Geographic Variation in Progeny: Climatic and Soil Changes in Offspring Size and Colour in Four Sorbus spp. (Rosaceae)
Abstract
:1. Introduction
- (1)
- How do the seed and fruit mass of four Sorbus spp. vary across different environmental and soil conditions?
- (2)
- How does the seed-to-fruit mass ratio of four Sorbus spp. vary across different environmental and soil conditions?
- (3)
- How does the fruit colour of four Sorbus spp. vary across different environmental and soil conditions?
2. Materials and Methods
2.1. Territory Studied
2.2. Material Collection
2.3. Fruit Parameter Measurement
2.4. Seed Parameter Measurement
2.5. Measurement of Fruit Peel Pigment Parameters
2.6. Extraction and Screening of Environmental Factors
2.7. Data Statistics and Analysis
2.8. Maxent Model Prediction Analysis
3. Results
3.1. Phenotypic Variations in Seed and Fruit Traits
3.2. Variations in Peel Pigment and Fruit Colour
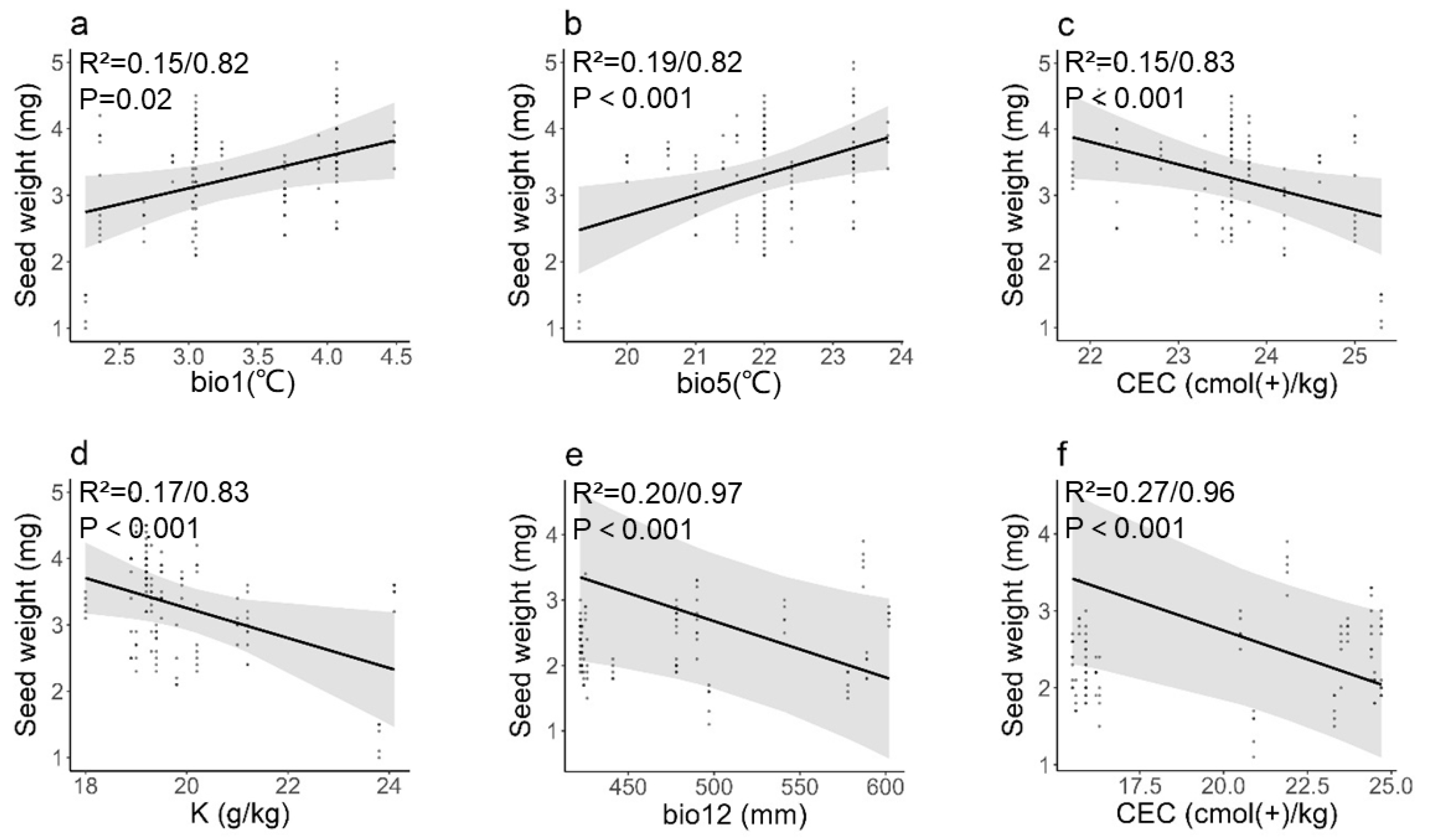
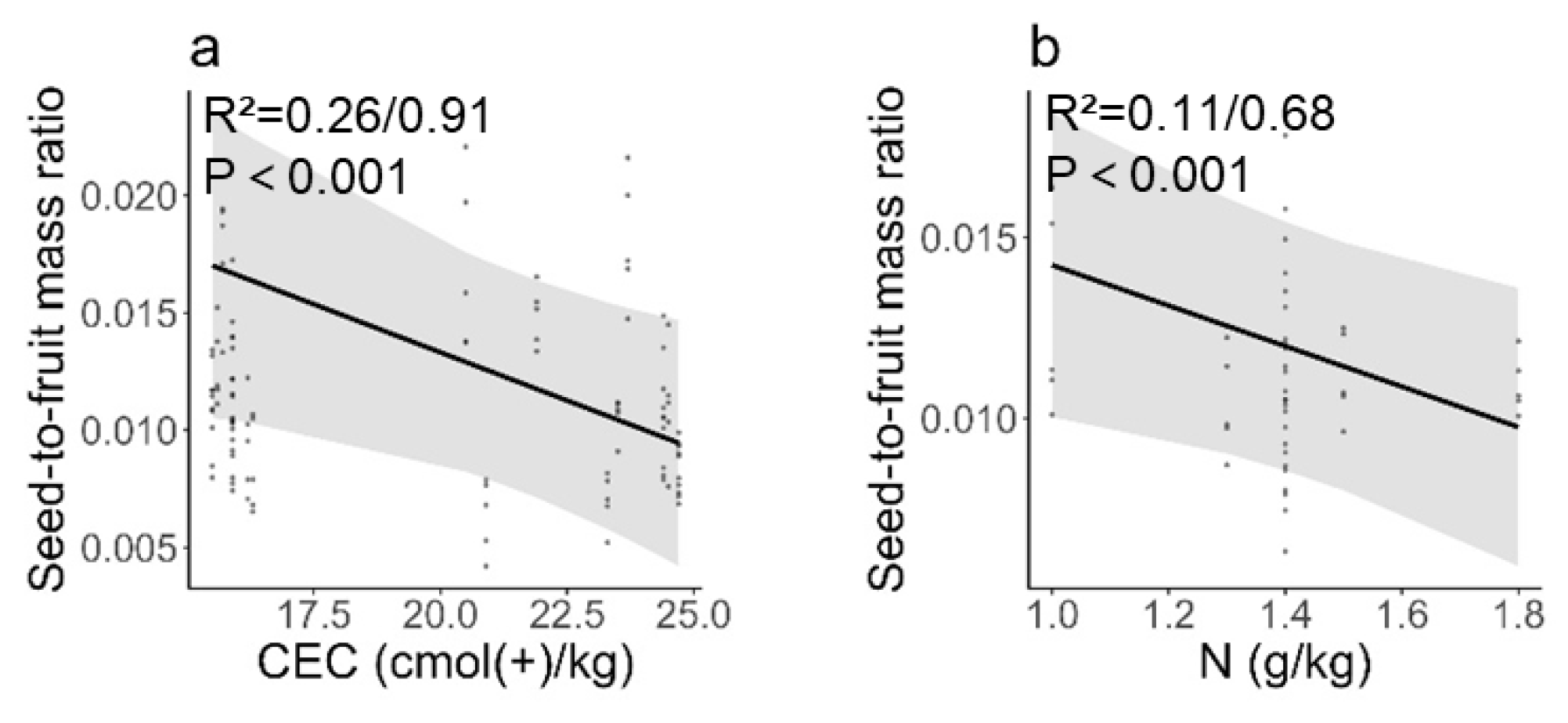
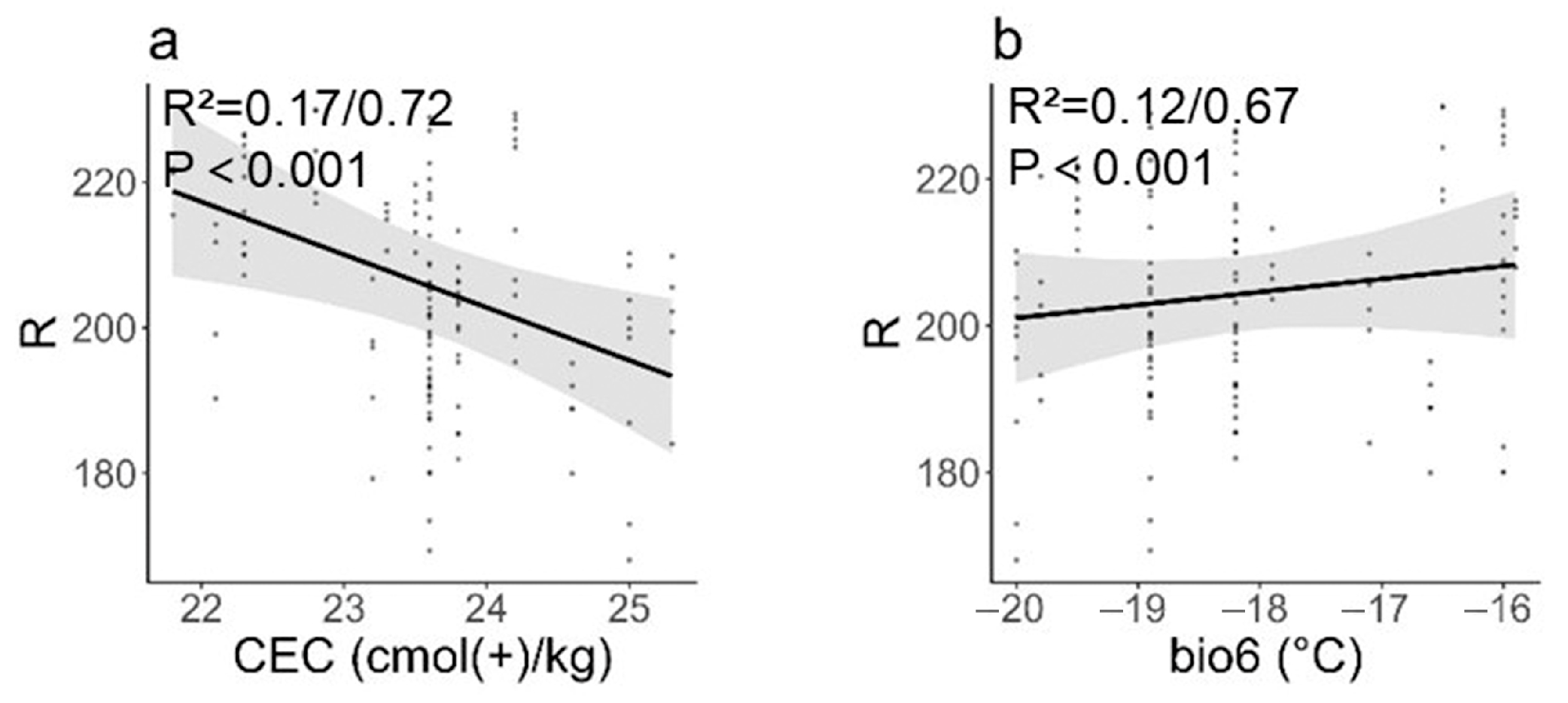
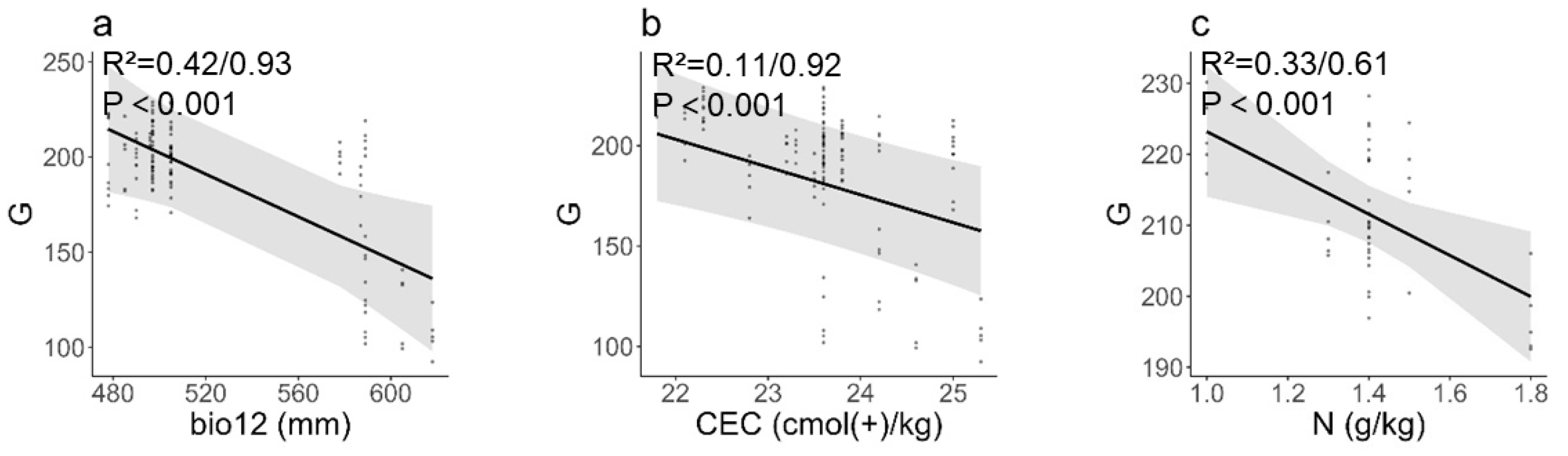
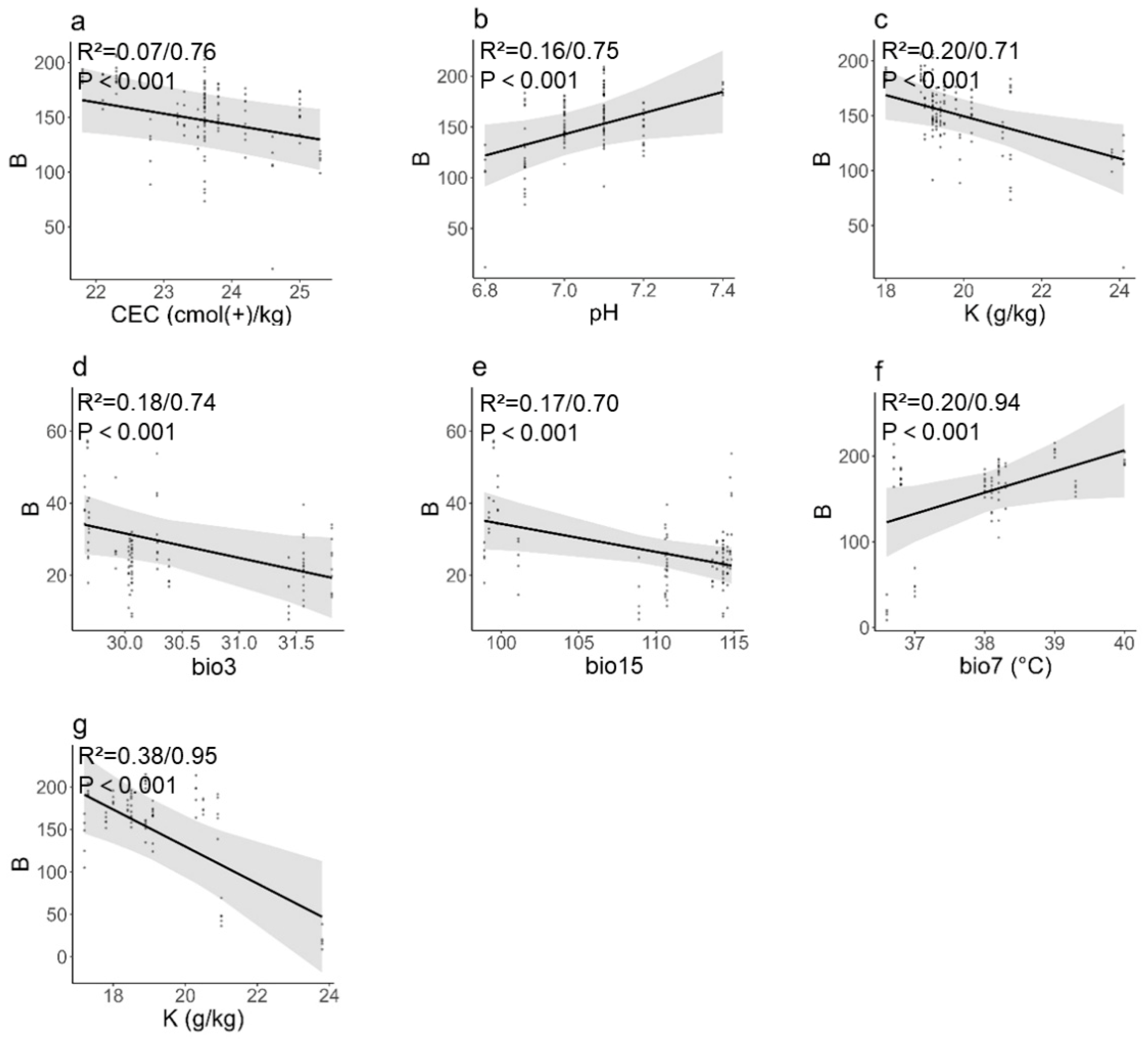
3.3. Influence of Environmental and Soil Factors on Seed and Fruit Phenotypic Traits
3.4. Predicted Potential Distribution Zones
4. Discussion
4.1. Environmental Stresses Enable Seeds to Gain More Mass Allocation Than Fruits
4.2. Long-Term Adaptation Affects Fruit and Seed Mass
4.3. Impact on Fruit Colour
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heide, O.M. Temperature rather than photoperiod controls growth cessation and dormancy in Sorbus species. J. Exp. Bot. 2011, 62, 5397–5404. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.N.; Beuerle, T.; Müller, A.; Ernst, L.; Bhavanam, V.B.; Liu, B.; Beerhues, L. Biosynthesis of the biphenyl phytoalexin aucuparin in Sorbus aucuparia cell cultures treated with Venturia inaequalis. Phytochemistry 2013, 96, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Huntley, B. Species-richness in north-temperate zone forests. J. Biogeogr. 1993, 20, 163–180. [Google Scholar] [CrossRef]
