Soil Microbial Community in Relation to Soil Organic Carbon and Labile Soil Organic Carbon Fractions under Detritus Treatments in a Subtropical Karst Region during the Rainy and Dry Seasons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Soil Properties and Enzyme Activities
2.3. Soil Microbial Community Analysis
2.4. Statistical Analysis
3. Results
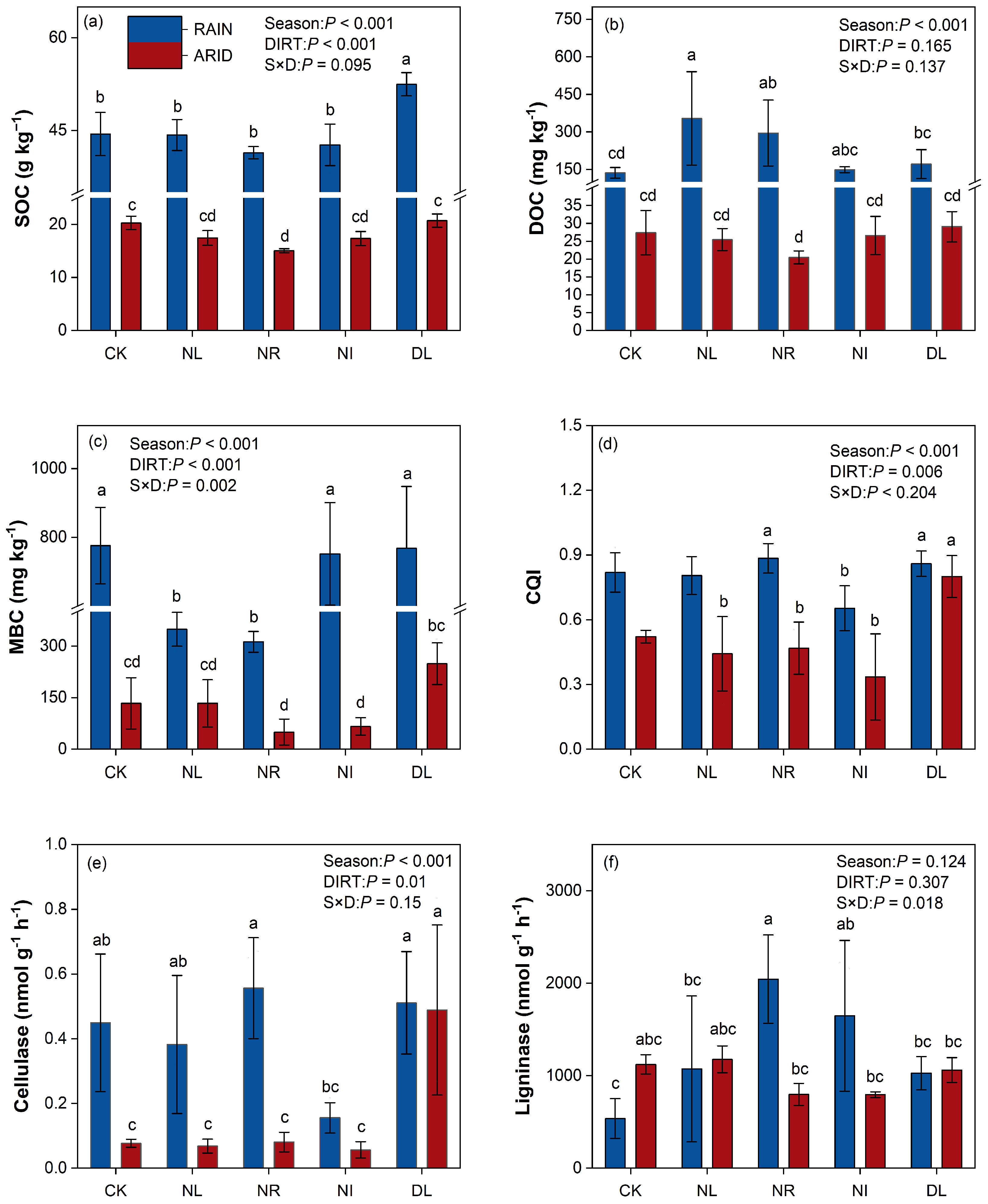
3.1. SOC and Soil Physicochemical Properties
3.2. Soil DOC, MBC, C-Degrading Enzymes, and CQI
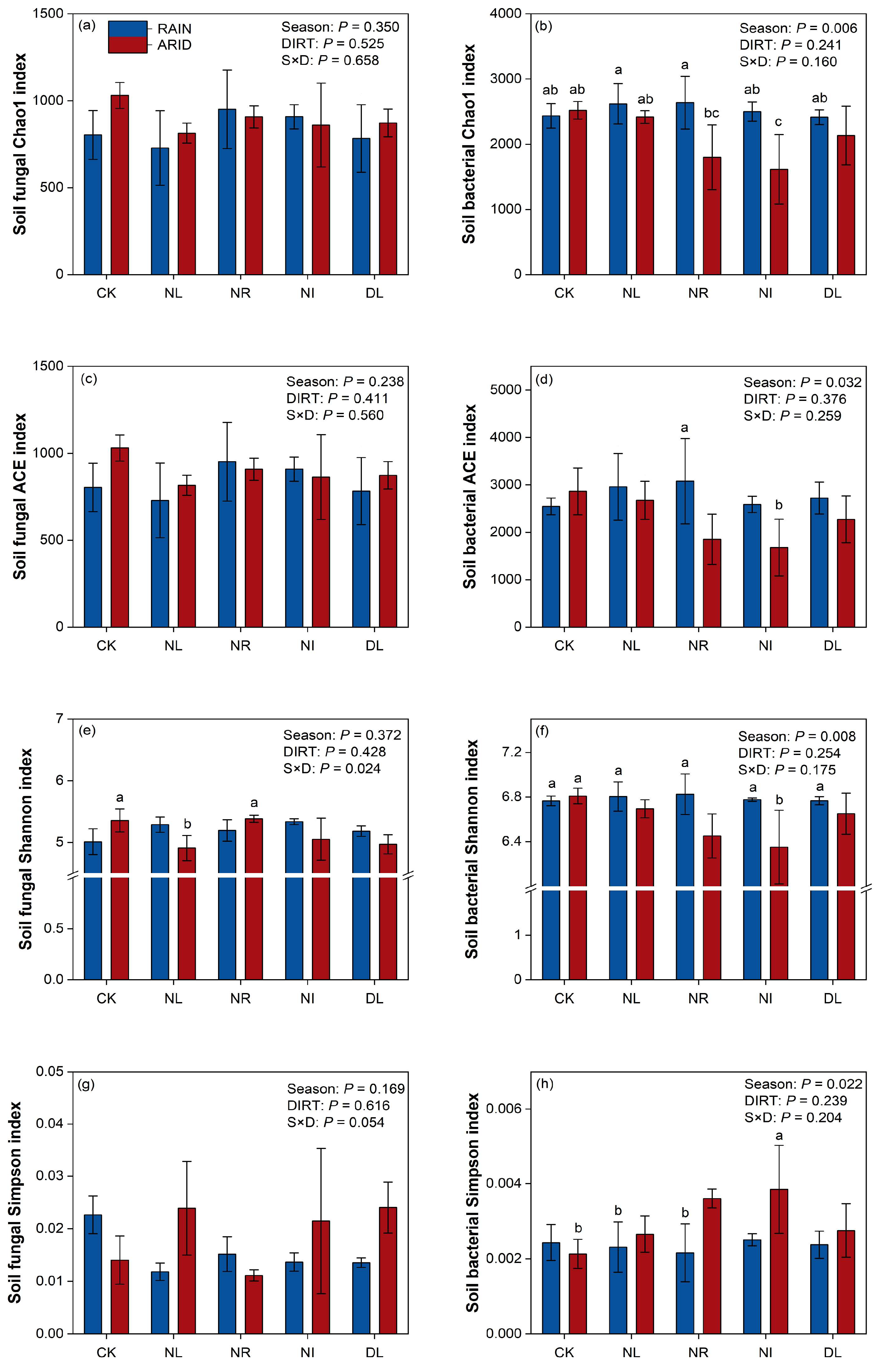
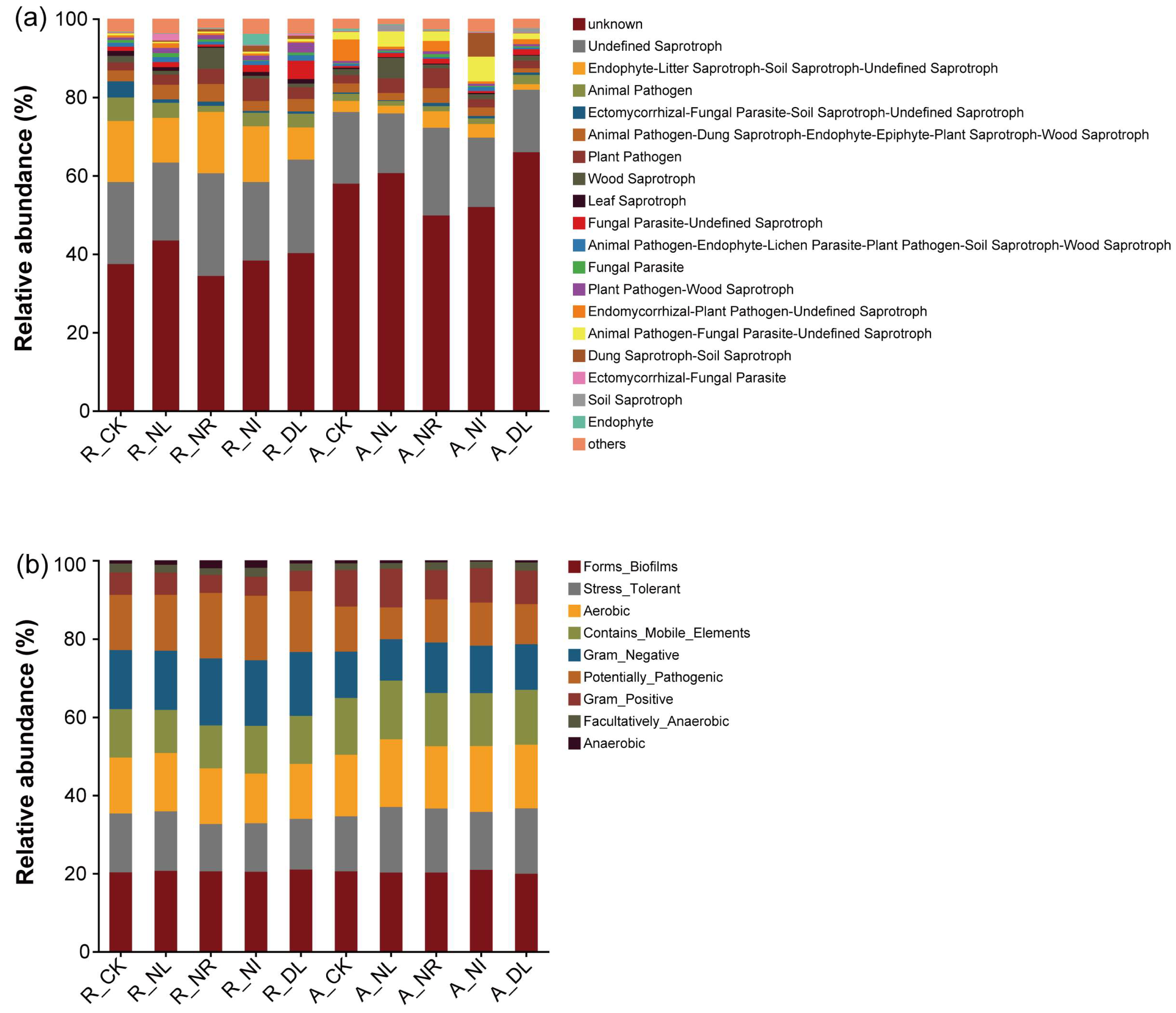
3.3. Soil Bacterial and Fungal Community Diversities and Compositions
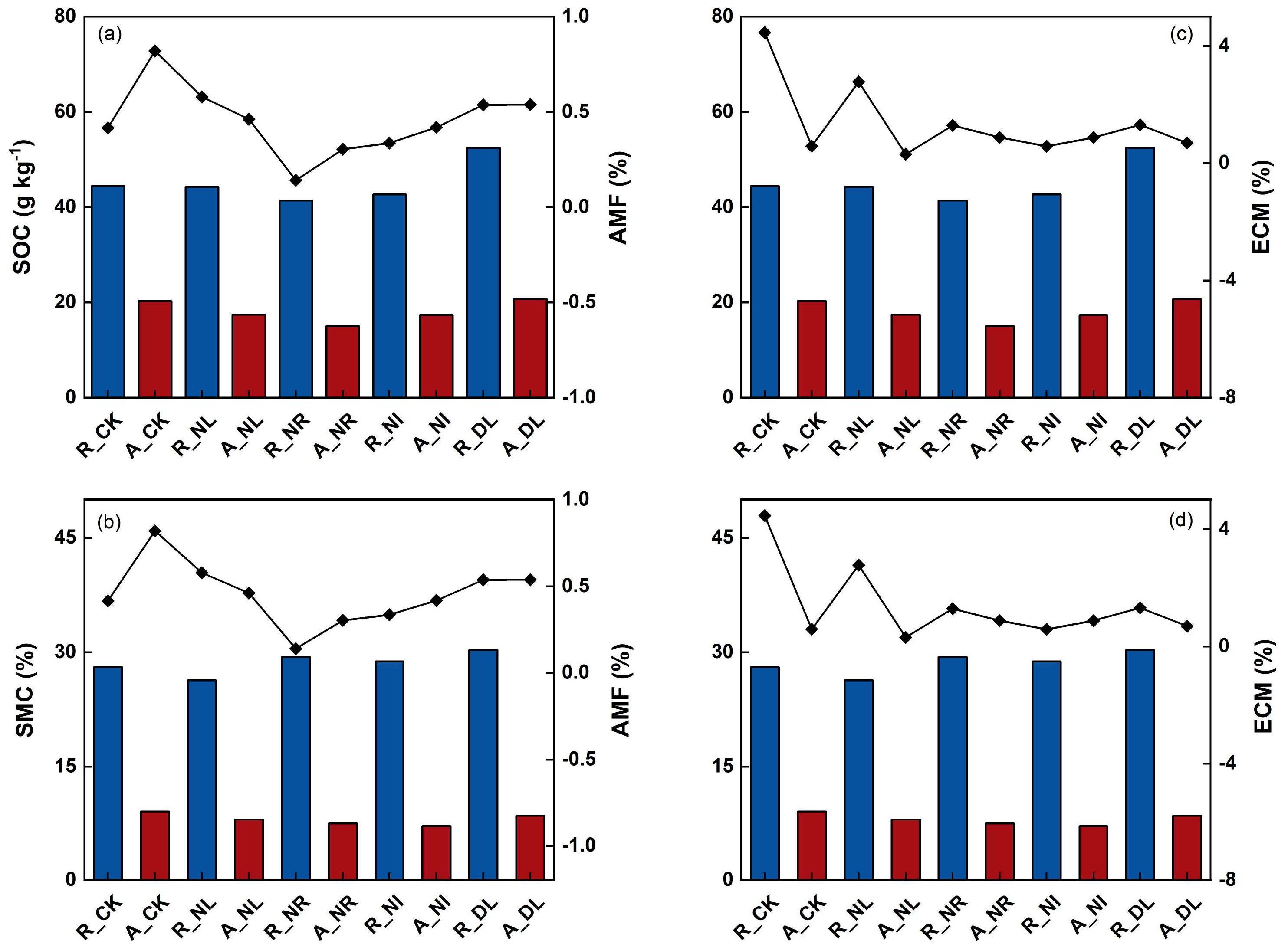
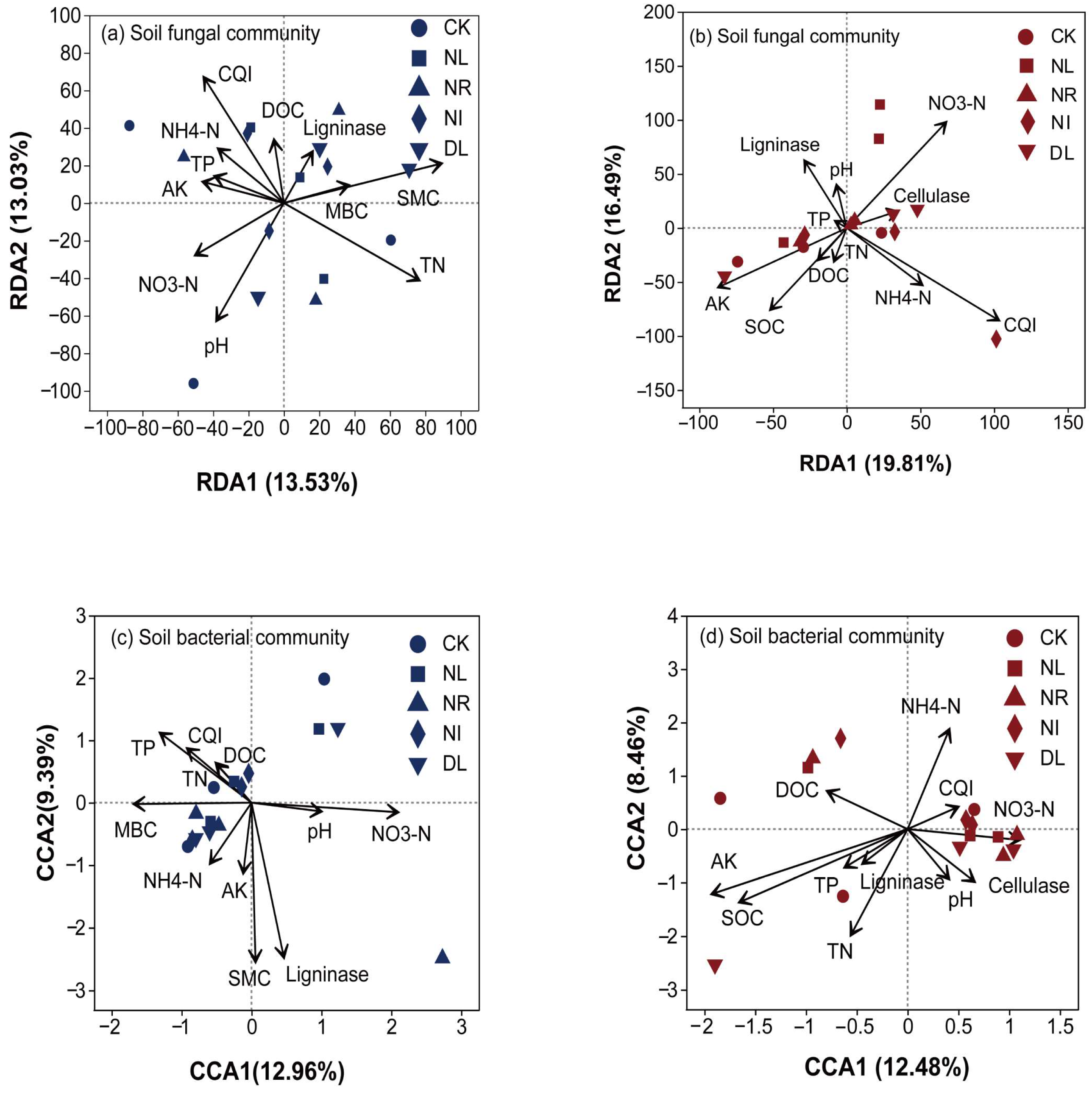
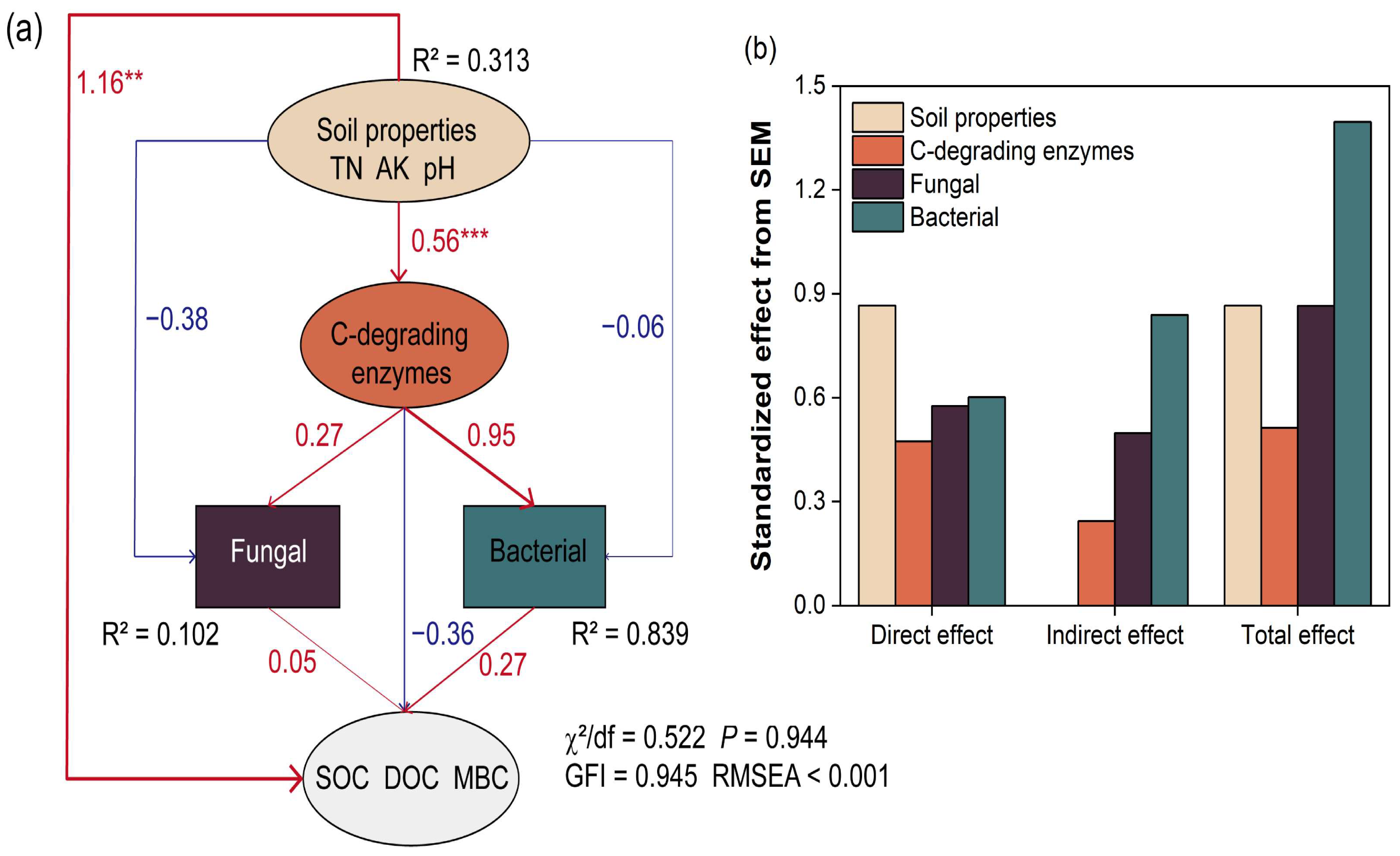
3.4. Soil Properties, Microbial Communities, and Soil Enzyme Activities
4. Discussion
4.1. Effects of Detritus Treatments on Soil Properties during Different Seasons
4.2. Effects of Detritus Treatments on Labile SOC Fractions, Soil C-Degrading Enzymes, and Functional Genes during Different Seasons
4.3. Effects of Detritus Treatments on Soil Microbial Community Diversities and Structures during Different Seasons
4.4. Driving Factors for Soil Microbial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradford, M.A.; Carey, C.J.; Atwood, L.; Bossio, D.; Fenichel, E.P.; Gennet, S.; Fargione, J.; Fisher, J.R.B.; Fuller, E.; Kane, D.A.; et al. Soil carbon science for policy and practice. Nat. Sustain. 2019, 2, 1070–1072. [Google Scholar] [CrossRef]
- Sowerby, A.; Emmett, B.A.; Williams, D.; Beier, C.; Evans, C.D. The response of dissolved organic carbon (DOC) and the ecosystem carbon balance to experimental drought in a temperate shrubland. Eur. J. Soil Sci. 2010, 61, 697–709. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, P.B.; Fuentes-Alburquenque, S.; Díez, B.; Vargas, I.; Bonilla, C.A. Soil microbial community responses to labile organic carbon fractions in relation to soil type and land use along a climate gradient. Soil Biol. Biochem. 2020, 141, 107692. [Google Scholar] [CrossRef]
