Abstract
Explaining the mechanism of the coexistence of sympatric species is an important goal of ecology. Five species of woodpeckers coexist in the broadleaved Korean pine forest of Liangshui National Nature Reserve, including the Black Woodpecker (Dryocopus martius), Great Spotted Woodpecker (Dendrocopos major), Lesser Spotted Woodpecker (Dendrocopos minor), Three-toed Woodpecker (Picoides tridactylus), and White-backed Woodpecker (Dendrocopos leucotos). Woodpeckers are considered to be keystone species because of their role as ecosystem engineers, creating breeding and shelter sites for many vertebrate and invertebrate taxa. As woodpeckers are predominant in primary forests, they are sensitive to changes in forest ecosystems. To understand their coexistence mechanisms and propose conservation strategies, it is necessary to investigate their foraging niche differentiation. This study aimed to identify the foraging behavior parameters and foraging tree parameters of five woodpecker species in Liangshui Reserve from October to December. The foraging niches of five woodpecker species were observed, including the type of foraging techniques, foraging height, foraging site, foraging duration, tree species being foraged upon, diameter of the foraging tree at breast height, foraging tree height, and decay status of trees. Our results identified that there were significant differences in the overall foraging ecology of the five species of woodpecker at Liangshui Reserve. The Great Spotted Woodpecker and Lesser Spotted Woodpecker had more diverse foraging patterns and preferred to forage on live trees. The Black Woodpecker and Three-toed Woodpecker excavated and pecked at the trunks of decaying spruce and fir trees. The White-backed Woodpecker preferred to forage in broadleaved trees. The choice of foraging sites was complicated. The size of the foraging trees and decay status of trees were important bases for woodpeckers when choosing trees to forage from. Different woodpeckers achieve stable coexistence through the separation of their foraging niches. This information regarding foraging behavior and foraging tree characteristics provides a basis to study the coexistence patterns of woodpeckers. Our research into woodpecker foraging should be used to inform forest management practices, protect forest ecosystem diversities, and maintain woodpecker community diversity.
1. Introduction
The mechanism of the coexistence of species in communities is the core of ecology and biodiversity research [1,2]. The niche concept is often used to explain the coexistence of sympatric species [3,4]. The competitive exclusion principle (Gause’s principle) states that two species cannot have exactly the same niche in a habitat and stably coexist, which proves that niche separation is a necessary condition to maintain the coexistence of species [5].
Many studies have identified that resource differentiation helps to reduce the number of niche overlaps between species according to multiple dimensions of niches [6,7,8]. Foraging niche separation has been extensively studied; species of the same population that coexist in the same domain differ in their foraging niche [9,10]. Understanding the foraging niche needs of species can help us to understand this differentiation and explore the mechanisms of the coexistence of species. Foraging niche differentiation has been extensively studied in bird populations. The foraging ecological separation of bird communities can be described using a number of methods, including the choice of foraging substrate and the foraging strategies used. For example, Palearctic migrants and resident birds in Northern Ghana coexist due to the spatial partitioning of foraging niches and the use of different foraging techniques [11]. In a mixed population of trees in mature coniferous forests, the spatial niche separation of foraging sites among different tit species was observed [12]. Differentiation in foraging may be a mechanism for the coexistence of sympatric woodpeckers [13].
Research on foraging niche differentiation is particularly important for woodpeckers, which have different degrees of specialization in foraging patterns [14,15,16]. Some woodpeckers can create rows of holes in trees to extract the sap, such as the Three-toed Woodpecker (Picoides tridactylus), the Great Spotted Woodpecker (Dendrocopos major), and a few sapsuckers. The Great Spotted Woodpecker (Dendrocopos major) can extract seeds from cones using anvils after removing them from a tree [17].
Woodpeckers are known ecosystem engineers, excavating tree cavities that are subsequently used by many other cavity-nesting animals such as mammals, birds, and invertebrates [18,19,20]. This results in the formation of a nest web, which provides important resources for the formation and maintenance of forest biodiversity [21,22]. The dependence of many woodpecker species on specific characteristics such as dead wood for nesting and large trees for foraging makes woodpeckers an indicator species for the management of forest quality [23,24]; they interact with wood-rotting fungi and are important for the functioning of forest ecosystems [25]. Woodpeckers are closely related to the forest environment due to a variety of morphological and ecological specializations [26]; they have been used to guide forest management methods and forest biodiversity conservation [27,28]. Studying the ecological characteristics of woodpeckers and understanding their foraging needs are of significance for the protection of forest ecosystems and to maintain biodiversity.
Foraging niche separation between sympatric woodpecker species allows several species to steadily coexist in the same type of habitat [13,29,30]. Several studies around the world have observed niche differentiation in sympatric woodpeckers, for example, in European forests, where Hogstad (1971) discovered the stratification of winter foraging on the part of the Great Spotted Woodpecker and Three-toed Woodpecker in the Boreal forest area near Oslo in southern Norway [31]. Kumar (2020) studied the foraging niche differentiation of 10 species of woodpeckers coexisting in Northwest India from six niche dimensions [13]. Bull (1986) surveyed eight species of woodpeckers in Oregon, USA, and observed differences in their foraging sites, foraging strategies, substrate types, and preference for obstacles [32]. Nevertheless, the foraging ecology of woodpeckers in Asia has not been studied in detail.
There are many species of woodpeckers distributed in the original Korean pine needle broadleaved mixed forests in the temperate zone of Northeast China [33], a suitable location to evaluate the distribution of foraging ecological niches among woodpeckers. We studied the foraging ecology and differentiation of sympatric woodpeckers in the broadleaved Korean pine forests of Northeast China. The purpose of this study was to improve our understanding of the foraging ecology of sympatric woodpeckers in the forests of this region, to understand their foraging needs, and to provide the necessary basis for the development of sustainable forest management and species conservation strategies.
