Abstract
Impressive wooden objects from past cultures can last for centuries or millennia in waterlogged soil. The aim of conservation is to bring the more or less degraded waterlogged archaeological wooden (WAW) finds to a stable state without altering the wood structure through shrinkage, collapse, and deformation. In this study, the most used methods in the conservation practice, such as the alcohol-ether resin method, conservation with the melamine formaldehyde resin Kauramin 800, a mixture of lactitol and trehalose, saccharose, silicone oil, and three different conservation methods with polyethylene glycol followed by freeze-drying were tested. The effects of the conservation agents on the structure of archaeological pine were investigated using optical light microscopy (reflected light microscopy, RLM), scanning electron microscopy (SEM), and X-ray computed tomography (XCT). Through the examinations, most conservation agents could be identified in the structure and their impact on conservation could be analyzed. In particular, it was possible to trace the incorporation of the conservation agents in the lumen, which was influenced by factors, such as wood anatomy, degree of degradation, and drying process. Differences in the mode of action of the conservation processes could also be identified in the composition of the cell wall tracheids.
1. Introduction
Wooden relicts crafted by cultures of the past survived in environments where microbial activity is decelerated or inhibited. Such extreme conditions with low temperatures and limited oxygen availability prevail, e.g., in aquatic ecosystems where waterlogged archaeological wood (WAW) can be preserved [1,2,3,4,5,6]. Besides physical and chemical degradation, biological degradation by wood degraders, such as insects, marine borers, fungi, and bacteria, plays a major role [7,8,9].
Softwood (coniferous wood) is phylogenetically older than hardwood (dicotyledonous wood) and therefore characterized by a simpler and more regular structure [10]. The main part of the cells (90–95%) are the longitudinal tracheids [11]. The cell wall of a tracheid is composed of a middle lamella, the primary wall, and the secondary wall. The individual layers have different thicknesses and chemical compositions (proportions of cellulose, hemicellulose, and lignin). The primary cell wall is attached to the middle lamella and consists of cellulose microfibrils in crossing layers embedded in pectin, followed by the secondary cell wall. The secondary cell wall consists of three different layers (S1, S2, S3). The compound middle lamella (middle lamella and primary wall) and the cell corners have, in general, a higher lignin concentration and the cellulose and hemicellulose are mainly concentrated in the secondary cell wall. Because of its thickness, the secondary cell wall is very important for the stability of the wood [12,13]. Compared to modern wood, WAW exhibits a different chemical composition and morphology [4,9]. Due to degradation mechanisms, a wide range of preservation conditions of the individual tracheid are also exhibited. In a mixed condition, both sound tracheids and degraded tracheids are present [9].
As a result of the surface tension of water and the capillary forces occurring during drying, WAW is damaged [14]. Low degraded cell walls are shrinking when water evaporates to a high degree due to the loss of water in the cell walls [15,16,17]. In the advanced state of deterioration, where the compound middle lamella is not degraded and an amorphous residual material remains [10], the structure is highly susceptible to collapsing when air-drying [18]. The loss of volume of the cell is reflected in shrinkage and warping in the whole structure of the sample [19].
The goal of conservation is to transform the wet, fragile object into a dry, stable state so that it is available for exhibition/research. The objects should be affected as little as possible by conservation, e.g., by changes in volume or dimensions, cracks, or cell collapse. They should meet aesthetic requirements and achieve sufficient mechanical stabilization. By penetrating the wood structure and cell walls, the conservation agent should strengthen the structure and prevent it from shrinking [20]. Conservation of WAW is usually done by cell wall bulking and/or lumen filling in order to stabilize the degraded structure [17]. It is obvious that the decomposed wood structure should be uniformly stabilized to counteract material fatigue and fractures. Here, porous, network-like structures are advantageous, which distribute acting forces evenly [21]. The disadvantage of a completely saturated structure is that it is difficult to be re-conserved, when necessary [22].
The measures usually consist of two main steps, firstly introducing conservation agents into the wood and then drying the object. In the case of solvent-based conservation methods, the water is being exchanged as a first step [17]. A variety of materials [17,23,24], such as melamine formaldehyde resins (MF) [25,26], sugars [27,28,29,30,31,32,33], silicone oil [34,35,36], polyethylene glycols (PEG) [24,37,38], or specific mixtures of oils and resins [23,39] are applied to conserve waterlogged wood, which is air-dried or freeze-dried [18,40,41] after impregnation.
Between 2008 and 2011, a sample collection was built up at Leibniz-Zentrum für Archäologie (LEIZA, former Römisch-Germanisches Zentralmuseum, RGZM) to compare common conservation practices [42]. Currently, the volume stabilization of these commonly used conservation methods is analyzed by 3D-scanning and X-ray computed tomography (XCT) [16]. This study showed varying conservation outcomes where the conservation methods with polyethylene glycol and freeze-drying, melamine formaldehyde (Kauramin 800, BASF), and alcohol-ether-resin performed overall very well in terms of stabilization and preventing collapse, shrinkage, and crack formation. Other methods, such as silicone oil or sugar, tended to produce poorer results in terms of volume stability. Microscopic studies on these samples are necessary to understand these results. Since the prevention of cell collapse and shrinkage cannot be achieved by filling the lumen alone, the study of the conservation processes at the microscopic level is crucial [43]. Therefore, the understanding of the interaction between the degraded wood structure and conservation agents is the key question in understanding the conservation procedure in depth.
This study aims to assess how the more or less degraded structure of WAW is stabilized in the current conservation practice. In order to avoid misinterpretation, different microscopic methods were used here, which allowed the observations to be compared [44]. First of all, the samples are to be examined with optical light microscopy, being the most frequently used technique to study wooden objects and wooden structures [45]. The advantages of scanning electron microscopy (SEM) include the high resolution (up to few nanometers) and three-dimensional (3D) information of the conserved wood structure generated [1,34,44,45,46]. CT has already been used to analyze the wood anatomy [47] and to visualize conservation agents in the structure of archaeological wood [48,49,50]. Cross-sectional images can be extracted from the digital CT datasets and were used here to supplement the microscopic procedures, allowing verification of the observations. The great advantage here is that misinterpretations due to manipulations during preparation are avoided.
2. Materials and Methods
2.1. Samples
Between 2008 and 2011, a reference collection for conserved WAW samples was built up in a research project, coordinated by the LEIZA and funded by the Federal Cultural Foundation and the Cultural Foundation of German States with national and international participants [16,51] (see Appendix A Table A1). From this collection, a softwood test series was chosen. The samples derived from an undated water pipe from Stralsund, Germany. The wood species is defined as coniferous wood (Pinus sp.). The water pipe shows a little degraded heartwood and heavily degraded areas in the outer regions (cf. Figure 1). This kind of degradation is defined by de Jong as grade 2 [52]. This morphological gradient change towards the core is characteristic for decay caused by erosion bacteria: a soft outer layer in which the secondary cell wall is strongly eroded [9]. Both the maximum water content (MWC) and the basic density (BD) were calculated to assess the overall condition of the sample [20,44,53,54].

Figure 1.
Air-dried reference sample. A top view of the cross section. Two different zones of the sample are visible indicating heavily degraded outer areas (hd) and low degraded inner areas (ld). Core samples were taken from both parts of the sample.
Inclusions of inorganic crystals are spread over the whole sample (cf. Figure 2a,b). This could be a ferrous phosphate hydrate, such as vivianite Fe2+3(PO4)2·8H2O. These pale blue compounds are formed when organic phosphate-containing materials decompose in the soil and can become embedded in archaeological porous materials, as shown in Figure 2a,b. When stored in air, the bivalent or trivalent iron oxidizes to trivalent iron and the iron phosphate loses its blue color [55].
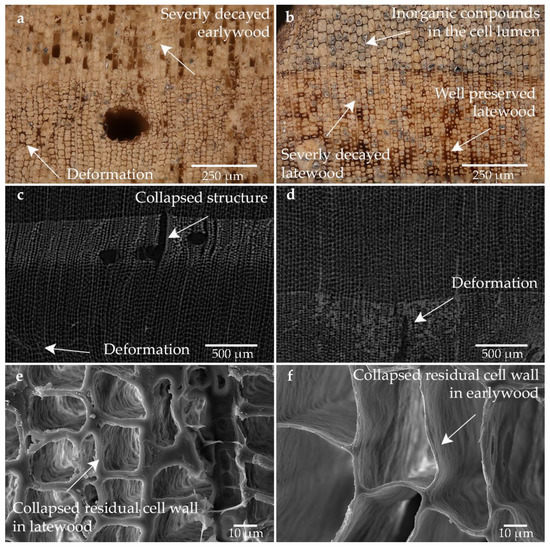
Figure 2.
Microscopic wood structure of the untreated, air-dried sample (Pi1-Air) (a) RLM image from the high degraded area (b) RLM image from the low degraded area; where the inorganic inclusions are visible; (c) CT image of the cross section of the heavily degraded sample; (d) CT image of the cross section of the low degraded sample; (e) SEM image of the latewood tracheids; (f) SEM image of the earlywood tracheids in the heavily degraded area.
In 2009, additional analyses of the composition of the wood sample were performed at the Hamburg University, Institute of Wood Science. After a two-stage acid hydrolysis in which the polysaccharides are split into their monomers, the hydrolysate is analyzed qualitatively and quantitatively for the sugars typically found in wood using HPLC (in this case borate ion exchange chromatography). The filtered hydrolysis residue was weighed and can be used as a measure of the lignin contained in the sample [42]. Using the percentages of the wood ingredients holocellulose and lignin, their ratio (H/L) can be calculated. The H/L ratio is a valuable measurement to analyze the state of preservation [3,56,57]. This ratio was calculated from the available data. The low value indicates an advanced degree of degradation (Table 1).

Table 1.
Physical and chemical properties of the object.
Then, the object was divided into several parts and five samples each were conserved using the procedures in designated specialized laboratories (see Appendix A Table A1).
- 1.
- Reference
- 2.
- Alkohol-ether resin
- 3.
- Melamine formaldehyde (Kauramin 800®, BASF, Ludwigshafen, Germany)
- 4.
- Lactitol-trehalose
- 5.
- Polyethylene glycol (PEG 2000) one-step and freeze-drying
- 6.
- Polyethylene glycol (PEG 400 and 4000) two-step and freeze-drying
- 7.
- Polyethylene glycol (PEG 400, 1500 and 4000) three-step and freeze-drying
- 8.
- Saccharose
- 9.
- Silicone oil
For each conservation method, one sample was selected. In order to compare the different states of degradation and the nature of their correspondence to conservation methods, two core samples with a diameter of 5 mm were taken using an increment borer, one from the heavily degraded area (hd) and one from the low degraded area (ld) (cf. Figure 1).
