp. 65–87. [Google Scholar]
- Larson, D.W. Differential wetting in some lichens and mosses: The role of morphology. Bryologist 1981, 84, 1. [Google Scholar] [CrossRef]
- Hoekstra, F.; Golovina, E.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, P.; Chu, L.; But, P.P. Three modes of asexual reproduction in the moss Octoblepharum albidum. J. Bryol. 2003, 25, 175–179. [Google Scholar] [CrossRef]
- Duckett, J.; Ligrone, R. A survey of diaspore liberation mechanisms and germination patterns in mosses. J. Bryol. 1992, 17, 335–354. [Google Scholar] [CrossRef]
- Duckett, J.; Goode, J.; Matcham, H. Studies of protonemal morphogenesis in mosses. VIII. The gemmiferous protonemata of Orthodontium and Dicranoweisia. J. Bryol. 2001, 23, 181–193. [Google Scholar] [CrossRef]
- Mogensen, G. The biological significance of morphological characters in bryophytes: The Spore. Bryologist 1981, 84, 187. [Google Scholar] [CrossRef]
- Renzaglia, K.; Lopez, R.; Welsh, R.; Owen, H.; Merced, A. Callose in sporogenesis: Novel composition of the inner spore wall in hornworts. Plant Syst. Evol. 2020, 306, 16. [Google Scholar] [CrossRef]
- Ellinger, D.; Voigt, C.A. Callose biosynthesis in arabidopsis with a focus on pathogen response: What we have learned within the last decade. Ann. Bot. 2014, 114, 1349–1358. [Google Scholar] [CrossRef]
- Yim, K.; Bradford, K.J. Callose deposition is responsible for apoplastic semipermeability of the endosperm envelope of muskmelon seeds. Plant Physiol. 1998, 118, 83–90. [Google Scholar] [CrossRef]
- Johri, B.M. (Ed.) Embryology of Angiosperms; Springer: Berlin/Heidelberg, Germany, 1984; ISBN 978-3-642-69304-5. [Google Scholar]
- Mogensen, G. The Spore. New Man. Bryol. 1983, 325–342. [Google Scholar]
- Oliveira, B.A.; Pereira, A.F.d.N.; Pôrto, K.C.; Maciel-Silva, A.S. Spore germination and young gametophyte development of the endemic Brazilian hornwort Notothylas vitalii Udar & Singh (Notothyladaceae—Anthocerotophyta), with insights into sporeling evolution. Acta Bot. Bras. 2017, 31, 313. [Google Scholar] [CrossRef]
- López-Pozo, M.; Fernández-Marín, B.; García-Plazaola, J.I.; Ballesteros, D. Desiccation tolerance in ferns: From the unicellular spore to the multi-tissular sporophyte. In Current Advances in Fern Research; Fernández, H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 401–426. ISBN 978-3-319-75103-0. [Google Scholar]
- Maciel-Silva, A.; Pôrto, K. Reproduction in Bryophytes. In Reproductive Biology of Plants; Ramawat, K., Mérillon, J.-M., Shivanna, R., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 57–84. ISBN 9781482201321. [Google Scholar]
- Peñaloza-Bojacá, G.; Oliveira, B.; Araujo, C.A.T.; Villarreal, J.C.; Fantecelle, L.B.; Maciel-Silva, A. Dendrocerotaceae in Flora Do Brasil 2020. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB97157 (accessed on 19 March 2021).
- Ligrone, R.; Renzaglia, K. The sporophyte–gametophyte junction in the hornwort, Dendroceros tubercularis Hatt (Anthocerotophyta). New Phytol. 1990, 114, 497–505. [Google Scholar] [CrossRef] [PubMed]
- López-Pozo, M.; Gasulla, F.; García-Plazaola, J.I.; Fernández-Marín, B. Unraveling metabolic mechanisms behind chloroplast desiccation tolerance: Chlorophyllous fern spore as a new promising unicellular model. Plant Sci. 2019, 281, 251–260. [Google Scholar] [CrossRef]
- Testo, W.; Watkins, J. Influence of plant size on the ecophysiology of the epiphytic fern Asplenium auritum (Aspleniaceae) from Costa Rica. Am. J. Bot. 2012, 99, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Stark, L.; Oliver, M.; Mishler, B.; McLetchie, N. Generational differences in response to desiccation stress in the desert moss Tortula inermis. Ann. Bot. 2007, 99, 53–60. [Google Scholar] [CrossRef]
- Sato, T.; Sakai, A. Freezing resistance of gametophytes of the temperate fern, Polystichum retroso-paleaceum. Can. J. Bot. 1980, 58, 1144–1148. [Google Scholar] [CrossRef]
- Mendieta-Leiva, G.; Ramos, F.N.; Elias, J.P.C.; Zotz, G.; Acuña-Tarazona, M.; Alvim, F.S.; Barbosa, D.E.F.; Basílio, G.A.; Batke, S.P.; Benavides, A.M.; et al. EpIG-DB: A database of vascular epiphyte assemblages in the Neotropics. J. Veg. Sci. 2020, 31, 518–528. [Google Scholar] [CrossRef]
