Impact of Simulated Acid Rain on Soil Base Cations Dissolution between Eucalyptus Pure Plantations and Eucalyptus–Castanopsis fissa Mixed Plantations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site
2.2. Sampling Method
2.3. Experimental Design
2.3.1. Experimental Preparation
2.3.2. Soil Column Leaching Experiment
2.3.3. Isothermal Adsorption and Desorption of Base Cations in Soil
2.4. Data Analysis
3. Results
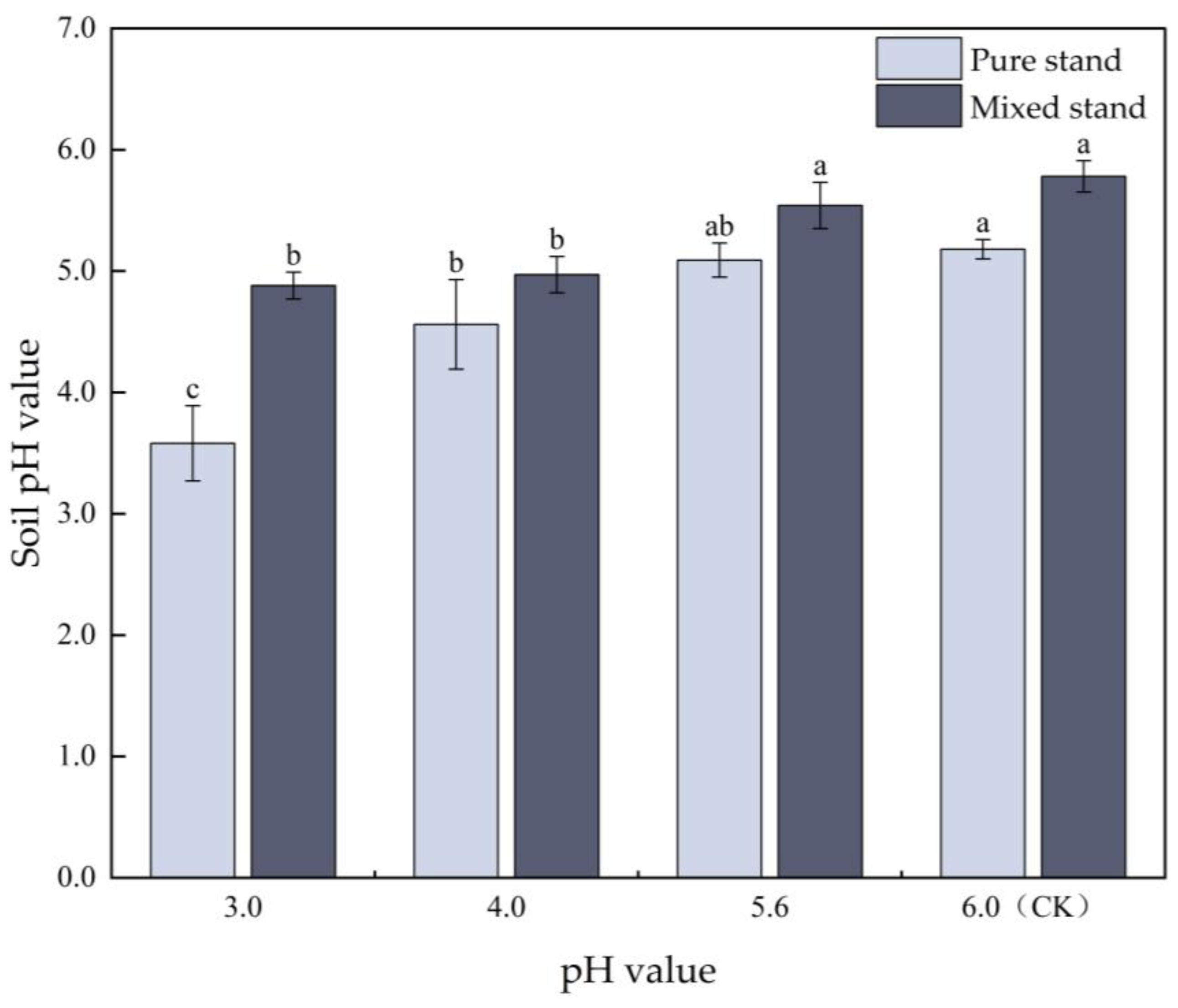
3.1. Effects of Simulated Acid Deposition on the Changes in Soil pH Value in Pure and Mixed Eucalyptus Plantations
3.2. Effects of Simulated Acid Deposition on the Content of Soil Base Cations in Pure and Mixed Eucalyptus Plantations
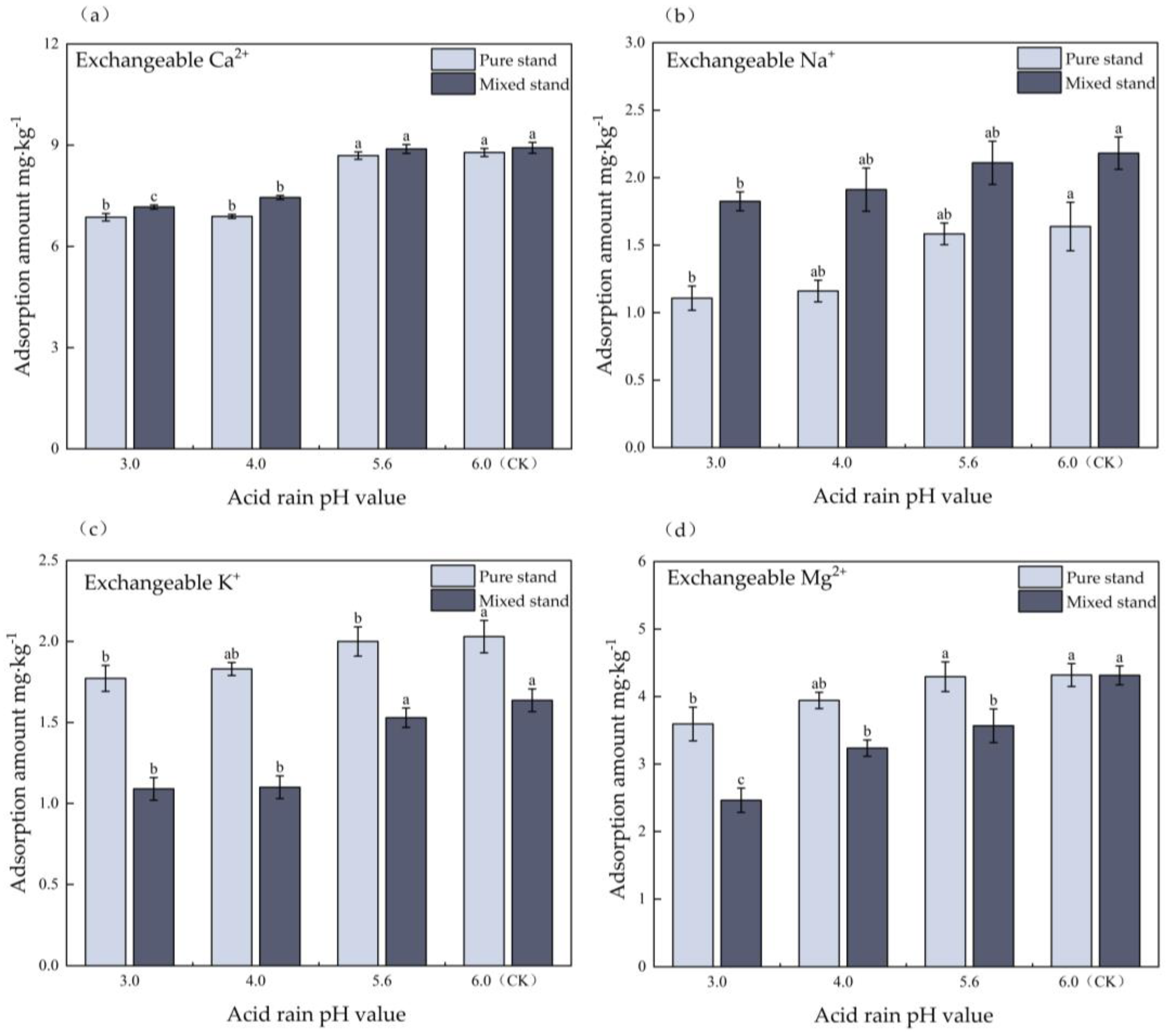
3.3. Effects of Simulated Acid Deposition on Isothermal Adsorption of Soil Base Cations in Pure and Mixed Eucalyptus Plantations
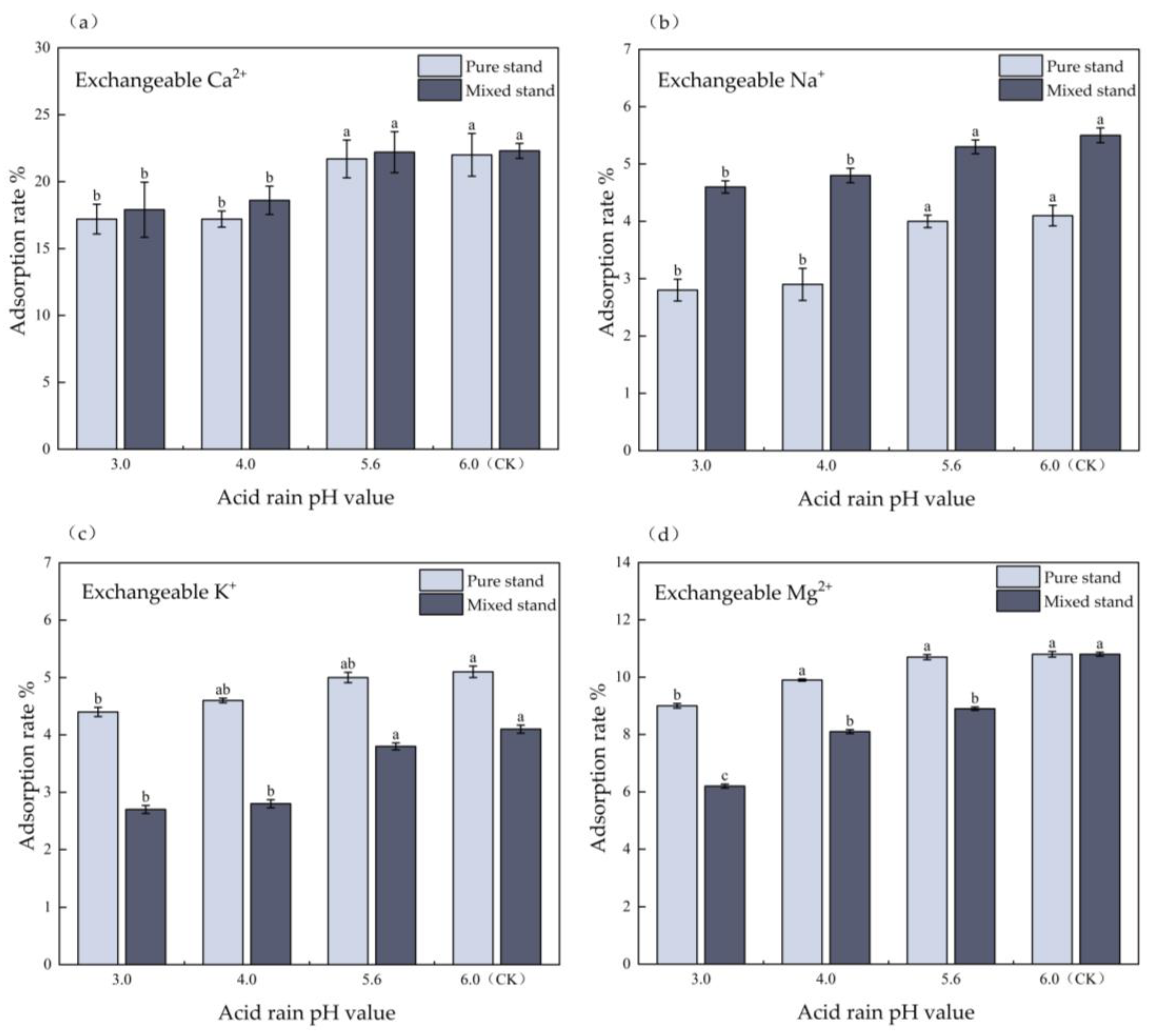
3.4. Effects of Simulated Acid Deposition on Adsorption Rate of Soil Base Cations in Pure and Mixed Eucalyptus Plantations
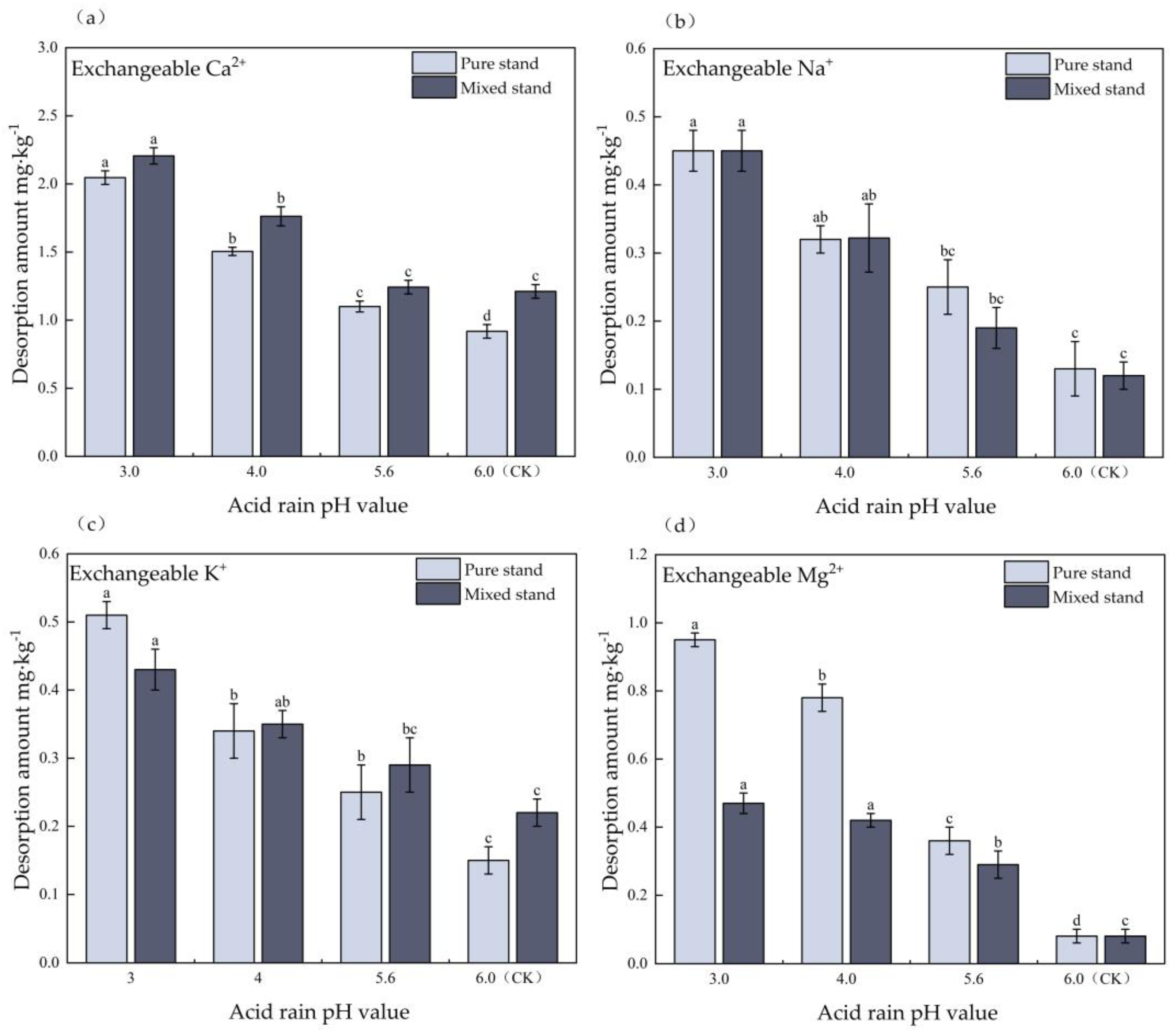
3.5. Effect of Simulated Acid Deposition on Isothermal Desorption of Base Cations in Pure and Mixed Eucalyptus Plantations
3.6. Effect of Simulated Acid Deposition on the Average Desorption Rate of Base Cations in the Soil of Eucalyptus Plantations
4. Discussion
4.1. Influence of Acid Rain on the Soil Acidity of Eucalyptus and Castanopsis fissa Mixed Plantations
4.2. Effect of Soil Salt Ions on Acid Rain Buffering in Pure Eucalyptus Forests and Mixed Forests
4.3. Characteristics of Adsorption and Desorption of Soil Base Ions in Pure and Mixed Eucalyptus Forests under Acid Deposition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Shan, X.R.; Shi, Z.J.; Zhang, J.E.; Qin, Z.; Xiang, H.M.; Wei, H. Analysis of the Spatio-temporal Changes in Acid Rain and Their Causes in China (1998–2018). J. Resour. Ecol. 2021, 12, 593–599. [Google Scholar]