2.2. Methods
Methodologically, the samples were first examined under the optical microscope, analyzing the influence of the conservation measures on both states, the heavily degraded and the low degraded areas. Both states of degradation were also analyzed with X-ray computed tomography (XCT) where no further preparation of the core samples is necessary. The state of preservation of the core samples varies in particular in the number of degraded tracheids or collapsed cells. Therefore, for the higher magnification SEM examination, only the sample from the heavily degraded area was further examined in detail (see Appendix A Table A2).
2.2.1. Optical Microscope
Subsequently, the core samples were examined with reflected light microscopy (RLM). The examinations with RLM were carried out on the core samples. Approximately 5-mm-wide slices were taken and polished with MICRO-MESHTM (Micro-Surface Finishing Products Inc., Wilton, IA, USA, grade 1500 to 12,000). Analyses were performed with the Axioscop A1 microscope (Carl Zeiss GmbH, Jena, Germany) and documented at a magnification of 200×. Adobe Photoshop software (Version 23.5.2 (2022), San Jose, CA, USA) was used for image processing where necessary.
2.2.2. Computed Tomography
In order to visualize the conserved wooden structure on a microscopic level using XCT, a high resolution is necessary. Since the resolution depends on the object size, it is necessary to take small core samples from the wood samples [47]. At the Lucerne University of Applied Sciences and Arts, the core samples were examined using an in-house XCT system (Diondo d2, Hattingen, Germany). Measurements were performed by setting the X-ray source (XWT-225 TCHE+ from X-RAY WorX, Garbsen, Germany) in the microfocus mode and selecting an acceleration voltage of 80 kV and a tube current of 125 μA with a 1 mm aluminum filter. Radiographic projections were acquired homogeneous over a 360° rotation with a 4343 CT X-ray detector (Varex, Salt Lake City, UT, USA) with a pixel size of 150 μm. There was a distance of 8 mm between the sample and the focal spot and 600 mm between the focal spot and the detector center. These distances resulted in a magnification of 30 and a nominal voxel size of 5 µm. Based on the filtered back-projection algorithm of Feldkamp [59] as implemented in CERA from Siemens Healthineer, a 3D data set consisting of approximately 3000 × 3000 × 3000 voxels was reconstructed from the individual projections. The 3D datasets were visualized using VGSTUDIO MAX (Version 3.4, Volume Graphics, Heidelberg, Germany).
2.2.3. Scanning Electron Microscope
For scanning electron microscope (SEM) analyses, slices of the core samples were taken with the scalpel. These were fixed on a sample plate and then sputtered with gold (sputter coater SCD 040, Balzers, Liechtenstein). The thickness of the gold achieved approximately 10 nm. The examination was then carried out in a SEM (Evo 60, Carl Zeiss GmbH, Jena, Germany) at TraCEr (Laboratory for Traceology and Controlled Experiments at LEIZA) in high vacuum mode (1.18 × 10−04 Pa) and an accelerating voltage of 10 kV.
3. Results
3.1. Air-Dried Reference Sample
Figure 2 shows the images of the microscopic analyses and CT of the untreated and air-dried sample. This sample served as a reference for the interpretation of the conserved samples discussed below.
The sample shows a high proportion of inorganic inclusions, which was described in the Section 2.1 samples. The condition of the individual tracheids is very inhomogeneous: Besides very well-preserved cells, tracheids that have severely degraded cell walls can also be seen here (cf. Figure 2b). This hints to anaerobic decay: Under near anoxic environments, e.g., erosion bacteria decompose the holocellulose (cellulose and hemicellulose) in the cell wall very effectively, whereas the lignin structure is hardly changed. Thus, next to healthy tracheids with intact holocellulose, degraded tracheids occur. These degraded tracheids show an eroded secondary cell wall and intact compound middle lamella and cell corners composed predominantly of lignin [4,7,9,60,61,62,63,64,65].
The structure of the sample showed clear differences in the strength of the cell walls between the severely degraded outer areas and the low degraded inner areas (cf. Figure 2a,b). They differ in the number of collapsed and degraded tracheids. The remaining cell walls of the low degraded areas are darker and thicker. These cell walls were able to sustain air-drying without damage. This resistance is especially visible in the latewood, where the cell walls are naturally thicker. The heavily degraded cell walls were seriously shrunken and compressed. In some cases, cracks and deformation are also evident here. The proportion of heavily degraded tracheids is higher in the severely degraded outer area than in the low degraded inner area. The overall picture shows a high degree of degradation clearly visible on the very thin cell walls of the latewood tracheids. Empty tracheids and distortions in the structure are apparent (cf. Figure 2a,c,d). Under the optical microscope, the grounded surface can be seen. In most cases, the lumen is filled with grinding dust. The heavily degraded cell walls have been collapsed and shrunken due to air-drying (cf. Figure 2a–f). Obviously, air drying results in the highest shrinkage, lower porosity, and lower number of free hydroxyl groups in the wood cell wall, indicating the formation of strong intra- and intermolecular interactions in the collapsed state [15].
3.2. Alcohol-Ether-Resin
Figure 3 shows the images of the wood structure, which is overall not highly affected by the conservation agent (cf. Figure 3c–f). The most obvious difference between degradation levels was observed in this sample. The cell walls of the tracheids in the latewood of the inner, well-preserved areas seem to be well stabilized (cf. Figure 3b). In contrast to good stabilization, in the outer, heavily degraded area, the wood structure is only very barely visible (cf. Figure 3a). In the latewood, the degraded S2 layers of the fibrous tracheids are partially visible. The heavily degraded secondary cell walls are nevertheless kept in shape, although they are shrunken and have detached from the compound middle lamella. Small gaps between compound middle lamella and secondary cell wall are apparent (cf. Figure 3a,e), indicating the shrinkage of the secondary cell wall. Compared to the untreated reference, deposits can be seen on the cell walls of the earlywood. In the latewood, isolated lumen could also be filled. These deposits could be addressed as conservation agents, but a clear identification was not possible (cf. Figure 3b–d). However, it does not appear here that the gaps between compound middle lamella and secondary cell walls were filled with the conservation agent (cf. Figure 3e).
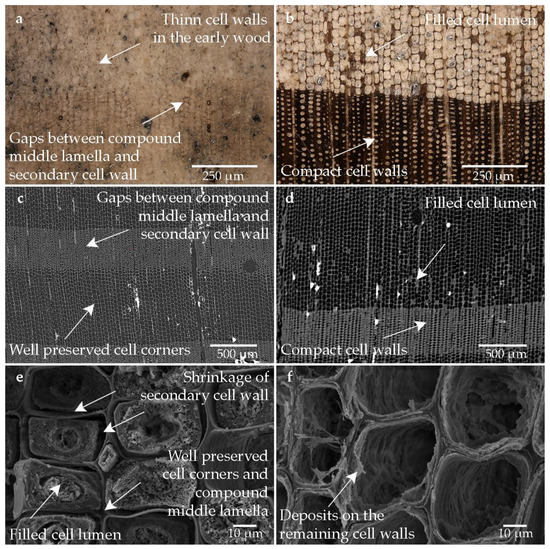
Figure 3.
Microscopic wood structure of sample conserved with alcohol-ether-resin (Pi1-AlEt) (a) RLM image from the high degraded area; (b) RLM image from the low degraded area; (c) CT image of the cross section of the heavily degraded sample (d) CT image of the cross section of the low degraded sample (e) SEM image of the latewood tracheids; (f) SEM image of the earlywood tracheids in the heavily degraded area.
3.3. Melamine Formaldehyde (Kauramin 800)
The microstructure of the sample conserved with the melamine formaldehyde Kauramin 800 can be seen in Figure 4. A significant difference between the state of degradation and the effect of the melamine formaldehyde Kauramin 800 was not evident (cf. Figure 4a,b). The melamine formaldehyde Kauramin 800 seems to stabilize both states, sound and degraded tracheids which maintained their shape. Possibly the well-preserved areas are less stabilized as the cell walls appear lighter and thinner. In addition, little deformation can be seen in the sample taken from the well-preserved area (cf. Figure 4d). Further analyses are necessary here. The cell walls have a very clear and compact appearance (cf. Figure 4a,b). Especially the latewood cells are very compact. Since the wood rays and the latewood cells are particularly conspicuous in the degraded area, the conservation agent may have accumulated there to a considerable extent (cf. Figure 4a–c).
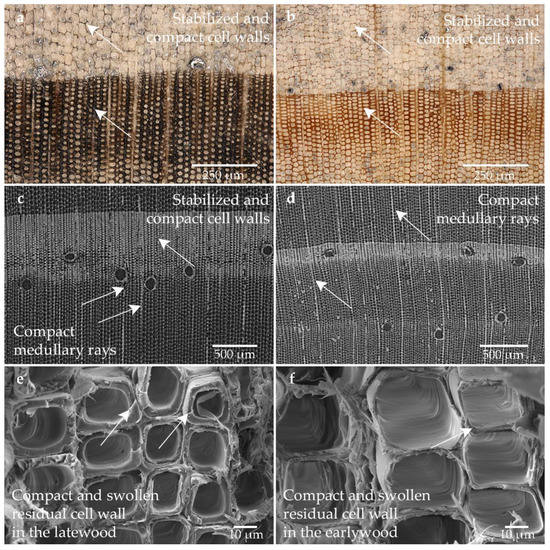
Figure 4.
Microscopic wood structure of the sample conserved with the melamine formaldehyde Kauramin 800 (Pi1-K800) (a) RLM image from the high degraded area; (b) RLM image from the low degraded area; (c) CT image of the cross section of the heavily degraded sample; (d) CT image of the cross section of the low degraded sample; (e) SEM image of the latewood tracheids; (f) SEM image of the earlywood tracheids in the heavily degraded area.
3.4. Lactitol/Trehalose
Figure 5 shows the images of the microscopic analyses and CT of the sample treated with lactitol/trehalose. The mixture of lactitol and trehalose could be well localized in sample, since, in contrast to the non-conserved sample, the lumen especially of the latewood was filled (cf. Figure 5a-e). It is striking that the lumen of the earlywood tracheids are largely empty (cf. Figure 5a–d,f). However, stabilized tracheids and non-stabilized tracheids could be observed (cf. Figure 5a,c). In the heavily degraded sample, the cell walls of the earlywood are well defined and are remarkably thick (cf. Figure 5a,b) compared to the core sample taken from the well-preserved area (cf. Figure 5b,d). The conservation agents appear also to deposit on the cell wall and seem to significantly strengthen it (cf. Figure 5a,e,f). However, many cracks are apparent, some of which also run through the cell wall, indicating the brittleness of the conservation agent (cf. Figure 5e,f). SEM examination also showed the attachment of a shaft-like, spiky substance to the cell wall. It was deposited on the existing cell walls of the tracheids (cf. Figure 5e,f). In the heavily degraded areas, deformation is visible (cf. Figure 5c).
