Exposure to Forest Air Monoterpenes with Pulmonary Function Tests in Adolescents with Asthma: A Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Design of the Study, Cohort, and Respiratory Measurements
2.3. Measurement of Volatile Organic Compounds
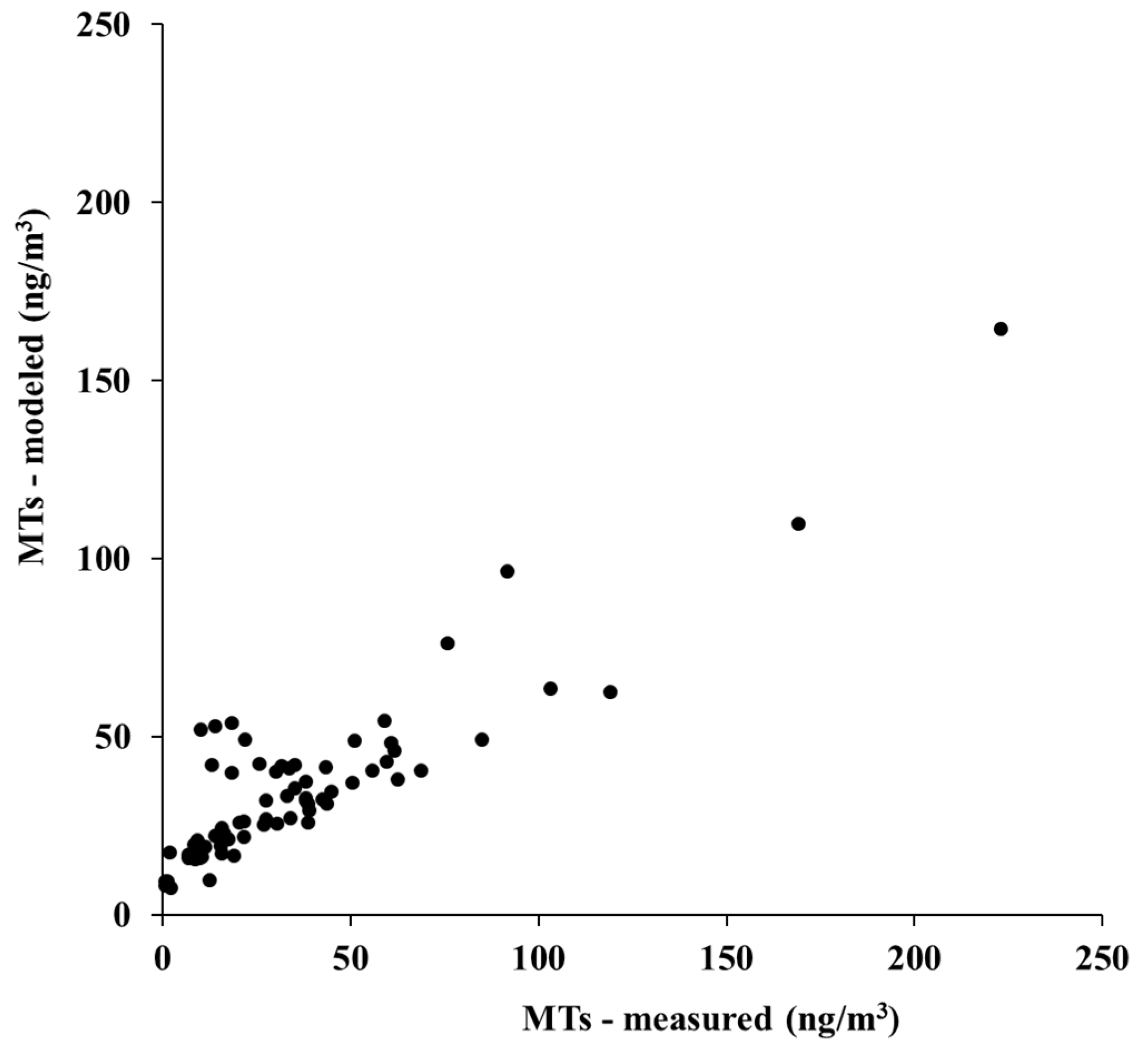
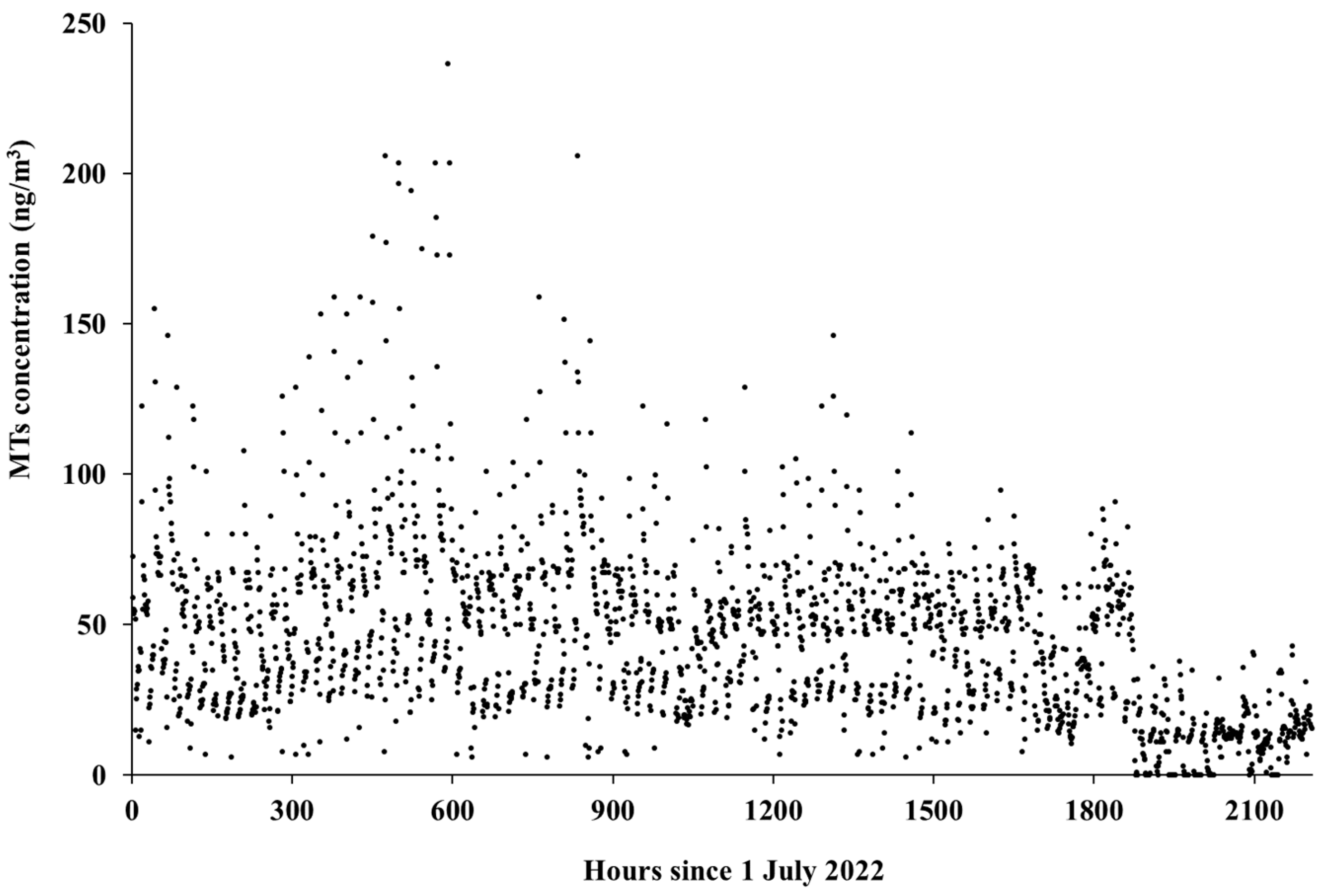
2.4. Reconstruction of a Continuous Series of Hourly MT Concentrations
2.5. Individual Exposure to MTs
2.6. Statistical Analysis
3. Results
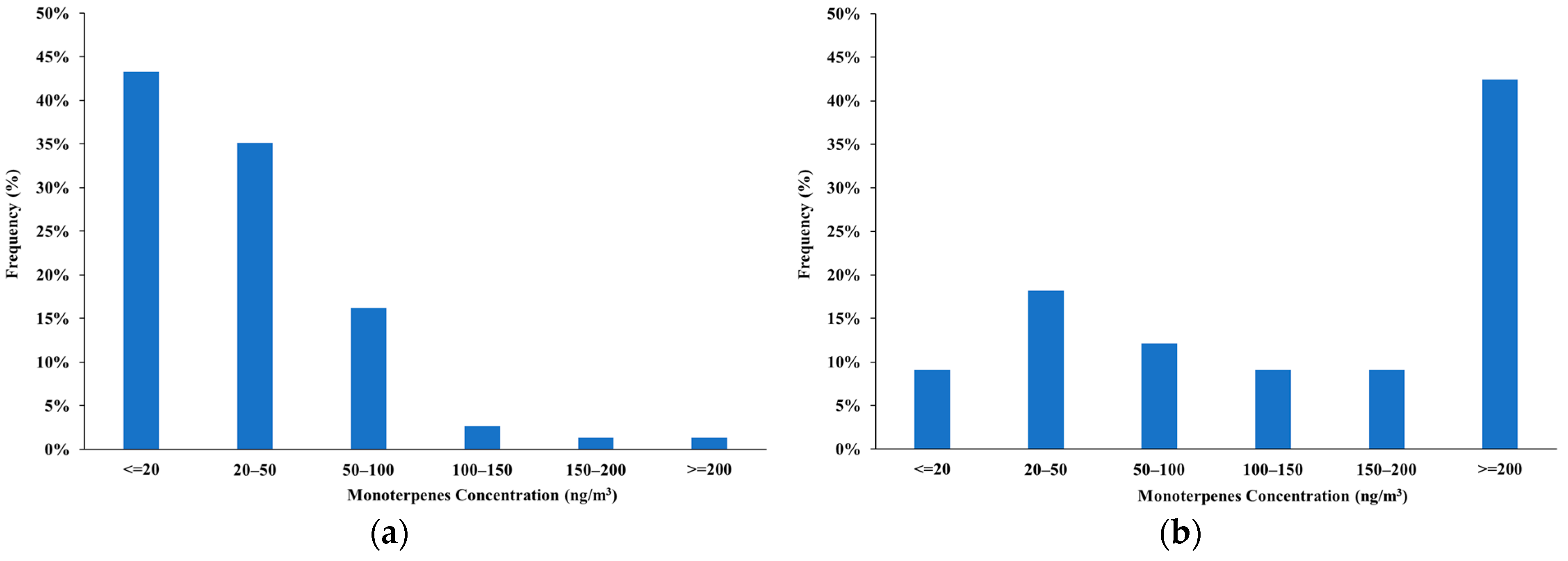
3.1. Characterization of the Forest Atmosphere
3.2. Association of MT Exposure with Pulmonary Functions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adewuyi, F.A.; Knobel, P.; Gogna, P.; Dadvand, P. Health Effects of Green Prescription: A Systematic Review of Randomized Controlled Trials. Environ. Res. 2023, 236, 116844. [Google Scholar] [CrossRef]
- Chae, Y.; Lee, S.; Jo, Y.; Kang, S.; Park, S.; Kang, H. The Effects of Forest Therapy on Immune Function. Int. J. Environ. Res. Public Health 2021, 18, 8440. [Google Scholar] [CrossRef]
- Bikomeye, J.C.; Beyer, A.M.; Kwarteng, J.L.; Beyer, K.M.M. Greenspace, Inflammation, Cardiovascular Health, and Cancer: A Review and Conceptual Framework for Greenspace in Cardio-Oncology Research. Int. J. Environ. Res. Public Health 2022, 19, 2426. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.-S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef]
- Stier-Jarmer, M.; Throner, V.; Kirschneck, M.; Immich, G.; Frisch, D.; Schuh, A. The Psychological and Physical Effects of Forests on Human Health: A Systematic Review of Systematic Reviews and Meta-Analyses. Int. J. Environ. Res. Public Health 2021, 18, 1770. [Google Scholar] [CrossRef]
- Lee, K.J.; Hur, J.; Yang, K.S.; Lee, M.K.; Lee, S.J. Acute Biophysical Responses and Psychological Effects of Different Types of Forests in Patients With Metabolic Syndrome. Environ. Behav. 