Abstract
Somatic embryogenesis is currently the most promising technique for the large-scale production and breeding of conifer species. Nonetheless, the low explant induction rate in this process hampers the development of improved materials. In this study, three immature Korean pine (Pinus koraiensis) embryos capable of induction were used as experimental materials. Various concentrations of brassinolide (BL), melatonin (Mel), salicylic acid (SA), glutathione (GSH), and ascorbic acid (AsA) were added to induce embryogenic callus tissue. The results show that BL had the most significant positive effect on promoting induction and increasing explant survival. Mel was also found to slightly increase the induction and survival rates of explants. When 1.00 mg·L−1 BL was added to the explants derived from stock tree I, which had strong self-induction ability, for 30 days, the callus induction rate rose to 400% compared to the control group. Following the addition of 25 mg·L−1 Mel to stock tree I for 30 days, the callus induction rate further increased to 450% of the control group. The effect of GSH on callus induction was insignificant. The addition of 0.10 g·L−1 to stock tree I for 30 days resulted in only a 150% higher induction rate compared to the control group. When 1.00 mg·L−1 BL was applied to explants with weak self-induction ability (stock tree III) for 15 days, the callus induction rate increased to 600% of that of the control group. The callus induction rate further increased to 800% of the control group after 50 mg·L−1 BL were added to stock tree III for 15 days. This study presents a method to improve the induction of embryogenic callus tissue in Korean pine.
1. Introduction
Korean pine (Pinus koraiensis Sieb. et Zucc) is a perennial tree species found in the broad-leaved Korean pine forest of Northeast China’s temperate zone. It holds immense economic and ecological significance. However, genetic variation when propagating this species using grafting and seed methods is unstable, and the process is both time-consuming and costly. The acquisition of superior germplasm resources is hindered by the limited history of breeding and the challenges in expanding breeding programs [1]. Somatic embryogenesis technology offers an opportunity for a high reproduction coefficient, which can broaden the utilization of limited germplasm resources and provide a novel approach to overcome the challenges associated with expanding superior varieties [2,3].
The development of superior varieties using somatic embryogenesis technology faces some obstacles, such as the low initial embryonic callus induction rate and significant genotype variations. To improve the Korean pine somatic embryogenesis system, extensive research concentrates on physiological and molecular biology aspects. The formation of somatic embryogenic callus in Korean pine involves asymmetric cell division. The preservation of this callus tissue relies heavily on intracellular antioxidant enzyme activity [4].
Previous studies on the induction culture systems of embryogenic callus tissues of Korean pine primarily used methods that altered the concentrations and ratios of auxins and cytokinins. In studies on Cuiminghamia lanceolata, thidiazuron was also included to enhance the induction rate of embryogenic callus [5]. However, it was ultimately discovered that its effect was comparable to that of cytokinin KT [6]. According to research on Chinese pine, it was also discovered that the ratio of NO3− to NH4+ had an impact on the induction of embryogenic callus and adventitious buds to some extent [7]. Some reports have suggested that the addition of small molecule substances during the induction stage can enhance the effect of embryogenic callus induction. For instance, in the somatic embryogenesis of Liriodendron, chitosan oligosaccharide was added to enhance the biological activity of the somatic embryogenesis process [8]. However, the use of similar small molecule substances in coniferous trees is uncommon, and the resulting effects have not been thoroughly evaluated. It has been discovered that the sterol hormone brassinolide (BL) has the ability to loosen the cell wall, increase the volume of the cell, and promote cell division and cell growth [9]. The presence of melatonin (Mel) and salicylic acid (SA) can modify the activity of antioxidant enzymes in plants [10], while glutathione (GSH) is known to maintain embryogenic callus at the proliferation stage [11]. Ascorbic acid (AsA) has been found to promote somatic embryogenesis in Fraxinus mandshurica [12]. These factors are significantly associated with the formation and maintenance of embryogenic callus. The present study investigated the effects of five novel plant growth regulators on explants of Korean pine derived from different stock trees (genotypes). This research aimed to assess the impact of treatment concentration and culture time on the induction of embryogenic callus. This research provides a scientific basis for improving the rate of embryogenic callus generation in different Korean pine genotypes.