- Ministry of Ecology and Environment of the People’s Republic of China. Report on the State of the Ecological Environment in China; [EB/OL]; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2022. [Google Scholar]
- Debnath, B.; Irshad, M.; Mitra, S.; Li, M.; Rizwan, H.M.; Liu, S.; Pan, T.; Qiu, D. Acid Rain Deposition Modulates Photosynthesis, Enzymatic and Non-enzymatic Antioxidant Activities in Tomato. Int. J. Environ. Res. 2018, 12, 203–214. [Google Scholar] [CrossRef]
- Johnson, J.; Pannatier, E.G.; Carnicelli, S.; Cecchini, G.; Clarke, N.; Cools, N.; Hansen, K.; Meesenburg, H.; Nieminen, T.M.; Pihl-Karlsson, G.; et al. The response of soil solution chemistry in European forests to decreasing acid deposition. Glob. Chang. Biol. 2018, 24, 3603–3619. [Google Scholar] [CrossRef] [PubMed]
- Sardar, M.F.; Younas, F.; Farooqi, Z.U.R.; Li, Y. Soil nitrogen dynamics in natural forest ecosystem: A review. Front. For. Glob. Change 2023, 6, 1144930. [Google Scholar] [CrossRef]
- Li, J.; Tong, X.; Awasthi, M.K.; Wu, F.; Ha, S.; Ma, J.; Sun, X.; He, C. Dynamics of soil microbial biomass and enzyme activities along a chronosequence of desertified land revegetation. Ecol. Eng. 2018, 111, 22–30. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, J.; Zhou, Y.; Li, S.; Peng, Y.; Chen, G. Influence of simulated acid rain on the spatial distribution of nutrients, Cu, and Zn in mangrove sediments. Acta Ecol. Sin. 2016, 36, 6209–6217. [Google Scholar]
- Ren, X.; Zhu, J.; Liu, H.; Xu, X.; Liang, C. Response of antioxidative system in rice (Oryza sativa) leaves to simulated acid rain stress. Ecotoxicol. Environ. Saf. 2018, 148, 851–856. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, W. Impact of simulated acid rain on the composition of soil microbial communities and soil respiration in typical subtropical forests in Southwest China. Ecotoxicol. Environ. Saf. 2021, 215, 112152. [Google Scholar] [CrossRef]