2018, 50, 298–323. [Google Scholar] [CrossRef]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Park, B.J.; et al. Effect of Phytoncide from Trees on Human Natural Killer Cell Function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Tsao, T.M.; Tsai, M.J.; Hwang, J.S.; Cheng, W.F.; Wu, C.F.; Chou, C.C.K.; Su, T.C. Health Effects of a Forest Environment on Natural Killer Cells in Humans: An Observational Pilot Study. Oncotarget 2018, 9, 16501–16511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, J.; Wu, Q.; Chen, Z.; Wang, G. Seasonal Dynamics of VOCs Released from Cinnamomun Camphora Forests and the Associated Adjuvant Therapy for Geriatric Hypertension. Ind. Crops. Prod. 2021, 174, 114131. [Google Scholar] [CrossRef]
- Imamura, C.; Sakakibara, K.; Arai, K.; Ohira, H.; Yamaguchi, Y.; Yamada, H. Effect of Indoor Forest Bathing on Reducing Feelings of Fatigue Using Cerebral Activity as an Indicator. Int. J. Environ. Res. Public Health 2022, 19, 6672. [Google Scholar] [CrossRef]
- Donelli, D.; Meneguzzo, F.; Antonelli, M.; Ardissino, D.; Niccoli, G.; Gronchi, G.; Baraldi, R.; Neri, L.; Zabini, F. Effects of Plant-Emitted Monoterpenes on Anxiety Symptoms: A Propensity-Matched Observational Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 2773. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Heinbockel, T. The Effects of Essential Oils and Terpenes in Relation to Their Routes of Intake and Application. Int. J. Mol. Sci. 2020, 21, 1558. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α-and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Bharathi, M.; Kunaviktikul, W.; Klunklin, A.; Chanthapoon, C.; Chaiyasut, C. Essential Oils, Phytoncides, Aromachology, and Aromatherapy—A Review. Appl. Sci. 2022, 12, 4495. [Google Scholar] [CrossRef]
- Cui, J.; Li, M.; Wei, Y.; Li, H.; He, X.; Yang, Q.; Li, Z.; Duan, J.; Wu, Z.; Chen, Q.; et al. Inhalation Aromatherapy via Brain-Targeted Nasal Delivery: Natural Volatiles or Essential Oils on Mood Disorders. Front. Pharmacol. 2022, 13, 1214. [Google Scholar] [CrossRef]
- Schuh, A.; Immich, G. Forest Therapy—The Potential of the Forest for Your Health; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Antonelli, M.; Donelli, D.; Maggini, V.; Firenzuoli, F.; Bedeschi, E. Forest Exposure and Respiratory Function: A Literature Review. Environ. Sci. Proc. 2022, 13, 16. [Google Scholar] [CrossRef]