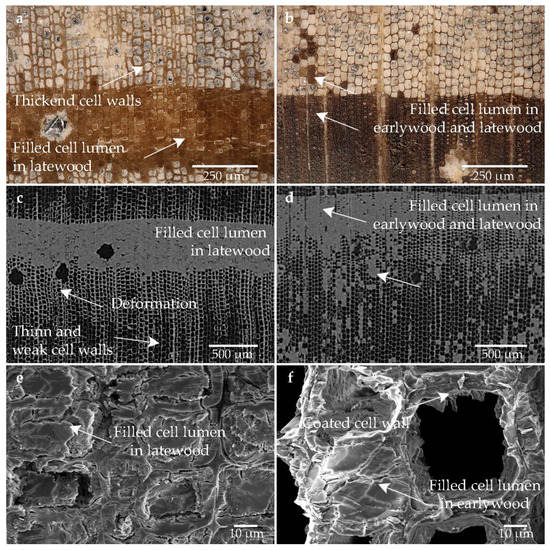
Figure 5.
Microscopic wood structure of sample conserved with lactitol/trehalose (Pi1-LaTr) (a) RLM image from the heavily degraded area; (b) RLM image from the low degraded area; (c) CT image of the cross section of the heavily degraded sample; (d) CT image of the cross section of the low degraded sample; (e) SEM image of the latewood tracheids; (f) SEM image of the earlywood tracheids in the heavily degraded area.
3.5. PEG 2000 and Freeze-Drying
Figure 6 shows the structure of the wood sample which was conserved with an aqueous solution of PEG 2000 and freeze-dried. In the heavily degraded area (cf. Figure 6a), more PEG has become incorporated into the lumen than in the less degraded areas (cf. Figure 6b). The cell walls of the latewood tracheids in the low degraded area (cf. Figure 6b) are partially compact, but the degraded secondary cell wall can also be seen, which in turn has shrunken and is detached from the compound middle lamella. The cell walls of the earlywood in the heavily degraded area (cf. Figure 6a) are exceptionally thin and are imaged only very lightly. The cell walls of the latewood are very compact, but there are several areas where the secondary cell wall has degraded and detached from the well-preserved compound middle lamella (cf. Figure 6a,b,e,f). However, it looks like some voids are filled with the PEG. The random distribution of PEG in the lumen of the wood tissue is visible (cf. Figure 6a–d). The porous structure of the freeze-dried porous PEG could be observed in the lumen of some tracheids. More PEG seems to be deposited in the heavily degraded sample. It seems that the residual cell wall was stabilized with PEG (cf. Figure 6e,f).
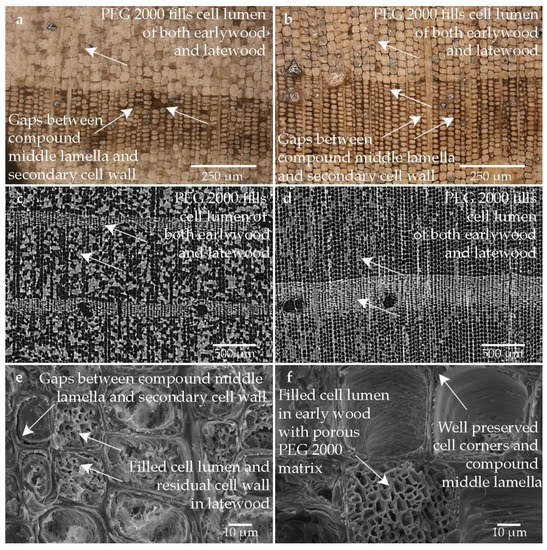
Figure 6.
Microscopic wood structure of sample conserved with PEG 2000 and freeze-drying (Pi1-PEG1) (a) RLM image from the heavily degraded area; (b) RLM image from the low degraded area; (c) CT image of the cross section of the heavily degraded sample; (d) CT image of the cross section of the low degraded sample; (e) SEM image of the latewood tracheids; (f) SEM image of the earlywood tracheids in the heavily degraded area.
3.6. PEG 400, PEG 4000 and Freeze-Drying
The structure of sample treated with an aqueous solution of PEG 400 and PEG 4000 is shown in Figure 7. Remarkably many large inorganic crystals are incorporated in the sample (cf. Figure 7a–c). The high degree of degradation is clearly seen (cf. Figure 7a), where in some cases only the scaffold of the compound middle lamella is visible and the secondary cell wall is degraded. This degraded structure appears to be stabilized. In CT (cf. Figure 7c,d), the random distribution of the freeze-dried high molecular weight PEG is visible as described in the previous sample. Especially in the high degraded area, the conservation agent has settled in the lumen of both early and latewood as well as in the cell wall of the tracheids (cf. Figure 7e,f). It seems that the compact secondary cell wall is filled with PEG (cf. Figure 7e). However, voids between the compound middle lamella and secondary cell wall are also seen here (cf. Figure 7e,f), which may have resulted from shrinkage of the secondary cell wall.
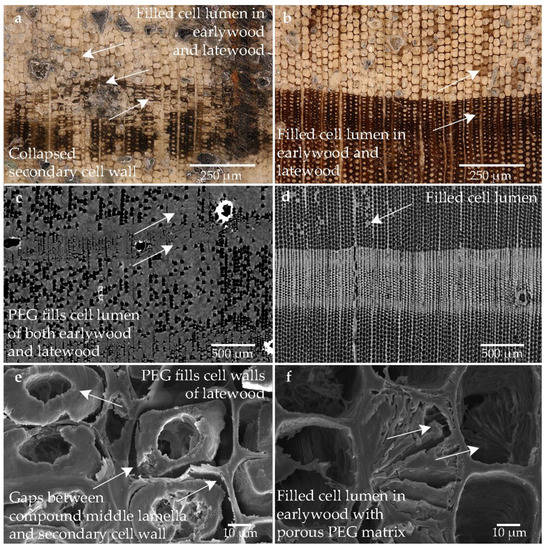
Figure 7.
Microscopic wood structure of sample conserved with PEG 400 and PEG 4000 and freeze-drying (Pi1-PEG2) (a) RLM image from the heavily degraded area; (b) RLM image from the low degraded area; (c) CT image of the cross section of the heavily degraded sample; (d) CT image of the cross section of the low degraded sample; (e) SEM image of the latewood tracheids; (f) SEM image of the earlywood tracheids in the heavily degraded area.
3.7. PEG 400, PEG 1500, PEG 4000 and Freeze-Drying
Figure 8 shows the wood sample that was conserved with an aqueous PEG solution (PEG 400, 1500 and 4000) and then freeze-dried. As with the other two PEG conserved samples (Pi-PEG1 and Pi-PEG2), the structure is very well stabilized (cf. Figure 8a,b). The degree of degradation can be well differentiated especially in the latewood cells, but also in the earlywood where the cell structure is only very weakly displayed (cf. Figure 8a). However, cells that seemed not to be stabilized at all can also be seen in the severely degraded areas. The low degraded tracheids are well preserved. The PEG mixture seems to be deposited in the lumen (cf. Figure 8c,d) and in the degraded cell wall (cf. Figure 8e,f). However, the cavities are not entirely filled with the conservation agent, leaving gaps between the compound middle lamella and the secondary wall. The secondary cell wall may have shrunk.
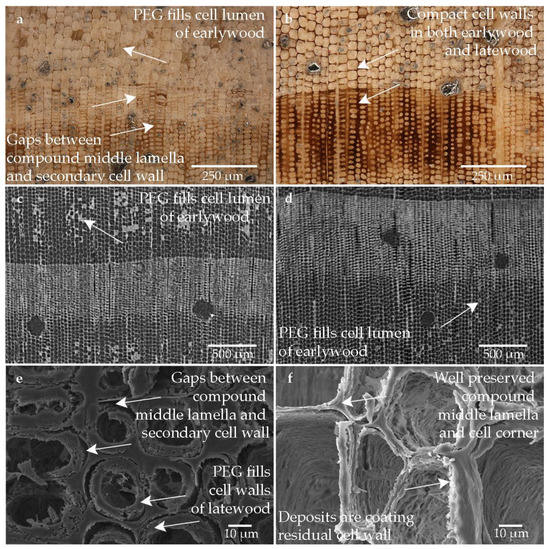
Figure 8.
Microscopic wood structure of sample conserved with PEG 400, PEG 1500 and PEG 4000 and freeze-drying (Pi1-PEG3) (a) RLM image from the heavily degraded area; (b) RLM image from the low degraded area; (c) CT image of the cross section of the heavily degraded sample; (d) CT image of the cross section of the low degraded sample; (e) SEM image of the latewood tracheids; (f) SEM image of the earlywood tracheids in the heavily degraded area.
3.8. Saccharose
The sample conserved with saccharose is shown in Figure 9. Saccharose is absorbed differently by the structure. It was stored predominantly in the latewood tracheids, but partially in the earlywood (cf. Figure 9a–f). Considerably more saccharose is deposited in the low degraded area, where the lumen of the earlywood tracheids is filled as well (cf. Figure 9b,d). The individual layers in the cell walls are also visible (cf. Figure 9b,e–f). Some of the secondary cell walls appear to have shrunk, leaving gaps visible between the secondary cell wall and the compound middle lamella (cf. Figure 9b,e–f). Because the sample was very hard and brittle, there are also many splinters visible that were created during preparation (cf. Figure 9d–f). The sharp-edged matrix is of saccharose is also visible in the SEM (cf. Figure 9d–f). Deformations can be seen in the structure (cf. Figure 9a,c).
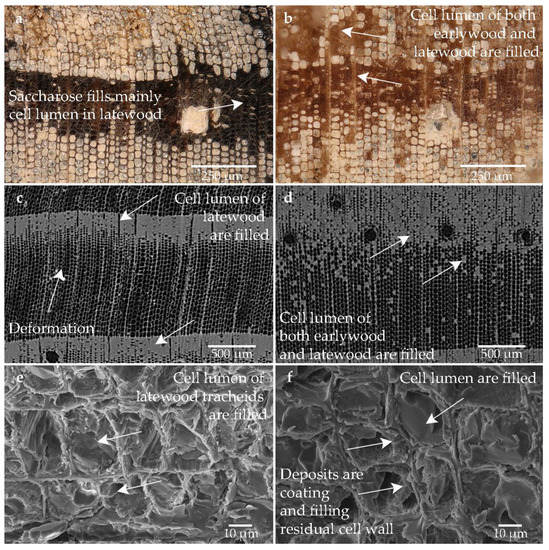
Figure 9.