- MMenge, D.N.L.; Wolf, A.A.; Funk, J.L. Diversity of nitrogen fixation strategies in Mediterranean legumes. Nat. Plants 2015, 1, 15064. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, X.; Yang, Q.; Li, H.; Luo, Y.; Fang, C.; Chen, J.; Yang, X.; Li, B. Responses of ecosystem carbon cycle to experimental warming: A meta-analysis. Ecology 2013, 94, 726–738. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, L.; Nie, Y.; Fu, Y.; Du, Z.; Shao, J.; Zheng, Z.; Wang, X. Similar responses of soil carbon storage to drought and irrigation in terrestrial ecosystems but with contrasting mechanisms: A meta-analysis. Agric. Ecosyst. Environ. 2016, 228, 70–81. [Google Scholar] [CrossRef]
- Aanderud, Z.T.; Richards, J.H.; Svejcar, T.; James, J.J. A Shift in Seasonal Rainfall Reduces Soil Organic Carbon Storage in a Cold Desert. Ecosystems 2010, 13, 673–682. [Google Scholar] [CrossRef]
- Hopkins, F.M.; Filley, T.R.; Gleixner, G.; Lange, M.; Top, S.M.; Trumbore, S.E. Increased belowground carbon inputs and warming promote loss of soil organic carbon through complementary microbial responses. Soil Biol. Biochem. 2014, 76, 57–69. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, Y.; Zhang, H.; An, S. Fast bacterial succession associated with the decomposition of Quercus wutaishanica litter on the Loess Plateau. Biogeochemistry 2019, 144, 119–131. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Severi, J.; Butterly, C.R.; Baldock, J.A. Rhizosphere priming effect on soil organic carbon decomposition under plant species differing in soil acidification and root exudation. New Phytol. 2016, 211, 864–873. [Google Scholar] [CrossRef]
- Crow, S.E.; Lajtha, K.; Filley, T.R.; Swanston, C.W.; Bowden, R.D.; Caldwell, B.A. Sources of plant-derived carbon and stability of organic matter in soil: Implications for global change. Glob. Chang. Biol. 2009, 15, 2003–2019. [Google Scholar] [CrossRef]
- Cao, J.; He, X.; Chen, Y.; Chen, Y.; Zhang, Y.; Yu, S.; Zhou, L.; Liu, Z.; Zhang, C.; Fu, S. Leaf litter contributes more to soil organic carbon than fine roots in two 10-year-old subtropical plantations. Sci. Total Environ. 2020, 704, 135341. [Google Scholar] [CrossRef] [PubMed]