- Soriano, J.B.; Abajobir, A.A.; Abate, K.H.; Abera, S.F.; Agrawal, A.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; Eddine Aichour, M.T.; Alam, K.; et al. Global, Regional, and National Deaths, Prevalence, Disability-Adjusted Life Years, and Years Lived with Disability for Chronic Obstructive Pulmonary Disease and Asthma, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef]
- Kocot, K.; Barański, K.; Melaniuk-Wolny, E.; Zajusz-Zubek, E.; Kowalska, M. Exercise under Exposure to Air Pollution and Spirometry in Healthy Adults with and without Allergy. Atmosphere 2021, 12, 1168. [Google Scholar] [CrossRef]
- Sinharay, R.; Gong, J.; Barratt, B.; Ohman-Strickland, P.; Ernst, S.; Kelly, F.J.; Zhang, J.; Collins, P.; Cullinan, P.; Chung, K.F. Respiratory and Cardiovascular Responses to Walking down a Traffic-Polluted Road Compared with Walking in a Traffic-Free Area in Participants Aged 60 Years and Older with Chronic Lung or Heart Disease and Age-Matched Healthy Controls: A Randomised, Crossover Study. Lancet 2018, 391, 339–349. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, N.; Zhang, G.; Zhang, Y.; Wang, Z.; Lu, P.; Yang, W.; Geng, C.; Wang, X.; Zhang, L.; et al. Short-Term Effects of the Toxic Component of Traffic-Related Air Pollution (TRAP) on Lung Function in Healthy Adults Using a Powered Air Purifying Respirator (PAPR). Environ. Res. 2022, 214, 113745. [Google Scholar] [CrossRef]
- Guarnieri, M.; Balmes, J.R. Outdoor Air Pollution and Asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- de Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A Review on Anti-Inflammatory Activity of Monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- Quintans, J.S.S.; Shanmugam, S.; Heimfarth, L.; Araújo, A.A.S.; Almeida, J.R.G.d.S.; Picot, L.; Quintans-Júnior, L.J. Monoterpenes Modulating Cytokines—A Review. Food Chem. Toxicol. 2019, 123, 233–257. [Google Scholar] [CrossRef] [PubMed]
