Abstract
The beach vitex (Vitex rotundifolia L.), a member of the Lamiaceae family, is a salt-tolerant, woody perennial common in coastal areas worldwide. Plant–fungal association was monitored in the shoreline forest area of Wando Island in Korea in 2020, and leaf samples showing leaf spot disease were collected. Eight fungal isolates were recovered from the samples on PDA and identified based on the morphological characteristics and multilocus molecular phylogeny. Among the eight isolates, four were identified as Alternaria alternata, two as Fusarium humuli, one as Colletotrichum aenigma, and one as Stagonosporopsis caricae. Pathogenicity tests of the fungal isolates on the detached leaves of beach vitex revealed that S. caricae CMML20–2 and A. alternata (CMML20–7, CMML20–8, CMML20–9, and CMML20–10) caused disease lesions while the other species did not. The fungal species S. caricae, C. aenigma, and F. humuli are the first reported in the host worldwide, and S. caricae and F. humuli are first reported in Korea. In vitro fungicide sensitivity assays were carried out based on a measurement of diametrical mycelial growth on media amended with different doses of the fungicides fludioxonil, metconazole, and fluxapyroxad. Fungicide sensitivity varied significantly among the species, and the A. alternata and S. caricae isolates were more sensitive to fluxapyroxad than the other isolates. Our study contributes to the understanding of fungal diversity in forest mycology and demonstrates that pathogenic fungi including A. alternata and S. caricae might accelerate decline in leaf health.
1. Introduction
Beach vitex (Vitex rotundifolia L.) is a low-growing woody shrub in the subfamily Viticoideae of the family Lamiaceae and grows in dune ecosystems in temperate and tropical countries [1,2,3]. It is also known as a medicinal plant for anti-inflammatory and allergic reactions and used to treat headaches, eye pain, asthma, and bronchitis. The plant has a long history of ethnopharmacological use in European and Asian countries including China, Korea, Japan, and Vietnam [4,5]. Functional research of secondary metabolites in beach vitex has shown its possible efficacy for anti-hyperlipidemia [6] and anti-cancer [7,8] activity. However, increased use of the plant for medical and erosion reduction purposes has severely destroyed its natural vegetation and even threatened the very existence of the species [9]. In China and Japan, for example, the plant has been identified as an important wild woody plant species to be conserved. Therefore, it is necessary to provide an effective plant management strategy for the species both in the world as well as in Korea.
Fungal diseases of this host plant have been reported to cause severe losses in plants. To date, six pathogens have been reported from beach vitex: Corynespora cassiicola from Korea and Taiwan, Cryptostictis inaequalis from the USA, Corynespora viticus from China, Exosporium vicitis from Taiwan, and Mycosphaerella viticis and Pseudocercospora viticola from Japan Fungal Databases, U.S. National Fungus Collections (https://nt.ars-grin.gov/fungaldatabases, accessed on 19 December 2022). C. cassiicola has been reported to cause severe leaf spot disease in Korea and Taiwan [3,10]. In addition to these pathogens, a total of 1052 endophytic fungal isolates were recovered from the roots, leaves, and branches of the plant in Taiwan and the species with the three highest isolation frequencies were A. alternata, Aspergillus terreus, and Alpestrisphaeria sp. [4].
The marine habitat is the host of a wide variety of microorganisms, especially fungi, which offer a rich source of novel metabolites [11]. Modern technological and molecular agriculture advances have opened new frontiers in natural product research, leading to new discoveries from nature [12]. Therefore, we investigated the fungal assemblages in leaves of beach vitex in Korea. The study aimed to characterize the fungi using morphological and molecular methods, and to test their pathogenicity. Additionally, we used the fungicide sensitivity assays of these isolated fungi against fludioxonil, metconazole, and fluxapyroxad.
2. Materials and Methods
2.1. Fungal Isolation
In May 2020, a total of 10 diseased leaf samples showing leaf spot near the roadside of Wando Island’s mudflat in the Republic of Korea (latitude 34°22′16.9″ N, longitude 126°38′31.6″ E) were collected in plastic polyethylene bags (Figure 1). The leaves were brought to the laboratory and stored in a refrigerator prior to the isolation of the fungi. For fungal isolation, samples were surface-sterilized with 1% NaOCl solution for 1 min, rinsed with sterilized distilled water three times, and then air-dried on filter paper in a laminar airflow chamber. Then, the disease lesions were cut into small pieces (0.5 × 0.5 cm) and placed onto potato dextrose agar (PDA, BD Difco, Franklin Lakes, NJ, USA) supplemented with 50 μg mL−1 of streptomycin and ampicillin (MB Cell, Seoul, Republic of Korea) to inhibit bacterial growth. After incubation at 25 °C for 3–10 days, individual hyphal tips of the developing fungal colonies were excised and then transferred to fresh PDA in order to obtain pure culture isolates, before being stored in a 20% glycerol stock solution at −80 °C. A total of eight isolates were obtained, assigned identification numbers (CMML20–2, CMML20–7, CMML20–8, CMML20–9, CMML20–10, CMML20–11, CMML20–12, and CMML20–13), and preserved in the Molecular Microbiology Laboratory, Department of Integrative Food, Bioscience, and Biotechnology, Chonnam National University, Gwangju, Republic of Korea.
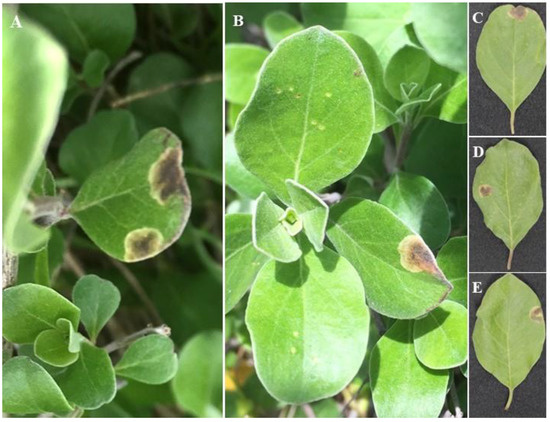
Figure 1.
Disease symptoms on leaves of beach vitex plants. Natural disease symptoms (A,B). Harvested individual leaves showing symptoms (C–E).