- Mayzelle, M.M.; Krusor, M.L.; Lajtha, K.; Bowden, R.D.; Six, J. Effects of Detrital Inputs and Roots on Carbon Saturation Deficit of a Temperate Forest Soil. Soil Sci. Soc. Am. J. 2014, 78, S76–S83. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Cleveland, C.C.; Wieder, W.R.; Washenberger, C.L.; Townsend, A.R. Plot-scale manipulations of organic matter inputs to soils correlate with shifts in microbial community composition in a lowland tropical rain forest. Soil Biol. Biochem. 2010, 42, 2153–2160. [Google Scholar] [CrossRef]
- Zhao, M.; Xue, K.; Wang, F.; Liu, S.; Bai, S.; Sun, B.; Zhou, J.; Yang, Y. Microbial mediation of biogeochemical cycles revealed by simulation of global changes with soil transplant and cropping. Isme J. 2014, 8, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Orwin, K.H.; Dickie, I.A.; Holdaway, R.; Wood, J.R. A comparison of the ability of PLFA and 16S rRNA gene metabarcoding to resolve soil community change and predict ecosystem functions. Soil Biol. Biochem. 2018, 117, 27–35. [Google Scholar] [CrossRef]
- Wei, H.; Man, X. Increased Litter Greatly Enhancing Soil Respiration in Betula platyphylla Forests of Permafrost Region, Northeast China. Forests 2021, 12, 89. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Hu, X.-F.; Wan, S.; Wang, H.; Liang, C.; Chen, F.-S. Litter manipulation effects on microbial communities and enzymatic activities vary with soil depth in a subtropical Chinese fir plantation. For. Ecol. Manag. 2021, 480, 118641. [Google Scholar] [CrossRef]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Xue, Y.; Tian, J.; Quine, T.A.; Powlson, D.; Xing, K.; Yang, L.; Kuzyakov, Y.; Dungait, J.A.J. The persistence of bacterial diversity and ecosystem multifunctionality along a disturbance intensity gradient in karst soil. Sci. Total Environ. 2020, 748, 142381. [Google Scholar] [CrossRef]
- Liu, R.; Wang, D. C:N:P stoichiometric characteristics and seasonal dynamics of leaf-root-litter-soil in plantations on the loess plateau. Ecol. Indic. 2021, 127, 107772. [Google Scholar] [CrossRef]