2. Materials and Methods
2.1. Plant Materials
The plant materials gathered in this study adhered to international standards and were authorized by the cooperative institution of Lushuihe Seed Orchard, Jilin Province, China. On 14 July 2021, immature cones of Korean pine were collected based on the appropriate sampling timing determined by the preliminary experiments. The cone numbers of different mother trees were designated genotypes I, II, and III. The callus induction capacity was observed to be highest in genotype I, followed by genotype II and genotype III during the initial screening. Next, the cones were washed with running water, and immature seeds were extracted from them using a sterile knife and stored under aseptic conditions. Before the process, the cones were soaked in 75% alcohol for one hour on a super-clean bench.
2.2. Basal Medium
The induction medium was based on mLV medium (Litvay et al., [13], modified by Hargreaves et al., [14]) and supplemented with 2 mg·L−1 NAA, 1.5 mg·L−1 6-benzylamine-purine (6-BA), 30 g·L−1 sucrose, 500 mg·L−1 acid hydrolyzed complex protein, and 500 mg·L−1 L-glutamine. The solidifying agent was 4 g·L−1 gellan gum. The pH of the medium was adjusted to 5.8 before autoclaving, followed by autoclaving at 121 °C for 30 min. Cultures were incubated in the dark at 25 ± 2 °C.
2.3. Plant Growth Regulators
After sterilization, the medium was cooled to approximately 55–60 °C, and pre-configured exogenous plant growth regulators were added to the culture medium through a filter membrane (Millex-GP Syringe Filter Unit, with a pore size of 0.22 μm, Merck KGaA, Darmstadt, Germany). Table 1 displays the additional concentrations of the five exogenous plant growth regulators. The control group was the induction medium without any exogenous additives.

Table 1.
Concentration and types of exogenous additives.
2.4. Explant Inoculation and Induction of Embryogenic Callus
The seed coat was removed using a sterilized nut clipper, and the inner epidermis of the zygotic embryo was removed using sterilized tweezers on an ultra-clean bench. These treated seed kernels were then used as explants and implanted into a sterile culture using forceps. Each dish comprised five explant samples in duplicate. Each treatment was repeated five times. The culture was kept at 25 ± 2 °C in the dark. The induction rate was calculated every five days for a period of 30 days. A Zeiss Stemi 508 stereomicroscope was used to visually document the immature explant material at different stages of development.
2.5. Data Analysis
Statistical analyses of the data were conducted using Microsoft Excel 2019. Additionally, SPSS software (v26.0, SPSS Inc., Cary, NC, USA) was utilized to perform multiple comparisons using Duncan’s multiple range test and analysis of variance for one-way ANOVA of the callus proliferation rate. Significant differences were determined at a p-value of 0.05. The figures were generated in Origin Pro 2021(9.0.8.200).
3. Results
3.1. Induction and Development Process of Embryogenic Callus of Korean Pine
In this study, immature embryos of Korean pine were cultured in induction culture medium for varying periods. It was observed that after 5 days, the apex of the zygotic embryo displayed outward growth in a curved pattern (Figure 1a). Subsequently, after 10 days, a small quantity of embryogenic callus tissue developed from the micropylar end of the zygotic embryo (Figure 1b). After a 20-day culture period, the embryogenic callus tissue emerged from the apex of the zygotic embryo. At this point, it was transparent and colorless (see Figure 1c). Subsequently, immature embryos of Korean pine were cultured in induction culture medium for 30 days, resulting in the growth of a considerable amount of callus tissue. This tissue changed from colorless and transparent to a light shade of yellowish green (see Figure 1d). The highest embryogenic callus induction rates for immature Korean pine hybrid embryos were obtained in approximately 25 days without the use of any induction drugs. Nevertheless, some embryogenic calluses induced in the early stages demonstrated signs of necrosis as the induction time progressed (Figure 1e). In addition, non-embryogenic calluses were also formed during this process of induction (Figure 1f).
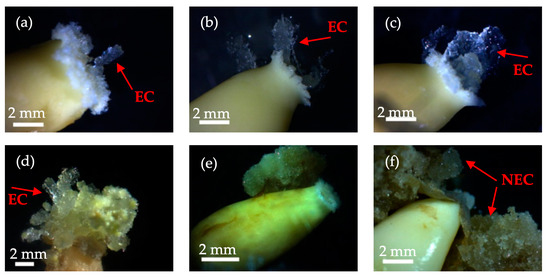
Figure 1.