- Alavinezhad, A.; Khazdair, M.R.; Boskabady, M.H. Possible Therapeutic Effect of Carvacrol on Asthmatic Patients: A Randomized, Double Blind, Placebo-Controlled, Phase II Clinical Trial. Phytother. Res. 2018, 32, 151–159. [Google Scholar] [CrossRef]
- Hirota, R.; Nakamura, H.; Bhatti, S.A.; Ngatu, N.R.; Muzembo, B.A.; Dumavibhat, N.; Eitoku, M.; Sawamura, M.; Suganuma, N. Limonene Inhalation Reduces Allergic Airway Inflammation in Dermatophagoides Farinae-Treated Mice. Inhal. Toxicol. 2012, 24, 373–381. [Google Scholar] [CrossRef]
- Joy, L.; Heinrich, J.; Uwe, W.; Juergens, R.; Juergens, L.J.; Worth, H. New Perspectives for Mucolytic, Anti-Inflammatory and Adjunctive Therapy with 1,8-Cineole in COPD and Asthma: Review on the New Therapeutic Approach. Adv. Ther. 2020, 37, 1737–1753. [Google Scholar] [CrossRef]
- Worth, H.; Dethlefsen, U. Patients with Asthma Benefit from Concomitant Therapy with Cineole: A Placebo-Controlled, Double-Blind Trial. J. Asthma 2012, 49, 849–853. [Google Scholar] [CrossRef]
- Fieten, K.B.; Drijver-Messelink, M.T.; Cogo, A.; Charpin, D.; Sokolowska, M.; Agache, I.; Taborda-Barata, L.M.; Eguiluz-Gracia, I.; Braunstahl, G.J.; Seys, S.F.; et al. Alpine Altitude Climate Treatment for Severe and Uncontrolled Asthma: An EAACI Position Paper. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 1991–2024. [Google Scholar] [CrossRef]
- Cogo, A.; Piazza, M.; Costella, S.; Appodia, M.; Aralla, R.; Zanconato, S.; Carraro, S.; Piacentini, G. A Positive Effect of a Short Period Stay in Alpine Environment on Lung Function in Asthmatic Children. Pediatr. Pulmonol. 2022, 57, 2116–2121. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- King, G.G.; Bates, J.; Berger, K.I.; Calverley, P.; de Melo, P.L.; Dellacà, R.L.; Farré, R.; Hall, G.L.; Ioan, I.; Irvin, C.G.; et al. Technical Standards for Respiratory Oscillometry. Eur. Respir. J. 2020, 55, 1900753. [Google Scholar] [CrossRef] [PubMed]