Microscopic wood structure of sample conserved with saccharose (Pi1-Sac) (a) RLM image from the heavily degraded area; (b) RLM image from the low degraded area; (c) CT image of the cross section of the heavily degraded sample; (d) CT image of the cross section of the low degraded sample; (e) SEM image of the latewood tracheids; (f) SEM image of the earlywood tracheids in the heavily degraded area.
3.9. Silicone Oil
Figure 10 shows the structure of the wood impregnated with silicone. The structure is well preserved. Apparently, more silicone is concentrated in the high degraded sample (cf. Figure 10c) than in the sample taken from the low degraded area (cf. Figure 10d). Silicone is predominantly deposited in the lumen of the latewood and only a few earlywood tracheids are filled (cf. Figure 10c,d). While no structures are visible in the latewood of the sample conserved with silicone, the cell walls in the earlywood are completely saturated with silicone. Structural details of the latewood are no longer visible (cf. Figure 10c–e). The high contrast in the cell walls gives evidence that the cell walls are saturated with silicone (cf. Figure 10d,f).
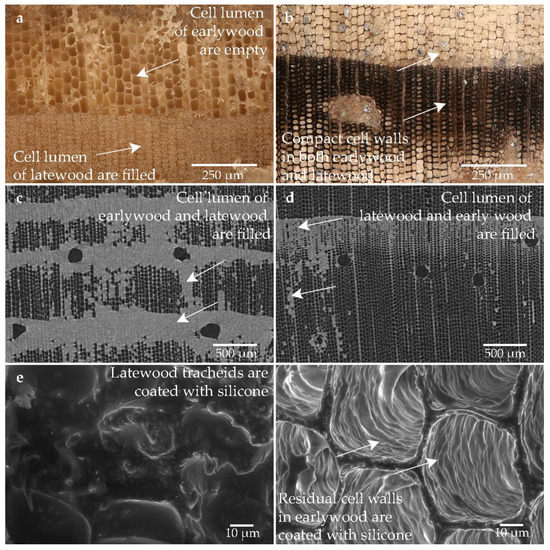
Figure 10.
Microscopic wood structure of sample conserved with silicone oil (Pi1-Sil) (a) RLM image from the high degraded area; (b) RLM image from the low degraded area; (c) CT image of the cross section of the high degraded sample; (d) CT image of the cross section of the low degraded sample; (e) SEM image of the latewood tracheids; (f) SEM image of the earlywood tracheids in the heavily degraded area.
4. Discussion
4.1. Distribution of the Conservation Agent
The microscopic examinations on the cell wall level of WAW that were conserved with the methods used to date showed significant differences.
The alcohol–ether–resin mixture was not clearly visible in the samples analyzed in the study. This might be due to the rather low concentration of the conservation agent of approximately 26% (cf. Appendix A Table A1). Further investigations are necessary here. In addition, shrinkage of the secondary cell wall was observed in the heavily degraded area. This was also reported previously [23,66].
Hardly any differences could be observed in the treatment with the melamine formaldehyde (Kauramin 800). The conservation method performed well in consolidating highly degraded tracheids. The compact appearance of the cell walls suggests that melamine formaldehyde (Kauramin 800) is strengthening the residual cell wall of WAW. This was also reported from previous used products in wood conservation: Arigal C, Ciba Geigy and Lyofix DML, Pfersee Augsburg [23,66,67]. The melamine formaldehyde Kauramin 800 is irreversibly fixed in the cell walls keeping the cell wall in a permanently swollen state. This effect is called “cell wall bulking”. As the resin fills the voids between the wood polymers and interacts with them [43]. Therefore, a rather low concentration of 25% resin is used (cf. Appendix A Table A1). By solid state NMR, it was possible to show that more melamine formaldehyde was present in the highly degraded outer regions and that, in addition to a network of hydrogen bonds, interactions between the triazine rings of the melamine resin and the aromatic rings of the lignin also formed, stabilizing the wood structure [68]. By interacting with the cell wall, the absorption of liquid water and water vapor into the cell wall is reduced. This also limits the dimensional changes of the wood [43].
The stabilization of lactitol/trehalose was higher in the heavily degraded areas. Lactitol/trehalose showed good stabilization of the well and poorly preserved areas, respectively. However, some inhomogeneity was evident in the heavily degraded areas. Here, lactitol/trehalose was shown to accumulate in the lumen where a high proportion of conservation agents were seen in the small-lumen latewood tracheids. This might be due to the relatively high concentration of the impregnating solution of 70% (cf. Appendix A Table A1). Crystal-like structures were observed to provide stability to the cell walls. In particular, the earlywood cells in the heavily degraded area, the cell walls are clearly reinforced. After impregnation, trehalose crystallizes in the wood structure. Then, the stabilized wood is allowed to be air-dried. When drying an aqueous lactitol solution, three different crystal types can be formed: lactitol mono-, di-, and trihydrate. The formation of the lactitol MC trihydrate, which precipitates on the surface as a white foam, is accompanied by an increase in volume, so that cracks may appear in the object [33]. The crystal structure is also observed here. Trehalose is used to prevent the formation of trihydrates. In addition, the solubility of lactitol MC is increased when trehalose is added [30,33]. The conserved wood shows the filled lumen of the fibers and parenchyma, but the vessels remain empty [69]. Crystallization and cracks could be documented here on a microscopic level, as well as cracks that also run through the fragile structure of degraded wood.
Freeze-dried polyethylene glycols were also found in the lumen, but they were randomly distributed in the wood structure although the concentration of the solution was between 27% and 40% (cf. Appendix A Table A1). The cell walls of the samples conserved with PEG (Pi-PEG1, Pi-PEG2, Pi-PEG3) are shrunken and in some cases have no connection to the compound middle lamella. When the lower molecular weight PEGs, e.g., PEG 400 and PEG 1500 (Sample Pi-PEG2 and Pi-PEG3), are added, distinct structural changes can be seen, and the porous cell walls are filled. The cell walls of the samples conserved with low molecular weight PEG here are darker and thicker than in the Pi-PEG1 sample, which was only conserved with high molecular weight PEG. The concentration of the solution was also higher with the high molecular weight PEG (40% for PEG 1, cf. Appendix A Table A1) compared to the impregnation solutions with low molecular weight PEG (35% for PEG 2 and 27% for PEG 3, cf. Appendix A Table A1). The low molecular weight PEG appears to further stabilize the well-preserved cell walls in the samples (Pi-PEG2 and Pi-PEG3). The low molecular weight PEG with shorter chain molecules could diffuse better into the cell wall. Higher molecular weight PEG precipitates as a thin coating on the cell walls. At high concentrations, the lumen are also filled [70,71,72]. This mode of action was confirmed here. Water occupies the space in the wood that was degraded in the soil during aging. During impregnation, PEG penetrates the mesopores of the wood cell wall and replaces the water. Even sound cell walls are penetrated up to a molecular weight of 3000 g/mol [73]. It was also confirmed by NMR studies that these places are occupied by PEG. According to the chemical structure, PEG is not involved in covalent bonds. Only a certain amount of PEG in wood has a close molecular interaction with the WAW, especially with lignin, resulting in mechanical stability [61,74]. The distribution of high molecular weight PEG in the sample (Pi-PEG1) is random, with significantly more PEG found in the highly degraded area than in the well-preserved sample. The low molecular weight PEG indicates that the better-preserved cell walls are stabilized. This was already reported [70,71,72,75].
The morphology of the conservation agents is decisively determined by the drying method [21,76]. This was shown ex-situ on potential conservation agents. Thereupon, it is hypothesized that the conservation agents are more likely to be deposited on the cell wall by air drying. This might be due to the development of capillary forces during air-drying and a film is created. In contrast, freeze-drying builds up a kind of scaffold, which has a positive effect on the mechanical stabilization of the wood [21]. In freeze-drying, the aqueous conservation solution is frozen, and the conservation agent is randomly distributed. In the subsequent primary drying, the frozen water is removed by sublimation and the porous conservation agent framework remains [21,77]. The fact that this also happens in the wood structure can be seen particularly clearly in the sample that was conserved with PEG 2000 and freeze-drying [18]. An aqueous solution of high molecular weight PEG (2000 or 4000) is, unlike aqueous solutions of low molecular weight PEG 400, solid at temperatures where freeze-drying equipment in conservation labs usually run [18,40,78,79].
In this study, the accumulation of saccharose in the structure of pine could be visualized. Here, it could be shown that saccharose precipitates more in the better-preserved inner areas of the sample. The fact that conservation with saccharose is better for well-preserved wood is also mentioned by Hoffmann [1,80]. It is assumed that the reason for this is also the high concentration of the saccharose solution of 60% (cf. Appendix A Table A1). The ability to fill the wood structure by bulking the vessels, lumina, and cell walls is mentioned [19]. It was observed that an amorphous matrix rather than a crystalline saccharose covers the cell walls [81]. Saccharose fills most of the tracheids [44] but an uneven uptake of saccharose was suspected [80]. The uptake of saccharose depends on the anatomical direction, which may be due to the different cells. In particular, the uptake is higher in the tangential direction due to the degradation of the pits and the higher permeability of the solution [82]. Here, we could see that saccharose accumulates in the lumen where a high proportion of conservation agents were seen in the small-lumen latewood tracheids. A lower uptake of saccharose was observed in the tylose-containing earlywood vessels was already observed [83]. Schmitt and Noldt assume the cause for this in the structure of the tylose cell wall, which contains an inner layer of suberin, a chemically very resistant hydrophobic material. In contrast, the accumulation of saccharose was seen in the small latewood vessels and in the parenchyma cells. It is assumed that the hydroxyl groups of cellulose interact with those of saccharose and hydroxyl bonds are formed [1,19].
The silicone oil mixture which was investigated here is composed of two kinds of silicone oil (SFD1 and SFD5), a crosslinker (methyltrimethoxysilane, MTMS) and a catalyst (dibutyltin diacetate/dibutyltin diacetate, DBDTA) [42]. The condensation product accumulates in the lumen, especially in the small-lumen tracheids of latewood, where high levels of conservation agent were found. This is due to the high concentration of approximately 100% of the conservation solution (cf. Appendix A Table A1). Currently, a lot of research on the treatment of silanes and siloxanes has been undertaken [34,84,85,86]. However, it could be shown that the stabilizing effectiveness and their deposition in the wood structure of particular organosilicons is dependent on their chemical composition [34]. SEM images of the treated wood show that alkoxysilanes coat the cell walls while the siloxane covers the cell walls but also fills the lumen. In the case of methyltrimethoxysilane, for example, the surface area and total pore volume decrease in proportion to the amount of methyltrimethoxysilane incorporated. In addition, depending on the degree of degradation and the type of wood, methyltrimethoxysilane shows a softening effect as well as the reduction of moisture content in the wood [84]. The deposition of the chemical into the cell wall and amorphous aggregates in the lumen areas was observed [86]. Due to its chemical structure, methyltrimethoxysilane bonds with wood polymers via hydrogen or ionic bonds involving amino groups. Alkoxysilanes can also react not only with the hydroxyl groups of the wood, but also condense with their own molecules. As a result, a potential spatial network is created that stabilizes the structure of the wood [85].