States of embryogenic callus (EC) formation from immature hybrid embryo explants of Korean pine. (a) Morphology of embryogenic callus induction on day 5; (b) morphology of embryogenic callus induction on day 10; (c) morphology of embryogenic callus induction on day 20; (d) morphology of embryogenic callus induction on day 30; (e) degradation of induced callus tissue after formation; (f) morphology of non-embryogenic callus (NEC) tissue.
3.2. Impact of Brassinolide on Embryogenic Callus Induction of Different Genotypes of Korean Pine
The results indicate that BL treatment improved the callus induction capacity in all three genotypes. Notably, the induction rate initially increased but later decreased with increasing BL concentration. The optimal induction treatment involved adding BL to genotypes Ⅰ and Ⅱ at a 1.0 mg·L−1 concentration. Similarly, the treatment with the highest induction effect was promoted by the addition of BL to genotype Ⅲ at a concentration of 0.50 mg·L−1. Additionally, it was observed that after the addition of BL, genotype Ⅰ, genotype Ⅱ, and genotype Ⅲ achieved their maximum induction rates at 25 d, 20 d, and 15 d, respectively.
At a concentration of 2.00 mg·L−1, the induction rate was lower for zygotic embryos of genotype Ⅱ in comparison to other treatments. Moreover, embryos of genotype Ⅰ and genotype Ⅲ could not be maintained at this concentration.
According to Table 2, the induction rate of genotype I was 67% higher than that of the control group when treated with 1.00 mg·L−1 for 10 days. Finally, after 15 days, the induction rate increased to 167% higher than that of the control group. However, after 25 days, the induction rate matched that of the control. Intriguingly, after 30 days, the induction rate was four times higher than that of the control group.

Table 2.
The induction rate of embryogenic callus from different stock tree explants of Korean pine under treatments of brassinolide/%.
For genotype Ⅱ, the induction rate was 300% higher than that of the control group after treatment with 0.05 mg·L−1 and 2.00 mg·L−1 on day 10 of induction. In addition, the induction rate was higher than that of the control group after treatment with 1.00 mg·L−1 on day 20 of induction. The control group was 125% higher, and after 25 days of induction, the induction rate was 20% higher than that of the control group after treatment with 0.25 mg·L−1.
The tissue induction rate for genotype Ⅲ was initially very low. However, after a 15-day treatment with BL, the tissue induction rate increased by 500%, 400%, 400%, and 200% for the 0.05 mg·L−1, 0.25 mg·L−1, 1.00 mg·L−1, and 2.0 mg·L−1 treatment groups, respectively. At the 20-day mark, the induction rate of the 0.5 mg·L−1 treatment group was three times higher than that of the control group. Unfortunately, the control group did not survive the 25-day induction process. The induction rates in the treatment groups receiving 0.5 mg·L−1 and 1.0 mg·L−1 were 25.0% and 8.0%, respectively, whereas the induction rate for the group receiving 1.0 mg·L−1 was 20.0%, considering the control group’s mortality after 30 days.
This study found that the maximum rate of embryogenic callus induction after 25 days of cultivation was achieved by 1.00 mg·L−1 BL to genotype Ⅰ with robust induction capacity. Likewise, the addition of 1.00 mg·L−1 BL to the induction medium of genotype Ⅱ caused the highest rate of embryogenic callus induction at 20 days. Conversely, the addition of 0.05 mg·L−1 BL to weakly inducible genotype Ⅲ induction medium for 15 days or 0.50 mg·L−1 BL for 25 days produced the highest rate of embryogenic callus induction. Nevertheless, most of the treatment groups displayed inhibition.
3.3. Impact of Melatonin on Embryogenic Callus Induction of Different Genotypes of Korean Pine
The embryogenic callus induction rate among the three tested genotypes of immature zygotic embryos varied, with genotype I exhibiting the highest rate, followed by genotype II and genotype III. Nevertheless, all three genotypes showed comparable rates during the early induction stage. Although certain immature zygotic embryos endured elevated Mel concentrations, they perished as induction progressed with Mel concentrations beyond 100 mg·L−1.