- Li, M.; Ohi-Toma, T.; Gao, Y.-D.; Xu, B.; Zhu, Z.-M.; Ju, W.-B.; Gao, X.-F. Molecular phylogenetics and historical biogeography of Sorbus sensu stricto (Rosaceae). Mol. Phylogenetics Evol. 2017, 111, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Šedivá, J.; Velebil, J.; Zahradník, D. Micropropagation as a Tool for the Conservation of Autochthonous Sorbus Species of Czechia. Plants 2023, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Schaal, B.; Leverich, W.J.; Rogstad, S. A Comparison of Methods for Assessing Genetic Variation in Plant Conservation Biology; Oxford University Press: New York, NY, USA, 1991; pp. 123–134. [Google Scholar]
- Larios, E.; Venable, D.L. Selection for seed size: The unexpected effects of water availability and density. Funct. Ecol. 2018, 32, 2216–2224. [Google Scholar] [CrossRef]
- Morales, A.; Teapal, J.; Ammerlaan, J.M.H.; Yin, X.; Evers, J.B.; Anten, N.P.R.; Sasidharan, R.; van Zanten, M. A high throughput method for quantifying number and size distribution of Arabidopsis seeds using large particle flow cytometry. Plant Methods 2020, 16, 27. [Google Scholar] [CrossRef]
- Drvodelić, D.; Oršanić, M.; Vuković, M.; Jatoi, M.A.; Jemrić, T. Correlation of Fruit Size with Morphophysiological Properties and Germination Rate of the Seeds of Service Tree (Sorbus domestica L.). South-East Eur. For. 2018, 9, 47–54. [Google Scholar] [CrossRef]
- Love, N.L.R.; Mazer, S.J. Geographic variation in offspring size: Long- and short-term climate affect mean seed mass of Streptanthus populations. Ecology 2022, 103, e3698. [Google Scholar] [CrossRef]
- Stromberg, J.C.; Boudell, J.A. Floods, drought, and seed mass of riparian plant species. J. Arid. Environ. 2013, 97, 99–107. [Google Scholar] [CrossRef]
- Yang, X.; Baskin, C.C.; Baskin, J.M.; Pakeman, R.J.; Huang, Z.; Gao, R.; Cornelissen, J.H.C. Global patterns of potential future plant diversity hidden in soil seed banks. Nat. Commun. 2021, 12, 7023. [Google Scholar] [CrossRef]
- Thomas, P.A. Biological Flora of the British Isles: Sorbus torminalis. J. Ecol. 2017, 105, 1806–1831. [Google Scholar] [CrossRef]
- Suhonen, J.; Jokimäki, J. Fruit removal from rowanberry (Sorbus aucuparia) trees at urban and rural areas in Finland: A multi-scale study. Landsc. Urban Plan. 2015, 137, 13–19. [Google Scholar] [CrossRef]
- Peng, S.; Zheng, Y.; Ma, M.; Zhang, C.; Li, T.; Ni, Y. Physiological adaptation of Sorbus pohuashanensis seedlings to heat stress. J. For. Res. 2011, 24, 602–608. [Google Scholar]