- Ren, C.; Chen, J.; Lu, X.; Doughty, R.; Zhao, F.; Zhong, Z.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Responses of soil total microbial biomass and community compositions to rainfall reductions. Soil Biol. Biochem. 2018, 116, 4–10. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, Y.; Zhang, B.; Luo, Y.; Yi, X.; Wu, J.; Chen, Y.; Sarker, T.C.; Cai, Y.; Chang, S.X. Land-use-driven change in soil labile carbon affects microbial community composition and function. Geoderma 2022, 426, 116056. [Google Scholar] [CrossRef]
- Yang, B.; Qi, K.; Bhusal, D.R.; Huang, J.; Chen, W.; Wu, Q.; Hussain, A.; Pang, X. Soil microbial community and enzymatic activity in soil particle-size fractions of spruce plantation and secondary birch forest. Eur. J. Soil Biol. 2020, 99, 103196. [Google Scholar] [CrossRef]
- Grace, J.B.; Keeley, J.E. A structural equation model analysis of postfire plant diversity in California shrublands. Ecol. Appl. A Publ. Ecol. Soc. Am. 2006, 16, 503–514. [Google Scholar] [CrossRef]
- Wang, L.X.; Deng, D.Z.; Feng, Q.H.; Xu, Z.J.R.; Pan, H.L.; Li, H.C. Changes in litter input exert divergent effects on the soil microbial community and function in stands of different densities. Sci. Total Environ. 2022, 845, 157297. [Google Scholar] [CrossRef]
- Feng, J.; He, K.; Zhang, Q.; Han, M.; Zhu, B. Changes in plant inputs alter soil carbon and microbial communities in forest ecosystems. Glob. Chang. Biol. 2022, 28, 3426–3440. [Google Scholar] [CrossRef]
- Sokol, N.W.; Kuebbing, S.E.; Karlsen-Ayala, E.; Bradford, M.A. Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic carbon. New Phytol. 2019, 221, 233–246. [Google Scholar] [CrossRef]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Pineiro, G. The Ecology of Soil Carbon: Pools, Vulnerabilities, and Biotic and Abiotic Controls. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 419–445. [Google Scholar] [CrossRef]