4.2. Relationship between Microscopic and Macroscopic Level
Stabilization at the microscopic level will be studied here in the context of the preservation result of the whole sample. In former work [16,51], the volume of the investigated sample before and after conservation was measured with an structured-light 3D scanning. The shrinkage was calculated in percentage terms on the basis of the volume data. The anti-shrink efficiency (ASE) is normalized on the volume data before conservation [87]. Table 2 shows the shrinkage of the entire samples before and after conservation as well as the anti-shrink efficiency. The data were taken from [16].

Table 2.
Volume stability expressed by the volume shrinkage and anti-shrink efficiency cf. [16,42].
The highest shrinkage and anti-shrink efficiency with a value of zero are shown by the air-dried reference. This result is also reflected on a microscopic level. Besides warping, shrinkage of the cell walls can be seen.
The sample conserved with alcohol-ether resin showed a high shrinkage and a low anti-shrink efficiency (76%). These results could be related to the shrinkage of the secondary cell walls. In addition, the poor consolidation of the degraded areas in this sample due to strong differences in the conservation results could be observed between the highly degraded wood with very little stabilization and the good stabilization of the low degraded sample. However, with the alcohol-ether-resin method, very good conservation results are achieved, especially of heavily degraded objects. But consolidation of the surface was necessary [39].
The samples that were conserved with melamine formaldehyde (Kauramin 800) showed a high anti-shrink efficiency of 87%. Cell wall bulking is probably the reason for this. In addition, hardly any difference in the consolidation of the outer area and the inner better-preserved areas could be observed. However, it is also reported that oak samples may split between well-preserved and poorly preserved areas [16]. These phenomena could not be observed here, indicating that further investigations on other wood species are necessary.
The conservation with lactitol/trehalose, however, showed a good conservation outcome with an anti-shrink efficiency of 89%. High dimensional stabilization is achieved with lactitol/trehalose as reported in literature [88,89]. The ability to stabilize the cell walls so well could be the explanation for this. The microscopic structure also shows good stabilization of the degraded areas, although the distribution is rather uneven.
The samples conserved with PEG and freeze-drying also showed good volume stabilization from 85% up to 91% anti-shrink efficiency, where the highest stability was achieved by PEG 2000. This cannot be explained on the basis of observations at the microscopic level because the low molecular weight PEG embeds itself well in the residual cell wall. The ability to maintain the cell wall in a swollen state would argue for higher volume stability of the low molecular weight PEG. Perhaps the reason for higher stabilization of PEG 2000 is due to freeze-drying, since no liquid phases occur during impregnation with high molecular weight PEG and the structure remains in its swollen extension [77]. The good ability of volume stabilization of PEG in general is known [44,75,90]. This result corresponds to the microscopic examinations here, where the degraded wood structure is stabilized relatively uniformly. However, freeze-drying can cause cracks [16,77].
The saccharose treated sample, showed a good conservation outcome with an anti-shrink efficiency of 90% [16]. Here, the cell walls were well stabilized by saccharose. Especially the latewood and the well-preserved samples are particularly well soaked by saccharose. However, collapse and deformation in the wood structure could also be detected here on a microscopic level, which contradicts the high values for the anti-shrink efficiency.
Silicone seemed also to stabilize both degradation states, although it achieved less good volume stabilization of the whole sample (anti-shrink efficiency 75%). In addition, relatively high voids were detected inside the sample [16]. This result cannot be explained by microscopic examination, since it appears that the silicone is found in the lumen and in the cell walls.
5. Conclusions
Wood-degrading organisms prefer the cellulose-rich components of the cell for their metabolism. Lignin-rich areas, such as the middle lamella and the primary cell wall, are left behind. What remains is a skeleton of woody tissue, along with degradation products and bacterial slime. The integrity of the WAW now depends on this very fragile network of composite middle lamella, which, unlike healthy wood, is not supported by a solid and strong secondary cell wall [7].
The aim of conservation is to transform the wet fragile object into a dry, stable state, whereby the object is changed as little as possible by the measures. Aesthetic considerations play a major role here, but above all the objects should be sufficiently stabilized. In addition to the previous studies on these samples on color or dimensional stabilization [16,42], the stabilization on a microscopic level was investigated here. The microscopic examinations have shown how the most diverse formulations used to date as standard conservation methods for archaeological wet wood are effective. As a limitation, it must also be mentioned that in the analysis of the microscopic structure, only a section of the entire heterogeneous structure at a certain depth of the sample is considered. Misinterpretations due to the preparation procedures could be avoided by using nondestructive examination by CT. Through the investigations, the following differences in the mode of action could be revealed:
In the sample conserved with the alcohol-ether method, the conservation agent was not clearly recognizable. The structure does not seem to be strongly stabilized. The ASE also showed the second lowest values here (76%). Some conservation methods, such as the melamine formaldehyde Kauramin 800, mainly stabilize the cell walls. The incorporation and stabilization of the cell wall are advantageous, as the structure and thus also the stabilization of the wood as a whole are also closest to that of recent wood. Moreover, the ASE value was relatively high (87%), which was achieved by cell wall bulking.
Other conservation agents, e.g., silicone oil, saccharose, and lactitol/trehalose, also fill the lumen, which may also be due to the amount of conservation agents used. With a full impregnation, the lumen and cell walls are completely filled with the conservation agent so that the degraded wood is supported and cannot collapse during air-drying. The samples treated with lactitol/trehalose (89%) and saccharose (90%) showed high ASE values. The wood sample conserved with silicone oil, on the other hand, showed the highest shrinkage (ASE 75%). This shows that filling the entire structure does not guarantee high volume stability. In addition, it could be shown that visibly more conservation agent was stored in the lumen of the latewood, whereas rather less conservation agent was stored in the earlywood. This effect was particularly visible in the samples treated with silicone oil, saccharose and lactitol/trehalose. In air drying, the distribution of the conservation agent changes due to the occurrence of capillary forces during drying. The uneven distribution is rather disadvantageous, as it could lead to mechanical stresses. Complete impregnation also brings disadvantages, e.g., high material consumption, heavy objects, and often strong color changes. Moreover, post-treatment is rarely possible.
The PEG treated and subsequently freeze-dried samples showed very high ASE values (87–91%). In the freeze-dried samples, the conservation agent PEG was distributed over the entire wood structure (over earlywood and latewood). From this, it can be hypothesized the freeze-drying process can help prevent a predominant part of the conservation agent from precipitating in the late wood. The freeze-dried PEG remains randomly distributed as a kind of scaffold in the wood tissue. Since the degraded wood is more evenly stabilized, this structure could prove to be advantageous. Further studies on the mechanical properties would be interesting here.
Besides the viscosity, the surface charge and molecular weight affect the diffusion of molecules [21,91]. In addition, the affinity (polarity) of the conservation agent to cellulose components and to lignin seems to be crucial. However, the residual material present in the degraded cell wall consists primarily of modified lignin. Further studies will follow to investigate the mode of action of the conservation methods in the degraded structure of archaeological wet wood. Detailed studies, e.g., with hardwoods, are desirable to show differences, if any, in how the conservation methods work according to a different anatomy.
Author Contributions
Conceptualization, I.S.; methodology, I.S.; software, J.S., J.M.-G., D.G. and I.S.; validation, I.S.; formal analysis, I.S. and D.G.; investigation, I.S.; resources, I.S.; data curation, I.S.; writing—original draft preparation, I.S. and J.S. writing—review and editing, I.S.; visualization, I.S. and J.S.; supervision, I.S.; project administration, I.S. and P.S.; funding acquisition, I.S. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - 416877131 and the Swiss National Science Foundation (SNSF) - 200021E_183684. The acquisition of the CT instrument LuCi was supported by the Swiss National Science Foundation SNSF grant number- 206021_189608). The APC was funded by Swiss National Science Foundation (SNSF).
Data Availability Statement
All data is available open access (www.rgzm.de/kur, accessed on 1 December 2022).
Acknowledgments
The Federal Cultural Foundation and the Cultural Foundation of German States funded the development of the reference collection for archaeological wood. The authors thank M. Egg, M. Wittköpper and W. Muskalla, LEIZA and all other participants of the KUR project for their work in conserving the wooden samples. We also thank J. Odermatt, University Hamburg, Institute of Wood Science for wood analyses, Dipl.-Ing. Dieter Willer, Universität Stuttgart for sputter coating the samples and I. Calandra, LEIZA for his kind support with the SEM analyses.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Conservation methods and details [33].
Table A1.
Conservation methods and details [33].