2.2. DNA Extraction, PCR Amplification, and Molecular Phylogeny
The total genomic DNA of all examined isolates was extracted directly from the mycelia grown on PDA using the CTAB DNA-extraction method [13]. The following genomic regions were amplified: internal transcribed spacer (ITS) for all isolate and endopolygalacturonase (endoPG) and anonymous (OPA10-2) for Alternaria isolates; calmodulin (CAL) and chitin synthase (CHS) for Colletotrichum isolate; calmodulin (CaM) and translation elongation factor 1α (EF-1α) for Fusarium isolates; and RNA polymerase II subunit (RPB2) and beta-tubulin (BT) for Stagonosporopsis isolate. A total of three molecular markers were used for the identification of each isolate at the species level (Figure 2). Amplifications of the ITS, CAL, CaM, CHS, EF-1α, RPB2, BT, endoPG, and OPA10-2 were performed using the primer pairs of ITS1–ITS4 [14], CL1C–CL2C [15], CL1–CL2A [16], CHS-79F–CHS–345R [15], EF1–EF2 [17], RPB2 5F–RPB2 7cR [18], TUB2Fd–TUB4Rd [19], PG3–PG2b [20], and OPA10-2R–OPA10-2L [20], respectively. Polymerase chain reactions (PCRs) were carried out using a SimpliAmp™ PCR system (Thermo Fisher Scientific, Waltham, MA, USA) in a 25 μL reaction volume containing 0.25 μL of Takara Ex Taq® DNA polymerase (5 U/μL), 2.5 μL of 10× Ex Taq buffer, 2 μL of dNTP mixture (2.5 mM each), 1 μL of each primer (10 pmoles/μL), 1 μL of template DNA solution (100 ng/μL), and DNase/RNase-free water up to 25 μL. PCR amplification conditions were initial denaturation at 98 °C for 30 s, followed by 35 cycles of denaturation at 98 °C for 15 s. Annealing temperatures were as follows: 49 °C for RPB2, 50 °C for ITS, 52 °C for BT and OPA10-2, 54 °C for CHS, 55 °C for CAL and EF-1α, 59 °C for CaM, 62 °C for endoPG for 30 s, and a final extension at 72 °C for 2 min. PCR products were sequenced in both directions by a commercial sequencing service provider (Macrogen, Daejeon, Republic of Korea).
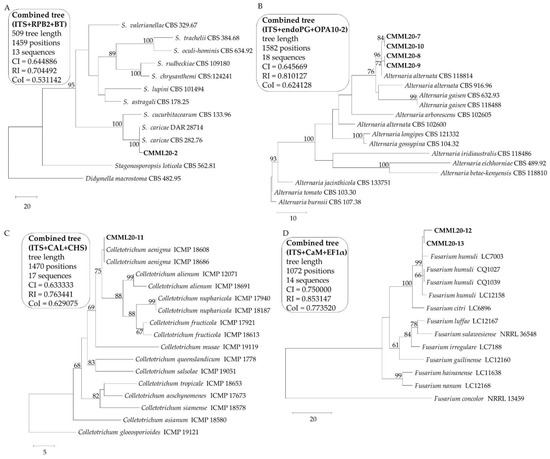
Figure 2.
Phylogenetic tree constructed by the maximum parsimony method from alignment of combined datasets (A) CMML20–2, ITS, RPB2, and BT, (B) CMML20–(7–10), ITS, endoPG, and OPA10-2, (C) CMML20–(11–12), ITS, CAL, and CHS, (D) CMML2–12 CAL, CHS, EF1α, RPB2, and BT. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. Bootstrap values >50% are indicated above branches. All positions containing gaps and missing data were eliminated. The isolates in this study are marked in bold.
The DNA sequences were retrieved from GenBank after BLASTN search analysis for molecular phylogenetic analysis (Table S1). All the sequences were aligned using the Clustal Omega implemented in the MEGA X program with manual adjusting by truncating the ends [21]. The evolutionary history was inferred by the maximum parsimony method in the MEGA X program with 1000 bootstrap replications.
2.3. Morphology
For morphological characterization, the isolates were cultured on PDA or V8 juice agar (20% (v/v) V8 juice, 3 g/L CaCO3 and 15 g/L Agar) at 25 °C in the dark for 7 days (Figure 3, Figure 4, Figure 5 and Figure 6). Colonies assumed to be Colletotrichum and Fusarium (CMML20–11, CMML 20–12, and CMML20–13) produced spores on PDA after culturing for 10–12 days at 25 °C. For the Stagonosporopsis isolate (CMML20–2), mycelia on PDA were scratched off and removed after 7 days of incubation. The remaining plate was placed under NUV (near-ultraviolet) light in alternating 12 h–12 h light–dark conditions [22]. After 3–5 days of culture, pycnidia were induced and observed under a microscope (OLYMPUS, Tokyo, Japan). The Alternaria isolates (CMML 20–7, CMML 20–8, CMML 20–9, and CMML 20–10) induced sporulation on the V8 juice agar. A square of the growing marginal colony of CMML20–11 was covered with a sterile cover glass. After 3 days of culture, appressoria were observed under the microscope. The size, shape, and septation of the conidia of all isolates were measured under the microscope.

Figure 3.
Anterior and posterior view of CMML20–2 colony on PDA at 25 °C for 7 days (A,B). The structure of pycnidia (C,D) and conidia (E) formed on PDA observed under the microscope. Scale bars in (C,D) = 100 μm, (E) = 10 μm.

Figure 4.
Colony morphology of the isolates of CMML20–12 and CMML–13 on PDA at 25° C for 7 days (A–D). Different conidial sizes and shapes are observed under the microscope (E,F). Scale bars (E,F) = 20 μm.
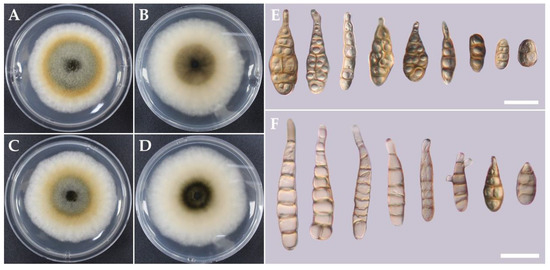
Figure 5.
Obverse and reverse colony characteristics of CMML20–7 and CMML20–8 isolates on PDA at 25 °C for 7 days (A–D). Different conidial sizes and shapes are observed under the microscope (E,F). Scale bars (E,F) = 20 μm.

Figure 6.
Colony characteristics of the isolate CMML20–11 on PDA at 25 °C for 7 days (A–D). A number of conidiomata under 20× magnification (C,D) and conidia (E) were observed under the microscope. Scale bars (E) = 10 μm.