- Villarino, S.H.; Pinto, P.; Jackson, R.B.; Pineiro, G. Plant rhizodeposition: A key factor for soil organic matter formation in stable fractions. Sci. Adv. 2021, 7, eabd3176. [Google Scholar] [CrossRef] [PubMed]
- Sokol, N.W.; Sanderman, J.; Bradford, M.A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Chang. Biol. 2019, 25, 12–24. [Google Scholar] [CrossRef]
- Chen, X.; Deng, Q.; Lin, G.; Lin, M.; Wei, H. Changing rainfall frequency affects soil organic carbon concentrations by altering non-labile soil organic carbon concentrations in a tropical monsoon forest. Sci. Total Environ. 2018, 644, 762–769. [Google Scholar] [CrossRef]
- Schuster, M.J. Increased rainfall variability and N addition accelerate litter decomposition in a restored prairie. Oecologia 2016, 180, 645–655. [Google Scholar] [CrossRef]
- Guo, X.; Meng, M.; Zhang, J.; Chen, H.Y.H. Vegetation change impacts on soil organic carbon chemical composition in subtropical forests. Sci. Rep. 2016, 6, 29607. [Google Scholar] [CrossRef] [PubMed]
- Hatton, P.-J.; Castanha, C.; Torn, M.S.; Bird, J.A. Litter type control on soil C and N stabilization dynamics in a temperate forest. Glob. Chang. Biol. 2015, 21, 1358–1367. [Google Scholar] [CrossRef]
- Ruiz-Colmenero, M.; Bienes, R.; Eldridge, D.J.; Marques, M.J. Vegetation cover reduces erosion and enhances soil organic carbon in a vineyard in the central Spain. Catena 2013, 104, 153–160. [Google Scholar] [CrossRef]
- Shi, L.; Feng, W.; Jing, X.; Zang, H.; Mortimer, P.; Zou, X. Contrasting responses of soil fungal communities and soil respiration to the above- and below-ground plant C inputs in a subtropical forest. Eur. J. Soil Sci. 2019, 70, 751–764. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Shi, L.; Marshall, M.R.; Kuzyakov, Y.; Blagodatskaya, E.; Zang, H. Strong priming of soil organic matter induced by frequent input of labile carbon. Soil Biol. Biochem. 2021, 152, 108069. [Google Scholar] [CrossRef]