2. Materials and Methods
2.1. Study Area
The fieldwork was conducted at Liangshui National Nature Reserve (128°48′8″–128°55′46″ E, 47°7′15″–47°14′38″ N) of the Xiao Xing′an mountains in Heilongjiang province, Northeast China. The geographical location of Liangshui Reserve is in the eastern margin of the Eurasia continent, which has obvious temperate continental climate characteristics. The average annual temperature is −0.3 °C and the average annual precipitation is 676 mm. The total area of Liangshui Reserve is 12,133 ha; the original Korean pine forests cover an area of approximately 2375 hm2 [34]. Liangshui Reserve has various forest types; these are predominantly mixed forest types, including large Korean pine forests and secondary birch broadleaved forests. The forests almost cover the entire Xiao Xing’an mountain range at different succession stages [35]. Liangshui Reserve is rich in tree resources and the dominant species is Korean pine (Pinus koraiensis). The other common trees include Khingan fir (Abies nephrolepis), scale spruce (Picea jezoensis), red spruce (Picea koraiensis), Xing’an larch (Laris gemlini), Mongolian scotch pine (Pinus sylvestris var. mongolica), Japanese birch (Betula platyphylla), Manchurian ash (Fraxinus mandschurica), elm (Ulmus pumila), Amur linden (Tilia amurensis), Amur cork tree (Phellodendron amurense), and Mono maple (Acer mono) [36]. There are six species of woodpecker at Liangshui Reserve; these include the Black Woodpecker (Dryocopus martius), Great Spotted Woodpecker, Lesser Spotted Woodpecker (Dendrocopos minor), Three-toed Woodpecker, White-backed Woodpecker (Dendrocopos leucotos), and Grey-headed Woodpecker (Picus canus). The Grey-headed Woodpecker is relatively rare in this location; only the other woodpeckers were observed in this study.
2.2. Data Collection
The fieldwork was performed in autumn and winter of 2020–2021; the data were collected from October to December each year. Foraging birds were observed via random walks in the study area, which usually began one hour after sunrise and lasted until sunset. Woodpecker individuals were located mainly by sound (pecking and calling) and using binoculars. Once a bird was detected, the time of its foraging, its species, the parameters of its foraging behavior, and its foraging site were recorded. The foraging duration was measured from when the woodpecker was located to when the bird flew to another tree. To ensure sample independence, multiple observations of the same individual were not usually considered. In rare cases, a second observation was permitted after the woodpecker moved at least 100 m from the previous position [37,38]. To avoid disturbing the birds, we maintained a viewing distance of no less than 10 m from them.
2.2.1. Foraging Behavior Variables
The following variables were recorded for each foraging behavior observation: foraging technique (FOT), foraging height (FOH), foraging site (FOS; trunk or branch), and foraging duration (FOD; min). We distinguished the foraging techniques into the following categories (Remsen and Robinson, 1990) [39]: (1) pecking, or pecking at a tree trunk or branch from which small pieces of bark or wood chips fall (to forage under the bark); (2) excavating, or making a hole with the beak from which large pieces of bark and wood fall (to forage on the wood layer); (3) scaling, or flaking off the bark; (4) picking, or picking up food from the surface of a tree trunk or branch; (5) probing, or poking and peering for food in a tree where there are holes or cracks; and (6) extracting, where woodpeckers remove cones from trees and place them in anvils, which they use to extract seeds from the cones [40]. In addition, the rare occurrence of woodpeckers accidentally foraging pests directly from cones falls into this category. Most often, crevices and cracks in bark or fractures formed in branches as a result of the breaking-off or decay of their parts become anvils. Under this foraging technique, the foraging of a cone is recorded as a foraging duration. We recorded the foraging height using the following four classes: canopy, upper trunk, lower trunk, and near-ground (including the base of the tree, the fall of the wood, and the ground).
2.2.2. Foraging Tree Characteristics
The foraging tree characteristics included the following: the tree species being foraged upon (TRS); the diameter of the foraging tree at breast height (DBH; cm); the height of the foraging tree (FTH; m); and the decay status of the tree (DST). The decay status of the tree was divided into the following four categories: (1) living; (2) sick, with dead or yellow leaves or fungal presence; (3) recently dead, with a bark cover of 80%–90% or leafless; and (4) long-dead, with <80% bark cover. To determine the trees preferred by the woodpeckers as foraging sites, we assessed whether specific species were selective in their choice of foraging trees by comparing foraging trees (i.e., foraging species) and control trees (i.e., trees of similar size to foraging trees that were randomly selected in the same stand).
2.3. Data Analysis
To eliminate multicollinearity, Spearman’s correlation coefficient was used to evaluate the correlation between all variables. If there was a strong correlation between two variables (Spearman’s rank correlation; > 0.70), only the more important parameters for woodpeckers in the literature were selected for further analyses [41]. To determine which foraging behavior variables and foraging tree characteristics of the five species of woodpecker were significantly different, a multiclassification logistic regression (MLR) analysis was performed using the bird species as the dependent variables and variables other than the foraging duration as the independent variables. A stepwise regression method was adopted to optimize the model. All categorical variables in the independent variables were converted into factors. For variables entered into the optimal model, a likelihood ratio test was used for a significance test. The variables with a significant test were selected for the subsequent analysis.
Each foraging variable was assessed for differences between species. For variables measured as continuous data (i.e., DBH, FTH, and FOD), these were obtained using Mann–Whitney U tests (α level = 0.05). For the categorical variables (i.e., TRS, DST, FOT, FOH, and FOS), chi-squared tests (α level = 0.05) were used. To examine which variables were associated with the woodpecker foraging duration, a generalized linear mixed model (GLMM) with a gamma error distribution and log link function was developed. The following parameters were used in the analysis as fixed categorical explanatory variables: the TRS, FOH, FOS, FOT, and DST. DBH and FTH were used as continuous explanatory variables.
To compare the differences in the diversity of the trees that the woodpeckers fed on, we calculated the Shannon–Wiener index (H′) [42]. The selectivity of five woodpecker species to foraging tree species was analyzed using Bailey’s method based on the use available method principle [43]. Statistical analyses were performed using R version 4.1.2 software [44]. Measurements were assigned as the mean ± SE.
