Effect of Gap Size and Elevation on the Regeneration and Coexistence of Abies, Betula, and Acer Tree Species in a Subalpine Coniferous Forest
Abstract
:1. Introduction
2. Materials and Methods
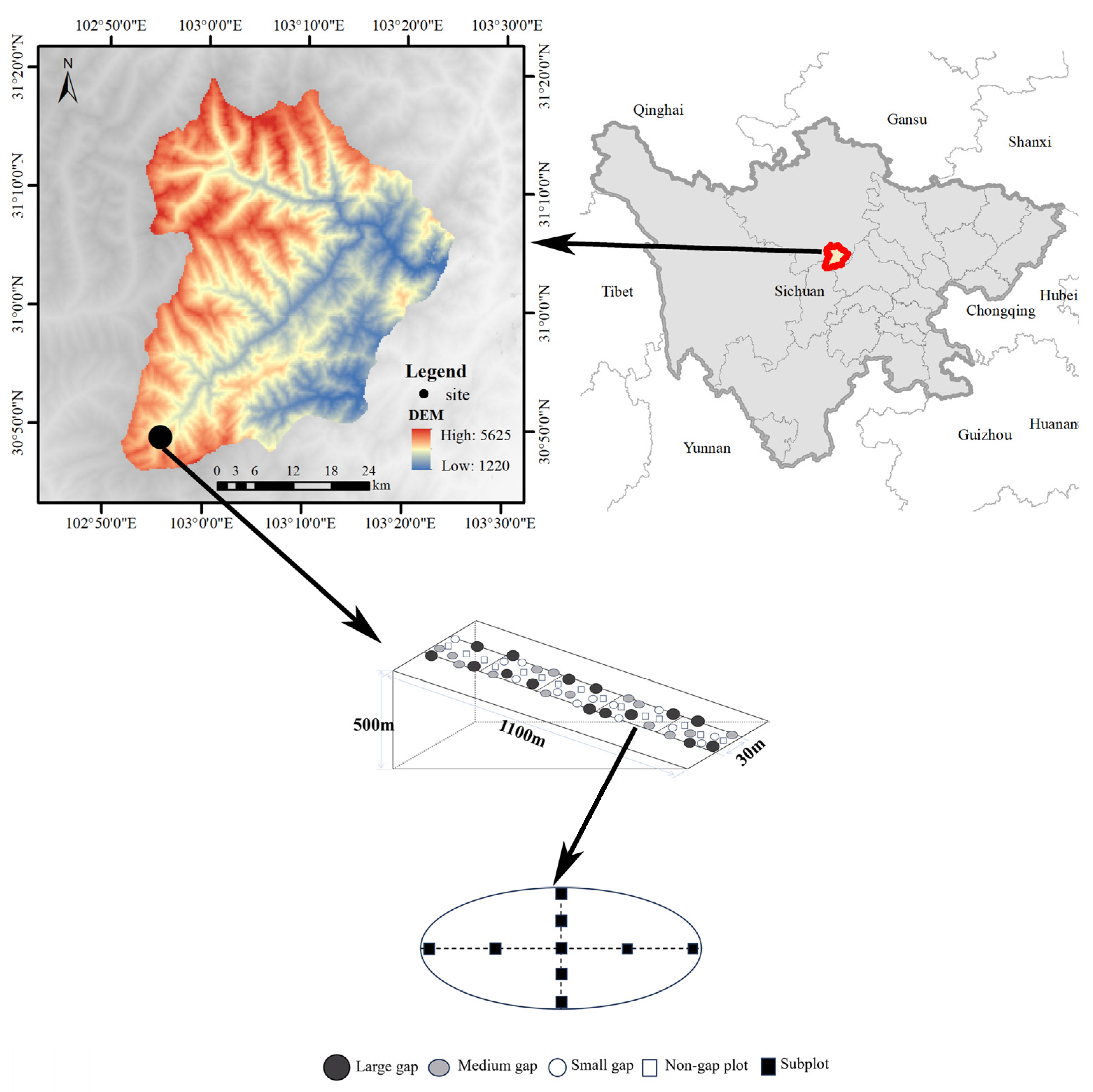
2.1. Study Site
2.2. Gap Selection and Experimental Design
2.3. Data Collection
2.3.1. Regeneration Characteristics
2.3.2. Environmental factors
2.4. Statistical Analysis
3. Results
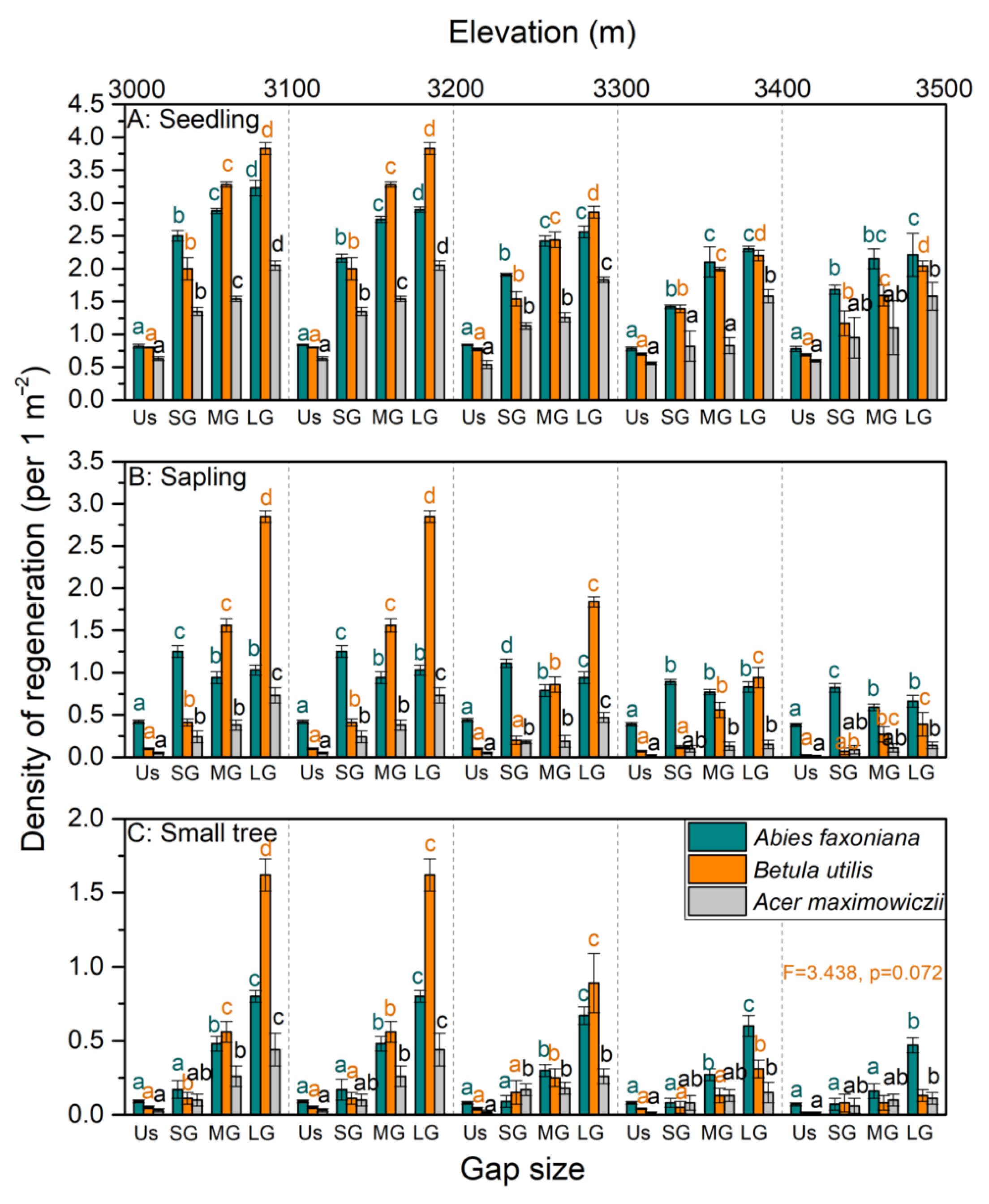
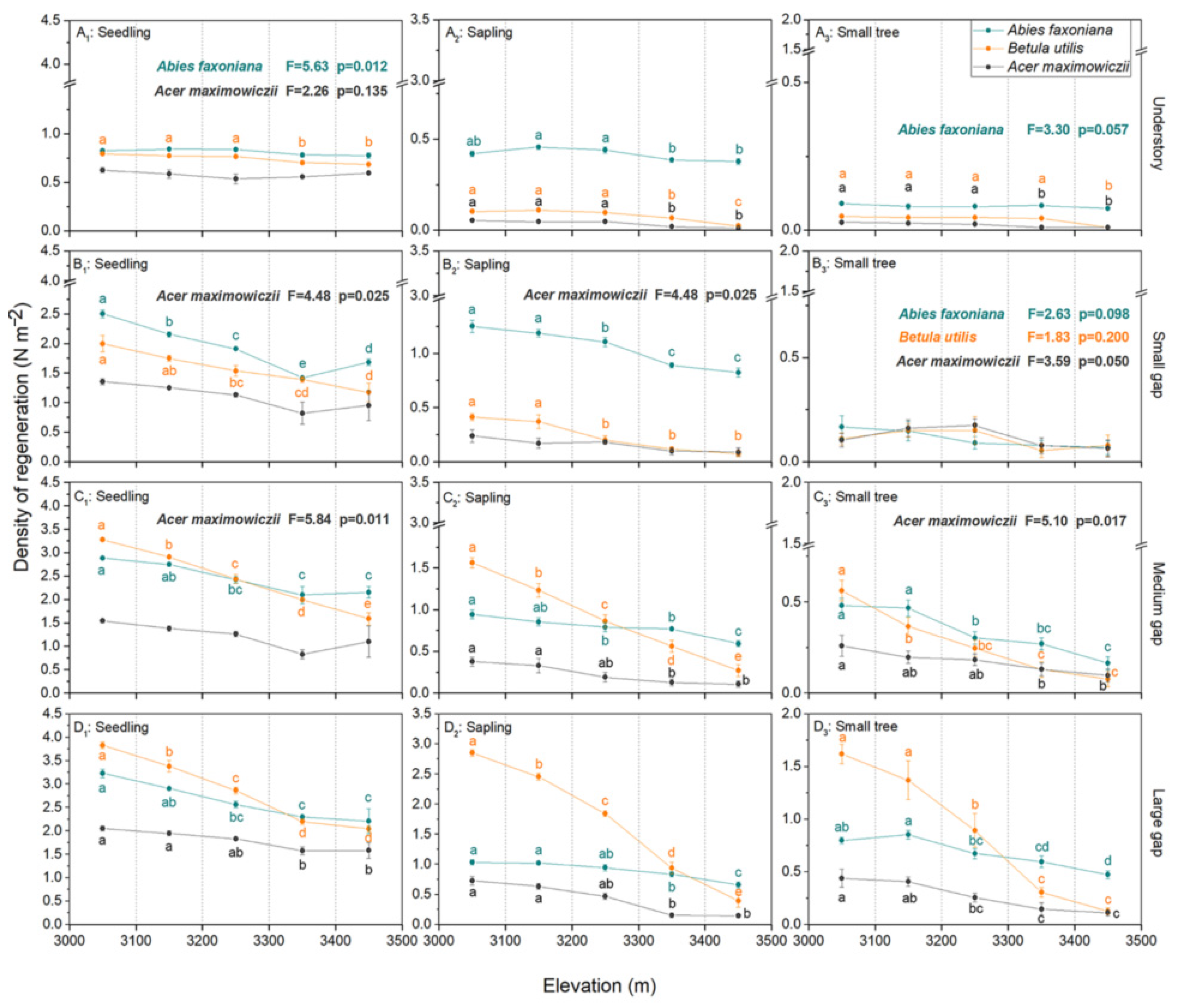
3.1. Variations in the Regeneration Density and Niche of the Three Species among Gaps
3.2. Effect of Gap Size and Elevation on Species Regeneration and Environmental Factors
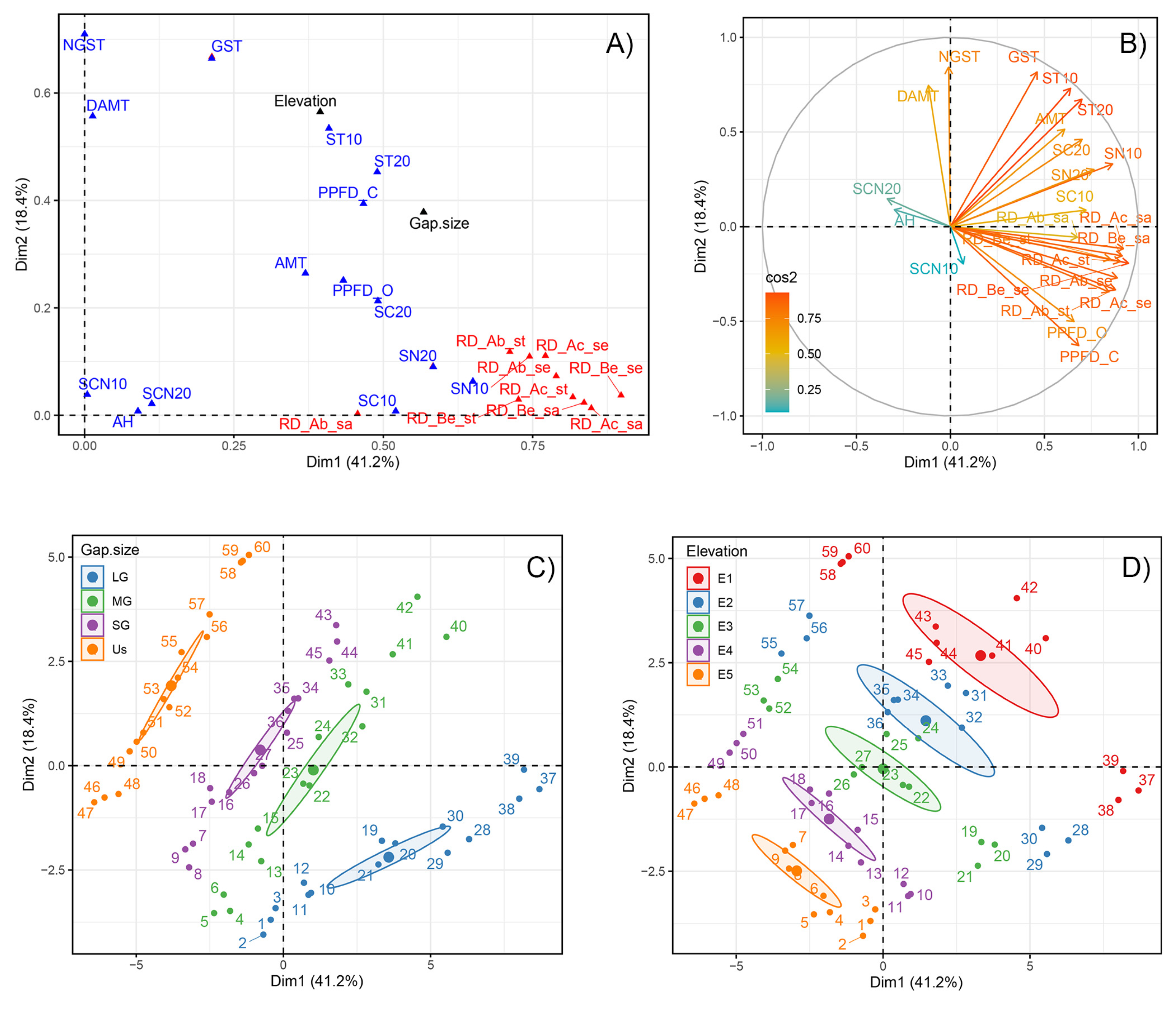
3.3. Relationships between Regeneration Density and Environmental Factors
4. Discussion
4.1. Effect of Gap Size and Elevation on the Regeneration Niche for Species Coexistence
4.2. Community Assembly Processes along Gap and Elevation Gradients
4.3. Forest Management Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar]
- Wang, Z.B.; Yang, H.J.; Dong, B.Q.; Zhou, M.M.; Ma, L.Y.; Jia, Z.K.; Duan, J. Effects of canopy gap size on growth and spatial patterns of Chinese pine (Pinus tabulaeformis) regeneration. For. Ecol. Manag. 2017, 385, 46–56. [Google Scholar]
- Zhu, J.J.; Lu, D.L.; Zhang, W.D. Effects of gaps on regeneration of woody plants: A meta-analysis. J. For. Res. 2014, 25, 501–510. [Google Scholar]
- Chen, L.; Liu, G.H.; Liu, D. How Forest Gap and Elevation Shaped Abies faxoniana Rehd. et Wils. Regeneration in a Subalpine Coniferous Forest, Southwestern China. Forests 2018, 9, 271. [Google Scholar] [CrossRef]
- Dirzo, R.; Horvitz, C.C.; Quevedo, H.; Lopez, M.A. The effects of gap size and age on the understorey herb community of a tropical Mexican rain forest. J. Ecol. 1992, 80, 809–822. [Google Scholar] [CrossRef]
- Barberis, I.M.; Tanner, E.V. Gaps and root trenching increase tree seedling growth in Panamanian semi-evergreen forest. Ecology 2005, 86, 667–674. [Google Scholar] [CrossRef]
- Lu, D.L.; Wang, G.G.; Zhang, J.X.; Fang, Y.T.; Zhu, C.Y.; Zhu, J.J. Converting larch plantations to mixed stands: Effects of canopy treatment on the survival and growth of planted seedlings with contrasting shade tolerance. For. Ecol. Manag. 2018, 409, 19–28. [Google Scholar]
- Lang, G.E.; Knight, D.H. Tree growth, mortality, recruitment, and canopy gap formation during a 10-year period in a tropical moist forest. Ecology 1983, 64, 1075–1080. [Google Scholar] [CrossRef]