- Lehtimäki, L.; Csonka, P.; Mäkinen, E.; Isojärvi, J.; Hovi, S.-L.; Ahovuo-Saloranta, A. Predictive Value of Exhaled Nitric Oxide in the Management of Asthma: A Systematic Review. Eur. Respir. J. 2016, 48, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.W.; Pandey, S.K.; Kim, K.H. Comparison of GC-MS Calibration Properties of Volatile Organic Compounds and Relative Quantification without Calibration Standards. J. Chromatogr. Sci. 2011, 49, 19–28. [Google Scholar] [CrossRef]
- Carriero, G.; Neri, L.; Famulari, D.; Di Lonardo, S.; Piscitelli, D.; Manco, A.; Esposito, A.; Chirico, A.; Facini, O.; Finardi, S.; et al. Composition and Emission of VOC from Biogas Produced by Illegally Managed Waste Landfills in Giugliano (Campania, Italy) and Potential Impact on the Local Population. Sci. Total Environ. 2018, 640–641, 377–386. [Google Scholar] [CrossRef]
- Karl, M.; Guenther, A.; Köble, R.; Leip, A.; Seufert, G. A New European Plant-Specific Emission Inventory of Biogenic Volatile Organic Compounds for Use in Atmospheric Transport Models. Biogeosciences 2009, 6, 1059–1087. [Google Scholar] [CrossRef]
- Guenther, A.; Zimmerman, P.R.; Harley, P.C.; Monson, R.K.; Fall, R. Isoprene and Monoterpene Emission Rate Variability: Model Evaluations and Sensitivity Analyses. J. Geophys. Res. 1993, 98, 12609–12617. [Google Scholar] [CrossRef]
- Hov, O.; Schjoldager, J.; Wathne, B.M. Measurement and Modeling of the Concentrations of Terpenes in Coniferous Forest Air (Norway). J. Geophys. Res. 1983, 88, 10679–10688. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L.; Bartolini, G.; Zabini, F. Temporal and Spatial Variability of Volatile Organic Compounds in the Forest Atmosphere. Int. J. Environ. Res. Public Health 2019, 16, 4915. [Google Scholar] [CrossRef]
- Borsdorf, H.; Bentele, M.; Müller, M.; Rebmann, C.; Mayer, T. Comparison of Seasonal and Diurnal Concentration Profiles of BVOCs in Coniferous and Deciduous Forests. Atmosphere 2023, 14, 1347. [Google Scholar] [CrossRef]
- Turner, D.B. A Diffusion Model for an Urban Area. J. Appl. Meteorol. 1964, 3, 83–91. [Google Scholar] [CrossRef]
- Regional Agency for Environmental Prevention and Protection of Veneto (ARPAV)—Hystorical Weather Data. Available online: https://www.arpa.veneto.it/dati-ambientali/dati-storici (accessed on 30 August 2023).