- Taulavuori, K.; Taulavuori, E.; Saravesi, K.; Jylänki, T.; Kainulainen, A.; Pajala, J.; Markkola, A.; Suominen, O.; Saikkonen, K. Competitive success of southern populations of Betula pendula and Sorbus aucuparia under simulated southern climate experiment in the subarctic. Ecol. Evol. 2017, 7, 4507–4517. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Krska, B.; Kiprovski, B.; Veberic, R. Bioactive components and antioxidant capacity of fruits from nine Sorbus genotypes. J. Food Sci. 2017, 82, 647–658. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Shu, Z.; Li, Z.; Ning, Y.; Yun, K.; Bai, H.; Liu, R.; Liu, W. Effect and mechanism of Sorbus pohuashanensis (Hante) Hedl. flavonoids protect against arsenic trioxide-induced cardiotoxicity. Biomed. Pharmacother. 2017, 88, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, D.; Liu, H.; Wei, C.; Wang, J.; Shen, H. Effects of a nitric oxide donor and nitric oxide scavengers on Sorbus pohuashanensis embryo germination. J. For. Res. 2018, 29, 631–638. [Google Scholar] [CrossRef]
- Ma, Q.; Jiao, Y.; Ren, Y. Linear mixed-effects models to describe length-weight relationships for yellow croaker (Larimichthys Polyactis) along the north coast of China. PLoS ONE 2017, 12, e0171811. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Potts, A.S.; Hunter, M.D. Unraveling the roles of genotype and environment in the expression of plant defense phenotypes. Ecol. Evol. 2021, 11, 8542–8561. [Google Scholar] [CrossRef]
- Westberg, E.; Ohali, S.; Shevelevich, A.; Fine, P.; Barazani, O. Environmental effects on molecular and phenotypic variation in populations of Eruca sativa across a steep climatic gradient. Ecol. Evol. 2013, 3, 2471–2484. [Google Scholar] [CrossRef]
- Wu, Z.; Raven, P.H.; Hong, D. Flora of China. Volume 9: Ulmaceae through Basellaceae; Science Press: Beijing, China, 2003. [Google Scholar]
- Lichtenthaler, F.W.; Cuny, E.; Weprek, S. Eine einfache und leistungsfähige Synthese acylierter Glyculosylbromide aus Hydroxyglycal-estern. Angew. Chem. 1983, 95, 906–908. [Google Scholar] [CrossRef]
- Pietrini, F.; Massacci, A. Leaf anthocyanin content changes in Zea mays L. grown at low temperature: Significance for the relationship between the quantum yield of PS II and the apparent quantum yield of CO2 assimilation. Photosynth. Res. 1998, 58, 213–219. [Google Scholar] [CrossRef]