- Tamura, A.; Nakajima, K. Effects of 10 years of fencing under a gap and closed canopy on the regeneration of tree seedlings in an old-growth Japanese fir (Abies firma) forest overbrowsed by sika deer. J. For. Res. 2017, 22, 224–232. [Google Scholar]
- Cole, E.M.; Bustamante, M.R.; Almeida-Reinoso, D.; Funk, W.C. Spatial and temporal variation in population dynamics of Andean frogs: Effects of forest disturbance and evidence for declines. Glob. Ecol. Conserv. 2014, 1, 60–70. [Google Scholar]
- Sizonenko, T.A.; Dubrovskiy, Y.A. Ectomycorrhiza of Larix sibirica Ledeb. along the gradients of main ecological factors and elevation at the Northern and Subpolar Urals. J. Mt. Sci. 2018, 15, 2603–2613. [Google Scholar]
- Eisenlohr, P.V.; Alves, L.F.; Bernacci, L.C.; Padgurschi, M.C.G.; Torres, R.B.; Prata, E.M.B.; Santos, F.D.; Assis, M.A.; Ramos, E.; Rochelle, A.L.C.; et al. Disturbances, elevation, topography and spatial proximity drive vegetation patterns along an altitudinal gradient of a top biodiversity hotspot. Biodivers. Conserv. 2013, 22, 2767–2783. [Google Scholar]
- Sun, X.Y.; Wang, G.X.; Huang, M.; Hu, Z.Y.; Song, C.L. Effect of climate change on seasonal water use efficiency in subalpine Abies fabri. J. Mt. Sci. 2017, 14, 142–157. [Google Scholar]
- Luo, S.Z.; Liu, G.H.; Li, Z.S.; Hu, C.J.; Gong, L.; Wang, M.; Hu, H.F. Soil respiration along an altitudinal gradient in a subalpine secondary forest in China. iForest-Biogeosci. For. 2015, 8, 526–532. [Google Scholar]
- Masaki, T.; Osumi, K.; Takahashi, K.; Hoshizaki, K.; Matsune, K.; Suzuki, W. Effects of microenvironmental heterogeneity on the seed-to-seedling process and tree coexistence in a riparian forest. Ecol. Res. 2007, 22, 724–734. [Google Scholar] [CrossRef]
- Quero, J.L.; Gómez-Aparicio, L.; Zamora, R.; Maestre, F.T. Shifts in the regeneration niche of an endangered tree (Acer opalus ssp. granatense) during ontogeny: Using an ecological concept for application. Basic Appl. Ecol. 2008, 9, 635–644. [Google Scholar]
- Guo, Y.; Zhao, P.; Zhou, Z.; Yi, J.; Chai, Y.; Yue, M. Coexistence of three common species in a temperate mixed forest: Linking seedling microhabitats and functional traits. For. Ecol. Manag. 2020, 465, 118057. [Google Scholar]
- Tang, C.Q.; Ohsawa, M. Coexistence mechanisms of evergreen, deciduous and coniferous trees in a mid-montane mixed forest on Mt. Emei, Sichuan, China. Plant Ecol. 2002, 161, 215–230. [Google Scholar] [CrossRef]
- Jin, Y.; Russo, S.E.; Yu, M.J. Effects of light and topography on regeneration and coexistence of evergreen and deciduous tree species in a Chinese subtropical forest. J. Ecol. 2018, 106, 1634–1645. [Google Scholar] [CrossRef]