2.4. Pathogenicity Tests
Pathogenicity tests of the isolates were performed in Vitex rotundifolia detached leaves with agar plugs to confirm whether the leaf-associated fungi produce disease. The isolates were grown on PDA for 5 days at 25 °C, and agar plugs (5 mm in diameter) were taken from the edge of the colony and placed on the wounded adaxial surface of the leaves; non-inoculated wounded leaves served as controls. Inoculated and non-inoculated leaves were placed on wet 90 mm filter paper in a 90 mm petri dish and maintained at 25 °C under a day–night cycle of 16 h light and 8 h darkness for 7 days with high-humidity conditions. After incubation, the width of the lesion was measured using a digital caliper (Mitutoyo, Kanagawa, Japan). Statistical significance of differences between means of lesion length was determined by Tukey’s honestly significant difference (HSD) test. Different letters above bars indicate significant difference (p < 0.05). The experiment was performed in triplicate on three replicate leaves for each of the isolates.
2.5. In Vitro Fungicide Sensitivity Assay
Sensitivities of the isolates were assayed to three commercial fungicides, fludioxonil (Syngenta, Basel, Switzerland), fluxapyroxad (BASF, Ludwigshafen, Germany), and metconazole (BASF, Ludwigshafen, Germany), which had been selected based on their mode of action and standard operating procedure. Fungicides were dissolved in distilled water to provide the desired concentration of the active ingredient and added to PDA after autoclaving when the media temperature was approximately 50 °C. Diluted fungicide solutions were added to achieve the medium at concentrations corresponding to 0.01, 0.1, 1, 10, or 100 μg mL−1 of each fungicide (fludioxonil, fluxapyroxad, and metconazole). Control plates were filled with unamended PDA (without fungicides). Isolates were cultured on PDA at 25 °C for 5 days, and agar plugs taken from a colony’s edge were placed on PDA plates. After culturing for 5 days, the colony diameter was measured using a digital caliper, and relative mycelial growth (RMG) percentage and half-maximal effective concentration (EC50) values of fludioxonil, fluxapyroxad, and metconazole for all 8 isolates were calculated using a user-developed tool in an R software package version 1.4 (R Foundation of Statistical Computing, Vienna, Austria) [23]. Statistical significance of differences between means was determined by Tukey’s honestly significant difference (HSD) test (p < 0.05). Two separate experiments and two replicates per experiment were performed.
3. Results
3.1. Fungal Isolation
Several fungal isolates with various colony morphologies were obtained from the leaves of beach vitex plants by the direct culture of fungi from surface-sterilized leaf samples showing leaf spot disease (Figure 1). All isolates, including CMML20–2 and CMML20–7 to CMML20–13, were grown on PDA for 7 days to observe colony morphology. Based on colony morphology, the isolates were grouped into four fungal groups: Alternaria sp. (CMML20–7, CMML20–8, CMML20–9, and CMML20–10), Colletotrichum sp. (CMML20–11), Fusarium sp. (CMML20–12 and CMML20–13), and Stagonosporopsis sp. (CMML20–2).
3.2. Phylogenetic Analysis
BLASTN search analysis of the sequences of CMML20–2 indicated that the three sequences from the study matched well with the reference sequences of Stagonosporopsis caricae CBS 282.76, with 100% sequence similarity in the ITS region, RPB2, and BT genes. Based on the sequences of these three loci, a combined phylogenetic tree was constructed. The tree revealed that the isolate CMML20–2 produced a single clade with the published reference strains of S. caricae (CBS 282.76 and DAR 28714) supported with a high bootstrap value (Figure 2A). This result from multilocus phylogenetic analysis confirmed the identification of the isolate CMML20–2 as S. caricae.
Sequences of the ITS region of the isolates CMML20–7 to CMML20–10 were subjected for BLASTN search analysis and found that the sequences were identical to the sequence of the reference strain Alternaria alternata CBS 118814. The sequence of the endoPG gene of CBS 118814 was identical to the sequences of CMML20–7 and CMML20–10 and the reference strain showed 99.8% sequence similarity with CMML20–8 and CMML20–9. The sequence of OPA10-2 genomic region of CBS 118814 was identical to the sequence of CMML20–8 and CMML20–9 and the reference strain showed 99.8% sequence similarity with CMML20–7 and CMML20–10. Combined phylogenetic analysis revealed that the four isolates formed a single clade with the strain A. alternata CBS 118814, with a 96% bootstrap value (Figure 2B). Therefore, the isolates CMML20-7 to CMML20-10 were identified as A. alternata.
A combined phylogenetic tree of the ITS, CAL, and CHS gene sequences of CMML20–11 was constructed by the maximum parsimony method which indicated that the isolates CMML20–11 and Colletotrichum aenigma ICMP 18608 and ICMP 18686 were grouped with a high bootstrap value (Figure 2C). BLASTN search analysis of all gene sequences of CMML20–11 also revealed 100% sequence similarity with sequences of ITS, CAL, and CHS with the reference C. aenigma strains including ICMP 18608 and ICMP 18686.
A combined phylogenetic tree was constructed for the isolates CMML20–12 and CMML20–13 using the maximum likelihood method. The isolates and reliable reference strains (Fusarium humuli LC7003, CQ1027, CQ1039, and LC12158) from GenBank formed a monophyletic clade with a 99% bootstrap value (Figure 2D). The ITS sequences of CMML20–12 and CMML20–13 showed 100% sequence similarity with the reference sequence of LC12158. The CaM sequences of CMML20–12 and CMML20–13 showed 99.7% and 99.8% sequence similarity with the sequence of LC12158. The EF1α sequences of CMML20–12 and CMML20–13 showed 99.5% and 100% sequence similarity with LC12158. Therefore, these two isolates were confirmed as Fusarium humuli.
A detailed phylogenetic tree indicated that there are four different fungal taxa recovered from the beach vitex plant leaves—A. alternata, C. aenigma, F. humuli, and S. caricae. All the sequences retrieved in the present study were submitted to GenBank for assigning accession numbers (Table S1). Among the fungi recovered, C. aenigma, F. humuli, and S. caricae are newly isolated from the host worldwide; F. humuli and S. caricae are newly recorded in Korea. Therefore, detailed taxonomical descriptions of the fungi are included.
3.3. Taxonomy
Stagonosporopsis caricae (Sydow & P. Sydow) Aveskamp, Gruyter & Verkley
The fast-growing colony of S. caricae isolate CMML20–2 was light or shady green to yellow-green with aerial mycelia observed. The isolate was transferred to a V8 agar medium and incubated at 25 °C for 7 days. Mycelia over the surface of the medium were removed by scratching with a needle and the petri dish was transferred under NUV light.