- Guo, Q.; Zhao, Y.; Shao, J.; Cao, S.; Wang, Q.; Wu, W.; Duan, X. Using Heart Rate to Estimate the Minute Ventilation and Inhaled Load of Air Pollutants. Sci. Total Environ. 2021, 763, 143011. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Carvalho, I.E.; Dal Corso, S.; Lanza, F.C. Reference Equation for Maximal Voluntary Ventilation in Children and Adolescents. Pediatr. Pulmonol. 2020, 55, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Kohara, J.; Dong, L.; Takeda, C.; Shiraki, A.; Fukagawa, H.; Mizota, T. Derivation and Validation of an Equation to Determine the Optimal Ventilator Setting in Children Undergoing Intracranial Revascularization Surgery: A Single-center Retrospective Study. Pediatr. Anesth. 2020, 30, 50–56. [Google Scholar] [CrossRef]
- Greenwald, R.; Hayat, M.J.; Dons, E.; Giles, L.; Villar, R.; Jakovljevic, D.G.; Good, N. Estimating Minute Ventilation and Air Pollution Inhaled Dose Using Heart Rate, Breath Frequency, Age, Sex and Forced Vital Capacity: A Pooled-Data Analysis. PLoS ONE 2019, 14, e0218673. [Google Scholar] [CrossRef]
- Kim, G.J.; Newth, C.J.L.; Khemani, R.G.; Wong, S.L.; Coates, A.L.; Ross, P.A. Does Size Matter When Calculating the “Correct” Tidal Volume for Pediatric Mechanical Ventilation? Chest 2018, 154, 77–83. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; ISBN 3-900051-07-0. [Google Scholar]
- Yáñez-Serrano, A.M.; Bach, A.; Bartolomé-Català, D.; Matthaios, V.; Seco, R.; Llusià, J.; Filella, I.; Peñuelas, J. Dynamics of Volatile Organic Compounds in a Western Mediterranean Oak Forest. Atmos. Environ. 2021, 257, 118447. [Google Scholar] [CrossRef]
- Quignon, P.; da Mata, P.; Faraj, F.; Guibert, S.; Bernard, A.; Léonardi, J.; Loundou, A.D.; Vitte, J.; Charpin, D. Altitude Healing Effect in Severe Asthmatic Children. Respir. Med. Res. 2021, 79, 100810. [Google Scholar] [CrossRef]
- Larsen, G.L.; Morgan, W.; Heldt, G.P.; Mauger, D.T.; Boehmer, S.J.; Chinchilli, V.M.; Lemanske, R.F.; Martinez, F.; Strunk, R.C.; Szefler, S.J.; et al. Impulse Oscillometry versus Spirometry in a Long-Term Study of Controller Therapy for Pediatric Asthma. J. Allergy Clin. Immunol. 2009, 123, 861–867.e1. [Google Scholar] [CrossRef]
- Shi, Y.; Aledia, A.S.; Galant, S.P.; George, S.C. Peripheral Airway Impairment Measured by Oscillometry Predicts Loss of Asthma Control in Children. J. Allergy Clin. Immunol. 2013, 131, 718–723. [Google Scholar] [CrossRef]
- Amazouz, H.; Bougas, N.; Thibaudon, M.; Lezmi, G.; Beydon, N.; Bourgoin-Heck, M.; Just, J.; Momas, I.; Rancière, F. Association between Lung Function of School Age Children and Short-Term Exposure to Air Pollution and Pollen: The PARIS Cohort. Thorax 2021, 76, 887–894. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Available online: https://ginasthma.org/ (accessed on 30 August 2023).