- Baraloto, C.; Goldberg, D.E.; Bonal, D. Performance trade-offs among tropical tree seedlings in contrasting microhabitats. Ecology 2005, 86, 2461–2472. [Google Scholar]
- Bannar-Martin, K.H.; Kremer, C.T.; Ernest, S.M.; Leibold, M.A.; Auge, H.; Chase, J.; Declerck, S.A.J.; Eisenhauer, N.; Harpole, S.; Hillebrand, H.; et al. Integrating community assembly and biodiversity to better understand ecosystem function: The Community Assembly and the Functioning of Ecosystems (CAFE) approach. Ecol. Lett. 2018, 21, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Padilha, D.L.; De Marco, P. A gap in the woods: Wood density knowledge as impediment to develop sustainable use in Atlantic Forest. For. Ecol. Manag. 2018, 424, 448–457. [Google Scholar] [CrossRef]
- Chen, L.; Mi, X.C.; Comita, L.S.; Zhang, L.W.; Ren, H.B.; Ma, K.P. Community-level consequences of density dependence and habitat association in a subtropical broad-leaved forest. Ecol. Lett. 2010, 13, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.H.; Qin, Z.S. Regeneration Patterns in Old-Growth Abies-Betula Forests in the Wolong Natural Reserve, Sichuan, China. J. Ecol. 1998, 76, 1204–1218. [Google Scholar] [CrossRef]
- Bossard, C.C.; Cao, Y.; Wang, J.; Rose, A.; Tang, Y. New patterns of establishment and growth of Picea, Abies and Betula tree species in subalpine forest gaps of Jiuzhaigou National Nature Reserve, Sichuan, southwestern China in a changing environment. For. Ecol. Manag. 2015, 356, 84–92. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, P.; Yue, M. Canopy disturbance and gap partitioning promote the persistence of a pioneer tree population in a near-climax temperate forest of the Qinling Mountains, China. Ecol. Evol. 2019, 9, 7676–7687. [Google Scholar] [CrossRef]
- Liu, D.; Liu, G.H.; Chen, L.; Wang, J.T.; Zhang, L.M. Soil pH determines fungal diversity along an elevation gradient in Southwestern China. Sci. China-Life Sci. 2018, 61, 718–726. [Google Scholar] [CrossRef]
- Chen, L.; Han, W.; Liu, D.; Liu, G. How forest gaps shaped plant diversity along an elevational gradient in Wolong National Nature Reserve. J. Geogr. Sci. 2019, 29, 1081–1097. [Google Scholar] [CrossRef]
- Kneeshaw, D.D.; Bergeron, Y. Canopy gap characteristics and tree replacement in the southeastern boreal forest. Ecology 1998, 79, 783–794. [Google Scholar] [CrossRef]
- Gao, X.M.; Ma, K.P.; Chen, L.Z. Species Diversity of Some Deciduous Broad-Leaved Forests in the Warm-Temperate Zone and its Relations Community Stability. Acta Phytoecol. Sin. 2001, 25, 283–290. (In Chinese) [Google Scholar]