After 3–5 days, sufficient conidia were observed under NUV. Conidiomata pycnidial formed in PDA, and the size varied from 131.0–(222.7)–287.6 × 127.7–(172.6)–210.4 μm, ostiolate, smooth-walled conidia cylindrical to slightly reniform, size (n = 30) ranging from 4.7–(6.2)–7.3 × 2.1–(2.4)–3.0 μm, (Figure 3). Morphologically, the fungus was identical to the descriptions previously outlined for S. caricae [24].
Isolate examined: CMML20–2
DNA barcode: ITS (MW149263), BT (MW149265), and RPB2 (MW149264)
Note: Morphological and microscopic characteristics of the isolate CMML20–2 were matched with reference strains (CBS 282.76 and DAR 28714) [24,25] of S. caricae and are closely related to S. cucurbitacearum. However, BLASTN search analysis of BT and RPB2 gene sequences showed base pair differences with S. cucurbitacearum, and the combined phylogenetic tree constructed in the study matched well with S. caricae with a high bootstrap value. The fungus S. caricae was reported for the first time in Korea and first reported in the host worldwide.
Fusarium humuli M.M. Wang, Q. Chen & L. Cai
Two isolates of F. humuli formed a white-colored colony with aerial mycelia on PDA with a 30–32 mm diam. Isolates produced orange-colored sporodochia on the same media. Sporodochia were directly harvested from the center region of the colony to observe the morphology. Macroconidia were straight to slightly curved and slender; apical cell hooked; size (n = 30) ranging from 15.8–(26.7)–36.4 µm in length and 2.9–(4.2)–6.8 µm in width (Figure 4).
Isolates examined: CMML20–12 and CMML20–13
DNA barcode: ITS (OL307777–78), CAL (OL331024–25), and EF1α (OL687237–38)
Note: Micromorphology and colony characters of the isolates conformed well with the descriptions of F. humuli [26]. The species is related to Fusarium citri but phylogenetically F. humuli represents a novel clade within its relatives, and high base pair differences were observed with F. citri. Morphologically, the two species are distinguished by the size of the macroconidia [26]. The fungus F. humuli is the first recorded in Korea and the first reported in the host worldwide.
Alternaria alternata (Fr.) Keissl.
The fast-growing colonies of A. alternata CMML20–7 to CMML20–10 isolates showed white, light-brown, or dark-green color with aerial mycelia. Four isolates were transferred to the V8 agar media and cultured for 7 days for sporulation. Abundant spores were grown and were obclavate in shape; color varied from pale brown to dark brown. The conidial size varied with the isolates. The sizes of the conidia (n = 30) of CMML20–7 and CMML20–9 were 18.6–(32.6)–50.3 × 5.6–(10.2)–15.0 and 21.0–(36.1)–72.9 × 7.9–(12.1)–17.5 μm, respectively (Figure 5).
Isolates examined: CMML20–7 to CMML20–10
DNA barcode: ITS (OL307772–75), OPA10–2 (OL687229–32), and endoPG (OL687233–36)
Note: The micromorphological characteristics explained in the isolates perfectly matched with the reference strain of CBS 118814. The fungus A. alternata was frequently isolated from the leaves of beach vitex in Taiwan and Korea as an endophyte [4,27].
Colletotrichum aenigma B. Weir & P.R. Johnst.
Colletotrichum aenigma isolate CMML20-11 was a fast-growing, white to pale colony with aerial mycelia and reverse pale to dark grey. Orange-colored conidiomata or ascomata were present at the center of the colony. Conidia were observed under a microscope and the shape was cylindrical with broadly rounded ends (Figure 6). The conidial sizes (n = 30) were 14.7–(17.3)–20.4 × 4.7–(5.6)–6.8 µm.
Isolate examined: CMML20–11
DNA barcode: ITS (OL307776), CAL (OL331023), and CHS-1 (OL687239)
3.4. Pathogenicity Tests
After incubation at 25 °C for 7 days, the disease occurrence was observed and used to calculate the disease severity. The isolates CMML20–2, CMML20–7, CMML20–8, CMML20–9, and CMML20–10 formed lesions of different sizes on detached leaves. Repeated experiments showed similar results and confirmed that the isolates of S. caricae and A. alternata caused disease. The isolate A. alternata CMML20–9 showed the longest diameter of lesions on beach vitex leaves (8.24 ± 0.80 mm). The other A. alternata isolates CMML20–7, CMML20–8, and CMML20–10 also produced large disease lesions ranging from 5.02 ± 0.97 to 6.85 ± 1.22 mm (Figure 7). The S. caricae CMML20–2 isolate showed significant disease lesions (5.67±0.72 mm) on detached leaves. The isolates of C. aenigma CMML20–11 and F. humuli CMML20–12 and CMML20–13 remained symptomless (Figure 7). The fungi from diseased leaves caused by A. alternata and S. caricae isolates were re-isolated from the lesions to fulfill Koch’s postulates; morphological characteristics were analyzed and found conidial morphology and colony characteristics similar to the original.

Figure 7.
Lesion length produced by fungal isolates on beach vitex detached leaves. The A. alternata isolates and S. caricae CMML20–2 showed significant lesion development compared to C. aenigma CMML20–11 and F. humuli isolates. Bars and error bars represent means and standard errors of length of lesion formed by the isolates (n = 9). Different letters above bars indicate statistically significant difference of the means.
3.5. In Vitro Fungicide Sensitivity Assay
Sensitivities of fungal isolates from beach vitex plant leaves to three fungicides were assayed to find a potential control agent. The C. aenigma isolate showed the highest EC50 values of fludioxonil and metconazole among all the isolates, and S. caricae CMML20-2 and F. humuli CMML20-13 showed the lowest EC50 values of the two fungicides (Table 1, Figure 8 and Figure 9). F. humuli and C. aenigma isolates showed tolerance against fluxapyroxad compared with A. alternata and S. caricae isolates (Table 1, Figure 10).

Table 1.
Fungicide sensitivity of all fungal isolates and their EC50 value calculated in a plate assay. The fluxapyroxad showed effective control activity against the pathogens but not endophytes identified in this study. Different letters indicate statistically significant difference of the values.

Figure 8.
Anterior view of colonies of all fungal isolates on non-supplemented PDA and supplemented with 0.01, 0.1, 1, 10, or 100 μg mL−1 of metconazole.
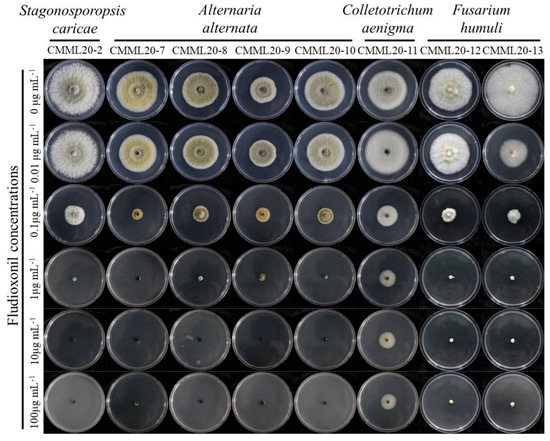
Figure 9.