- Liang, G.; Hui, D.; Wu, X.; Wu, J.; Liu, J.; Zhou, G.; Zhang, D. Effects of simulated acid rain on soil respiration and its components in a subtropical mixed conifer and broadleaf forest in southern China. Environ. Sci. Process. Impacts 2016, 18, 246–255. [Google Scholar] [CrossRef]
- Raben, G.; Andreae, H.; Meyer-Heisig, M. Long-term acid load and its consequences in forest ecosystems of Saxony (Germany). Water Air Soil Pollut. 2000, 122, 93–103. [Google Scholar] [CrossRef]
- Tomlinson, G.H. A possible mechanism relating increased soil temperature to forest decline. Water Air Soil Pollut. 1993, 66, 365–380. [Google Scholar] [CrossRef]
- Ullah, S.; Xu, Y.; Liao, C.; Li, W.; Cheng, F.; Ye, S.; Yang, M. Continuous planting Eucalyptus plantations in subtropical China: Soil phenolic acid accumulation and adsorption physiognomies. Front. For. Glob. Chang. 2023, 6, 1135029. [Google Scholar] [CrossRef]
- Wei, J.; Wang, C.; He, B.; You, Y.; Huang, X. Research progress on soil microorganisms in eucalypt forests. J. Zhejiang Af Univ. 2022, 39, 1144–1154. [Google Scholar]
- Jaiyeoba, I.A. Changes in soil properties related to conversion of savannah woodland into pine and Eucalyptus plantations, northern Nigeria. Land Degrad. Dev. 1998, 9, 207–215. [Google Scholar] [CrossRef]
- Grillo, G.; Tabasso, S.; Cravotto, G.; Ree, T.V. Burning Biomass: Environmental Impact on the Soil; Springer: Dordrecht, The Netherlands, 2020. [Google Scholar]