- Hurlbert, S.H. The measurement of niche overlap and some relatives. Ecology 1978, 59, 67–77. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zuo, J.M.; Liu, J.G. Study on regeneration niche metrics based on ecostate-ecorole theory. Sci. Silvae Sin. 2005, 41, 20–24. (In Chinese) [Google Scholar]
- Parent, S.; Messier, C. A simple and efficient method to estimate microsite light availability under a forest canopy. Can. J. For. Res. 1996, 26, 151–154. [Google Scholar] [CrossRef]
- Denslow, J.S.; Ellison, A.M.; Sanford, R.E. Treefall gap size effects on above- and below-ground processes in a tropical wet forest. J. Ecol. 1998, 86, 597–609. [Google Scholar] [CrossRef]
- He, Z.; Wang, L.; Jiang, L.; Wang, Z.; Liu, J.; Xu, D.; Hong, W. Effect of Microenvironment on Species Distribution Patterns in the Regeneration Layer of Forest Gaps and Non-Gaps in a Subtropical Natural Forest, China. Forests 2019, 10, 90. [Google Scholar] [CrossRef]
- Miao, N.; Liu, S.R.; Yu, H.; Shi, Z.M.; Moermond, T.; Liu, Y. Spatial analysis of remnant tree effects in a secondary Abies-Betula forest on the eastern edge of the Qinghai-Tibetan Plateau, China. For. Ecol. Manag. 2014, 313, 104–111. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Mercurio, R. Influence of gap size on organic matter decomposition, microbial biomass and nutrient cycle in Calabrian pine (Pinus laricio, Poiret) stands. For. Ecol. Manag. 2007, 242, 412–418. [Google Scholar] [CrossRef]
- Gray, A.N.; Spies, T.A.; Easter, M.J. Microclimate and soil moisture responses to gap formation in coastal Douglas-fir forests. Can. J. For. Res. 2002, 32, 332–343. [Google Scholar] [CrossRef]
- Denslow, J.S. Tropical rainforest gaps and tree species diversity. Annu. Rev. Ecol. Syst. 1987, 18, 431–451. [Google Scholar] [CrossRef]
- Adler, P.B.; Fajardo, A.; Kleinhesselink, A.R.; Kraft, N.J. Trait-based tests of coexistence mechanisms. Ecol. Lett. 2013, 16, 1294–1306. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Bergholz, K.; May, F.; Giladi, I.; Ristow, M.; Ziv, Y.; Jeltsch, F. Environmental heterogeneity drives fine-scale species assembly and functional diversity of annual plants in a semi-arid environment. Perspect. Plant Ecol. Evol. Syst. 2017, 24, 138–146. [Google Scholar] [CrossRef]
- Brokaw, N.V.L. Gap-phase regeneration of three pioneer tree species in a tropical forest. J. Ecol. 1987, 75, 9–19. [Google Scholar] [CrossRef]
- Hoffman, A.; Bataineh, M.; Adams, J.; Nelson, A. Partial harvesting effects on seedling growth and physiology of three hardwood species in mature pine (Pinus spp., Pinaceae)-hardwood mixtures. J. Torrey Bot. Soc. 2018, 145, 237–249. [Google Scholar] [CrossRef]