| Conservation Method | Institutions and Short Descriptions of the Methods | |
|---|---|---|
| Air dried reference | Institution: | Leibniz-Zentrum für Archäologie, Mainz, Germany |
| Treatment: | none, air-drying | |
| Impregnation solution: | none | |
| Alcohol-ether-resin | Institution: | Schweizerisches Nationalmuseum, Zürich, Switzerland. |
| Treatment: | Exchange of water with ethanol. Exchange of ethanol with diethyl ether. Soaking of wood with diethyl ether in resin-diethyl solution. Drying by evaporation of the diethyl ether in the vacuum vessel. Application of surface protection 3% Paraloid B72 solution in acetone. | |
| Impregnation solution: | 70.7% diethyl ether, 16.1% dammar resin, 6.4% rosin, 3.2% dienol D102, 3.2% rhizinus oil, 0.4% PEG 400 | |
| Kauramin 800 | Institution: | Leibniz-Zentrum für Archäologie, Mainz, Germany |
| Treatment: | Soaking in demineralized water. Bath impregnation at room temperature. Replacement of the solution when early polymerisation occurs. Curing of the impregnated wood in the heating cabinet at 60 °C. Afterwards slow, controlled air-drying. Dip in linseed oil varnish. | |
| Impregnation solution: | 25% Kauramin 800 solution (72 L resin + 210 L deionised water, 3.6 L urea, 7.2 L triethylene glycol) | |
| Lactitol-trehalose | Institution: | Brandenburgisches Landesamt für Denkmalpflege, Zossen, Germany |
| Treatment: | Starting with 30% concentration. Increasing monthly in 10% steps up to 70%. Bath temperature 55 °C. After removal from the bath, the surfaces were dusted with crystalline lactitol monohydrate and dried in a heating oven over a period of one week. After drying, the surface was cleaned by dabbing with damp cloths. | |
| Impregnation solution: | lactitol-trehalose solution (9:1) 30%–70%. Addition of biocide if necessary (0,1% Bioban 404) | |
| Polyethylene glycol (PEG 2000) one-step and freeze-drying | Institution: | Nationalmuseet, Copenhagen, Denmark |
| Treatment: | Starting with 10% PEG 2000 solution. Increasing the concentration up to 40% at room temperature. Freeze-drying in cooled chamber (approx. −30 °C). Removal of excess PEG from the surface with a soft brush and ethanol. Subsequent surface stabilisation with 25% PEG 2000 solution in ethanol. | |
| Impregnation solution: | PEG 2000, 10–40% solution with tap water | |
| Polyethylene glycol (PEG 400 and 4000) two-step and freeze-drying | Institution: | Brandenburgisches Landesamt für Denkmalpflege, Zossen, Germany |
| Treatment: | Soaking in demineralised water. Starting with 5% PEG 400 solution. Raising the concentration in 5% steps every 4 weeks. At its calculated final concentration, it was kept constant. Then the increase of PEG 4000 solution was continued in 5% steps up to its final concentration where it was kept for 10 weeks. Precooling of the wood to 5 °C then deep-freezing to −25 to −35 °C, freeze-drying in cooled chamber (approx. −30 °C). | |
| Impregnation solution: | PEG-solution in demineralised water (PEG 400 and PEG 4000) was adapted according to the condition of the wood (PEGcon): 35% PEG solution (of which 10% PEG 400 and 25% PEG 4000) in demineralized water, impregnation at room temperature | |
| Polyethylene glycol (PEG 400, 1500 and 4000) three-step and freeze-drying | Institution: | Archäologische Staatssammlung, Munich, Germany |
| Treatment: | Soaking in demineralised water. Starting with 11% increasing to 15% PEG 400 solution at room temperature. 16% increasing to 20.5% PEG 1500 solution at 40 °C. 20.5% increasing to 27.5% PEG 4000 solution at 40 °C. Washing of the wood and wrapping in cellulose tissues. Intermediate storage in freezer (−25 to −35 °C) until freeze-drying. Subsequent freeze-drying in a cooled chamber (approx. −30 °C). Excess of PEG was removed with a brush and ethanol. | |
| Impregnation solution: | PEG-solution in demineralised water: 15% PEG 400, 20.5% PEG 1500, 27.5% PEG 4000 | |
| Saccharose | Institution: | Sächsisches Landesamt für Archäologie, Dresden, Germany |
| Treatment: | Concentrated the solution in 10% steps, from 10% up to 60% sugar solution at room temperature. Slow, controlled air-drying in microperforated bags. Removal of crystallised sugar residues from the surface with damp sponge. | |
| Impregnation solution: | Aqueous saccharose solution 10%–60%. If necessary biocide addition composed of 0.6%, sodium benzoate (E211), 0.5% Parmetol K40, 0.5% Quartasept Plus and 0.02% Tallofin OT | |
| Silicone oil | Institution: | Texas University, Texas, USA |
| Treatment: | Exchange of water with ethanol. Exchange of ethanol with acetone. Placing the still dripping wet acetone-impregnated samples in impregnation solution under normal atmospheric conditions. Triggering the polymerisation of the impregnation solution by gaseous catalyst: DBTDA (dibutyl diacetate). | |
| Impregnation solution: | 80% silicone oil (SFD1 (66%) + SFD5 (34%)—silanol functional polydimethylsiloxanes “PDMS”) and 20% crosslinker MTMS (methyltrimethoxysilane) | |

Table A2.
Overview over the samples and methods of investigation. For each analysis (marked with x), one sample was prepared from a core sample. In all, five samples were analyzed for each conservation method.
Table A2.
Overview over the samples and methods of investigation. For each analysis (marked with x), one sample was prepared from a core sample. In all, five samples were analyzed for each conservation method.
| Sample | Database [33] | Conservation | Condition | Analysis | ||
|---|---|---|---|---|---|---|
| CT | SEM | LM | ||||
| Pi-Air-ld | V03-01 | None, air-drying | Low degraded | x | x | |
| Pi-Air-hd | heavily degraded | x | x | x | ||
| Pi-AlEt-ld | V03-17 | Alcohol-ether-resin | Low degraded | x | ||
| Pi-AlEt-hd | heavily degraded | x | x | x | ||
| Pi-K800-ld | V03-41 | Melamine formaldehyde | Low degraded | x | x | |
| Pi-K800-hd | heavily degraded | x | x | x | ||
| Pi-LaTr-ld | V03-28 | Lactitol-trehalose | Low degraded | x | x | |
| Pi-LaTr-hd | heavily degraded | x | x | x | ||
| Pi-PEG1-ld | V03-35 | PEG 2000 | Low degraded | x | x | |
| Pi-PEG1-hd | heavily degraded | x | x | x | ||
| Pi-PEG2-ld | V03-32 | PEG 400 and 4000 | Low degraded | x | x | |
| Pi-PEG2-hd | heavily degraded | x | x | x | ||
| Pi-PEG3-ld | V03-20 | PEG 400, 1500 and 4000 | Low degraded | x | x | |
| Pi-PEG3-hd | heavily degraded | x | x | x | ||
| Pi-Sac-ld | V03-45 | Saccharose | Low degraded | x | x | |
| Pi-Sac-hd | heavily degraded | x | x | x | ||
| Pi-Sil-ld | V03-42 | Silicone oil | Low degraded | x | x | |
| Pi-Sil-hd | heavily degraded | x | x | x | ||
References
- Broda, M.; Hill, C.A.S. Conservation of Waterlogged Wood—Past, Present and Future Perspectives. Forests 2021, 12, 1193. [Google Scholar] [CrossRef]
- Gregory, D.; Jensen, P. The Importance of Analysing Waterlogged Wooden Artefacts and Environmental Conditions When Considering Their In Situ Preservation. J. Wetl. Archaeol. 2006, 6, 65–81. [Google Scholar] [CrossRef]
- Lucejko, J.J.; Tamburini, D.; Modugno, F.; Ribechini, E.; Colombini, M.P. Analytical Pyrolysis and Mass Spectrometry to Characterise Lignin in Archaeological Wood. Appl. Sci. 2021, 11, 240. [Google Scholar] [CrossRef]
- Pournou, A. Biodeterioration of Wooden Cultural Heritage: Organisms and Decay Mechanisms in Aquatic and Terrestrial Ecosystems; Springer: Cham, Switzerland, 2020; ISBN 978-3-030-46503-2. [Google Scholar]
- Walsh-Korb, Z.; Avérous, L. Recent Developments in the Conservation of Materials Properties of Historical Wood. Prog. Mater. Sci. 2019, 102, 167–221. [Google Scholar] [CrossRef]
- Zisi, A. Forest Wood through the Eyes of a Cultural Conservator. Forests 2021, 12, 1001. [Google Scholar] [CrossRef]
- Björdal, C.G.; Nilsson, T.; Daniel, G. Microbial Decay of Waterlogged Archaeological Wood Found in Sweden Applicable to Archaeology and Conservation. Int. Biodeterior. Biodegrad. 1999, 43, 63–73. [Google Scholar] [CrossRef]
- Klaassen, R.K.W.M. Bacterial Decay in Wooden Foundation Piles—Patterns and Causes: A Study of Historical Pile Foundations in the Netherlands. Int. Biodeterior. Biodegrad. 2008, 61, 45–60. [Google Scholar] [CrossRef]
- Pedersen, N.B.; Łucejko, J.J.; Modugno, F.; Björdal, C. Correlation between Bacterial Decay and Chemical Changes in Waterlogged Archaeological Wood Analysed by Light Microscopy and Py-GC/MS. Holzforschung 2021, 75, 635–645. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, Y.S.; Chavan, R.R. Advances in Understanding Microbial Deterioration of Buried and Waterlogged Archaeological Woods: A Review. Forests 2022, 13, 394. [Google Scholar] [CrossRef]
- Grosser, D. Die Hölzer Mitteleuropas: Ein Mikrophotographischer Lehratlas; Springer: Berlin, Germany, 1977; ISBN 978-3-540-08096-1. [Google Scholar]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 2011; ISBN 978-3-11-083965-4. [Google Scholar]
- Zhang, X.; Li, L.; Xu, F. Chemical Characteristics of Wood Cell Wall with an Emphasis on Ultrastructure: A Mini-Review. Forests 2022, 13, 439. [Google Scholar] [CrossRef]
- Hawley, L.F. Wood-Liquid Relations. Tech. Bull. 1931, 248, 1–34. [Google Scholar]
- Broda, M.; Curling, S.F.; Frankowski, M. The Effect of the Drying Method on the Cell Wall Structure and Sorption Properties of Waterlogged Archaeological Wood. Wood Sci. Technol. 2021, 55, 971–989. [Google Scholar] [CrossRef]