- Neris Lino, I.A.; dos Santos, V.M.; Costa Escobar, I.E.; Alves da Silva, D.K.; Ferreira de Araujo, A.S.; Maia, L.C. Soil Enzymatic Activity in Eucalyptus Grandis Plantations of Different Ages. Land Degrad. Dev. 2016, 27, 77–82. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.; Li, X.; Wu, K.; Sun, J. Impact of simulated acid rain on growth and nutrient elements uptake by Eucalyptus urophylla and Pinus massoniana. Ecol. Environ. 2006, 15, 331–336. [Google Scholar]
- Yu, Y.C.; Ding, A.F.; Hu, J.; Meng, L. Effects of Simulated Acid Rain on Soil Acidification and Base Ions Transplant. J. Nanjing For. Univ. 2001, 44, 39. [Google Scholar]
- Mei, Y.; Xiaolu, H.; Wuming, Q.I.N.; Bin, H.E. Effect of planting conditions on soil nutrient contents in Eucalyptus plantations. J. Fujian Coll. For. 2011, 31, 234–238. [Google Scholar]
- Xu, Y.X.; Ren, S.Q.; Liang, Y.F.; Du, A.; Li, C.; Wang, Z.C.; Zhu, W.K.; Wu, L.C. Soil nutrient supply and tree species drive changes in soil microbial communities during the transformation of a multi-generation Eucalyptus plantation. Appl. Soil Ecol. 2021, 166, 103991. [Google Scholar] [CrossRef]
- Ai-Zhen, C. Spatial and Temporal Distribution and Seasonal Variation Analysis in Guangxi. J. Anhui Agric. Sci. 2010, 38, 4683–4685. [Google Scholar]
- Ren, S.; Xiang, D.; Xiao, W.; Chen, J.; Liang, Y.; Yang, Z.; Tang, Q.; Wu, Q. Rainfall redistribution of Eucalypt plantation in Nanning, Guangxi, China. Chin. J. Ecol. 2017, 36, 1473–1480. [Google Scholar]
- Tóth, J.A.; Nagy, P.T.; Krakomperger, Z.; Veres, Z.; Kotroczó, Z.; Kincses, S.; Fekete, I.; Papp, M.; Lajtha, K. Effect of Litter Fall on Soil Nutrient Content and pH, and its Consequences in View of Climate Change (Síkfőkút DIRT Project). Acta Silv. Lignaria Hung. 2011, 7, 75–86. [Google Scholar]