- Alexander, J.M.; Diez, J.M.; Levine, J.M. Novel competitors shape species’ responses to climate change. Nature 2015, 525, 515–518. [Google Scholar] [CrossRef] [PubMed]

| LG | MG | SG | Us | Total | ||
|---|---|---|---|---|---|---|
| Regeneration density | ||||||
| Abies faxoniana | Seedling | 2.64 ± 0.42 a | 2.46 ± 0.34 a | 1.93 ± 0.39 b | 0.81 ± 0.03 c | 1.96 ± 0.79 |
| Sapling | 0.90 ± 0.15 b | 0.79 ± 0.13 b | 1.05 ± 0.18 a | 0.42 ± 0.04 c | 0.79 ± 0.27 | |
| Small tree | 0.68 ± 0.15 a | 0.34 ± 0.13 b | 0.11 ± 0.06 c | 0.08 ± 0.01 c | 0.30 ± 0.26 | |
| Betula utilis | Seedling | 2.86 ± 0.71 a | 2.44 ± 0.63 a | 1.57 ± 0.32 b | 0.75 ± 0.05 c | 1.90 ± 0.96 |
| Sapling | 1.70 ± 0.95 a | 0.90 ± 0.48 b | 0.23 ± 0.14 c | 0.08 ± 0.03 c | 0.73 ± 0.83 | |
| Small tree | 0.86 ± 0.61 a | 0.28 ± 0.19 b | 0.11 ± 0.06 b | 0.04 ± 0.01 c | 0.32 ± 0.45 | |
| Acer maximowiczii | Seedling | 1.80 ± 0.22 a | 1.22 ± 0.30 b | 1.10 ± 0.25 b | 0.58 ± 0.05 c | 1.18 ± 0.49 |
| Sapling | 0.42 ± 0.25 a | 0.23 ± 0.13 b | 0.15 ± 0.07 b c | 0.04 ± 0.02 c | 0.21 ± 0.20 | |
| Small tree | 0.27 ± 0.15 a | 0.17 ± 0.07 b | 0.12 ± 0.06 b | 0.02 ± 0.01 c | 0.14 ± 0.13 | |
| VI | ||||||
| Abies faxoniana | Seedling | 4.087 | 4.421 | 5.224 | 2.572 | 16.306 |
| Sapling | 2.861 | 3.631 | 3.882 | 2.705 | 13.079 | |
| Small tree | 3.466 | 3.767 | 4.144 | 2.827 | 14.205 | |
| Betula utilis | Seedling | 3.202 | 4.130 | 5.022 | 2.124 | 14.477 |
| Sapling | 2.346 | 3.184 | 3.697 | 1.214 | 10.441 | |
| Small tree | 2.314 | 3.276 | 3.795 | 2.569 | 11.954 | |
| Acer maximowiczii | Seedling | 1.685 | 2.020 | 2.173 | 1.004 | 6.883 |
| Sapling | 0.861 | 1.215 | 1.441 | 0.893 | 4.410 | |
| Small tree | 1.860 | 2.161 | 2.435 | 1.789 | 8.244 | |
| Niche breadth | ||||||
| Abies faxoniana | Seedling | 0.989 | 0.958 | 0.987 | 0.989 | 0.969 |
| Sapling | 0.988 | 0.990 | 0.997 | 0.887 | 0.900 | |
| Small tree | 0.988 | 0.975 | 0.973 | 0.813 | 0.918 | |
| Betula utilis | Seedling | 0.946 | 0.905 | 0.940 | 1.325 | 0.977 |
| Sapling | 0.889 | 0.765 | 0.869 | 3.999 | 0.811 | |
| Small tree | 0.989 | 0.897 | 0.934 | 0.990 | 0.881 | |
| Acer maximowiczii | Seedling | 0.927 | 0.906 | 0.954 | 6.424 | 0.969 |
| Sapling | 0.990 | 0.888 | 0.919 | 8.198 | 0.891 | |
| Small tree | 0.997 | 0.988 | 0.985 | 2.034 | 0.976 | |
| Regeneration niche breadth | ||||||
| Abies faxoniana | Seedling | |||||
| Sapling | 0.612 | 0.662 | 0.611 | 0.105 | 0.651 | |
| Small tree | 0.575 | 0.584 | 0.559 | 0.179 | 0.584 | |
| Betula utilis | Seedling | |||||
| Sapling | 0.487 | 0.459 | 0.503 | 0.400 | 0.478 | |