- Stelzner, J.; Stelzner, I.; Martinez-Garcia, J.; Gwerder, D.; Wittkoepper, M.; Muskalla, W.; Cramer, A.; Heinz, G.; Egg, M.; Schuetz, P. Stabilisation of Waterlogged Archaeological Wood—The Application of Structured-Light 3D Scanning and Micro Computed Tomography for Analysing Dimensional Changes. Herit. Sci. 2022, 10, 1–25. [Google Scholar] [CrossRef]
- Grattan, D.W.; Clarke, R.W. Conservation of Waterlogged Wood. In Conservation of Marine Archaeological Objects; Pearson, C., Ed.; Butterworth-Heinemann: Oxford, UK, 1987; pp. 164–206. ISBN 978-0-408-10668-9. [Google Scholar]
- Schnell, U.; Jensen, P. Determination of Maximum Freeze Drying Temperature for PEG-Impregnated Archaeological Wood. Stud. Conserv. 2007, 52, 50–58. [Google Scholar] [CrossRef]
- Parrent, J.M. The Conservation of Waterlogged Wood Using Sucrose. Stud. Conserv. 1985, 30, 63–72. [Google Scholar] [CrossRef]
- Jensen, P.; Gregory, D.J. Selected Physical Parameters to Characterize the State of Preservation of Waterlogged Archaeological Wood: A Practical Guide for Their Determination. J. Archaeol. Sci. 2006, 33, 551–559. [Google Scholar] [CrossRef]
- Walsh-Korb, Z.; Stelzner, I.; Dos Santos Gabriel, J.; Eggert, G.; Avérous, L. Morphological Study of Bio-Based Polymers in the Consolidation of Waterlogged Wooden Objects. Mater. Basel Switz. 2022, 15, 681. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M. Developing New Consolidants for Archaeological Wood. Ph.D. Thesis, University of Oslo, Oslo, Norway, 2013. [Google Scholar]
- Bräker, O.U.; Bill, J.; Mühlethaler, B.; Schoch, W.; Schweingruber, F.H.; Haas, A.; Hug, B.; Kramer, W.; Elmer, J.T.; Tassigny, C.D.; et al. Zum derzeitigen Stand der Nassholzkonservierung. Diskussion der Grundlagen und Resultate eines von Fachlaboratorien 1976-1978 durchgeführten Methodenvergleiches. Z. Für Schweiz. Archaeol. Kunstgesch. 1979, 36, 97–145. [Google Scholar]
- Jensen, P.; Petersen, A.H.; Straetkvern, K. From the Skuldelev to the Roskilde Ships—50 Years of Shipwreck Conservation at the National Musem of Denmark. In Proceedings of the Shipwrecks 2011 Chemistry and Preservation of Waterlogged Wooden Shipwrecks; Ek, M., Ed.; ICOM-CC: Stockholm, Sweden, 2011; pp. 14–20. [Google Scholar]
- Müller-Beck, H.-J.; Haas, A. A Method for Wood Preservation Using Arigal C. Stud. Conserv. 1960, 5, 150–158. [Google Scholar]
- Wittköpper, M. Der aktuelle Stand der Konservierung archäologischer Naßhölzer mit Melamin/Aminoharzen am Römisch-Germanischen Zentralmuseum. Arbeitsblätter Restaur. 1998, 31, 227–283. [Google Scholar]
- Babiński, L. Dimensional Changes of Waterlogged Archaeological Hardwoods Pre-Treated with Aqueous Mixtures of Lactitol/Trehalose and Mannitol/Trehalose before Freeze-Drying. J. Cult. Herit. 2015, 16, 876–882. [Google Scholar] [CrossRef]
- Han, L.; Guo, J.; Tian, X.; Jiang, X.; Yin, Y. Evaluation of PEG and Sugars Consolidated Fragile Waterlogged Archaeological Wood Using Nanoindentation and ATR-FTIR Imaging. Int. Biodeterior. Biodegrad. 2022, 170, 105390. [Google Scholar] [CrossRef]
- Hoffmann, P. Zur Naßholzkonservierung mit Zucker am Deutschen Schiffahrtsmuseum eine Bilanz. Arbeitsblätter der Restauratoren 1996, 10, 231–240. [Google Scholar]
- Imazu, S.; Morgós, A. An Improvement on the Lactitol MC Conservation Method Used for the Conservation of Archaeological Waterlogged Wood (The Conservation Method Using Lactitol MC and Trehalose Mixture). In Proceedings of the 8th ICOM Group on Wet Organic Archaeological Materials Conference, Stockholm, Sweden, 11–15 June 2001; Hoffmann, P., Spriggs, J.A., Grant, T., Cook, C., Recht, A., Eds.; ICOM Committee for Conservation: Bremerhaven, Germany, 2002; pp. 413–428. [Google Scholar]
- Jensen, P.; Pedersen, N.B. Examination of D-Mannitol as an Impregnation Agent for Heavily Degraded Waterlogged Archaeological Wood. In Proceedings of the 12th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Istanbul, Turkey, 13–17 May 2013; Grant, T., Cook, C., Eds.; ICOM-CC: Istanbul, Turkey, 2016; pp. 118–125. [Google Scholar]
- Kennedy, A.; Pennington, E.R. Conservation of Chemically Degraded Waterlogged Wood with Sugars. Stud. Conserv. 2014, 59, 194–201. [Google Scholar] [CrossRef]
- Imazu, S.; Ito, K.; Fujita, H.; Morgos, A. The Rapid Trehalose Conservation Method for Archaeological Waterlogged Wood and Laquerware. In Proceedings of the 12th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Istanbul, Turkey, 13–17 May 2013; Grant, T., Cook, C., Eds.; ICOM-CC: Istanbul, Turkey, 2016; pp. 110–117. [Google Scholar]
- Broda, M.; Dąbek, I.; Dutkiewicz, A.; Dutkiewicz, M.; Popescu, C.-M.; Mazela, B.; Maciejewski, H. Organosilicons of Different Molecular Size and Chemical Structure as Consolidants for Waterlogged Archaeological Wood—A New Reversible and Retreatable Method. Sci. Rep. 2020, 10, 2188. [Google Scholar] [CrossRef]
- Hamilton, D.L. Methods of Conserving Archaeological Material from Underwater Sites, 2nd ed.; Anthropology; Department of Anthropology, Texas A&M University, Ed.; Conservation of Archaeological Resources I, Texas A&M University: Texas TX, USA, 2010. [Google Scholar]
- Smith, C.W. Archaeological Conservation Using Polymers: Practical Applications for Organic Artifact Stabilization; Texas A&M University Press: Texas TX, USA, 2003; ISBN 978-1-58544-217-1. [Google Scholar]
- Hocker, E.; Almkvist, G.; Sahlstedt, M. The Vasa Experience with Polyethylene Glycol: A Conservator’s Perspective. J. Cult. Herit. 2012, 13, S175–S182. [Google Scholar] [CrossRef]
- Hoffmann, P. A Rapid Method for the Detection of Polyethylene Glycols (Peg) in Wood. Stud. Conserv. 1983, 28, 189–193. [Google Scholar] [CrossRef]
- Schmidt-Ott, K.; André, C.; Bader, M. Fishing for Stability: Conserving a Fish Trap in a Block Excavation by the Alcohol-Ether-Resin Method. In Proceedings of the 14th ICOM-CC Wet Organic Archaeological Materials Working Group Interim Meeting, Portsmouth, UK, 20–24 May 2019; ICOM-CC: Portsmouth, UK, 2022; pp. 322–327. [Google Scholar]
- Jensen, P.; Strætkvern, K.; Schnell, U.; Jensen, J.B. Technical Specifications for Equipment for Vacuum Freeze-Drying of PEG Impregnated Waterlogged Organic Materials. In Proceedings of the 10th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Amsterdam, The Netherlands, 10–15 September 2007; ICOM-CC: Amsterdam, The Netherlands, 2010; pp. 417–438. [Google Scholar]
- Wiesner, I.; Gieseler, H. Freeze-Dry Microscopy—Real-Time Observation of the Drying Process. In Proceedings of the 12th ICOM-CC Wet Organic Archaeological Materials Conference, Istanbul, Turkey, 13–17 May 2013; Grant, T., Cook, C., Eds.; ICOM-CC: Istanbul, Turkey, 2016; pp. 417–424. [Google Scholar]
- Massenfunde in archäologischen Sammlungen. Available online: www.rgzm.de/kur (accessed on 1 December 2022).
- Altgen, M.; Awais, M.; Altgen, D.; Klüppel, A.; Mäkelä, M.; Rautkari, L. Distribution and Curing Reactions of Melamine Formaldehyde Resin in Cells of Impregnation-Modified Wood. Sci. Rep. 2020, 10, 3366. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P. Conservation of Archaeological Ships and Boats: Personal Experiences; Archetype Publications: London, UK, 2013; ISBN 978-1-904982-82-1. [Google Scholar]
- Daniel, G. Microscope Techniques for Understanding Wood Cell Structure and Biodegradation. In Secondary Xylem Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 309–343. ISBN 978-0-12-802185-9. [Google Scholar]
- Giachi, G.; Capretti, C.; Donato, I.D.; Macchioni, N.; Pizzo, B. New Trials in the Consolidation of Waterlogged Archaeological Wood with Different Acetone-Carried Products. J. Archaeol. Sci. 2011, 38, 2957–2967. [Google Scholar] [CrossRef]
- Stelzner, J.; Million, S. X-Ray Computed Tomography for the Anatomical and Dendrochronological Analysis of Archaeological Wood. J. Archaeol. Sci. 2015, 55, 188–196. [Google Scholar] [CrossRef]
- Bugani, S.; Modugno, F.; Łucejko, J.J.; Giachi, G.; Cagno, S.; Cloetens, P.; Janssens, K.; Morselli, L. Study on the Impregnation of Archaeological Waterlogged Wood with Consolidation Treatments Using Synchrotron Radiation Microtomography. Anal. Bioanal. Chem. 2009, 395, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Hansen, F.K.; Kutzke, H. Phenol Formaldehyde Revisited-Novolac Resins for the Treatment of Degraded Archaeological Wood: Novolac Resins for Treatment of Degraded Archaeological Wood. Archaeometry 2015, 57, 536–559. [Google Scholar] [CrossRef]
- Braovac, S.; Sahlstedt, M.; Wittköpper, M. Retreatment Testing of Alum-Treated Woods from the Oseberg Collection—Results from First Trials. Wet Organic Archaeological Materials 2019. In Proceedings of the 14th ICOM-CC Wet Organic Archaeological Materials Working Group Interim Meeting, Portsmouth, UK, 20–24 May 2019; Williams, E., Ed.; ICOM-CC: Portsmouth, UK, 2022; pp. 207–215. [Google Scholar]
- Wittköpper, M.; Muskalla, W.; Stephan, B.; Le Boedec-Moesgard, A.; Gebhadt, S.; Klonk, S.; André, C.; Schmidt-Ott, K.; Smith, W. The KUR (Conservation and Restauration) Project—A Comparison of Different Methods to Preserve Waterlogged Wood. In Proceedings of the 12th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Istanbul, Turkey, 13–17 May 2013; Grant, T., Cook, C., Eds.; ICOM-CC: Istanbul, Turkey, 2016; pp. 134–143. [Google Scholar]
- de Jong, J. The Conservation of Waterlogged Timber at Ketelhaven (Holland). In Proceedings of the 4th ICOM-CC Triennial Meeting, Venice, Italy, 13–18 October 1975; ICOM Committee for Conservation, Ed.; ICOM-CC: Paris, France, 1975; p. 75/8/1-1–9. [Google Scholar]