| Variable | Mean | SE Mean | StDev |
|---|---|---|---|
| Age | 14.6 | 0.31 | 2.01 |
| Height | 165.9 | 1.70 | 11.02 |
| Weight | 59.9 | 2.13 | 13.83 |
| Z-score | 0.3 | 0.15 | 1.00 |
| BMI | 21.6 | 0.57 | 3.72 |
| IBW | 52.5 | 1.45 | 9.41 |
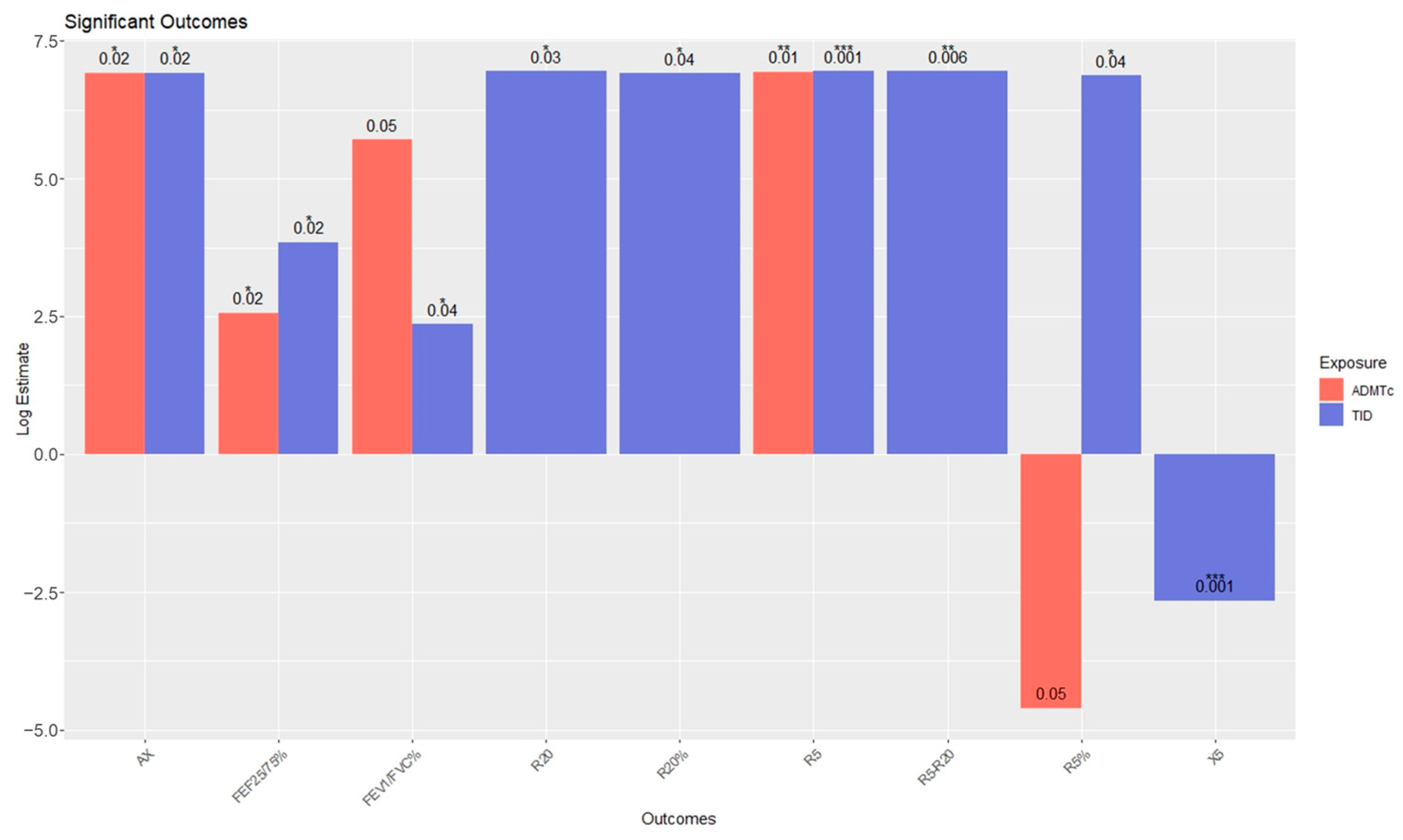
| Outcome | Estimate a | Std. Error | t | p Value | |
|---|---|---|---|---|---|
| ADMTc exposure | FEV1/FVC% | 302 | 147 | 2.05 | 0.05 |
| FEF25/75% | 13 | 558 | 2.36 | 0.02 * | |
| R5 | −5.76 | 2.21 | −2.61 | 0.01 ** | |
| R5% | −1038 | 532 | −1.95 | 0.05 | |
| AX | −28.6 | 11.7 | −2.45 | 0.02 * | |
| TID exposure | FEV1/FVC% | 10.6 | 4.9 | 2.2 | 0.04 * |
| FEF25/75% | 46.6 | 19.6 | 2.4 | 0.02 * | |
| R5 | −0.27 | 0.07 | −3.78 | <0.001 *** | |
| R5% | −70 | 34 | −2.1 | 0.04 * | |
| R20 | −0.14 | 0.06 | −2.32 | 0.03 * | |
| R20% | −39 | 19 | −2.1 | 0.04 * | |
| X5 | 0.07 | 0.03 | 2.4 | 0.02 * | |
| AX | −1.38 | 0.4 | −3.65 | <0.001 *** | |
| R5-R20 | −0.12 | 0.04 | −2.94 | 0.006 ** |
| Outcome | Estimate a | Std. Error | t | p Value | |
|---|---|---|---|---|---|
| TID exposure | FEF25/75% | 11.4 | 6.2 | 1.8 | 0.07 |
| 12.7 | 7.0 | 1.8 | 0.07 | ||
| R5% | −26 | 8.6 | −3 | 0.004 ** | |
| −24.2 | 8.9 | −2.7 | 0.01 ** | ||
| R20% | −15.8 | 7 | −2.3 | 0.03 * | |
| −17.8 | 7.4 | −2.4 | 0.02 * | ||
| X5% | −47.7 | 22.1 | −2.2 | 0.03 * | |
| −50.3 | 22.3 | −2.3 | 0.03 * | ||
| Fres% | −30.1 | 14.2 | −2.1 | 0.04 * | |