| Small tree | 0.470 | 0.454 | 0.487 | 0.462 | 0.457 | |
| Acer maximowiczii | Seedling | |||||
| Sapling | 0.414 | 0.403 | 0.400 | 1.626 | 0.393 | |
| Small tree | 0.460 | 0.469 | 0.458 | 1.138 | 0.465 |
| Seedling–Sapling | Sapling–Small Tree | |||||
|---|---|---|---|---|---|---|
| Total | ||||||
| Species | S1 | S2 | S3 | S1 | S2 | S3 |
| S1 | 1.00 | 1.00 | ||||
| S2 | 0.94 | 1.00 | 0.94 | 1.00 | ||
| S3 | 0.98 | 0.97 | 1.00 | 0.98 | 0.94 | 1.00 |
| Large gap | ||||||
| Species | S1 | S2 | S3 | S1 | S2 | S3 |
| S1 | 1.00 | 1.00 | ||||
| S2 | 0.97 | 1.00 | 0.98 | 1.00 | ||
| S3 | 0.98 | 0.96 | 1.00 | 0.99 | 0.98 | 1.00 |
| Medium gap | ||||||
| Species | S1 | S2 | S3 | S1 | S2 | S3 |
| S1 | 1.00 | 1.00 | ||||
| S2 | 0.94 | 1.00 | 0.94 | 1.00 | ||
| S3 | 0.97 | 0.98 | 1.00 | 0.98 | 0.97 | 1.00 |
| Small gap | ||||||
| Species | S1 | S2 | S3 | S1 | S2 | S3 |
| S1 | 1.00 | 1.00 | ||||
| S2 | 0.96 | 1.00 | 0.96 | 1.00 | ||
| S3 | 0.97 | 0.98 | 1.00 | 0.98 | 0.98 | 1.00 |
| Understory | ||||||
| Species | S1 | S2 | S3 | S1 | S2 | S3 |
| S1 | 1.00 | 1.00 | ||||
| S2 | 0.97 | 1.00 | 0.98 | 1.00 | ||
| S3 | 0.99 | 0.97 | 1.00 | 0.99 | 0.98 | 1.00 |
| Speices | Large Gap | Medium Gap | Small Gap | Understory | |||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | ||
| Abies faxoniana | seedling | 20.71 | <0.001 | 21.88 | <0.001 | 166.61 | <0.001 | 5.63 | 0.012 |
| sapling | 22.43 | <0.001 | 17.22 | <0.001 | 40.06 | <0.001 | 10.91 | <0.001 | |
| small tree | 25.06 | <0.001 | 25.36 | <0.001 | 2.63 | 0.098 | 3.30 | 0.057 | |
| Betula utilis | seedling | 170.88 | <0.001 | 155.74 | <0.001 | 17.74 | <0.001 | 29.19 | <0.001 |
| sapling | 339.97 | <0.001 | 99.05 | <0.001 | 29.25 | <0.001 | 57.50 | <0.001 | |
| small tree | 59.28 | <0.001 | 34.42 | <0.001 | 1.83 | 0.200 | 34.17 | <0.001 | |
| Acer maximowiczii | seedling | 10.85 | <0.001 | 5.84 | 0.011 | 4.48 | 0.025 | 2.26 | 0.135 |
| sapling | 56.30 | <0.001 | 8.91 | 0.002 | 4.48 | 0.025 | 54.67 | <0.001 | |
| small tree | 14.31 | <0.001 | 5.10 | 0.017 | 3.59 | 0.050 | 13.25 | <0.001 | |
| Species | Variables | Seedling | Sapling | Small Tree | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Abies faxoniana | Gap size | 867.28 | <0.001 | 483.17 | <0.001 | 631.14 | <0.001 |
| Elevation | 93.20 | <0.001 | 79.40 | <0.001 | 45.83 | <0.001 | |
| Gap size × Elevation | 10.07 | <0.001 | 7.40 | <0.001 | 8.78 | <0.001 | |
| Betula utilis | Gap size | 1458.34 | <0.001 | 1654.16 | <0.001 | 304.98 | <0.001 |
| Elevation | 265.93 | <0.001 | 448.71 | <0.001 | 83.67 | <0.001 | |
| Gap size × Elevation | 36.76 | <0.001 | 122.53 | <0.001 | 38.62 | <0.001 | |