- Wei, H.; Liu, Y.; Xiang, H.; Zhang, J.; Li, S.; Yang, J. Soil pH Responses to Simulated Acid Rain Leaching in Three Agricultural Soils. Sustainability 2020, 12, 280. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, X.; Wu, J.; Xu, J. Impacts of simulated acid rain on recalcitrance of two different soils. Environ. Sci. Pollut. Res. 2013, 20, 4216–4224. [Google Scholar] [CrossRef] [PubMed]
- Xinming, Z.; Junping, Z.; Suping, L.I.U.; Changwei, W.; Yanhong, L.I.U. Effects of Simulated Acid Rain on Nitrogen Transplant and Acidifying Potential in Litchi Orchard Soils. J. Soil Water Conserv. 2006, 20, 18–21. [Google Scholar]
- Muhammad, N.; Zvobgo, G.; Zhang, G.-P. A review: The beneficial effects and possible mechanisms of aluminum on plant growth in acidic soil. J. Integr. Agric. 2021, 18, 1518–1528. [Google Scholar] [CrossRef]
- Bakhshipour, Z.; Asadi, A.; Huat, B.B.K.; Sridharan, A.; Kawasaki, S. Effect of acid rain on geotechnical properties of residual soils. Soils Found. 2016, 56, 1008–1020. [Google Scholar] [CrossRef]
- Gratchev, I.; Towhata, I. Compressibility of natural soils subjected to long-term acidic contamination. Environ. Earth Sci. 2011, 64, 193–200. [Google Scholar] [CrossRef]
- Noble, A.D.; Zenneck, I.; Randall, P.J. Leaf litter ash alkalinity and neutralisation of soil acidity. Plant Soil 1996, 179, 293–302. [Google Scholar] [CrossRef]
- Dong, X.; Gao, P.; Zhou, R.; Li, C.; Dun, X.; Niu, X. Changing characteristics and influencing factors of the soil microbial community during litter decomposition in a mixed Quercus acutissima Carruth. and Robinia pseudoacacia L. forest in Northern China. Catena 2021, 196, 104811. [Google Scholar] [CrossRef]
- Ukonmaanaho, L.; Starr, M.; Lindroos, A.-J.; Nieminen, T.M. Long-term changes in acidity and DOC in throughfall and soil water in Finnish forests. Environ. Monit. Assess. 2014, 186, 7733–7752, Erratum in Environ. Monit. Assess. Environ. Monit. Assess. 2014, 186, 7753. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Wu, J.; Xiong, X.; Wu, X.; Chu, G.; Zhou, G.; Zeng, R.; Zhang, D. Responses of Soil pH Value and Soil Microbial Biomass Carbon and Nitrogen to Simulated Acid Rain in Three Successional Subtropical Forests at Dinghushan Nature Reserve. Ecol. Environ. Sci. 2015, 24, 911–918. [Google Scholar]
- Ng, J.F.; Ahmed, O.H.; Jalloh, M.B.; Omar, L.; Kwan, Y.M.; Musah, A.A.; Poong, K.H. Soil Nutrient Retention and pH Buffering Capacity Are Enhanced by Calciprill and Sodium Silicate. Agronomy 2022, 12, 219. [Google Scholar] [CrossRef]
- Watmough, S.A.; Dillon, P.J. Major element fluxes from a coniferous catchment in central Ontario, 1983–1999. Biogeochemistry 2004, 67, 369–398. [Google Scholar] [CrossRef]
- Reuss, J.O.; Cosby, B.J.; Wright, R.F. Chemical processes governing soil and water acidification. Nature 1987, 329, 27–32. [Google Scholar] [CrossRef]
- Nawaz, R.; Parkpian, P.; Garivait, H.; Anurakpongsatorn, P.; DeLaune, R.D.; Jugsujinda, A. Impacts of Acid Rain on Base Cations, Aluminum, and Acidity Development in Highly Weathered Soils of Thailand. Commun. Soil Sci. Plant Anal. 2012, 43, 1382–1400. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Li, Y.; Zhang, Y.; Huang, X.; Yang, Y.; Zhu, H.; Xiong, H.; Jiang, T. Influence of Nitrogen Fertilizer Application on Soil Acidification Characteristics of Tea Plantations in Karst Areas of Southwest China. Agriculture 2023, 13, 849. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.-P.; Yu, M.; Cao, N.; Yan, J. Soil organic matter is important for acid buffering and reducing aluminum leaching from acidic forest soils. Chem. Geol. 2018, 501, 86–94. [Google Scholar] [CrossRef]
- Zhang, J.-E.; Ouyang, Y.; Ling, D.-J. Impacts of simulated acid rain on cation leaching from the Latosol in south China. Chemosphere 2007, 67, 2131–2137. [Google Scholar] [CrossRef]