Table 3 illustrates that embryogenic calluses were induced at all concentrations of genotype I after 10 days of Mel treatment. Embryogenic calluses appeared on all concentrations of genotype II after 20 days of induction and on all concentrations of genotype III after 15 days of induction. The effect of 25 mg·L−1 treatment on genotype I was significant (p < 0.05) at 30 days after an induction rate of 350% compared to the control group. The induction rate of genotype II was three times higher than that of the control group after 10 days of installation with 25 mg·L−1, and for genotype III, the induction rate was seven times higher than that of the control group after 15 days of induction with 50 mg·L−1 (p < 0.05).

Table 3.
The induction rate of embryogenic callus from different stock tree explants of Korean pine under treatments of melatonin/%.
Genotype I had strong inducibility, which resulted in the highest induction rate of embryogenic callus after 25 days without exogenous growth substances or 30 days with 25 mg·L−1 Mel. The induction rate of embryogenic callus was the highest with 50 mg·L−1 Mel after 15 days of induction in genotype III with weak inducibility. The other treatment groups mostly showed an inhibited induction rate of embryogenic callus.
3.4. Impact of Glutathione on Embryogenic Callus Induction of Different Genotypes of Korean Pine
Table 4 illustrates that the addition of GSH did not significantly enhance the induction rate of embryogenic callus for the three genotypes. However, after supplementation with 0.10 g·L−1 GSH for 30 days, genotype Ⅰ showed an increase in induction rate of up to 150% compared to the control group. Similarly, genotype Ⅲ displayed an increase in induction rate by 140% after adding 0.90 g·L−1 GSH for 20 days compared to the control group. Unfortunately, a significant number of zygotic embryos of genotypes II and III did not survive after GSH treatment. In addition, as the induction time increased at each stage, the induction rate of the embryogenic callus with genotype I progressed steadily. On the 30th day of induction, the induction rate of 0.10 g·L−1 exceeded that of the control group, possibly due to a reduced survival rate of the induced embryogenic callus in the control group.

Table 4.
The induction rate of embryogenic callus from different stock tree explants of Korean pine under treatments of glutathione/%.
3.5. Impact of Salicylic Acid on Embryogenic Callus Induction of Different Genotypes of Korean Pine
As shown in Table 5, the addition of SA to the culture medium resulted in only a few immature zygotic embryos in genotype Ⅰ showing strong inducibility. Notably, we observed that the addition of SA significantly inhibited the induction rate of embryogenic callus. However, the immature zygotic embryos of the other two genotypes were able to survive in the lower-concentration experimental group, although with an extremely low induction rate.

Table 5.
The induction rate of embryogenic callus from different stock tree explants of Korean pine under treatments of salicylic acid/%.
3.6. Impact of Ascorbic Acid on Embryogenic Callus Induction of Different Genotypes of Korean Pine
Table 6 shows that the addition of AsA to the medium led to a low induction of genotype I. Furthermore, the survival rates of immature zygotic embryos of genotypes II and III were also low. Additionally, the induction rate of embryogenic callus at each induction stage was lower than that of the control group. All zygotic embryos died when the concentration of AsA reached or exceeded 1.00 g·L−1.

Table 6.
The induction rate of embryogenic callus from different stock tree explants of Korean pine under treatments of ascorbic acid/%.
3.7. The Effects of Five Inducing Agents on the Induction of Callus Tissues from Different Genotypes of Korean Pine
This study investigated the induction rates of five different drugs on immature zygotic embryos of Korean pine during a 30-day induction period. BL was found to have a higher induction rate than the other drugs tested. Additionally, adding 1.00 mg·L−1 BL to zygotic embryos of all three genotypes led to higher induction rates of embryogenic callus (refer to Table 7). Similarly, the addition of 0.25 mg·L−1 BL to genotype II led to higher rates of induction. The promoting effects of adding low concentrations of GSH or Mel to genotypes with poor embryogenic callus induction ability were limited. Furthermore, AsA had an adverse effect on the survival rate of zygotic embryos, resulting in only a few embryos that induced embryogenic callus. Similarly, adding SA to the medium was found to decrease the survival and induction rates of zygotic embryos. In contrast, only BL and Mel showed a significant promoting effect on callus induction in immature Korean pine zygotic embryos.