3. Results
A total of 477 foraging records for five woodpecker species and 477 control trees were collected, including 94 for the Black Woodpecker, 150 for the Great Spotted Woodpecker, 59 for the Lesser Spotted Woodpecker, 104 for the Three-toed Woodpecker, and 70 for the White-backed Woodpecker.
3.1. Separation of Foraging Niches of Five Woodpecker Species
According to Spearman’s correlation test, there was no strong correlation between all variables; thus, all variables were included in the subsequent analysis. The stepwise multinomial logistic regression results indicated that all seven variables involved in the analysis of the foraging tree characteristics and foraging behavior variables had significant effects on the separation of the foraging niches of the five woodpecker species (Table 1).

Table 1.
Stepwise multinomial logistic regression results of forage habitat factors of five woodpecker species in broadleaved Korean pine forests of Northeast China.
3.2. Differences in Foraging Behavior of Five Woodpecker Species
The proportional use of the height of a foraging tree for foraging significantly differed among species (χ2 = 66.2; df = 1; p < 0.001). All five woodpecker species foraged more often in the upper trunks of trees and rarely at a near-ground level (Figure 1). The Lesser Spotted Woodpecker rarely foraged in the lower trunks and demonstrated a clear preference for canopy-height foraging (38.6%) compared with the other birds (Black Woodpecker, 2.3%; Great Spotted Woodpecker, 9.7%; Three-toed Woodpecker, 11.7%; and White-backed Woodpecker, 8.2%). When comparing between-species pairs (Table 2), there were significant differences in foraging height between the Lesser Spotted Woodpecker and the other four species.
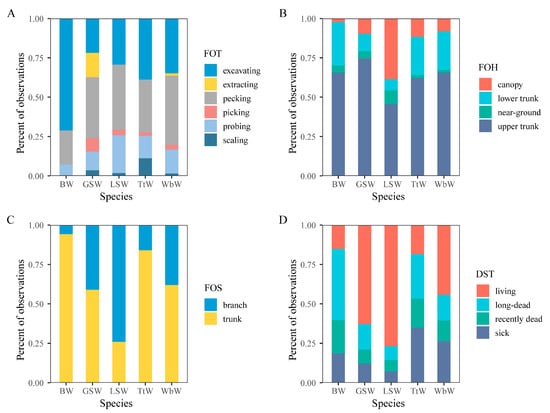
Figure 1.
Relationship between foraging frequency and foraging technique: (A), foraging height (B), foraging site (C), and decay status of trees (D) of five woodpecker species in broadleaved Korean pine forests of Northeast China. BW: Black Woodpecker (Dryocopus martius); GSW: Great Spotted Woodpecker (Dendrocopos major); LSW: Lesser Spotted Woodpecker (Dendrocopos minor); TtW: Three-toed Woodpecker (Picoides tridactylus); WbW: White-backed Woodpecker (Dendrocopos leucotos).

Table 2.
Inter-species differences between species pairs for foraging site (below diagonal) and foraging height (above diagonal) in broadleaved Korean pine forests of Northeast China. Figures indicate p-values from chi-squared tests. Differences were significant at α = 0.05 and are shown in bold.
An analysis of the foraging sites demonstrated clear differences between the five woodpeckers (χ2 = 92.2; df = 4; p < 0.001). Compared to the Lesser Spotted Woodpecker, the other four species of woodpecker made greater use of the trunks (Black Woodpecker, 94.3%; Great Spotted Woodpecker, 60.0%; Three-toed Woodpecker, 84.0%; and White-backed Woodpecker, 61.9%) (Figure 1). The Black Woodpecker was conspicuous in its preferential use of trunks. There was no difference between the Great Spotted Woodpecker and White-backed Woodpecker, but there was a significant difference between the other species pairs (Table 2).
The use of foraging techniques significantly differed between the five woodpecker species (χ2 = 122.6; df = 20; p < 0.001). The Black Woodpecker used only three foraging techniques; the most common was excavating (71.1%), followed by pecking (21.7%), and then intermittent probing (7.2%) (Figure 1). The Great Spotted Woodpecker used a variety of foraging techniques; the most common was pecking (38.7%), followed by excavating (21.8%) and extracting (15.5%). Extracting has only been observed once in the White-backed Woodpecker (1.5%). There were significant differences between species pairs, except for between the White-backed Woodpecker and the Lesser Spotted Woodpecker and between the White-backed Woodpecker and the Three-toed Woodpecker (Table 3). Pecking was the technique most used by the White-backed Woodpecker and Lesser Spotted Woodpecker (43.9% and 41.4%, respectively). The Three-toed Woodpecker mostly used excavating (38.8%) and pecking (33.7%), then intermittent probing (14.3%) and scaling (11.2%).

Table 3.
Inter-species differences between species pairs for foraging techniques (below diagonal) in broadleaved-Korean pine forests of Northeast China. Figures indicate p-values from chi-squared tests. Differences were significant at α = 0.05 and are shown in bold.
The foraging duration was significantly different among the five species of woodpecker (H = 67.9; df = 4; p < 0.001). The longest foraging duration was recorded for the Black Woodpecker (10.5 ± 1.5 min), followed by the Three-toed Woodpecker (7.1 ± 0.7 min); for the remaining three species of woodpeckers, the foraging duration was less than 5 min (Great Spotted Woodpecker, 3.7 ± 0.4 min; Lesser Spotted Woodpecker, 4.8 ± 0.6 min; and White-backed Woodpecker, 4.7 ± 0.7 min) (Figure 2). The foraging durations for the Black Woodpecker and Great Spotted Woodpecker were significantly different from all other species (Figure 2). The GLMM analysis indicated that the foraging duration was associated with the foraging techniques and the decay status of trees (Table 4).