- Liu, T.; Yin, G.; Liu, J.; Liang, G.; Wu, J. Impact of Acid Deposition on the Main Nutrients of Subtropical Forest Soil. Chin. J. Appl. Environ. Biol. 2013, 19, 255–261. [Google Scholar] [CrossRef]
- Qiu, Q.; Chen, X.; Liang, G.; Zhou, G.; Zhang, D. Effect of simulated acid deposition on chemistry of surface runoff in monsoon evergreen broad-leaved forest in Dinghushan. Acta Ecol. Sin. 2013, 33, 4021–4030. [Google Scholar] [CrossRef]
- Li, W.; Ullah, S.; Xu, Y.; Bai, T.; Ye, S.; Jiang, W.; Yang, M. Effects of Elevated Aluminum Concentration and Distribution on Root Damage, Cell Wall Polysaccharides, and Nutrient Uptake in Different Tolerant Eucalyptus Clones. Int. J. Mol. Sci. 2022, 23, 13438. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Su, Y.B. Research on the action of Mechanism of lime on the coagulation of coal slime water. Environ. Eng. 1999, 17, 26–30. [Google Scholar]
- Fox, T.R. Nitrogen mineralization following fertilization of Douglas-fir forests with urea in western Washington. Soil Sci. Soc. Am. J. 2004, 68, 1720–1728. [Google Scholar] [CrossRef]
- Li, W.; Ali, I.; Han, X.; Ullah, S.; Yang, M. Soil C, N, P, K and Enzymes Stoichiometry of an Endangered Tree Species. Parashorea chinensis of Different Stand Ages Unveiled Soil Nutrient Limitation Factors. Forests 2023, 14, 624. [Google Scholar] [CrossRef]
- Yang, F.; Xu, Z.; Huang, Y.; Tsang, D.C.W.; Ok, Y.S.; Zhao, L.; Qiu, H.; Xu, X.; Cao, X. Stabilization of dissolvable biochar by soil minerals: Release reduction and organo-mineral complexes formation. J. Hazard. Mater. 2021, 412, 125213. [Google Scholar] [CrossRef]


| Stand Type | Terrain | Stand Conditions | Soil Physical Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stand Age (a) | Slope Aspect | Slope Position | Stand Density (Trees·ha−1) | Average DBH /(cm) | Average Height /(m) | Natural Water Content /(%) | Soil Bulk Density /(g·cm−3) | Maximum Water-Holding Capacity /(%) | Total Porosity /(%) | Soil Aeration /(%) | |