Table 7.
Average induction rate of embryogenic callus after adding different concentrations of exogenous additives.
4. Discussion
During somatic embryogenesis, the induction of callus in plant tissues and organs is significantly affected by plant hormone regulators. These regulators, including auxin and cytokinin, not only stimulate cell growth and division but also alter cell differentiation and morphology. Previous studies on Korean pine embryogenic callus have focused on the enhancement of induction by the adjustment of auxin and cytokinin hormone concentrations. However, despite some increase in the induction rate of embryogenic callus, this approach has not proven to be very effective. The formation of Korean pine embryogenic callus involves the asymmetric division of cells. The interplay between intracellular active oxygen, superoxide dismutase, superoxide anion, and H2O2 directly affects cell differentiation and callus embryogenesis.
Based on these physiological processes, our experiment aimed to increase the induction rate of embryogenic callus by introducing exogenous drugs to interfere with the callus formation process. Our studies show that the optimal time for callus production with a conventional induction medium was approximately 25 days. However, some of the callus formed in the early stages died in the induction medium, which contributed to the low callus induction rate in Korean pine. Therefore, our study focused on the use of new plant growth substances to improve both the induction rate and the survival rate of callus during the induction phase.
Our research revealed that the application of BL significantly increased the callus induction rate of Korean pine throughout the process. In addition, BL also showed a promoting effect on callus tissue formation in various plants such as Brassica napus [15], Arabidopsis thaliana [16], Artemisia lactiflora [17], and Spartina patens [18]. The addition of BL can increase the efficiency of the induction process by relaxing the cell wall of the explants. This relaxation leads to an increase in cell volume, promotes cell division and growth, and ultimately improves callus induction [19]. In our experiment, we found that the induction rate of Korean pine zygotic embryos was the same in all experimental groups when 1.0 mg·L−1 BL was added during the induction phase. After the addition of 1.0 mg·L−1 BL to the immature Korean pine zygotic embryos, the induction rate of zygotic embryos was high in all experimental groups during the induction stage. The results show that the addition of BL successfully induced robust embryogenic callus and significantly improved the induction rate of embryogenic tissue [20]. The addition of 1.0 mg·L−1 BL during the induction of immature Korean pine zygotic embryos resulted in a shorter callus induction time. In addition, the survival rate of callus was higher in the later stage of induction, suggesting that the addition of BL may accelerate callus formation. These results are consistent with previous research on adventitious root callus formation in poplar (Populus Sieboldii × P. Grandidentata) [21]. In callus induction in Korean pine, it was observed that the addition of BL during the induction process increased the survival rate of callus in genotypes with strong inducibility. Similarly, the addition of BL during the induction period improved the induction rate of callus in genotypes with weak inducibility.
In the process of callus induction of immature embryos of Korean pine embryos, the addition of 25 mg·L−1 Mel during the callus induction stage of the genotype with higher induction ability inducibility resulted in a higher callus survival rate. Similarly, in a study on Kandelia obovata, it was suggested that the addition of Mel during the induction process could enhance the CAT activity of the callus during the in vitro differentiation process, thereby improving explant survival [22]. It is believed that adding an appropriate amount of Mel during induction is beneficial for early embryonic development and the differentiation of embryogenic cells [23,24]. Our findings indicate that the addition of 50 mg·L−1 Mel to the callus induction stage phase of genotypes with low inducibility promoted callus formation to a certain extent, and it is indeed beneficial for the differentiation of embryogenic cells in the early-stage phase. Additionally, we observed that the addition of Mel in the early phase of induction led to faster callus formation in a short period of time during the early stage of induction. This observation aligns with a previous report that suggested that adding a low concentration of Mel during the installation of somatic embryos in Vitis vinifera seeds can shorten the germination time of somatic embryos [25].
In a study of Korean pine embryogenic callus, GSH was observed to promote the growth of embryogenic callus and maintain higher embryogenicity during the proliferation stage [11]. In a study of Araucaria angustifolia, it was found that the addition of GSH reduced the production of NO in cells and improved the quantity, quality, and development of early somatic embryos [26]. However, it was found that the addition of GSH in the induction phase of immature Korean pine zygotic embryos did not produce satisfactory results.