- Hollister, E.B.; Schadt, C.W.; Palumbo, A.V.; Ansley, R.J.; Boutton, T.W. Structural and functional diversity of soil bacterial and fungal communities following woody plant encroachment in the southern Great Plains. Soil Biol. Biochem. 2010, 42, 1816–1824. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Morreale, S.J.; Schneider, R.L.; Li, Z.; Wu, G.-L. Contributions of plant litter to soil microbial activity improvement and soil nutrient enhancement along with herb and shrub colonization expansions in an arid sandy land. Catena 2023, 227, 107098. [Google Scholar] [CrossRef]
- Beimforde, C.; Feldberg, K.; Nylinder, S.; Rikkinen, J.; Tuovila, H.; Doerfelt, H.; Gube, M.; Jackson, D.J.; Reitner, J.; Seyfullah, L.J.; et al. Estimating the Phanerozoic history of the Ascomycota lineages: Combining fossil and molecular data. Mol. Phylogenetics Evol. 2014, 78, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global patterns in belowground communities. Ecol. Lett. 2009, 12, 1238–1249. [Google Scholar] [CrossRef]
- Wu, B.; Umer, M.; Guo, Y.; He, M.; Han, X.; Shen, K.; Xia, T.; He, Y.; He, X. Positive responses of soil nutrients and enzyme activities to AM fungus under interspecific and intraspecific competitions when associated with litter addition. Rhizosphere 2023, 27, 100728. [Google Scholar] [CrossRef]
- Hodge, A. Interactions between Arbuscular Mycorrhizal Fungi and Organic Material Substrates. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 89, pp. 47–99. [Google Scholar]
- Zhu, H.; Gong, L.; Luo, Y.; Tang, J.; Ding, Z.; Li, X. Effects of Litter and Root Manipulations on Soil Bacterial and Fungal Community Structure and Function in a Schrenk’s Spruce (Picea schrenkiana) Forest. Front. Plant Sci. 2022, 13, 849483. [Google Scholar] [CrossRef]
- Dou, Y.; Liao, J.; An, S. Importance of soil labile organic carbon fractions in shaping microbial community after vegetation restoration. Catena 2023, 220, 106707. [Google Scholar] [CrossRef]
- Ridgeway, J.R.; Morrissey, E.M.; Brzostek, E.R. Plant litter traits control microbial decomposition and drive soil carbon stabilization. Soil Biol. Biochem. 2022, 175, 108857. [Google Scholar] [CrossRef]



| Detritus Treatment | SMC (%) | pH | SOC (g kg−1) | TN (g kg−1) | TP (g kg−1) | NH4-N (mg kg−1) | NO3-N (mg kg−1) | AK (mg kg−1) | |