- Zhang, K.M.; Li, Z.; Li, Y.; Kong, D.Z.; Wu, R.H. Carbohydrate accumulation may be the proximate trigger of anthocyanin biosynthesis under autumn conditions in Begonia semperflorens. Plant Biol. 2013, 15, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, H.; Zhao, Y.; Li, D.; Yang, J.-L.; Song, X.; Shi, Z.; Zhu, A.-X.; Zhang, G.-L. Mapping high resolution national soil information grids of China. Sci. Bull. 2022, 67, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, G.-L.; Song, X.; Li, D.; Zhao, Y.; Yang, J.; Wu, H.; Yang, F. High-resolution and three-dimensional mapping of soil texture of China. Geoderma 2020, 361, 114061. [Google Scholar] [CrossRef]
- Puijalon, S.; Piola, F.; Bornette, G. Abiotic stresses increase plant regeneration ability. Evol. Ecol. 2008, 22, 493–506. [Google Scholar] [CrossRef]
- Westoby, M.; Wright, I.J. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 2006, 21, 261–268. [Google Scholar] [CrossRef]
- Cunha, H.F.V.; Andersen, K.M.; Lugli, L.F.; Santana, F.D.; Aleixo, I.F.; Moraes, A.M.; Garcia, S.; Di Ponzio, R.; Mendoza, E.O.; Brum, B.; et al. Direct evidence for phosphorus limitation on Amazon forest productivity. Nature 2022, 608, 558–562. [Google Scholar] [CrossRef]
- Ďurkovič, J.; Lengyelová, A.; Čaňová, I.; Kurjak, D.; Hladká, D. Photosynthetic performance and stomatal characteristics during ex vitro acclimatisation of true service tree (Sorbus domestica L.). J. Hortic. Sci. Biotechnol. 2009, 84, 223–227. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Zornoza, R.; Acosta, J.A.; Bastida, F.; Domínguez, S.G.; Toledo, D.M.; Faz, A. Identification of sensitive indicators to assess the interrelationship between soil quality, management practices and human health. J. Soil 2015, 1, 173–185. [Google Scholar] [CrossRef]
- Guo, K.; Peng, L.; Hong, Y.; Qiao, G. Optimizing Nitrogen, Phosphorus, and Potassium Fertilization Rates for Fruit Performance of Chinese Cherry (Prunus pseudocerasus Lindl.). Int. J. Fruit Sci. 2022, 22, 769–778. [Google Scholar] [CrossRef]
- Morugán-Coronado, A.; Linares, C.; Gómez-López, M.D.; Faz, Á.; Zornoza, R. The impact of intercropping, tillage and fertilizer type on soil and crop yield in fruit orchards under Mediterranean conditions: A meta-analysis of field studies. Agric. Syst. 2020, 178, 102736. [Google Scholar] [CrossRef]