| PE | 5 years | South | Middle | 1400 | 28.7 | 21.6 | 21.78 | 1.48 | 32.75 | 48.06 | 16.14 |
| MED | 5 years | South | Middle | 700/700 | 30.2/8.45 | 22.8/7.14 | 18.65 | 1.62 | 26.47 | 42.75 | 12.65 |
| Stand Type | Soil Chemical Properties | ||||||||||
| pH Value | Total Nitrogen (g·kg−1) | Nitrate Nitrogen (mg·kg−1) | Ammoniacal Nitrogen (mg·kg−1) | Available Phosphorus (mg·kg−1) | |||||||
| PE | 5.22 | 1.02 | 20.25 | 22.05 | 7.4 | ||||||
| MED | 5.89 | 1.21 | 22.03 | 24.40 | 9.0 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Ullah, S.; Zhong, L.; Xu, Y.; Wei, G.; Yang, M. Impact of Simulated Acid Rain on Soil Base Cations Dissolution between Eucalyptus Pure Plantations and Eucalyptus–Castanopsis fissa Mixed Plantations. Forests 2023, 14, 2159. https://doi.org/10.3390/f14112159
Wu T, Ullah S, Zhong L, Xu Y, Wei G, Yang M. Impact of Simulated Acid Rain on Soil Base Cations Dissolution between Eucalyptus Pure Plantations and Eucalyptus–Castanopsis fissa Mixed Plantations. Forests. 2023; 14(11):2159. https://doi.org/10.3390/f14112159
Chicago/Turabian StyleWu, Tong, Saif Ullah, Lianxiang Zhong, Yuanyuan Xu, Guoyu Wei, and Mei Yang. 2023. "Impact of Simulated Acid Rain on Soil Base Cations Dissolution between Eucalyptus Pure Plantations and Eucalyptus–Castanopsis fissa Mixed Plantations" Forests 14, no. 11: 2159. https://doi.org/10.3390/f14112159
APA StyleWu, T., Ullah, S., Zhong, L., Xu, Y., Wei, G., & Yang, M. (2023). Impact of Simulated Acid Rain on Soil Base Cations Dissolution between Eucalyptus Pure Plantations and Eucalyptus–Castanopsis fissa Mixed Plantations. Forests, 14(11), 2159. https://doi.org/10.3390/f14112159