|---|---|---|---|---|---|---|---|---|---|
| CK | Rainy | 0.28 ± 0.02 ab | 6.78 ± 0.10 a | 44.43 ± 3.28 b | 7.93 ± 1.48 ab | 0.21 ± 0.06 ab | 104.37 ± 8.58 d | 129.67 ± 53.85 c | 5.33 ± 0.11 a |
| Dry | 0.09 ± 0.01 c | 6.45 ± 0.14 ab | 20.28 ± 1.19 c | 2.23 ± 0.24 d | 0.29 ± 0.01 b | 305.57 ± 62.83 b | 255.73 ± 95.96 abc | 2.82 ± 0.20 b | |
| NL | Rainy | 0.26 ± 0.002 b | 6.68 ± 0.12 a | 44.27 ± 2.37 b | 7.40 ± 0.29 bc | 0.19 ± 0.07 ab | 65.07 ± 16.36 d | 145.60 ± 12.63 bc | 5.23 ± 0.15 a |
| Dry | 0.08 ± 0.004 c | 6.38 ± 0.16 ab | 17.46 ± 1.30 cd | 2.10 ± 0.16 d | 0.28 ± 0.01 b | 285.27 ± 15.50 bc | 590.83 ± 6.91 a | 2.59 ± 0.15 b | |
| NR | Rainy | 0.29 ± 0.02 a | 6.65 ± 0.16 a | 41.40 ± 0.94 b | 6.40 ± 0.22 c | 0.17 ± 0.06 ab | 153.57 ± 37.75 cd | 194.87 ± 27.97 bc | 5.24 ± 0.13 a |
| Dry | 0.07 ± 0.01 c | 6.05 ± 0.61 b | 15.03 ± 0.37 d | 1.73 ± 0.05 d | 0.23 ± 0.02 ab | 493.93 ± 149.61 a | 204.47 ± 34.21 abc | 2.57 ± 0.31 b | |
| NI | Rainy | 0.29 ± 0.02 a | 6.49 ± 0.18 ab | 42.67 ± 3.18 b | 7.40 ± 0.51 bc | 0.20 ± 0.02 ab | 113.77 ± 25.42 d | 201.27 ± 6.41 abc | 5.31 ± 0.10 a |
| Dry | 0.07 ± 0.01 c | 6.50 ± 0.28 ab | 17.34 ± 1.25 cd | 1.83 ± 0.21 d | 0.25 ± 0.05 b | 599.57 ± 60.73 a | 307.80 ± 63.29 abc | 2.74 ± 0.33 b | |
| DL | Rainy | 0.30 ± 0.02 a | 6.39 ± 0.14 ab | 52.47 ± 1.76 a | 9.37 ± 0.84 a | 0.19 ± 0.06 b | 77.70 ± 17.60 d | 168.30 ± 21.40 bc | 5.34 ± 0.05 a |
| Dry | 0.09 ± 0.01 c | 6.42 ± 0.14 ab | 20.73 ± 1.18 c | 2.53 ± 0.17 d | 0.32 ± 0.04 a | 294.00 ± 136.33 bc | 357.23 ± 89.35 ab | 3.33 ± 0.24 a | |
| p-value | <0.001 | 0.285 | <0.001 | <0.001 | 0.069 | <0.001 | 0.048 | <0.001 | |
| Rainy Season | Dry Season | |||||||
|---|---|---|---|---|---|---|---|---|
| Fungal Chao1 | Bacterial Chao1 | Fungal Shannon | Bacterial Shannon | Fungal Chao1 | Bacterial Chao1 | Fungal Shannon | Bacterial Shannon | |
| SMC (%) | 0.42 | −0.14 | 0.20 | −0.17 | 0.40 | 0.68 ** | 0.11 | 0.70 ** |
| pH | −0.24 | 0.20 | −0.09 | 0.05 | 0.24 | 0.11 | 0.13 | 0.23 |
| TN (g kg) | −0.08 | −0.49 | 0.14 | −0.24 | −0.05 | 0.54 * | −0.33 | 0.49 |