| Acer maximowiczii | Gap size | 176.92 | <0.001 | 140.60 | <0.001 | 75.26 | <0.001 |
| Elevation | 18.23 | <0.001 | 56.76 | <0.001 | 20.37 | <0.001 | |
| Gap size × Elevation | 1.95 | 0.057 | 13.17 | <0.001 | 5.89 | <0.001 | |
| Elevation | Gap Size | Elevation× Size | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| PPFD (μ mol m−2 s−1) | ||||||
| Cloudless | 0.50 | 0.485 | 1.49 | 0.229 | 0.75 | 0.393 |
| Overcast | 0.68 | 0.615 | 27.57 | <0.001 | 0.27 | 0.971 |
| Air Temperature (°C) | ||||||
| Annual mean temperature | 134.52 | <0.001 | 13.59 | <0.001 | 5.07 | <0.001 |
| DAMT | 139.05 | <0.001 | 510.80 | <0.001 | 50.97 | <0.001 |
| Temperature in growing season | 151.53 | <0.001 | 2.09 | 0.141 | 5.87 | <0.001 |
| Temperature in non-growing season | 44.82 | <0.001 | 85.31 | <0.001 | 39.98 | <0.001 |
| Air Humidity (%) | ||||||
| Annual mean humidity | 7.39 | <0.001 | 51.40 | <0.001 | 15.15 | <0.001 |
| Soil property | ||||||
| Soil temperature (°C) 0–10 cm | 127.26 | <0.001 | 0.09 | 0.914 | 0.51 | 0.836 |
| 10–20 cm | 106.56 | <0.001 | 1.86 | 0.173 | 0.82 | 0.588 |
| STC (%) 0–10 cm | 11.68 | <0.001 | 8.54 | 0.002 | 2.33 | 0.044 |
| 10–20 cm | 371.40 | <0.001 | 298.50 | <0.001 | 21.80 | <0.001 |
| STN (%) 0–10 cm | 31.93 | <0.001 | 12.23 | <0.001 | 1.17 | 0.347 |
| 10–20 cm | 155.63 | <0.001 | 134.36 | <0.001 | 22.49 | <0.001 |
| Soil C/N ratio (%) 0–10 cm | 2.48 | 0.065 | 1.68 | 0.203 | 2.36 | 0.042 |
| 10–20 cm | 26.16 | <0.001 | 29.73 | <0.001 | 6.47 | <0.001 |
| Environmental Variables | Abies faxoniana | Betula utilis | Acer maximowiczii | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seedling | Sapling | Small Tree | Seedling | Sapling | Small Tree | Seedling | Sapling | Small Tree | ||||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| PPFD_O | 0.87 | <0.001 | 0.78 | <0.001 | 0.58 | <0.001 | 0.75 | <0.001 | 0.52 | <0.001 | 0.43 | 0.001 | 0.76 | <0.001 | 0.55 | <0.001 | 0.63 | <0.001 |
| PPFD_C | 0.86 | <0.001 | 0.65 | <0.001 | 0.72 | <0.001 | 0.78 | <0.001 | 0.61 | <0.001 | 0.53 | <0.001 | 0.83 | <0.001 | 0.60 | <0.001 | 0.66 | <0.001 |
| AMT | 0.35 | 0.006 | 0.43 | 0.001 | 0.26 | 0.043 | 0.39 | 0.002 | 0.41 | 0.001 | 0.40 | 0.002 | 0.32 | 0.011 | 0.47 | <0.001 | 0.44 | <0.001 |
| DAMT | −0.20 | 0.122 | 0.18 | 0.168 | −0.45 | <0.001 | −0.19 | 0.139 | −0.26 | 0.047 | −0.27 | 0.035 | −0.30 | 0.019 | −0.17 | 0.194 | −0.20 | 0.126 |
| GST | 0.16 | 0.208 | 0.23 | 0.073 | 0.16 | 0.219 | 0.30 | 0.019 | 0.37 | 0.004 | 0.33 | 0.009 | 0.15 | 0.250 | 0.39 | 0.002 | 0.30 | 0.020 |