| −27.5 | 14.6 | −1.9 | 0.04 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donelli, D.; Antonelli, M.; Baraldi, R.; Corli, A.; Finelli, F.; Gardini, F.; Margheritini, G.; Meneguzzo, F.; Neri, L.; Lazzeroni, D.; et al. Exposure to Forest Air Monoterpenes with Pulmonary Function Tests in Adolescents with Asthma: A Cohort Study. Forests 2023, 14, 2012. https://doi.org/10.3390/f14102012
Donelli D, Antonelli M, Baraldi R, Corli A, Finelli F, Gardini F, Margheritini G, Meneguzzo F, Neri L, Lazzeroni D, et al. Exposure to Forest Air Monoterpenes with Pulmonary Function Tests in Adolescents with Asthma: A Cohort Study. Forests. 2023; 14(10):2012. https://doi.org/10.3390/f14102012
Chicago/Turabian StyleDonelli, Davide, Michele Antonelli, Rita Baraldi, Anna Corli, Franco Finelli, Federica Gardini, Giovanni Margheritini, Francesco Meneguzzo, Luisa Neri, Davide Lazzeroni, and et al. 2023. "Exposure to Forest Air Monoterpenes with Pulmonary Function Tests in Adolescents with Asthma: A Cohort Study" Forests 14, no. 10: 2012. https://doi.org/10.3390/f14102012
APA StyleDonelli, D., Antonelli, M., Baraldi, R., Corli, A., Finelli, F., Gardini, F., Margheritini, G., Meneguzzo, F., Neri, L., Lazzeroni, D., Ardissino, D., Piacentini, G., Zabini, F., & Cogo, A. (2023). Exposure to Forest Air Monoterpenes with Pulmonary Function Tests in Adolescents with Asthma: A Cohort Study. Forests, 14(10), 2012. https://doi.org/10.3390/f14102012