- Paganová, V. Ecology and distribution of service tree Sorbus domestica (L.) in Slovakia. Ekológia (Bratislava) 2008, 27, 152–167. [Google Scholar]
- Ma, X.; Su, Z.; Ma, H. Molecular genetic analyses of abiotic stress responses during plant reproductive development. J. Exp. Bot. 2020, 71, 2870–2885. [Google Scholar] [CrossRef] [PubMed]
- Mazer, S.J. Ecological, taxonomic, and life history correlates of seed mass among indiana dune angiosperms: Ecological archives M059-001. Ecol. Monogr. 1989, 59, 153–175. [Google Scholar] [CrossRef]
- Jensen, K.; Gutekunst, K. Effects of litter on establishment of grassland plant species: The role of seed size and successional status. Basic Appl. Ecol. 2003, 4, 579–587. [Google Scholar] [CrossRef]
- Stromberg, J.C.; Butler, L.; Hazelton, A.F.; Boudell, J.A. Seed size, sediment, and spatial heterogeneity: Post-flood species coexistence in dryland riparian ecosystems. Wetlands 2011, 31, 1187–1197. [Google Scholar] [CrossRef]
- Salama, A.-M.; Ezzat, A.; El-Ramady, H.; Alam-Eldein, S.M.; Okba, S.K.; Elmenofy, H.M.; Hassan, I.F.; Illés, A.; Holb, I.J. Temperate fruit trees under climate change: Challenges for dormancy and chilling requirements in warm winter regions. Horticulturae 2021, 7, 86. [Google Scholar] [CrossRef]
- Azcárate, F.M.; Manzano, P.; Peco, B. Testing seed-size predictions in Mediterranean annual grasslands. Seed Sci. Res. 2010, 20, 179–188. [Google Scholar] [CrossRef]
- Murray, B.R.; Brown, A.H.D.; Dickman, C.R.; Crowther, M.S. Geographical gradients in seed mass in relation to climate. J. Biogeogr. 2004, 31, 379–388. [Google Scholar] [CrossRef]
- Wu, H.; Meng, H.; Wang, S.; Wei, X.; Jiang, M. Geographic patterns and environmental drivers of seed traits of a relict tree species. For. Ecol. Manag. 2018, 422, 59–68. [Google Scholar] [CrossRef]
- Deren’ko, S.A. Carotenoids of the fruit of Sorbus aucuparia. Chem. Nat. Compd. 1978, 14, 454–455. [Google Scholar] [CrossRef]
- Yu, X.-D.; Zhang, C.-H.; Zheng, Y.-Q.; Xia, X.-H.; Huang, L. Relationship between carotenoid component and fruit color of two Sorbus species. For. Res. 2021, 34, 71–79. [Google Scholar]