Anterior view of colonies of all fungal isolates on non-supplemented PDA and supplemented with 0.01, 0.1, 1, 10, or 100 μg mL−1 of fludioxonil.

Figure 10.
Anterior view of colonies of all fungal isolates on non-supplemented PDA and supplemented with 0.01, 0.1, 1, 10, or 100 μg mL−1 of fluxapyroxad.
4. Discussion
In this study, four different fungal species were isolated from leaves of beach vitex—the isolates were identified as Alternaria alternata, Colletotrichum aenigma, Fusarium humuli, and Stagonosporopsis caricae based on multigene molecular phylogeny and morphological character analysis. In addition, their pathogenicity and sensitivity to fungicides were evaluated to further characterize the fungal species.
In beach vitex plants, several fungal species including plant pathogens and endophytes have been previously reported [3,4]. Pathogenicity tests of the four fungal species from the current study revealed that the isolates of A. alternata and S. caricae caused leaf spot symptoms whereas F. humuli, and C. aenigma were nonpathogenic to the host. A. alternata and S. caricae might be the pathogens causing leaf spot disease on beach vitex, and F. humuli and C. aenigma are, potentially, endophytes. F. humuli has frequently been isolated only from leaves of different plants as endophytes [26,28], and C. aenigma was reported from Achyranthes aspera plants as endophytes in India [29]. A. alternata is one of the Alternaria species that is widely recognized to be an endophyte and pathogen in various plants [30]. The fungus has been reported as a pathogen in Aloe vera and Pyrus sikiangensis [31,32], indicating its extreme host diversity. In Vitex rotundifolia, A. alternata was reported as an endophyte [27], but we identified it as a pathogen in this study, indicating that A. alternata can be an opportunistic pathogen. The fungus S. caricae was previously considered an aggressive pathogen in papaya. Later, it was found that the pathogen infects cucurbits in the genera Cucurbita, Cucumis, and Citrullus [33]. Three species of Stagonosporopsis (S. cucurbitacearum, S. citrulli, and S. caricae) were reported pathogenic to cucurbits, causing gummy stem blights, and the aggressiveness of these pathogens was tested in representative hosts of Citrullua lanatus, Cucurvita moschata, Cucumis sativus, and Carica papaya [33,34,35,36]. There were no differences in the aggressiveness of the three fungal species on cucurbit hosts, and S. caricae was more aggressive on C. papaya than the other species. As S. caricae has been reported from plants belonging to other families from the previous [24] and present studies, it may have high host diversity.
Morphological characteristics of fungal species can be a factor in identifying isolates at the species level. Reports about Stagonosporopsis cucurbitacearum, a fungal species closely related with the isolate CMML20–2, have described similar colonies, conidia, and conidiomata morphology [24,37,38,39,40] with S. caricae [41,42]. The isolates CMML20–7 and CMML20–8 showed identical morphological characteristics with A. alternata. There are similarities with Alternaria gaisen as described by Nishikawa and Nakashima [43], except for colony color, which matched well with A. alternata. Colony morphology of CMML20–11 showed similarity to both Colletotrichum alienum ICMP 12071 and C. aenigma ICMP 18608, including irregular dark grey patches and pale orange-colored pigmentation on the reverse surface of the colony, but conidial characteristics of the isolate were more similar to the description of C. aenigma ICMP 18608 [15]. Isolates of CMML20–12 and CMML20–13 showed unique colony morphology as also seen with F. humuli CQ1039 and Fusarium citri LC6896. However, the micromorphology of conidia was very similar to F. humuli [26]. Although S. caricae and S. cucurbitacearum showed similar or substantially identical morphological characteristics, they are genetically distinct species. Similarly, A. gaisen and A. arborescens, which are retained as separate species from A. alternata [20], are hardly distinguishable except for a few morphological characteristics [44]. Therefore, identifying Alternaria species requires multilocus molecular phylogeny. Morphological characteristics, such as pigment, conidia, or production of aerial mycelia, matched well with the F. humuli reference isolates (Figure 4 and Figure 5).
In this study, we employed multilocus molecular phylogenetic analyses combined with morphological characteristics to confirm the identities of fungal species associated with beach vitex leaves. The combined phylogenetic tree constructed with ITS, RPB2, and BT gene sequences of Stagonosporopsis species identified the isolate CMML20–2 at the species level, as suggested [45], and was confirmed as S. caricae. For phylogenetic analysis of Alternaria isolates, we selected the endoPG gene and anonymous region OPA10-2 known as a proper molecular marker for identification of the small-spored Alternaria species [44,46]—it proved suitable for molecular identification of Alternaria isolates as A. alternata. The combined phylogeny of the CAL and CHS sequence was employed to identify Colletotrichum sp. based on a previous study [15], and our isolate CMML20–11 was confirmed as C. aenigma. The EF1α and CaM markers were reported as highly informative loci for identifying Fusarium species-complexes [47]. Phylogenetically, F. humuli represents a novel clade within the Fusarium incarnatum-equiseti species complex (FIESC 33) and is closely related to F. citri, which supports the identification of the isolates of CMML20–12 and CMML20–13 as F. humuli [26].