- High, K.E.; Penkman, K.E.H. A Review of Analytical Methods for Assessing Preservation in Waterlogged Archaeological Wood and Their Application in Practice. Herit. Sci. 2020, 8, 83. [Google Scholar] [CrossRef]
- Macchioni, N.; Pecoraro, E.; Pizzo, B. The Measurement of Maximum Water Content (MWC) on Waterlogged Archaeological Wood: A Comparison between Three Different Methodologies. J. Cult. Herit. 2018, 30, 51–56. [Google Scholar] [CrossRef]
- Scott, D.A.; Eggert, G. The Vicissitudes of Vivianite as Pigment and Corrosion Product. Stud. Conserv. 2007, 52, 3–13. [Google Scholar] [CrossRef]
- Macchioni, N.; Pizzo, B.; Capretti, C.; Giachi, G. How an Integrated Diagnostic Approach Can Help in a Correct Evaluation of the State of Preservation of Waterlogged Archaeological Wooden Artefacts. J. Archaeol. Sci. 2012, 39, 3255–3263. [Google Scholar] [CrossRef]
- Zborowska, M.; Babiński, L.; Gajewska, J.; Waliszewska, B.; Prądzyński, W. Pysical and Chemical Properties of Contemporary Pine Wood (Pinus Sylvestris L.) in Conditions of a Wet Archaeolocical Site in Biskupin. Folia For. Pol. 2007, 38, 13–26. [Google Scholar]
- McLean, P. Wood Properties and Uses of Scots Pine in Britain. Research Report; Forestry Commission: Edinburgh, UK, 2019. [Google Scholar]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical Cone-Beam Algorithm. JOSA A 1984, 1, 612–619. [Google Scholar] [CrossRef]
- Čufar, K.; Gričar, J.; Zupančič, M.; Koch, G.; Schmitt, U. Anatomy, Cell Wall Structure and Topochemistry of Water-Logged Archaeological Wood Aged 5,200 and 4,500 Years. IAWA J. 2008, 29, 55–68. [Google Scholar] [CrossRef]
- Bardet, M.; Gerbaud, G.; Trân, Q.-K.; Hediger, S. Study of Interactions between Polyethylene Glycol and Archaeological Wood Components by 13C High-Resolution Solid-State CP-MAS NMR. J. Archaeol. Sci. 2007, 34, 1670–1676. [Google Scholar] [CrossRef]
- Björdal, C.G.; Nilsson, T. Waterlogged Archaeological Wood—A Substrate for White Rot Fungi during Drainage of Wetlands. Int. Biodeterior. Biodegrad. 2002, 50, 17–23. [Google Scholar] [CrossRef]
- Björdal, C.G.; Nilsson, T. Observations on Microbial Growth during Conservation Treatment of Waterlogged Archaeological Wood. Stud. Conserv. 2001, 46, 211–220. [Google Scholar] [CrossRef]
- Guo, J.; Xiao, L.; Han, L.; Wu, H.; Yang, T.; Wu, S.; Yin, Y. Deterioration of the Cell Wall in Waterlogged Wooden Archeological Artifacts, 2400 Years Old. IAWA J. 2019, 40, 820–844. [Google Scholar] [CrossRef]
- Pedersen, N.B. Microscopic and Spectroscopic Characterisation of Waterlogged Archaeological Softwood from Anoxic Environments. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, 2015. [Google Scholar]
- Schweingruber, F.H. Mikroskopische Holzanatomie; 3. Auflage; Eidgenössische Forschungsanstalt für Wald, Schnee und Landschaft: Birmensdorf, Switzerland, 1990. [Google Scholar]
- Haas, A.; Müller-Beck, H.-J.; Schweingruber, F.H. Erfahrungen Bei Der Konservierung von Feuchthölzern Mit Arigal C. In Jahrbuch des Bernischen Historischen Museums in Bern; 1961/62; 41/42; 1961; pp. 509–537. [Google Scholar]
- Spinella, A.; Chillura Martino, D.F.; Saladino, M.L.; Sammartino, F.; Caruso, F.; Caponetti, E. Solid State NMR Investigation of the Roman Acqualadroni Rostrum: Tenth Year Assessment of the Consolidation Treatment of the Wooden Part. Cellulose 2021, 28, 1025–1038. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Kohdzuma, Y.; Endo, R.; Sugiyama, J. Evaluation of Chemical Treatments on Dimensional Stabilization of Archeological Waterlogged Hardwoods Obtained from the Thang Long Imperial Citadel Site, Vietnam. J. Wood Sci. 2018, 64, 436–443. [Google Scholar] [CrossRef]
- Bilz, M.; Grant, T.; Young, G. Treating Waterlogged Basketry: A Study of Polyethylene Glycol Penetration into the Inner Bark of Western Red Cedar. In Proceedings of the 7th ICOM Group on Wet Organic Archaeological Materials Conference, Grenoble, France, 19–23 October 1998; Bonnot-Diconne, C., Hiron, X., Tran, Q.K., Hoffmann, P., Eds.; ICOM-CC: Grenoble, France, 1999; pp. 249–253. [Google Scholar]
- Cook, C.; Grattan, D. A Method of Calculation the Concentration of PEG for Freeze-Drying Waterlogged Wood. In Proceedings of the 4th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Bremerhaven, Germany, 20–24 August 1990; Hoffmann, P., Ed.; ICOM-CC: Bremerhaven, Germany, 1990; pp. 239–252. [Google Scholar]
- Hoffmann, P.; Riens, R.; Eckstein, D. Zur Gefriertrocknung schwer zu konservierender Naßhölzer. Arbeitsblätter der Restauratoren 1991, 13, 193–205. [Google Scholar]
- Penttilä, P.A.; Altgen, M.; Awais, M.; Österberg, M.; Rautkari, L.; Schweins, R. Bundling of Cellulose Microfibrils in Native and Polyethylene Glycol-Containing Wood Cell Walls Revealed by Small-Angle Neutron Scattering. Sci. Rep. 2020, 10, 20844. [Google Scholar] [CrossRef]
- Bardet, M.; Gerbaud, G.; Giffard, M.; Doan, C.; Hediger, S.; Le Pape, L. 13C High-Resolution Solid-State NMR for Structural Elucidation of Archaeological Woods. Prog. Nucl. Magn. Reson. Spectrosc. 2009, 55, 199–214. [Google Scholar] [CrossRef]
- Hoffmann, P. On the Stabilization of Waterlogged Oakwood with PEG. II. Designing a Two-Step Treatment for Multi-Quality Timbers. Stud. Conserv. 1986, 31, 103–113. [Google Scholar] [CrossRef]
- Thill, A.; Spalla, O. Aggregation Due to Capillary Forces during Drying of Particle Submonolayers. Colloids Surf. Physicochem. Eng. Asp. 2003, 217, 143–151. [Google Scholar] [CrossRef]
- Stelzner, I. Transfer into Praxis. Evaluation of Consolidants for Freeze-Drying Archaeological Wood. In Proceedings of the 13th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Florence, Italy, 16–20 May 2016; Williams, E., Hocker, E., Eds.; ICOM-CC: Florence, Italy, 2018; pp. 325–332. [Google Scholar]
- Jensen, P.; Jørgensen, G.; Schnell, U. Dynamic LV-SEM Analyses of Freeze Drying Processes for Waterlogged Wood. In Proceedings of the 8th ICOM Group on Wet Organic Archaeological Materials Conference, Stockholm, Sweden, 11–15 June 2001; Hoffmann, P., Grant, T., Spriggs, J.A., Cook, C., Recht, A., Eds.; ICOM-CC: Bremerhaven, Germany, 2002; pp. 319–333. [Google Scholar]
- Stelzner, I. Zur Nassholzkonservierung Bestimmung Prozessrelevanter Eigenschaften für Die Gefriertrocknung; Staatliche Akademie der Bildenden Künste: Stuttgart, Germany, 2017. [Google Scholar]
- Hoffmann, P. Sucrose for Waterlogged Wood—Not so Simple at All. In Proceedings of the ICOM Committee for Conservation 11th Triennial Meeting Edinburgh, Scotland, 1–6 September 1996; ICOM-CC: Paris, France, 1996; pp. 657–662. [Google Scholar]
- Hoffmann, P.; Perez de Andres, C.; Sierra Mendes, J.L.; Ramiere, R.; Tran, Q.K.; Weber, U.M. European Inter-Laboratory Study on the Conservation of Waterlogged Wood with Sucrose. In Proceedings of the 5th ICOM Group on Wet Organic Archaeological Materials Conference, Portland, Maine, 16–20 August 1993; Hoffmann, P., Daley, T.W., Grant, T., Eds.; ICOM-CC: Bremerhaven, Germany, 1994; pp. 309–335. [Google Scholar]
- Hoffmann, P. Sucrose for Stabilizing Waterlogged Wood. 2 Stabilization and the Degree of Degradation. In Proceedings of the 5th ICOM Group on Wet Organic Archaeological Materials Conference, Portland, Maine, 16–20 August 1993; Hoffmann, P., Daley, T.W., Grant, T., Eds.; ICOM-CC: Bremerhaven, Germany, 1995; pp. 357–379. [Google Scholar]
- Schmitt, U.; Noldt, U. Scanning Electron Microscopic Observations on Saccharose Impregnation of Waterlogged Archaeological Wood; Hoffmann, P., Ed.; ICOM-CC: Bremerhaven, Germany, 1994; pp. 381–390. [Google Scholar]
- Broda, M.; Spear, M.J.; Curling, S.F.; Ormondroyd, G.A. The Viscoelastic Behaviour of Waterlogged Archaeological Wood Treated with Methyltrimethoxysilane. Materials 2021, 14, 5150. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.-M.; Broda, M. Interactions between Different Organosilicons and Archaeological Waterlogged Wood Evaluated by Infrared Spectroscopy. Forests 2021, 12, 268. [Google Scholar] [CrossRef]
- Broda, M.; Curling, S.F.; Spear, M.J.; Hill, C.A.S. Effect of Methyltrimethoxysilane Impregnation on the Cell Wall Porosity and Water Vapour Sorption of Archaeological Waterlogged Oak. Wood Sci. Technol. 2019, 53, 703–726. [Google Scholar] [CrossRef]
- Grattan, D.W. A Practical Comparative Study of Several Treatments for Waterlogged Wood. Stud. Conserv. 1982, 27, 124–136. [Google Scholar] [CrossRef]
- Imazu, S.; Morgos, A. Conserving Waterlogged Wood Using Sugar Alcohols and Comparison the Effectiveness of Lactitol MC, Saccharose and PEG 4000 Treatment. In Proceedings of the 6th ICOM Group on Wet Organic Archaeological Materials Conference, York, UK, 9–13 September 1996; Hoffmann, P., Daley, T., Grant, T., Spriggs, J.A., Eds.; ICOM-CC: Bremerhaven, Germany, 1997; pp. 235–255. [Google Scholar]
- Imazu, S.; Morgos, A. Lactitol Conservation of a 6 m Long Waterlogged Timber Coffin. In Proceedings of the 7th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Grenoble, France, 19–23 October 1998; Bonnot-Diconne, C., Hiron, X., Tran, K., Hoffmann, P., Eds.; ICOM-CC: Grenoble, France, 1999; pp. 210–214. [Google Scholar]
- Stamm, A.J. Effect of Polyethylene Glycol on The Dimensional Stability of Wood. For. Prod. J. 1959, 9, 375–381. [Google Scholar]
- Janeček, E.-R.; Walsh-Korb, Z.; Bargigia, I.; Farina, A.; Ramage, M.H.; D’Andrea, C.; Nevin, A.; Pifferi, A.; Scherman, O.A. Time-Resolved Laser Spectroscopy for the in Situ Characterization of Methacrylate Monomer Flow within Spruce. Wood Sci. Technol. 2017, 51, 227–242. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).