- Zymone, K.; Raudone, L.; Raudonis, R.; Marksa, M.; Ivanauskas, L.; Janulis, V. Phytochemical profiling of fruit powders of twenty Sorbus L. cultivars. Molecules 2018, 23, 2593. [Google Scholar] [CrossRef]
- Yue, C.; Wang, Z.; Yang, P. The effect of light on the key pigment compounds of photosensitive etiolated tea plant. Bot. Stud. 2021, 62, 21. [Google Scholar] [CrossRef]
- Hu, C.; Shang, H.; Xia, X.; Li, A. A brief description of the coloration mechanism of color-leaved plants. J. South China Agric. 2014, 8, 162–163. [Google Scholar]
- Hörtensteiner, S.; Kräutler, B. Chlorophyll breakdown in higher plants. Biochim. Biophys. Acta (BBA) Bioenerg. 2011, 1807, 977–988. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, L.; Wen, B.; Wang, K.; Liu, Z.; Huang, J.A.; Li, J. Advances in leaf color variation of tea plant (Camellia sinensis). J. Plant Physiol. J. 2020, 56, 643–653. [Google Scholar]
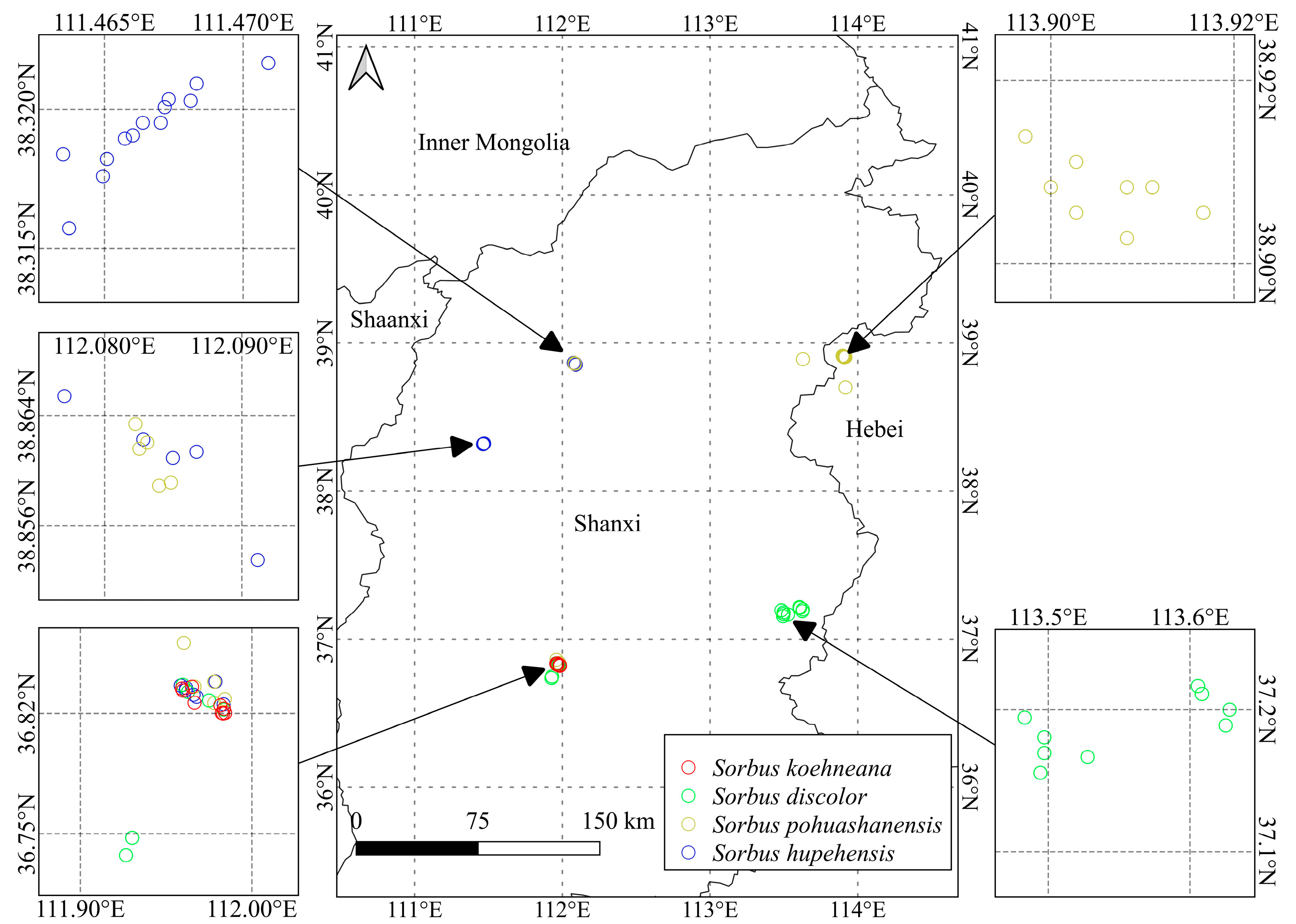
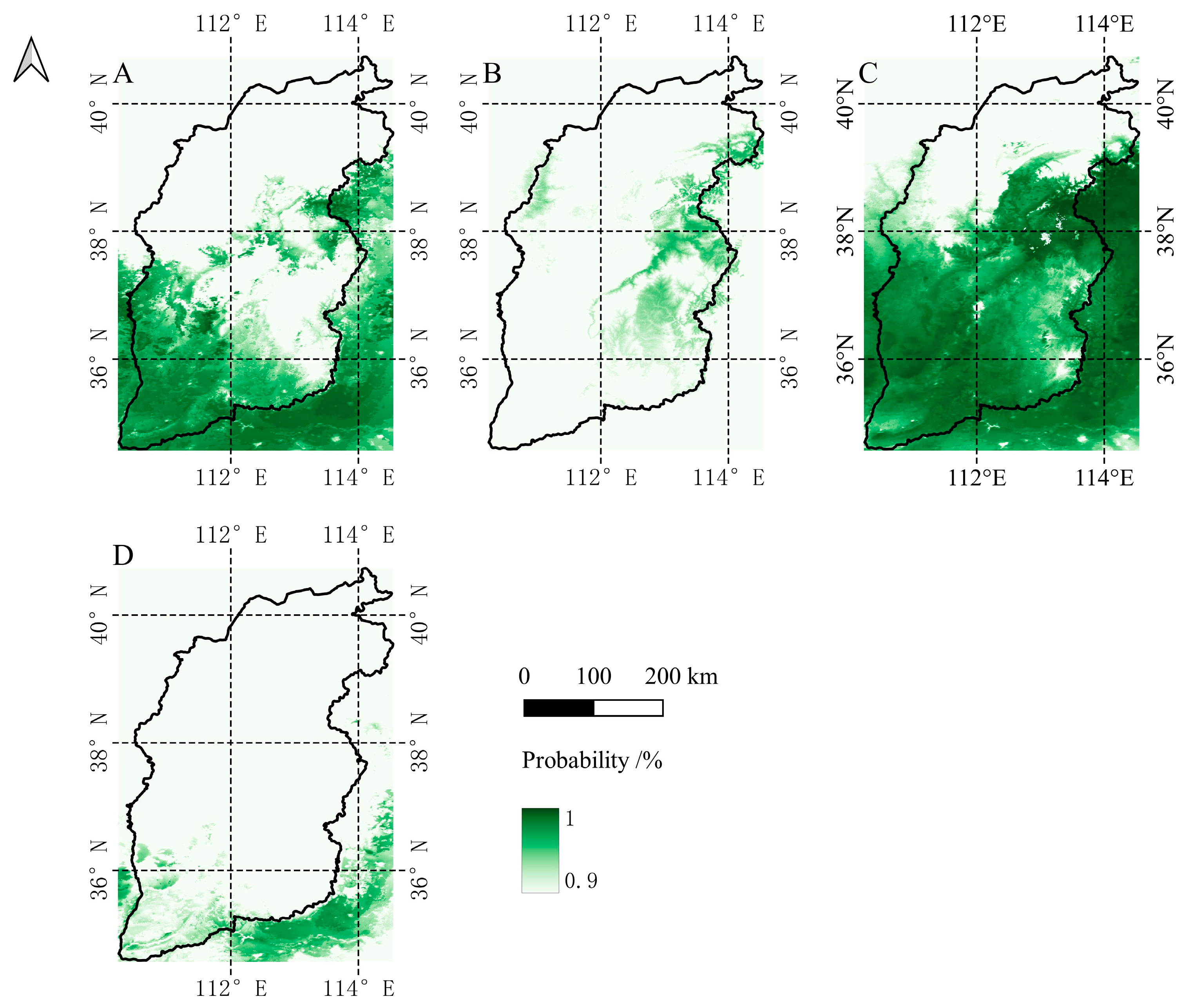


| Species | Family | Longitude | Latitude | Altitude/m | Annual Mean Temperature/°C | Annual Mean Precipitation/mm |
|---|---|---|---|---|---|---|
| A | HC1~HC13 | 111.46~111.48 | 38.31~38.33 | 1738~2049 | 3.05~4.48 | 496~505 |
| LYS1~LYS5 | 112.07~112.09 | 38.85~38.87 | 1829~1986 | 2.35~3.03 | 478~490 | |
| TY1~TY4 | 111.96~111.98 | 36.7~36.9 | 2139~2434 | 2.23~3.69 | 587~618 | |
| LS1~LS3 | 111.95~111.98 | 36.81~36.8 | 2026~2139 | 3.69~3.93 | 578~589 | |
| B | LYS6~LYS10 | 112.08~112.09 | 38.85~38.87 | 1829~1986 | 2.35~3.03 | 478~490 |
| TY5~TY6 | 111.97~111.98 | 36.82~36.85 | 2141~2308 | 2.66~3.23 | 587~602 | |
| LS4~LS6 | 111.96~111.98 | 36.83~36.8 | 1626~2026 | 3.69~6.13 | 541~589 | |
| WT1~WT11 | 113.63~113.92 | 38.7~38.9 | 727~1688 | 3.67~9.31 | 422~497 | |
| C | ZQ1~ZQ9 | 113.48~113.63 | 37.15~37.2 | 1391~1919 | 4.3~6.86 | 579~632 |
| TY7~TY10 | 111.97~111.99 | 36.82~36.84 | 2285~2312 | 2.53~2.88 | 602~605 | |
| LS7 | 111.95 | 36.83 | 2139 | 3.69 | 589 | |
| SG1~SG2 | 111.92~111.93 | 36.73~36.75 | 1238~1367 | 7.57~8.07 | 514~522 | |
| D | TY11~TY12 | 111.98~111.99 | 36.82~36.84 | 2285 | 2.24~2.53 | 604~613 |
| LS8~LS14 | 111.95~111.98 | 36.81~36.86 | 2139~2385 | 2.24~3.69 | 589~613 |
| Code | Mean | Unit | Explanation |
|---|---|---|---|
| BIO1 | Annual Mean Temperature | °C | Mean annual daily mean air Temperatures averaged over 1 year |