To identify the optimal fungicide to control fungal pathogens, fungicide sensitivity assays were conducted against all fungi obtained in the study. In a previous study, the baseline sensitivity of 146 isolates of S. cucurbitacearum was tested, and the EC50 value for fludioxonil ranged from 0.02 to 0.024 [48]. The EC50 value of CMML20–2 to fludioxonil was in this range, indicating that baseline sensitivity to fludioxonil of S. caricae may be similar to S. cucurbitacearum. In the study of Li et al., the sensitivities of S. caricae isolates to tebuconazole, boscalid, and fluopyram, which are demethylation inhibitors (DMI) or succinate dehydrogenase inhibitor (SDHI) fungicides, were tested [49]. However, there is no report on the fungicide sensitivity to metconazole or fluxapyroxad of Stagonosporopsis spp. The EC50 values for fludioxonil, metconazole, and fluxapyroxad of A. alternata isolates were in the range of the values for the respective fungicides from previous reports [50,51,52,53]. Thus, the isolates can be considered relatively sensitive to each fungicide. As one of the species in the C. gloeosporioides species complex, C. aenigma showed similar fungicide sensitivity as other species in this species complex. The inherent tolerance of C. gloeosporioides to fludioxonil was confirmed [54], corresponding with our result that showed a consistent level of mycelial growth of CMML20–11 on PDA supplemented with up to 100 μg mL−1 of fludioxonil. The EC50 values of metconazole and fluxapyroxad of CMML20–11 were similar with the values of other species belonging to C. gloeosporioides [55,56], which also corresponds with our result. This is the first report of fungicide sensitivity of F. humuli isolates to fludioxonil, metconazole, and fluxapyroxad, and the sensitivity showed a similar tendency to the Fusarium graminearum species complex (FGSC), despite their taxonomical difference. The sensitivity to fludioxonil [57,58], metconazole [59], and fluxapyroxad [60,61] of isolates of FGSC species and other Fusarium species were evaluated, and their range of EC50 values was similar to F. humuli CMML20–12 and CMML20–13, including over 100 mg L−1 of the EC50 value of fluxapyroxad. The two leaf spot pathogens, S. caricae and A. alternata, showed to be highly sensitive to the SDHI fungicide, fluxapyroxad, compared with the potential endophytes C. aenigma and F. humuli. This SDHI fungicide may represent a good option for controlling both pathogens.
5. Conclusions
We investigated the foliar fungi associated with beach vitex plants through molecular and morphological characterization to better understand the microbial diversity and potential effects on the forest ecosystem. The study described two fungal isolates of S. caricae and F. humuli, which have never been found in Korea. Moreover, S. caricae, F. humuli, and C. aenigma existing in the host are the first reported worldwide. Sensitivity results of the fungal isolates to three fungicides can aid the selection of fungicides to control foliar diseases of this plant. In the future, recognizing the large-scale association of fungi in different tissues of beach vitex is necessary to understand fungal diversity. Also, further study on leaf disease control in field conditions is warranted to determine the management strategy of beach vitex leaf diseases in Korea.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14020220/s1, Table S1: Gene sequence information and GenBank accession numbers were used to construct phylogenetic trees in this study. Isolates from this study were bolded.
Author Contributions
Conceptualization, H.S.; methodology, S.-W.P. and N.C.P.; molecular data analysis, S.-W.P. and N.C.P.; morphology, DIC photographs, microscopy, and pathogenicity, S.-W.P., N.C.P., K.-H.L. and G.H.H.; writing—original draft preparation, S.-W.P. and N.C.P.; writing—review and editing, H.S. and H.-J.K.; supervision, H.S.; project administration & funding acquisition, H.S. and H.-J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from National Research Foundation of Korea (2020R1C1C1010108) and the Korea Forest Service (Grant No. 2020183C10-2022-AA02), Republic of Korea. This work was also carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ014897)” Rural Development Administration, Republic of Korea.
Data Availability Statement
GenBank accession numbers for the microorganism’s sequence are available in Table S1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cousins, M.M.; Briggs, J.; Gresham, C.; Whetstone, J.; Whitwell, T. Beach Vitex (Vitex rotundifolia): An Invasive Coastal Species. Invasive Plant Sci. Manag. 2010, 3, 340–345. [Google Scholar] [CrossRef]
- Schmid, R.; Kubitzki, K.; Kubitzki, K.; Kadereit, J.W. The Families and Genera of Vascular Plants. Taxon 2005, 54, 574. [Google Scholar] [CrossRef]
- Park, J.H.; Park, M.J.; Lee, S.H.; Shin, H.D. First Report of Corynespora Leaf Spot on Beach Vitex Caused by Corynespora cassiicola in Korea. Plant Dis. 2013, 97, 1512. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-H.; Kirschner, R. Diversity of Endophytic Fungi of the Coastal Plant Vitex Rotundifolia in Taiwan. Microb. Environ. 2019, 34, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Azizul, N.; Amir, W.; Laili, N.; Aniq, M.; En, C.; Wini, N.; Assaw, S. The Coastal Medicinal Plant Vitex rotundifolia: A Mini-Review on Its Bioactive Compounds and Pharmacological Activity. Tradit. Med. Res. 2021, 6, 11. [Google Scholar] [CrossRef]
- Wang, W.; Yin, Y.; Jun, L.; Xuan, L. Halimane-Type Diterpenoids from Vitex rotundifolia and Their Anti-Hyperlipidemia Activities. Phytochemistry 2018, 146, 56–62. [Google Scholar] [CrossRef]
- Chaudhry, G.-S.; Jan, R.; Mohamad, H.; Muhammad, T.T. Vitex rotundifolia Fractions Induce Apoptosis in Human Breast Cancer Cell Line, MCF-7, via Extrinsic and Intrinsic Pathways. Res. Pharma. Sci. 2019, 14, 273. [Google Scholar] [CrossRef]