Previous studies have shown that SA, as an endogenous signaling molecule, can induce a specific peroxidase (POD) isoenzyme. This induced POD isoenzyme plays a crucial role in improving the stress resistance of plant explants by effectively scavenging reactive oxygen species [27]. The addition of SA during callus induction can reduce the detrimental effects of O2- toxicity on explants. SA plays a role in promoting the production of coumarin and influences cell wall formation through its involvement in phenylpropane metabolism in plants [28]. It also increases the probability of asymmetric cell division during the induction of embryogenic callus. To increase the survival and induction rates of immature zygotic embryos, we performed an experiment with the addition of SA. However, during the induction of embryogenic callus in Korean pine, we found that the addition of exogenous SA significantly inhibited the callus induction of immature zygotic embryos. Moreover, when the SA concentration exceeded 1.0 mg·L−1, the survival of zygotic embryos was severely impaired.
In Picea glauca, the addition of AsA was found to enhance the ability of somatic embryogenesis to some extent, especially in cell lines with low AsA content [29], but in Korean pine, AsA had an inhibitory effect on the induction of embryogenic callus. When the AsA concentration added to the induction medium exceeded 1.0 g·L−1, the survival of zygotic embryos was significantly impaired.
5. Conclusions
Based on previous studies, this study attempted to optimize the conditions for the induction culture of Korean pine embryogenic callus. It was found that the addition of BL and Mel to the induction medium improved the induction rate and survival rate of embryogenic callus. However, it was observed that the different genotypes showed significant differences in the induction of embryogenic callus. Therefore, to increase the yield of embryogenic callus, it is recommended to evaluate the genotypes of different families of mother trees individually during actual production and classify them based on their ability to induce embryogenic callus. This helps to select the appropriate induction culture system for different genotypes with different induction potentials. For mother tree genotypes with strong induction ability, it is recommended to add 1.00 mg·L−1 BL to the culture medium and induce the culture for 25 days. For genotypes with moderate inducibility, the addition of 1.00 mg·L−1 BL to the induction culture on day 20 is recommended. In contrast, for genotypes with poor inducibility, it is recommended to add 0.50 mg·L−1 BL to the induction medium and induce the culture for 25 days. The study found that GSH, SA, and AsA did not play a significant role in the induction phase of Korean pine embryogenic callus. Although this culture program effectively increased the induction rate of embryogenic callus, further studies are needed to determine the optimal type and concentration of inducers for the maintenance and somatic embryo transformation of induced embryogenic callus.
Author Contributions
H.S. designed and conceived the experiments. S.N. performed the experiments. Y.Y. and Y.W. curated the data. S.L. and W.G. analyzed the data. L.Y. revised the manuscript. H.S. and S.N. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the Innovation Project of the State Key Laboratory of Tree Genetics and Breeding (2021B01) and the National Key R&D Program of China (2017YFD0600600).
Data Availability Statement
The data presented in this study are available in this manuscript.