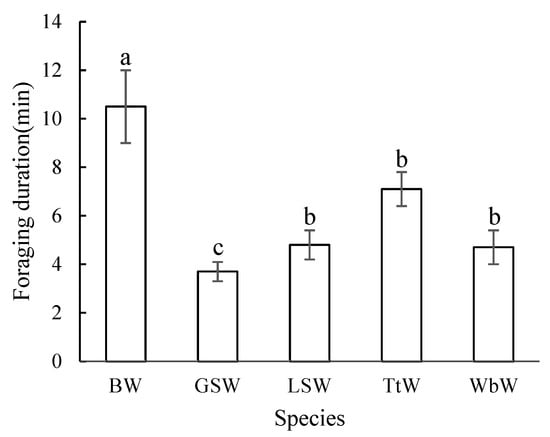
Figure 2.
Differences in foraging duration per tree among five woodpecker species in broadleaved Korean pine forests of Northeast China. Different letters above the boxes illustrate significant differences in the foraging duration of the five woodpecker species. BW: Black Woodpecker; GSW: Great Spotted Woodpecker; LSW: Lesser Spotted Woodpecker; TtW: Three-toed Woodpecker; WbW: White-backed Woodpecker.

Table 4.
GLMM analysis evaluating the influence of variables on the foraging duration of five woodpecker species in broadleaved Korean pine forests of Northeast China.
3.3. Differences in the Foraging Tree Characteristics of the Five Woodpecker Species
We observed significant differences in the frequency of the use of foraging tree species among the five woodpecker species (χ2 = 164.8; df = 24; p < 0.001). The Black Woodpecker preferred to forage on Khingan fir and avoided Korean pine and Xing’an larch trees, with random use of the other tree species (Table 5). The Great Spotted Woodpecker and Lesser Spotted Woodpecker demonstrated random use of foraging tree species. The Three-toed Woodpecker preferred scale spruce and Khingan fir; they avoided Japanese birch, Xing’an larch, and the other tree species. The White-backed Woodpecker preferred elm, avoided Xing’an larch trees, and demonstrated random use of the other tree species. There were significant differences between each species pair (Table 6). The Great Spotted Woodpecker (H′ = 2.03) and Lesser Spotted Woodpecker (H′ = 2.01) used the widest diversity of trees for foraging, followed by the White-backed Woodpecker (H′ = 1.75), Black Woodpecker (H′ = 1.34), and Three-toed Woodpecker (H′ = 1.08).

Table 5.
Selectivity in tree species used for foraging by five woodpecker species in broadleaved Korean pine forests of Northeast China.

Table 6.
Inter-species differences between species pairs for foraging tree species (below diagonal) and decay status of trees (above diagonal) in broadleaved Korean pine forests of Northeast China. Figures indicate p-values from chi-squared tests. Differences were significant at α = 0.05 and are shown in bold.
The five woodpecker species differed in the decay status of trees (χ2 = 111.1; df = 12; p < 0.001). The Black Woodpecker mostly used long-dead trees (45.3%) (Figure 1), and recently dead trees (20.9%). The Great Spotted Woodpecker and Lesser Spotted Woodpecker demonstrated similar choices in the different status of foraging trees, mainly foraging on live trees (62.9% and 76.8%, respectively), followed by dead trees (16.1% and 8.9%, respectively). The White-backed Woodpecker most frequently foraged on living trees (44.3%), followed by sick trees (26.2%). The Three-toed Woodpecker was often observed foraging on sick trees (34.8%) and long-dead trees (28.3%). There were significant differences among the other species pairs, except for one species pair (Great Spotted Woodpecker and Lesser Spotted Woodpecker) (Table 6).
We recorded tree diameters ranging from 5 cm to 107 cm. The foraging tree diameters were significantly different among the five woodpecker species (H = 20.8; df = 4; p < 0.001). The average diameter of the foraged trees of the Great Spotted Woodpecker was the largest (42.1 ± 1.8 cm), and was significantly larger than that of the other woodpeckers (Figure 3). There was no significant difference between the White-backed Woodpecker, Black Woodpecker, and Three-toed Woodpecker in the foraging tree diameters (35.5 ± 2.4, 35.5 ± 1.5 cm, and 33.4 ± 1.3 cm, respectively). The Lesser Spotted Woodpecker foraged on smaller-diameter trees (30.0 ± 1.8 cm). The five woodpecker species foraged on trees ranging in height from 3 to 30 m. The foraging tree height was significantly different among the species (H = 26.0; df = 4; p < 0.001). The mean height of foraging trees was higher for the Great Spotted Woodpecker (18.6 ± 0.3 m) and Black Woodpecker (17.4 ± 0.3 m), followed by the Three-toed Woodpecker (16.9 ± 0.5 m). The average heights of foraging trees for the White-backed Woodpecker and Lesser Spotted Woodpecker were 15.0 ± 0.6 m and 14.9 ± 0.6 m, respectively; these were significantly lower than those of the other woodpeckers (Figure 3).
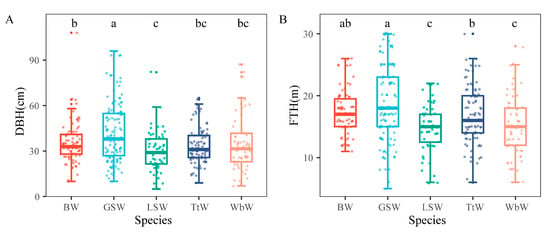
Figure 3.
Comparison of DBH (A) and FTH (B) among five woodpecker species in broadleaved Korean pine forests of Northeast China. See Figure 1 for species codes. In the boxplot, the boxes represent the middle 50% of scores, the horizontal lines represent the medians, observed values are represented using translucent dots, and whiskers include the range of distribution without outliers. Different letters above the boxes illustrate significant differences in DBH and FTH among the five woodpecker species. BW: Black Woodpecker; GSW: Great Spotted Woodpecker; LSW: Lesser Spotted Woodpecker; TtW: Three-toed Woodpecker; WbW: White-backed Woodpecker.