- Chaudhry, G.-S.; Jan, R.; Naveed Zafar, M.; Mohammad, H.; Muhammad, T.S.T. Vitex rotundifolia Fractions Induced Apoptosis in Human Breast Cancer T-47D Cell Line via Activation of Extrinsic and Intrinsic Pathway. Asian Pac. J. Cancer. Prev. 2019, 20, 3555–3562. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, H.; Zhang, Q.; Qin, L.; Li, P.; Lee, J.; Chen, S.; Rahman, K.; Kang, T.; Jia, M. Genetic Diversity and Its Conservation Implications of Vitex rotundifolia (Lamiaceae) Populations in East Asia. PeerJ 2019, 7, e6194. [Google Scholar] [CrossRef]
- Yeh, Y.H.; Kirschner, R. First Report of Corynespora Leaf Spot on Vitex rotundifolia Caused by Corynespora cassiicola in Taiwan. Plant Dis. 2017, 101, 1550. [Google Scholar] [CrossRef]
- Paliany, A.S.; Yasodha, S.; Khalijah, A.; Mohammed, R.-I.; Siti Aisyah, A. Marine Derived Fungi of Peninsular Malaysia—A Biochemical Perspective. Chiang Mai J. Sci. 2014, 41, 1–16. [Google Scholar]
- Berkov, S.; Mutafova, B.; Christen, P. Molecular Biodiversity and Recent Analytical Developments: A Marriage of Convenience. Biotechnol. Adv. 2014, 32, 1102–1110. [Google Scholar] [CrossRef]
- Cubero, O.F.; Crespo, A.; Fatehi, J.; Bridge, P.D. DNA Extraction and PCR Amplification Method Suitable for Fresh, Herbarium-Stored, Lichenized, and Other Fungi. Plant Syst. Evol. 1999, 216, 243–249. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Academic Press: New York, NY, USA, 1990; pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides Species Complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A Method for Designing Primer Sets for Speciation Studies in Filamentous Ascomycetes. Mycologia 1999, 91, 553. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple Evolutionary Origins of the Fungus Causing Panama Disease of Banana: Concordant Evidence from Nuclear and Mitochondrial Gene Genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- Staats, M. Molecular Phylogeny of the Plant Pathogenic Genus Botrytis and the Evolution of Host Specificity. Mol. Biol. Evol. 2004, 22, 333–346. [Google Scholar] [CrossRef]
- Aveskamp, M.M.; Verkley, G.J.M.; de Gruyter, J.; Murace, M.A.; Perelló, A.; Woudenberg, J.H.C.; Groenewald, J.Z.; Crous, P.W. DNA Phylogeny Reveals Polyphyly of Phoma Section Peyronellaea and Multiple Taxonomic Novelties. Mycologia 2009, 101, 363–382. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Seidl, M.F.; Groenewald, J.Z.; de Vries, M.; Stielow, J.B.; Thomma, B.P.H.J.; Crous, P.W. Alternaria Section Alternaria: Species, Formae Speciales or Pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Chilvers, M.I.; Jones, S.; Meleca, J.; Peever, T.L.; Pethybridge, S.J.; Hay, F.S. Characterization of Mating Type Genes Supports the Hypothesis That Stagonosporopsis chrysanthemi Is Homothallic and Provides Evidence That Stagonosporopsis tanaceti Is Heterothallic. Curr. Genet. 2014, 60, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Aveskamp, M.M.; de Gruyter, J.; Woudenberg, J.H.C.; Verkley, G.J.M.; Crous, P.W. Highlights of the Didymellaceae: A Polyphasic Approach to Characterise Phoma and Related Pleosporalean Genera. Stud. Mycol. 2010, 65, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Iturrieta-González, I.; García, D.; Gené, J.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Quaedvlieg, W.; Schumacher, R.K.; et al. Genera of Phytopathogenic Fungi: GOPHY 3. Stud. Mycol. 2019, 94, 1–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Chen, Q.; Diao, Y.Z.; Duan, W.J.; Cai, L. Fusarium incarnatum-equiseti Complex from China. Persoonia 2019, 43, 70–89. [Google Scholar] [CrossRef]
- Lee, C.; Li, W.; Bang, S.; Lee, S.J.; Kang, N.; Kim, S.; Kim, T.I.; Go, Y.; Shim, S.H. Secondary Metabolites of The Endophytic Fungus Alternaria alternata JS0515 Isolated from Vitex rotundifolia and Their Effects on Pyruvate Dehydrogenase Activity. Molecules 2019, 24, 4450. [Google Scholar] [CrossRef]
- Wang, M.M.; Crous, P.W.; Sandoval-Denis, M.; Han, S.L.; Liu, F.; Liang, J.M.; Duan, W.J.; Cai, L. Fusarium and Allied Genera from China: Species Diversity and Distribution. Persoonia 2022, 48, 1–53. [Google Scholar] [CrossRef]
- John, R.; Mathew, L. Genetic Diversity of Endophytic Colletotrichum Spp. in Achyranthes aspera Linn. In Proceedings of the 23rd Swadeshi Science Congress, Kottayam, Kerala, 5 November 2013; p. 653. [Google Scholar]
- DeMers, M. Alternaria alternata as Endophyte and Pathogen. Microbiology 2022, 168, 001153. [Google Scholar] [CrossRef]
- Bajwa, R.; Mukhtar, I.; Mushtaq, S. New Report of Alternaria alternata Causing Leaf Spot of Aloe vera in Pakistan. Can. J. Plant Pathol. 2010, 32, 490–492. [Google Scholar] [CrossRef]
- Aung, S.L.L.; Liu, H.F.; Pei, D.F.; Lu, B.B.; Oo, M.M.; Deng, J.X. Morphology and Molecular Characterization of a Fungus from the Alternaria alternata Species Complex Causing Black Spots on Pyrus sinkiangensis (Koerle Pear). Mycobiology 2020, 48, 233–239. [Google Scholar] [CrossRef]
- Stewart, J.E.; Turner, A.N.; Brewer, M.T. Evolutionary History and Variation in Host Range of Three Stagonosporopsis Species Causing Gummy Stem Blight of Cucurbits. Fungal Biol. 2015, 119, 370–382. [Google Scholar] [CrossRef]
- Chen, Q.; Hou, L.W.; Duan, W.J.; Crous, P.W.; Cai, L. Didymellaceae Revisited. Stud. Mycol. 2017, 87, 105–159. [Google Scholar] [CrossRef]
- Garampalli, R.H. Two Stagonosporopsis Species Identified as Causal Agents of Gummy Stem Blight Epidemics of Gherkin Cucumber (Cucumis sativus) in Karnataka, India. Eur. J. Plant Pathol. 2016, 145, 507–512. [Google Scholar] [CrossRef]
- Li, H.-X.; Gottilla, T.M.; Brewer, M.T. Organization and Evolution of Mating-Type Genes in Three Stagonosporopsis Species Causing Gummy Stem Blight of Cucurbits and Leaf Spot and Dry Rot of Papaya. Fungal Biol. 2017, 121, 849–857. [Google Scholar] [CrossRef]
- Moumni, M.; Mancini, V.; Allagui, M.B.; Murolo, S.; Romanazzi, G. Black Rot of Squash (Cucurbita moschata) Caused by Stagonosporopsis cucurbitacearum Reported in Italy. Phytopathol. Miediterr. 2019, 58, 379–383. [Google Scholar] [CrossRef]