Acknowledgments
We express our gratitude to our team members for their valuable contributions. We would also like to extend our thanks to Yuxin Song and Di Wang for their assistance during the experiment.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
References
- Nørgaard, J.V.; Duran, V.; Johnsen, Ø.; Krogstrup, P.; Baldursson, S.; Arnold, S.V. Variations in Cryotolerance of Embryogenic Picea abies Cell Lines and the Association to Genetic, Morphological, and Physiological Factors. Can. J. For. Res. 1993, 23, 2560–2567. [Google Scholar] [CrossRef]
- Attree, S.M.; Fowke, L.C. Embryogeny of Gymnosperms: Advances in Synthetic Seed Technology of Conifers. Plant Cell Tissue Organ. Cult. 1993, 35, 1–35. [Google Scholar] [CrossRef]
- Bozhkov, P.V.; Ahn, I.S.; Park, Y.G. Two Alternative Pathways of Somatic Embryo Origin from Polyembryonic Mature Stored Seeds of Pinus koraiensis Sieb et Zucc. Can. J. Bot. 1997, 75, 509–512. [Google Scholar] [CrossRef]
- Peng, C.; Gao, F.; Wang, H.; Shen, H.; Yang, L. Optimization of Maturation Process for Somatic Embryo Production and Cryopreservation of Embryogenic Tissue in Pinus koraiensis. Plant Cell Tiss. Organ. Cult. 2021, 144, 185–194. [Google Scholar] [CrossRef]
- Wu, X.L.; Han, C.; Sun, Y.H.; Cao, S.; Hu, R.Y.; Xu, J.i.L.; Zheng, H.Q.; Li, Y. Optimization of induction conditions for embryogenic callus of somatic embryogenesis in Cunninghamia lanceolata. J. Beijing For. Univ. 2020, 42, 79–86. [Google Scholar] [CrossRef]
- Han, D.Y.; Li, D.; Zhao, J.; Li, H.; Zhang, J.F. Factors Affecting Induction of Embryogenic Callus of Larix principis-rupprechtii. For. Res. 2013, 26, 454–458. [Google Scholar] [CrossRef]
- Dong, L.F.; Xiao, Y.; Shao, C.B. The Effect of Form and Concentration of Nitrogen, Phosphorus, Potassium on Inducing Embryos of Chinese Pine. J. Northwest For. Univ. 2006, 21, 64–66. [Google Scholar]
- Ali, A.; Zhang, J.; Zhou, M.; Chen, T.; Shah, L.; Rehman, S.U.; Hayat, S.; Shi, J.; Chen, J. Chitosan Oligosaccharides Stimulate the Efficacy of Somatic Embryogenesis in Different Genotypes of the Liriodendron Hybrid. Forests 2021, 12, 557. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Li, J. Brassinosteroids Regulate Root Growth, Development, and Symbiosis. Mol. Plant 2016, 9, 86–100. [Google Scholar] [CrossRef]
- Nazir, S.; Jan, H.; Zaman, G.; Ahmed, N.; Drouet, S.; Hano, C.; Abbasi, B.H. Synergistic Effects of Salicylic Acid and Light Stress on Bioactive Metabolites in Basil Callus Cultures. Biocatal. Agric. Biotechnol. 2021, 37, 102176. [Google Scholar] [CrossRef]
- Gao, F.; Wang, R.; Shi, Y.; Shen, H.; Yang, L. Reactive Oxygen Metabolism in the Proliferation of Korean Pine Embryogenic Callus Cells Promoted by Exogenous GSH. Sci. Rep. 2023, 13, 2218. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, T.Y.; Yang, L.; Shen, H.L. Effects of Ascorbic Acid on Physiological Characteristics during Somatic Embryogenesis of Fraxinus mandshurica. Int. J. Mol. Sci. 2022, 24, 289. [Google Scholar] [CrossRef]
- Litvay, J.D.; Verma, D.C.; Johnson, M.A. Influence of a Loblolly Pine (Pinus taeda L.). Culture Medium and Its Components on Growth and Somatic Embryogenesis of the Wild Carrot (Daucus carota L.). Plant Cell Rep. 1985, 4, 325–328. [Google Scholar] [CrossRef]
- Hargreaves, C.L.; Reeves, C.B.; Find, J.I.; Gough, K.; Josekutty, P.; Skudder, D.B.; van der Maas, S.A.; Sigley, M.R.; Menzies, M.I.; Low, C.B.; et al. Improving Initiation, Genotype Capture, and Family Representation in Somatic Embryogenesis of Pinus Radiata by a Combination of Zygotic Embryo Maturity, Media, and Explant Preparation. Can. J. For. Res. 2009, 39, 1566–1574. [Google Scholar] [CrossRef]