4. Discussion
We conducted a systematic analysis on the foraging behavior and foraging tree characteristics of five woodpecker species at Liangshui National Nature Reserve. The seven variables involved in the analysis of the foraging tree characteristics and foraging behavior variables had significant effects on the foraging niche separation of the five woodpecker species. We observed a notable differentiation in the overall foraging ecology of the five woodpecker species at Liangshui Reserve. Species pairs also demonstrated different degrees of differentiation along different ecological dimensions. The diameter of the foraging trees emerged as an important factor in niche segregation. The selection of the foraging tree species and the decay status of trees were important factors affecting the ecological niche separation of the five woodpecker species.
4.1. Multinomial Logistic Regression Results of Forage Habitat Factors of Five Woodpecker Species
In past studies, the methods of calculating the niche width and niche overlap index to analyze the degree of niche separation among species were used, and many important results were obtained [45,46]. However, in the calculation process of this kind of index, each variable is actually averaged, which may hide the information contained in the variable. We used multinomial logistic regression, to maximize the use of the information contained in the data and improve the sensitivity in identifying the niche differentiation. The results of multinomial logistic regression showed that the five woodpecker species had distinct foraging niche separation in terms of their foraging behavior and the characteristics of the foraging trees.
4.2. Differences in Foraging Behavior of the Five Woodpecker Species
The Black Woodpecker is a large woodpecker; it exhibited a high proportion of excavating, which was consistent with the foraging techniques primarily used by other larger woodpeckers [47]. Due to its large size, the Black Woodpecker requires greater energy to supplement its physical strength; thus, it must obtain large wood-living beetle larvae buried deep within the tree trunks by using long-term excavation [48,49]. Excavating and pecking allows the Black Woodpecker to expose the wood-living beetle larvae that live in the borehole. The other woodpeckers observed in our study fed using excavating and pecking in considerable proportion, suggesting that these two foraging techniques are the main techniques used by the woodpeckers at Liangshui Reserve. Studies have identified that woodpeckers can prey on Lepidoptera larvae and bark beetles by pecking them out of their galleries [50,51]. Many studies have identified that the Great Spotted Woodpecker can extract conifer seeds, especially when food is scarce [40]. We observed an occasional occurrence where a White-backed Woodpecker used extraction. So far, no studies have reported that the White-backed Woodpecker can extract seeds, so the possibility that it forages for pests inside the cone by chance cannot be ruled out. In winter, there is almost no food on the surface of plants; thus, the proportion of foraging using picking is relatively small. With the exception of the Lesser Spotted Woodpecker, the woodpeckers in our study fed more on the tree trunks. Compared with the trunk, the branches of trees are softer; these may be more suitable for the smaller, weaker beak of the Lesser Spotted Woodpecker. Charman (2012) et al. studied the foraging behavior of the Lesser Spotted Woodpecker in mature woodland blocks in England and observed that the small branches of live oak trees were most commonly used for foraging. This was consistent with our study [52].
The foraging duration of a single tree for woodpeckers may be related to the foraging techniques used by woodpeckers and the decay status of trees. The foraging duration of a single tree for species with a higher proportion of excavation was also longer, which means excavating takes longer. According to our field observations, the Black Woodpecker could form large and deep burrows when excavating; this required the longest time, which was consistent with published studies [30]. Trees that are in the process of decline may have more abundant prey inside, making it take longer for woodpeckers to forage.
All five species rarely forage near the ground, one possible reason being snow cover, where there may be a lack of food, and another possible reason being to avoid predators. A high stratification of foraging maximizes the utilization of resources by birds, and the vertical stratification of foraging by woodpeckers has also been noted in European and North American studies [31,53,54]. Hogstad (1971) demonstrated that the Great Spotted Woodpecker used the higher parts of trees for foraging more often than the Three-toed Woodpecker, which was consistent with our study [30]. Kumar (2020) et al. identified that foraging height was an important differentiation factor among sub-Himalayan woodpeckers [13]. All these results suggest that particular woodpeckers use different techniques or collect food in different locations to rationalize resources to achieve coexistence.
4.3. Differences in Foraging Tree Characteristics of Five Woodpecker Species
The five species of woodpecker demonstrated different preferences for the foraging of trees. The Black Woodpecker and Three-toed Woodpecker demonstrated a preference for fir and spruce; both are birds from northern spruce and fir forests [55]. Their main diet is the larvae of wood and bark-boring beetles, which are found in abundance in spruce and fir [56]. The Great Spotted Woodpecker and Lesser Spotted Woodpecker randomly used different tree species for foraging. Michalek and Miettinen (2003) identified that the Great Spotted Woodpecker is an omnivorous bird; its foraging techniques and food collection sites are extensive. Other studies have observed that the White-backed Woodpecker forages on broadleaved trees such as elm, poplar, and birch [57,58].
Dead wood is considered to be an important resource for many woodpecker species. During its decay, various wood-boring arthropods inhabit various layers of the dead wood, providing a food resource for birds [55,59,60]. Many studies have observed that the Black Woodpecker and Three-toed Woodpecker often forage on dead and soft wood that is relatively easy to excavate [61,62], which was consistent with our results. The Great Spotted Woodpecker and the Lesser Spotted Woodpecker use living trees more frequently. Previous studies have demonstrated that they are often associated with living trees [63,64]. We observed that the White-backed Woodpecker made use of both live and dead trees. Similar to our results, Czeszczewik (2009) demonstrated that woodpeckers mainly ate dead trees or the dead parts of live trees [58]. Compared with the other woodpeckers, the Great Spotted Woodpecker chose to forage on trees with a larger DBH and higher trees. The Black Woodpecker chose to forage on trees with a larger DBH. Other studies have also demonstrated segregation among woodpeckers by foraging substrate size [65,66]. Although woodpeckers use different-sized substrates differently, large trees are important for each species of woodpecker. Kumar (2014) et al. noted that an abundance of large trees is an important determinant of woodpecker diversity [67]. Larger trees can provide hidden foraging conditions for woodpeckers, which reduces the risk of predation.