- Mahapatra, S.; Rao, E.S.; Sandeepkumar, G.M.; Sriram, S. Stagonosporopsis cucurbitacearum the Causal Agent of Gummy Stem Blight of Watermelon in India. Australas. Plant Dis. Notes 2020, 15, 7. [Google Scholar] [CrossRef]
- Keinath, A.P.; Farnham, M.W.; Zitter, T.A. Morphological, Pathological, and Genetic Differentiation of Didymella bryoniae and Phoma Spp. Isolated from Cucurbits. Phytopathology 1995, 85, 364–369. [Google Scholar] [CrossRef]
- Savitha, R.S.; Garampalli, R.H. Identification And Molecular Characterization of Stagonosporopsis cucurbitacearum Causes Gummy Stem Blight Disease on Coccinia Grandis- A First Report in Karnataka, India. J. Adv. Sci. Res. 2022, 13, 70–78. [Google Scholar] [CrossRef]
- Sivanesan, A. CMI Description Sheets: Set 99. Mycopathologia 1990, 109, 47–48. [Google Scholar] [CrossRef]
- Bracale, M.F.; Nóbrega, T.F.; Barreto, R.W. Fungal Diseases of Non-Conventional Food Plants: First Report of Stagonosporopsis caricae Causing Leaf Spots on Vasconcellea monoica. Australas. Plant Dis. Notes 2020, 15, 20. [Google Scholar] [CrossRef]
- Nishikawa, J.; Nakashima, C. Japanese Species of Alternaria and Their Species Boundaries Based on Host Range. Fungal Syst. Evol. 2020, 5, 197–282. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.; Peever, T.L.; Pryor, B.M. An Expanded Multilocus Phylogeny Does Not Resolve Morphological Species within the Small-Spored Alternaria Species Complex. Mycologia 2009, 101, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.W.; Groenewald, J.Z.; Pfenning, L.H.; Yarden, O.; Crous, P.W.; Cai, L. The Phoma-like Dilemma. Stud. Mycol. 2020, 96, 309–396. [Google Scholar] [CrossRef] [PubMed]
- Armitage, A.D.; Barbara, D.J.; Harrison, R.J.; Lane, C.R.; Sreenivasaprasad, S.; Woodhall, J.W.; Clarkson, J.P. Discrete Lineages within Alternaria alternata Species Group: Identification Using New Highly Variable Loci and Support from Morphological Characters. Fungal Biol. 2015, 119, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Gueidan, C.; Crous, P.W.; Geiser, D.M. Novel Multilocus Sequence Typing Scheme Reveals High Genetic Diversity of Human Pathogenic Members of the Fusarium Incarnatum–F. equiseti and F. chlamydosporum Species Complexes within the United States. J. Clin. Microbiol. 2009, 47, 3851–3861. [Google Scholar] [CrossRef]
- Keinath, A.P. Baseline Sensitivity of Didymella bryoniae to Cyprodinil and Fludioxonil and Field Efficacy of These Fungicides Against Isolates Resistant to Pyraclostrobin and Boscalid. Plant Dis. 2015, 99, 815–822. [Google Scholar] [CrossRef]
- Li, H.; Nuckols, T.A.; Harris, D.; Stevenson, K.L.; Brewer, M.T. Differences in Fungicide Resistance Profiles and Multiple Resistance to a Quinone-outside Inhibitor (QoI), Two Succinate Dehydrogenase Inhibitors (SDHI), and a Demethylation Inhibitor (DMI) for Two Stagonosporopsis Species Causing Gummy Stem Blight of Cucurbits. Pest. Manag. Sci. 2019, 75, 3093–3101. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Detection of Isolates of Alternaria alternata with Multiple-Resistance to Fludioxonil, Cyprodinil, Boscalid and Pyraclostrobin in California Pistachio Orchards. Crop Prot. 2015, 78, 214–221. [Google Scholar] [CrossRef]
- Fonseka, D.L.; Gudmestad, N.C. Spatial and Temporal Sensitivity of Alternaria Species Associated With Potato Foliar Diseases to Demethylation Inhibiting and Anilino-Pyrimidine Fungicides. Plant Dis. 2016, 100, 1848–1857. [Google Scholar] [CrossRef]
- Avenot, H.F.; van den Biggelaar, H.; Morgan, D.P.; Moral, J.; Joosten, M.; Michailides, T.J. Sensitivities of Baseline Isolates and Boscalid-Resistant Mutants of Alternaria alternata from Pistachio to Fluopyram, Penthiopyrad, and Fluxapyroxad. Plant Dis. 2014, 98, 197–205. [Google Scholar] [CrossRef]
- Budde-Rodriguez, S.; Pasche, J.S.; Mallik, I.; Gudmestad, N.C. Sensitivity of Alternaria Spp. from Potato to Pyrimethanil, Cyprodinil, and Fludioxonil. Crop Prot. 2022, 152, 105855. [Google Scholar] [CrossRef]
- Schnabel, G.; Tan, Q.; Schneider, V.; Ishii, H. Inherent Tolerance of Colletotrichum gloeosporioides to Fludioxonil. Pestic. Biochem. Physiol. 2021, 172, 104767. [Google Scholar] [CrossRef]
- Chen, S.N.; Luo, C.X.; Hu, M.J.; Schnabel, G. Sensitivity of Colletotrichum Species, Including C. fioriniae and C. nymphaeae, from Peach to Demethylation Inhibitor Fungicides. Plant Dis. 2016, 100, 2434–2441. [Google Scholar] [CrossRef]
- Ishii, H.; Zhen, F.; Hu, M.; Li, X.; Schnabel, G. Efficacy of SDHI Fungicides, Including Benzovindiflupyr, against Colletotrichum Species: Efficacy of Benzovindiflupyr against Colletotrichum Species. Pest. Manag. Sci. 2016, 72, 1844–1853. [Google Scholar] [CrossRef]
- Broders, K.D.; Lipps, P.E.; Paul, P.A.; Dorrance, A.E. Evaluation of Fusarium graminearum Associated with Corn and Soybean Seed and Seedling Disease in Ohio. Plant Dis. 2007, 91, 1155–1160. [Google Scholar] [CrossRef]
- Zhou, F.; Li, D.X.; Hu, H.Y.; Song, Y.L.; Fan, Y.C.; Guan, Y.Y.; Song, P.W.; Wei, Q.C.; Yan, H.F.; Li, C.W. Biological Characteristics and Molecular Mechanisms of Fludioxonil Resistance in Fusarium graminearum in China. Plant Dis. 2020, 104, 2426–2433. [Google Scholar] [CrossRef]
- Tateishi, H.; Miyake, T.; Mori, M.; Kimura, R.; Sakuma, Y.; Saishoji, T. Sensitivity of Japanese Fusarium graminearum Species Complex Isolates to Metconazole. J. Pestic. Sci. 2010, 35, 419–430. [Google Scholar] [CrossRef]
- Mayorquin, J.S.; Carrillo, J.D.; Twizeyimana, M.; Peacock, B.B.; Sugino, K.Y.; Na, F.; Wang, D.H.; Kabashima, J.N.; Eskalen, A. Chemical Management of Invasive Shot Hole Borer and Fusarium Dieback in California Sycamore (Platanus racemosa) in Southern California. Plant Dis. 2018, 102, 1307–1315. [Google Scholar] [CrossRef]
- Xu, C.; Li, M.; Zhou, Z.; Li, J.; Chen, D.; Duan, Y.; Zhou, M. Impact of Five Succinate Dehydrogenase Inhibitors on DON Biosynthesis of Fusarium asiaticum, Causing Fusarium Head Blight in Wheat. Toxins 2019, 11, 272. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).