- Laube, S.; Zotz, G. Neither host-specific nor random: Vascular epiphytes on three tree species in a panamanian lowland forest. Ann. Bot. 2006, 97, 1103–1114. [Google Scholar] [CrossRef]
- Zotz, G.; Bogusch, W.; Hietz, P.; Ketteler, N. Growth of epiphytic bromeliads in a changing world: The effects of CO2, water and nutrient supply. Acta Oecologica 2010, 36, 659–665. [Google Scholar] [CrossRef]
- Benzing, D.H. Vulnerabilities of tropical forests to climate change: The significance of resident epiphytes. Clim. Chang. 1998, 39, 519–540. [Google Scholar] [CrossRef]
- Pardow, A.; Lakatos, M. Desiccation tolerance and global change: Implications for tropical bryophytes in lowland forests. Biotropica 2013, 45, 27–36. [Google Scholar] [CrossRef]
- Walsworth, T.E.; Schindler, D.E.; Colton, M.A.; Webster, M.S.; Palumbi, S.R.; Mumby, P.J.; Essington, T.E.; Pinsky, M.L. Management for network diversity speeds evolutionary adaptation to climate change. Nat. Clim. Chang. 2019, 9, 632–636. [Google Scholar] [CrossRef]
- Jump, A.S.; Penuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef] [PubMed]



| Source | Degree of Freedom (d.f.) | Deviance | Resid. Df | Resid.Dev | F | Pr(>F) |
|---|---|---|---|---|---|---|
| Chlorophyll fluorescence parameters for hornwort gametophytes | ||||||
| Null model | 323 | 184.989 | ||||
| Species | 3 | 82.696 | 320 | 102.293 | 2.130.616 | <0.001 |
| RHeq | 2 | 21.851 | 318 | 80.443 | 844.457 | <0.001 |
| Duration dry | 2 | 12.203 | 316 | 68.240 | 471.616 | <0.001 |
| Postrehydration | 2 | 12.289 | 314 | 55.951 | 474.911 | <0.001 |
| Species:RHeq | 6 | 12.782 | 308 | 43.169 | 164.665 | <0.001 |
| Species:duration dry | 6 | 0.2100 | 302 | 41.069 | 27.054 | 0.014 |
| Species:postrehydration | 6 | 0.2773 | 296 | 38.296 | 35.723 | <0.001 |
| Model: AIC: −460.44; Residual deviance: 3.8296; 296 degrees of freedom (df) | ||||||
| Survival spores | ||||||
| Null model | 134 | 55441 | ||||
| Species | 4 | 19447.3 | 130 | 35993 | 190.645 | <0.001 |
| RHeq | 2 | 967.2 | 128 | 35026 | 18.963 | 0.154 |
| Duration dry | 2 | 9.7 | 126 | 35016 | 0.0189 | 0.981 |
| Species:RHeq | 8 | 4295.7 | 118 | 30721 | 21.056 | 0.041 |
| Species:dry duration | 8 | 2668.6 | 110 | 28052 | 13.080 | 0.246 |
| Model: AIC: 1155.5; Residual deviance: 28052; 110 degrees of freedom (df) | ||||||
| Sporeling Size | ||||||
| Null model | 6749 | 580.75 | ||||
| Species | 4 | 198.547 | 6745 | 382.21 | 1.132.689 | <0.001 |
| RHeq | 2 | 17.994 | 6743 | 364.21 | 205.306 | <0.001 |
| Duration dry | 2 | 22.908 | 6741 | 341.30 | 261.377 | <0.001 |
| Species:RHeq | 8 | 26.078 | 6733 | 315.23 | 74.386 | <0.001 |
| Species:duration dry | 8 | 20.523 | 6725 | 294.70 | 58.540 | <0.001 |
| Model: AIC: −1928.8; residual deviance: 294.70; 6725 degrees of freedom (df) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñaloza-Bojacá, G.F.; Vilas-Boas, T.; Villarreal A, J.C.; Maciel-Silva, A.S. Differential Effects of Desiccation on Hornworts with Contrasting Life Histories in Tropical Montane Forests: A Functional Trait—Based Perspective. Forests 2023, 14, 255. https://doi.org/10.3390/f14020255
Peñaloza-Bojacá GF, Vilas-Boas T, Villarreal A JC, Maciel-Silva AS. Differential Effects of Desiccation on Hornworts with Contrasting Life Histories in Tropical Montane Forests: A Functional Trait—Based Perspective. Forests. 2023; 14(2):255. https://doi.org/10.3390/f14020255
Chicago/Turabian StylePeñaloza-Bojacá, Gabriel F., Tiago Vilas-Boas, Juan C. Villarreal A, and Adaíses S. Maciel-Silva. 2023. "Differential Effects of Desiccation on Hornworts with Contrasting Life Histories in Tropical Montane Forests: A Functional Trait—Based Perspective" Forests 14, no. 2: 255. https://doi.org/10.3390/f14020255
APA StylePeñaloza-Bojacá, G. F., Vilas-Boas, T., Villarreal A, J. C., & Maciel-Silva, A. S. (2023). Differential Effects of Desiccation on Hornworts with Contrasting Life Histories in Tropical Montane Forests: A Functional Trait—Based Perspective. Forests, 14(2), 255. https://doi.org/10.3390/f14020255