5. Conclusions
Our results demonstrated that the Great Spotted Woodpecker and the Lesser Spotted Woodpecker had a wider range of foraging sites and preferred to forage on live trees, but the Great Spotted Woodpecker preferred larger trees. The Black Woodpecker and Three-toed Woodpecker excavated and pecked more the trunks of decaying spruce and fir trees. The White-backed Woodpecker preferred to forage on broadleaved trees. The selection of foraging locations was also complicated. These results suggest that to achieve stable coexistence, there was a significant niche differentiation among the woodpecker species at Liangshui Reserve. This differentiation may be a potential mechanism for woodpecker community coexistence in northern broadleaved Korean pine forests. The factors that cause this separation remain to be studied, and they may also be related to resource availability, such as nesting and roosting sites, as well as food abundance. Further collection of these data is needed to explain the mechanisms of woodpecker community coexistence in detail to protect the diversity of woodpecker species, as well as the diversity of tree species in forests, preserving trees of different diameters, as well as dead wood.
Author Contributions
Conceptualization, Y.S., H.Z. (Hongfei Zou) and K.R.; methodology, Y.S. and K.R.; software, Y.S., D.M. and H.Z. (Han Zhong); validation, D.M., Z.Z. and H.Z. (Han Zhong); formal analysis, Y.S., D.M. and Z.Z.; investigation, Y.S., H.Z. (Hongfei Zou) and K.R.; resources, H.Z. (Hongfei Zou) and K.R.; data curation, Y.S. and D.M.; writing—original draft preparation, Y.S.; writing—review and editing, Y.S. and D.M.; visualization, Y.S., D.M. and K.R.; supervision, H.Z. (Hongfei Zou) and K.R.; project administration, K.R.; funding acquisition, Y.S. and K.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities, China, (2572021AW03) and the National Natural Science Foundation of China (No. 31970385).
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. The data are not publicly available due to they involve other unpublished studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amarasekare, P.; Hoopes, M.; Mouquet, N.; Holyoak, M. Mechanisms of coexistence in competitive metacommunities. Am. Nat. 2004, 164, 310–326. [Google Scholar] [CrossRef]
- Johnson, C.A.; Bronstein, J.L. Coexistence and competitive exclusion in mutualism. Ecology 2019, 100, e02708. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of Maintenance of Species Diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Leibold, M.A.; Mcpeek, M.A. Coexistence of the niche and neutral perspectives in community ecology. Ecology 2006, 87, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Gause, G.F. The Struggle for Existence; The Williams & Wilkins Company: Baltimore, MD, USA, 1934. [Google Scholar]
- Churchfield, S.; Rychlik, L. Diets and coexistence in Neomys and Sorex shrews in Bialowieza forest, eastern Poland. J. Zool. 2006, 269, 381–390. [Google Scholar] [CrossRef]
- Mckane, R.B.; Johnson, L.C.; Shaver, G.R.; Nadelhoffer, K.J.; Rastetter, E.B.; Fry, B.; Giblin, A.E.; Kielland, K.; Kwiatkowski, B.L.; Laundre, J.A. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 2002, 415, 68–71. [Google Scholar] [CrossRef]
- Rothhaupt, K.O.; Hanselmann, A.J.; Yohannes, E. Niche differentiation between sympatric alien aquatic crustaceans: An isotopic evidence. Basic Appl. Ecol. 2014, 15, 453–463. [Google Scholar] [CrossRef]
- Hogstad, O. Differentiation of foraging niche among tits, Parus spp. in norway during winter. IBIS 2010, 120, 139–146. [Google Scholar] [CrossRef]
- Hoskins, A.J.; Schumann, N.; Costa, D.P.; Arnould, J.P.Y. Foraging niche separation in sympatric temperate-latitude fur seal species. Mar. Ecol. Prog. Ser. 2017, 566, 229–241. [Google Scholar] [CrossRef]
- Gbemiga, A.E. Foraging Ecology and Resource Partitioning among Palearctic Migrants and Resident Bird Species in Northern Ghana. Master Thesis, University of Ghana, Accra, Ghana, 2014. [Google Scholar]
- Krams, I.A. Predation Risk and Shifts of Foraging Sites in Mixed Willow and Crested Tit Flocks. J. Avian Biol. 1996, 27, 153–156. [Google Scholar] [CrossRef]
- Kumar, R.; Shahabuddin, G.; Kumar, A. Foraging niche differentiation among sympatric woodpecker species in forests of north-western India. Acta Ornithol. 2020, 55, 88–100. [Google Scholar] [CrossRef]
- Versluijs, M.; Mikusiński, G.; Roberge, J.-M. Foraging behaviour of the Eurasian Three-toed Woodpecker Picoides tridactylus in its peak abundance after wildfire. Ardea 2022, 110, 1–14. [Google Scholar] [CrossRef]
- Lemaître, J.; Villard, M.-A. Foraging patterns of pileated woodpeckers in a managed Acadian forest: A resource selection function. Can. J. For. Res. 2005, 35, 2387–2393. [Google Scholar] [CrossRef]
- Mangini, G.; Thomas, O. Observations on foraging behaviour of Rufous-headed Woodpecker Celeus spectabilis in the Ecuadorian Amazon. Cotinga 2020, 42, 73–76. [Google Scholar]