| BIO3 | Isothermality | - | Ratio of diurnal variation to annual Variation in temperatures |
| BIO5 | Max Temperature of Warmest Month | °C | The highest temperature of any monthly daily mean maximum temperature |
| BIO6 | Min Temperature of Coldest Month | °C | The lowest temperature of any monthly daily mean maximum temperature |
| BIO7 | Temperature Annual Range | °C | The difference between the Maximum Temperature of Warmest month and the Minimum Temperature of Coldest month |
| BIO12 | Annual Precipitation | mm | Accumulated precipitation amount over 1 year |
| BIO15 | Precipitation Seasonality | - | The Coefficient of Variation is the standard deviation of the monthly precipitation estimates expressed as a percentage of the mean of those estimates (i.e., the annual mean) |
| N | Nitrogen | g/kg | The nitrogen content in the soil |
| P | Phosphorus | g/kg | The phosphorus content in the soil |
| K | Potassium | g/kg | The potassium content in the soil |
| CEC | Cation Exchange Capacity | cmol(+)/kg | The ability of soil to retain and exchange positively charged ions affects soil fertility and nutrient availability for plant growth |
| pH | Potential of hydrogen | - | The acidity and alkalinity of soil or other media |
| Species | Index | Seed Mass/mg | Fruit Mass/mg | Fruit Mass/Seed Mass | DNR | DNG | DNB |
|---|---|---|---|---|---|---|---|
| Sorbus L. | Average | 3.15 ± 0.87 | 213.67 ± 76.01 | 0.02 ± 0.01 | 201 ± 17 | 151 ± 17 | 120 ± 68 |
| F-value | 65.26 ** | 83.30 ** | 64.80 ** | 17.70 ** | 925.01 ** | 442.77 ** | |
| CV/% | 27.65 | 35.57 | 45.73 | 8.47 | 48.76 | 57.11 | |
| A | Average | 3.1 ± 0.7 | 188 ± 45.35 | 0.02 ± 0.01 | 206.17 ± 13.4 | 178.95 ± 23.86 | 145.61 ± 23.27 |
| F-value | 8.73 ** | 14.86 ** | 19.78 ** | 4.18 ** | 59.21 ** | 35.11 ** | |
| CV/% | 20.75 | 24.24 | 30.89 | 6.54 | 14.45 | 16.89 | |
| B | Average | 2.5 ± 0.4 | 220.1 ± 46.8 | 0.01 ± 0.00 | 194.7 ± 16.1 | 44.4 ± 13.6 | 28.3 ± 8.4 |
| F-value | 15.66 ** | 11.01 ** | 10.25 ** | 6.44 ** | 3.64 * | 11.88 ** | |
| CV/% | 18.21 | 21.92 | 24.20 | 8.32 | 30.02 | 29.92 | |
| C | Average | 3.4 ± 0.5 | 223.5 ± 39.8 | 0.02 ± 0.00 | 203.5 ± 13.2 | 192.2 ± 15.6 | 136.1 ± 26.8 |
| F-value | 9.06 ** | 11.55 ** | 9.00 ** | 1.99 | 7.93 ** | 15.78 ** | |