| NGST | −0.14 | 0.272 | 0.06 | 0.672 | −0.30 | 0.020 | −0.13 | 0.320 | −0.24 | 0.068 | −0.33 | 0.010 | −0.21 | 0.101 | −0.19 | 0.145 | −0.23 | 0.079 |
| AH | −0.15 | 0.265 | −0.13 | 0.331 | −0.31 | 0.016 | −0.24 | 0.065 | −0.33 | 0.010 | −0.41 | 0.001 | −0.32 | 0.014 | −0.37 | 0.003 | −0.33 | 0.009 |
| ST10 | 0.34 | 0.007 | 0.39 | 0.002 | 0.27 | 0.039 | 0.43 | 0.001 | 0.47 | <0.001 | 0.45 | <0.001 | 0.30 | 0.019 | 0.50 | <0.001 | 0.45 | <0.001 |
| ST20 | 0.41 | 0.001 | 0.40 | 0.001 | 0.35 | 0.006 | 0.51 | <0.001 | 0.55 | <0.001 | 0.52 | <0.001 | 0.37 | 0.004 | 0.57 | <0.001 | 0.52 | <0.001 |
| SC10 | 0.53 | <0.001 | 0.29 | 0.023 | 0.56 | <0.001 | 0.64 | <0.001 | 0.71 | <0.001 | 0.72 | <0.001 | 0.51 | <0.001 | 0.69 | <0.001 | 0.67 | <0.001 |
| SC20 | 0.57 | <0.001 | 0.31 | 0.014 | 0.51 | <0.001 | 0.64 | <0.001 | 0.58 | <0.001 | 0.44 | <0.001 | 0.49 | <0.001 | 0.54 | <0.001 | 0.50 | <0.001 |
| SN10 | 0.67 | <0.001 | 0.50 | <0.001 | 0.63 | <0.001 | 0.76 | <0.001 | 0.74 | <0.001 | 0.67 | <0.001 | 0.62 | <0.001 | 0.72 | <0.001 | 0.67 | <0.001 |
| SN20 | 0.62 | <0.001 | 0.46 | <0.001 | 0.52 | <0.001 | 0.64 | <0.001 | 0.53 | <0.001 | 0.43 | 0.001 | 0.56 | <0.001 | 0.53 | <0.001 | 0.53 | <0.001 |
| SCN10 | 0.01 | 0.939 | −0.15 | 0.241 | 0.13 | 0.340 | 0.07 | 0.609 | 0.21 | 0.107 | 0.29 | 0.025 | 0.02 | 0.888 | 0.19 | 0.143 | 0.20 | 0.122 |
| SCN20 | −0.32 | 0.013 | −0.42 | 0.001 | −0.17 | 0.196 | −0.23 | 0.079 | −0.11 | 0.413 | −0.12 | 0.379 | −0.28 | 0.029 | −0.17 | 0.199 | −0.22 | 0.091 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, W.; Chen, L.; Liu, J.; Wang, G.G.; Liu, D.; Liu, G. Effect of Gap Size and Elevation on the Regeneration and Coexistence of Abies, Betula, and Acer Tree Species in a Subalpine Coniferous Forest. Forests 2023, 14, 2099. https://doi.org/10.3390/f14102099
Han W, Chen L, Liu J, Wang GG, Liu D, Liu G. Effect of Gap Size and Elevation on the Regeneration and Coexistence of Abies, Betula, and Acer Tree Species in a Subalpine Coniferous Forest. Forests. 2023; 14(10):2099. https://doi.org/10.3390/f14102099
Chicago/Turabian StyleHan, Wangya, Li Chen, Jingyang Liu, G. Geoff Wang, Dan Liu, and Guohua Liu. 2023. "Effect of Gap Size and Elevation on the Regeneration and Coexistence of Abies, Betula, and Acer Tree Species in a Subalpine Coniferous Forest" Forests 14, no. 10: 2099. https://doi.org/10.3390/f14102099
APA StyleHan, W., Chen, L., Liu, J., Wang, G. G., Liu, D., & Liu, G. (2023). Effect of Gap Size and Elevation on the Regeneration and Coexistence of Abies, Betula, and Acer Tree Species in a Subalpine Coniferous Forest. Forests, 14(10), 2099. https://doi.org/10.3390/f14102099