| TP (g kg) | −0.05 | −0.74 ** | −0.28 | −0.46 | 0.19 | 0.28 | −0.23 | 0.26 |
| NH4-N (mg kg) | 0.37 | −0.12 | 0.01 | −0.21 | 0.15 | −0.67 ** | 0.39 | −0.64 * |
| NO3-N (mg kg) | −0.08 | 0.41 | 0.10 | 0.42 | −0.59 * | 0.20 | −0.64 * | 0.10 |
| AK (mg kg) | −0.23 | 0.03 | −0.19 | −0.18 | 0.38 | 0.10 | 0.01 | 0.19 |
| SOC (g kg) | −0.20 | −0.38 | −0.10 | −0.06 | 0.16 | 0.48 | −0.25 | 0.55 * |
| DOC | 0.05 | −0.33 | 0.08 | −0.07 | 0.25 | 0.10 | −0.16 | 0.14 |
| MBC (mg kg) | 0.13 | −0.28 | −0.07 | −0.33 | −0.15 | 0.41 | −0.36 | 0.30 |
| Cellulase | −0.13 | 0.08 | −0.17 | 0.27 | 0.20 | 0.18 | 0.10 | 0.15 |
| Ligninase | 0.31 | 0.18 | 0.37 | 0.08 | 0.21 | 0.68 ** | −0.04 | 0.67 ** |
| CQI | 0.15 | −0.11 | 0.10 | −0.31 | −0.04 | −0.28 | 0.10 | −0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Ding, S.; Liu, N.; Mo, Y.; Liang, Y.; Ma, J. Soil Microbial Community in Relation to Soil Organic Carbon and Labile Soil Organic Carbon Fractions under Detritus Treatments in a Subtropical Karst Region during the Rainy and Dry Seasons. Forests 2023, 14, 2291. https://doi.org/10.3390/f14122291
Liu P, Ding S, Liu N, Mo Y, Liang Y, Ma J. Soil Microbial Community in Relation to Soil Organic Carbon and Labile Soil Organic Carbon Fractions under Detritus Treatments in a Subtropical Karst Region during the Rainy and Dry Seasons. Forests. 2023; 14(12):2291. https://doi.org/10.3390/f14122291
Chicago/Turabian StyleLiu, Peiwen, Suya Ding, Ning Liu, Yanhua Mo, Yueming Liang, and Jiangming Ma. 2023. "Soil Microbial Community in Relation to Soil Organic Carbon and Labile Soil Organic Carbon Fractions under Detritus Treatments in a Subtropical Karst Region during the Rainy and Dry Seasons" Forests 14, no. 12: 2291. https://doi.org/10.3390/f14122291
APA StyleLiu, P., Ding, S., Liu, N., Mo, Y., Liang, Y., & Ma, J. (2023). Soil Microbial Community in Relation to Soil Organic Carbon and Labile Soil Organic Carbon Fractions under Detritus Treatments in a Subtropical Karst Region during the Rainy and Dry Seasons. Forests, 14(12), 2291. https://doi.org/10.3390/f14122291