- Cramp, S.; Simmons, K. The birds of the western palearctic: Terns to woodpeckers. In Handbook of the Birds of Europe, the Middle East and North Africa; Oxford University Press: Oxford, UK, 1988; Volume 4. [Google Scholar]
- Delhey, K. Nest webs beyond woodpeckers: The ecological role of other nest builders. Ecology 2018, 99, 985–988. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Schachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Aitken, K.E.; Martin, K. The importance of excavators in hole-nesting communities: Availability and use of natural tree holes in old mixed forests of western Canada. J. Ornithol. 2007, 148, 425–434. [Google Scholar] [CrossRef]
- Martin, K. Nest webs and woodpecker ecological services: The role of woodpeckers in tree cavity-using wildlife communities in North America. Denisia 2015, 36, 77–86. [Google Scholar]
- Perumal, M.; Paramasivam, B. Sequential Use of Tree Cavities by Birds and Nest Web in a Riparian Forest in Southwest India. Acta Ornithol. 2018, 53, 48–60. [Google Scholar]
- Drever, M.C.; Aitken, K.E.; Norris, A.R.; Martin, K. Woodpeckers as reliable indicators of bird richness, forest health and harvest. Biol. Conserv. 2008, 141, 624–634. [Google Scholar] [CrossRef]
- Menon, T.; Shahabuddin, G. Assessing woodpeckers as indicators of bird diversity and habitat structure in managed forests. Biodivers. Conserv. 2021, 30, 1689–1704. [Google Scholar] [CrossRef]
- Jackson, J.A.; Jackson, B.J. Ecological relationships between fungi and woodpecker cavity sites. Condor 2004, 106, 37–49. [Google Scholar] [CrossRef]
- Ilsøe, S.K.; Kissling, W.D.; Fjeldså, J.; Sandel, B.; Svenning, J.C. Global variation in woodpecker species richness shaped by tree availability. J. Biogeogr. 2017, 44, 1824–1835. [Google Scholar] [CrossRef]
- St-Amand, J.; Tremblay, J.A.; Martin, K. Stand-level forest management for foraging and nesting of Williamson’s sapsuckers. For. Ecol. Manag. 2021, 492, 119223. [Google Scholar] [CrossRef]
- Pasinelli, G. Nest site selection in middle and great spotted woodpeckers Dendrocopos medius & D. major: Implications for forest management and conservation. Biodivers. Conserv. 2007, 16, 1283–1298. [Google Scholar] [CrossRef]
- Winkler, H.; Christie, D.A. Family Picidae (woodpeckers). In Handbook of the Birds of the World: Jacamars to Woodpeckers; del Hoyo, J., Elliot, A., Sargatal, J., Eds.; Lynx Edicions: Barcelona, Spain, 2002; Volume 7, pp. 296–555. [Google Scholar]
- Rong, K.; Si, Y.H.; Pan, Q.Y.; Wang, H. Forage niche differentiation of three sympatric woodpecker species in winter. Acta Ecol. Sin. 2018, 38, 8314–8323. [Google Scholar]
- Hogstad, O. Stratification in Winter Feeding of the Great Spotted Woodpecker Dendrocopos major and the Three-Toed Woodpecker Picoides tridactylus. Ornis Scand. 1971, 2, 143–146. [Google Scholar] [CrossRef]
- Bull Evelyn, L.; Peterson, S.R.; Thomas, J.W. Resource Partitioning among Woodpeckers in Northeastern Oregon; Res. Note PNW-RN-444; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1986; 20p. [Google Scholar] [CrossRef]
- Zheng, G.M. Taxonomy and Distribution of Birds in China, 3rd ed.; Science Press: Beijing, China, 2017. [Google Scholar]
- Sun, Y.M.; Ao, Z.Q.; Jia, W.W.; Chen, Y.; Xu, K. A geographically weighted deep neural network model for research on the spatial distribution of the down dead wood volume in Liangshui National Nature Reserve (China). iForest-Biogeosciences For. 2021, 14, 353–361. [Google Scholar] [CrossRef]
- Zhen, Z.; Li, F.R.; Liu, Z.G.; Liu, C.; Zhao, Y.L.; Ma, Z.H.; Zhang, L.J. Geographically local modeling of occurrence, count, and volume of downwood inNortheast China. Appl. Geogr. 2013, 37, 114–126. [Google Scholar] [CrossRef]
- Ma, J.Z.; Zong, C.; Wu, Q.M.; Zou, H.F.; Zheng, X. Hoarding habitat selection of squirrels (Sciurus vulgaris) in Liangshui Nature Reserve, China. Acta Ecol. Sin. 2006, 26, 3542–3548. [Google Scholar] [CrossRef]
- Pechacek, P. Foraging Behavior of Eurasian Three-Toed Woodpeckers (Picoides Tridactylus Alpinus) in Relation to Sex and Season in Germany. Auk 2006, 123, 235–246. [Google Scholar] [CrossRef]
- Swihart, R.K.; Slade, N.A. Testing For Independence of Observations in Animal Movements. Ecology 1985, 66, 1176–1184. [Google Scholar] [CrossRef]
- Remsen, J.V.; Robinson, S.K. A classification scheme for foraging behavior of birds in terrestrial habitats. Stud. Avian Biol. 1990, 13, 144–160. [Google Scholar]
- Stański, T.; Czeszczewik, D.; Stańska, M.; Walankiewicz, W. Anvils of the Great Spotted Woodpecker (Dendrocopos major) in primeval oak-lime-hornbeam stands of the Białowieża National Park. Eur. Zool. J. 2021, 88, 1–8. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; Wiley: New York, NY, USA, 2000; p. 159. [Google Scholar]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Rong, K.; Zong, C.; Ma, J. A Method for Analysis of Habitat Selection Data: Bailey’s Interval. Zool. Res. 2009, 30, 215–220. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 1 May 2023).