- Cai, D.; Chang, J.; Zhu, H.; Luo, B. Tne Dual Effects of BR in Promoting Calluse Formation and Differentiation in Brassica napus. J. Huazhong Agric. 1989, 8, 10–15. [Google Scholar] [CrossRef]
- Chen, J.C.; Xu, M.D.; Zhao, Y.J. Effect of Epibrassinolide on Cell Differentiation of Arabidopsis thaliana. Acta Phytophysiol. Sin. 1996, 22, 399–403. [Google Scholar]
- He, Y.J.; Xu, R.J.; Huang, D.F. Effect of Epibrassinolide on Induction of Calli from Artemisia lactiflora Leaves. Chin. J. Appl. Environ. Biol. 1900, 3, 95–99. [Google Scholar]
- Lu, Z.; Huang, M.; Ge, D.P.; Yang, Y.H.; Cai, X.N.; Qin, P.; She, J.M. Effect of Brassinolide on Callus Growth and Regeneration in Spartina patens (Poaceae). Plant Cell Tissue Organ. Cult. 2003, 73, 87–89. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, N.; Wang, M.; Zhou, W.; Guo, J.; Han, C.; Zhou, C.; Wang, W.; Wu, S.; Tang, W.; et al. Integrated Regulation of Periclinal Cell Division by Transcriptional Module of BZR1-SHR in Arabidopsis Roots. New Phytol. 2022, 233, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H. Brassinolide Promotes Adventitious Shoot Regeneration from Cauliflower Hypocotyl Segments. Plant Cell Tissue Organ. Cult. 2002, 71, 111–116. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Yoshimoto, J.; Uchiyama, H.; Nabeshima, E.; Nakaba, S.; Watanabe, U.; Funada, R. In Vitro Induction of Secondary Xylem-like Tracheary Elements in Calli of Hybrid Poplar (Populus sieboldii × P. grandidentata). Planta 2013, 237, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.F.; Liu, W.C.; Wei, L.; Chen, J.N.; Zhang, C.N.; Qiu, J.B.; Ding, W.Y.; Zheng, Q.S. Melatonin Regulates Photosynthesis and Ascorbate-Glutathione Cycle in a Mangrove Kandelia obovata under Low Temperature Stress. Plant Physiol. J. 2019, 55, 1211–1221. [Google Scholar] [CrossRef]
- Zhou, R.; Cai, Y.; Lin, T.Y.; Chai, M.L. Effects of melatonin and epibrassinolide on the regeneration of long-term subcultured callus of Zoysia matrella (L.) Merr. under simulated drought stress. J. Zhejiang Univ. (Agric. Life Sci.) 2022, 48, 36–44. [Google Scholar] [CrossRef]
- Siamak, S.B.; Parisa, S. Strigolactone and Methyl Jasmonate-Induced Antioxidant Defense and the Composition Alterations of Different Active Compounds in Dracocephalum kotschyi Boiss Under Drought Stress. J. Plant Growth Regul. 2020, 40, 878–889. [Google Scholar] [CrossRef]
- Zhang, Y.; Ya, R.; Xu, W.R.; Wang, J.H.; Cui, Y.; Li, J.D. Role of melatonin in the induction of somatic embryogenesis from seeds of Vitis vinifera ‘Chardonnay’. J. Fruit. Sci. 2021, 38, 922–933. [Google Scholar] [CrossRef]
- do Nascimento Vieira, L.; Santa-Catarina, C.; de Freitas Fraga, H.P.; dos Santos, A.L.W.; Steinmacher, D.A.; Schlogl, P.S.; Silveira, V.; Steiner, N.; Floh, E.I.S.; Guerra, M.P. Glutathione Improves Early Somatic Embryogenesis in Araucaria angustifolia (Bert) O. Kuntze by Alteration in Nitric Oxide Emission. Plant Sci. 2012, 195, 80–87. [Google Scholar] [CrossRef]
- Shan, Y.S.; Dai, H.H.; He, X.; Xin, Z.Q.; Wu, N.B. Effects of Exogenous Methyl Jasmonate and Salicylic Acid on Physiological Characteristics and Secondary Metabolism of Atropa belladonna under NaCl Stress. Plant Physiol. J. 2019, 55, 1335–1346. [Google Scholar] [CrossRef]
- Shang, J.; Wu, Z.W.; Ma, Y.G. Phenylpropanoid Metabolism Pathway in Plants. Chin. J. Biochem. Mol. Biol. 2022, 38, 1467–1476. [Google Scholar] [CrossRef]
- Stasolla, C.; Yeung, E.C. Cellular Ascorbic Acid Regulates the Activity of Major Peroxidases in the Apical Poles of Germinating White Spruce (Picea glauca) Somatic Embryos. Plant Physiol. Biochem. 2007, 45, 188–198. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).