| CV/% | 14.93 | 19.01 | 24.10 | 6.49 | 8.08 | 20.36 | |
| D | Average | 3.9 ± 0.8 | 355.0 ± 97.2 | 0.01 ± 0.00 | 205.7 ± 8.5 | 208.6 ± 8.4 | 176.5 ± 11.1 |
| F-value | 15.74 ** | 2.46 | 5.377 * | 9.075 * | 12.23 ** | 3.89 | |
| CV/% | 20.80 | 26.82 | 19.49 | 4.12 | 4.01 | 6.29 |
| Species | Family | Carotenoids Content | Anthocyanin Content |
|---|---|---|---|
| Sorbus | 0.0533 ± 0.0164 bB | 0.0251 ± 0.0076 bB | |
| 1.1016 ± 0.2264 aA | 0.2943 ± 0.0709 aA | ||
| 0.0254 ± 0.0083 bB | 0.0061 ± 0.0020 bB | ||
| 0.0361 ± 0.0112 bB | 0.0123 ± 0.0027 bB | ||
| S. hupehensis | HC | 0.0460 ± 0.0143 aA | 0.0236 ± 0.0078 cC |
| LYS | 0.0591 ± 0.0168 aA | 0.0311 ± 0.0020 cC | |
| TY | 0.0555 ± 0.0117 aA | 0.1721 ± 0.0056 aA | |
| LS | 0.0558 ± 0.0173 aA | 0.0847 ± 0.0008 bB | |
| S. pohuashanensis | LYS | 1.3784 ± 0.0546 aA | 0.3446 ± 0.0125 bB |
| TY | 1.2238 ± 0.0160 bB | 1.1607 ± 0.0278 aA | |
| LS | 0.6808 ± 0.0290 dD | 0.3792 ± 0.0275 bB | |
| WT | 1.0306 ± 0.0129 cC | 0.2402 ± 0.0432 cC | |
| S. discolor | ZQ | 0.0252 ± 0.0077 aA | 0.0029 ± 0.0004 cB |
| TY | 0.0261 ± 0.0073 aA | 0.0057 ± 0.0016 bAB | |
| LS | − | 0.0090 ± 0.0011 aA | |
| SG | − | − | |
| S. koehneana | TY | 0.0168 ± 0.0022 bB | 0.0048 ± 0.0031 aA |
| LS | 0.0397 ± 0.0084 aA | 0.0148 ± 0.0049 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Wang, J.; Zhou, S.; Zhai, Y.; Wu, X. Geographic Variation in Progeny: Climatic and Soil Changes in Offspring Size and Colour in Four Sorbus spp. (Rosaceae). Forests 2023, 14, 2390. https://doi.org/10.3390/f14122390
Liu T, Wang J, Zhou S, Zhai Y, Wu X. Geographic Variation in Progeny: Climatic and Soil Changes in Offspring Size and Colour in Four Sorbus spp. (Rosaceae). Forests. 2023; 14(12):2390. https://doi.org/10.3390/f14122390
Chicago/Turabian StyleLiu, Ting, Jin Wang, Shuai Zhou, Yu Zhai, and Xiaogang Wu. 2023. "Geographic Variation in Progeny: Climatic and Soil Changes in Offspring Size and Colour in Four Sorbus spp. (Rosaceae)" Forests 14, no. 12: 2390. https://doi.org/10.3390/f14122390
APA StyleLiu, T., Wang, J., Zhou, S., Zhai, Y., & Wu, X. (2023). Geographic Variation in Progeny: Climatic and Soil Changes in Offspring Size and Colour in Four Sorbus spp. (Rosaceae). Forests, 14(12), 2390. https://doi.org/10.3390/f14122390