- Chazarreta, L.; Ojeda, V.; Lammertink, M. Morphological and foraging behavioral differences between sexes of the Magellanic Woodpecker (Campephilus magellanicus). Ornitol. Neotrop. 2012, 23, 529–544. [Google Scholar]
- Stenberg, I.; Hogstad, O. Sexual dimorphism in relation to winter foraging in the White-backed Woodpecker (Dendrocopos leucotos). J. Ornithol. 2004, 145, 321–326. [Google Scholar] [CrossRef]
- Newell, P.; King, S.; Kaller, M. Foraging behavior of Pileated Woodpeckers in partial cut and uncut bottomland hardwood forest. For. Ecol. Manag. 2009, 258, 1456–1464. [Google Scholar] [CrossRef]
- Rolstad, J.; Rolstad, E. Influence of large snow depths on Black Woodpecker Dryocopus martius foraging behavior. Ornis Fenn. 2000, 77, 65–70. [Google Scholar] [CrossRef]
- Rolstad, J.; Majewski, P.; Rolstad, E. Black Woodpecker use of habitats and feeding substrates in a managed Scandinavian forest. J. Wildl. Manag. 1998, 62, 11–23. [Google Scholar] [CrossRef]
- Allegro, G. Il Picchio rosso maggiore (Picoides major) nella limitazione naturale delle popolazioni della Saperda maggiore del pioppo (Saperda carcharias). Avocetta 1991, 15, 33–41. [Google Scholar]
- Kotliar, N.B.; Reynolds, E.W.; Deutschman, D.H. American Three-toed Woodpecker response to burn severity and prey availability at multiple spatial scales. Fire Ecol. 2008, 4, 26–45. [Google Scholar] [CrossRef]
- Charman, E.C.; Smith, K.W.; Dodd, S.; Gruar, D.J.; Dillon, I.A. Pre-breeding foraging and nest site habitat selection by Lesser Spotted Woodpeckers Dendrocopos minor in mature woodland blocks in England. Ornis Fenn. 2012, 89, 182–196. [Google Scholar] [CrossRef]
- Villard, P. Foraging behavior of Black-backed and Three-toed woodpeckers during spring and summer in a Canadian boreal forest. Can. J. Zool. 1994, 72, 1957–1959. [Google Scholar] [CrossRef]
- Stański, T.; Czeszczewik, D.; Stańska, M.; Walankiewicz, W. Foraging behaviour of the Great Spotted Woodpecker Dendrocopos major in relation to sex in primeval stands of the Białowieża National Park. Acta Ornithol. 2020, 55, 120–128. [Google Scholar] [CrossRef]
- Spiering, D.J. Woodpeckers at Tifft Nature Preserve (and beyond): A brief review of the habitats and conservation of the woodpeckers in eastern North America. Bull. Buffalo Soc. Nat. Sci. 2009, 38, 55–66. [Google Scholar]
- Werner, R.A.; Holsten, E.H.; Matsuoka, S.M.; Burnside, R.E. Spruce beetles and forest ecosystems in south-central Alaska: A review of 30 years of research. For. Ecol. Manag. 2006, 227, 195–206. [Google Scholar] [CrossRef]
- Aulén, G.; Lundberg, A. Sexual Dimorphism and Patterns of Territory Use by the White-Backed Woodpecker Dendrocopus leucotos. Ornis Scand. 1991, 22, 60–64. [Google Scholar] [CrossRef]
- Czeszczewik, D. Foraging behaviour of White-backed Woodpeckers Dendrocopos leucotos in a primeval forest (Białowieża National Park, NE Poland): Dependence on habitat resources and season. Acta Ornithol. 2009, 44, 109–118. [Google Scholar] [CrossRef]
- Nappi, A.; Drapeau, P.; Leduc, A. How important is dead wood for woodpeckers foraging in eastern North American boreal forests? For. Ecol. Manag. 2015, 346, 10–21. [Google Scholar] [CrossRef]
- Aszalós, R.; Szigeti, V.; Harmos, K.; Csernák, S.; Frank, T.; Ónodi, G. Foraging activity of woodpeckers on various forms of artificially created deadwood. Acta Ornithol. 2020, 55, 63–76. [Google Scholar] [CrossRef]
- Versluijs, M.; Eggers, S.; Mikusiński, G.; Roberge, J.M.; Hjältén, J. Foraging behavior of the Eurasian Three-toed Woodpecker (Picoides tridactylus) and its implications for ecological restoration and sustainable boreal forest management. Avian Conserv. Ecol. 2020, 15, 6. [Google Scholar] [CrossRef]
- Olano, M.; Aierbe, T.; Beñaran, H.; Hurtado, R.; Ugarte, J.; Urruzola, A.; Vázquez, J.; Ansorregi, F.; Galdos, A.; Gracianteparaluceta, A. Black woodpecker Dryocopus martius (L., 1758) distribution, abundance, habitat use and breeding performance in a recently colonized region in SW Europe. Munibe Cienc. Nat. 2015, 63, 2172–4547. [Google Scholar] [CrossRef]
- Ónodi, G.; Csörgö, T. Habitat Preference of Great-Spotted Woodpecker (Dendrocopos major Linnaeus, 1758) and Lesser-Spotted Woodpecker (Dendrocopos minor Linnaeus, 1758) in the Presence of Invasive Plant Species-Preliminary Study. Ornis Hung. 2014, 22, 50–64. [Google Scholar] [CrossRef]
- Charman, E.C.; Smith, K.W.; Gruar, D.J.; Dodd, S.; Grice, P.V. Characteristics of woods used recently and historically by Lesser Spotted Woodpeckers Dendrocopos minor in England. IBIS 2010, 152, 543–555. [Google Scholar] [CrossRef]
- Figarski, T.; Kajtoch, Ł. Differences in Habitat Requirements between Two Sister Dendrocopos Woodpeckers in Urban Environments: Implication for the Conservation of Syrian Woodpecker. Acta Ornithol. 2018, 53, 23–36. [Google Scholar] [CrossRef]
- Fernández, J.M.; Areta, J.I.; Lammertink, M. Does foraging competition drive plumage convergence in three look-alike Atlantic Forest woodpecker species? J. Ornithol. 2020, 161, 1105–1116. [Google Scholar] [CrossRef]
- Kumar, R.; Shahabuddin, G.; Kumar, A. Habitat Determinants of Woodpecker Abundance and Species Richness in Sub-Himalayan Dipterocarp Forests of North-West India. Acta Ornithol. 2014, 49, 243–256. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).