Abstract
Understanding seed characteristics, germination, and seedling establishment patterns is essential for formulating effective management strategies to control invasive species. Glossy buckthorn (Frangula alnus) is a shrub or small tree from Eurasia that has become invasive in North America, and which has negative impacts on plant communities and ecosystems. In this study, we analyzed the germination response of glossy buckthorn seeds to different temperatures (12, 14, 16, 18, 20, 24, and 28 °C), various stratification lengths (4 to 20 weeks), and scarification conditions to measure the impact on breaking seed dormancy, and the effect of light in triggering germination. Analysis using distinct time-to-event approaches, including the Kaplan–Meier estimator and Cox proportional hazard model, was employed to interpret germination data. Glossy buckthorn seeds exhibited physiological dormancy and required cold stratification to germinate. At 12 °C, only 14% of the seeds could germinate. At warmer temperatures, germination rates increased, reaching a peak of 70% at 20 °C. At 24 °C and 28 °C, germination declined, and seeds were probably induced into secondary dormancy. Scarified seeds had a higher probability of germination than non-scarified ones, even at the lowest temperatures. Darkness had a negative impact on germination at all tested temperatures. This study significantly advances our understanding of how temperature, light, stratification, and scarification impact glossy buckthorn seeds, elucidating the species’ seasonal germination patterns in North America. The results emphasize that glossy buckthorn utilizes seed banks as a primary strategy for invading and establishing in new habitats. The ungerminated seeds form persistent seed banks, ensuring F. alnus’ survival and bolstering its chances of successful establishment and invasion. As climate change drives temperature increases, it may affect seeds in the soil, altering stratification periods and consequently shifting the timing of germination.
1. Introduction
Seeds’ primary functions are dispersion, colonization of new areas, and the establishment of new plants. During germination, seeds transition from a heavily protected to a highly vulnerable phase where biotic and abiotic factors have a substantial impact on the survival, growth, and establishment of seedlings [1,2]. Germination plays a significant role in determining the traits that will manifest throughout the plant’s lifespan [3]. Consequently, comprehending the seed characteristics involved in invasiveness can aid in devising better management strategies to curb the spread and establishment of invasive species [4,5,6].
Another important factor to consider is timing of germination, as it has an important impact on seedlings’ survival due to determining in which conditions the seedling will grow [3]. Optimal germination timing helps reduce the risks linked to germinating too early and facing unstable or unfavorable environmental conditions, or germinating too late in the season and competing with earlier-germinated seedlings [1]. For this reason, germination timing is under highly selective pressure and many species have developed mechanisms to control it, enhancing their chances of survival [2]. One important mechanism that plays a major role in delaying germination is seed dormancy [1,7].
Dormancy is a seed trait and can define the range of conditions in which a seed can germinate [8]. Nondormant seeds will germinate over the broadest set of conditions, whereas dormant seeds will only do so when a narrow set of conditions are met [7,9]. Dormancy prevents seeds from germinating despite environmental conditions being suitable to germination when such conditions are unlikely to persist [7]. Several environmental factors, such as light and water availability, play an integral role in seed germination. However, temperature is the most important factor that regulates the seasonal changes in dormancy and germination [10]. Due to climate change, temperatures are projected to increase, and this may preclude, delay, or improve germination. Furthermore, these alterations will impact organisms, populations, and communities with very complex interactions and outcomes [9]. Another important factor impacting seed dormancy in several species is scarification, which may impact light and/or darkness requirements [11,12]. When buried, the seed coat can be damaged by friction with rocks, other soil particles, and/or watercourses [13]. The rupture of the seed coat or the removal of certain seed structures may be influenced or caused by several abiotic and biotic factors, including temperature (chilling and heating) [14], and chemical changes when seeds are ingested by frugivores and go through their digestive systems [15].
Frangula alnus Miller (Rhamnaceae), also known as glossy or alder buckthorn, is a shrub or small tree, native to Europe and Asia, that has become invasive and widespread in the United States and Canada [16,17]. It is typically found in wet terrestrial sites, poorly drained woodlands, riparian areas, and open and semi-open wetlands [18]. It is an aggressive invader of disturbed habitats, open areas, young forests, and plantations [19,20]. Like other invasive species, glossy buckthorn can impact species and communities [21,22], alter soil properties [23,24], reduce the abundance of native herbs, and inhibit the regeneration of trees [25], potentially reducing the production of harvestable species as white pine (Pinus strobus) [26,27], red oak (Quercus rubra L.), and sugar maple (Acer saccharum Marsh.) [28].
Identifying the environmental factors that affect an invasive species’ germination can be essential in understanding the species’ capacity to invade new sites. Despite this importance, there have been limited studies focusing on how abiotic conditions impact glossy buckthorn’s germination [8,9].
The main goal of this study was to investigate the germination response of glossy buckthorn to environmental factors under laboratory conditions. Specifically, we aimed to (i) evaluate the effect of cold stratification and scarification on breaking dormancy of glossy buckthorn seeds (ii), determine the optimum temperature for seed germination, and (iii) identify the effect of light on triggering germination. Finally, based on our results, we will discuss glossy buckthorn germination responses and how environmental conditions impact germination timing and establishment success.
2. Materials and Methods
2.1. Species and Study Area
In Québec, Frangula alnus Miller blooms from mid-May to mid-August and ripe fruits can be observed from the end of June till late September. The drupaceous fruits are globose (8.03 mm ± 0.557 long × 8894 mm ± 0.833 diameter), with a mean weight of 438 g (±102) and can contain two or three seeds (unpublished data).
The study was performed in the Ascot-Corner and Cookshire-Eaton region, in southern Québec, Canada (45°27′11.4″ N 71°41′01.2″ W). The average annual temperature is 5.6 °C and there is a total annual precipitation of 939 mm (as rain) and 206 cm (as snow) [29]. The individuals used in this study grew in three area plots located 2 to 3 km apart. These areas are predominantly covered by natural mixed forest, deciduous broadleaf, and spruce plantations. Glossy buckthorn plants grow in these areas associated with species such as Acer rubrum L., Acer saccharum Marsh, Abies balsamea Mill., Fraxinus spp., Betula alleghaniensis Britton., Picea rubens Sarg., Picea glauca (Moench) Voss., and Picea mariana Mill. Due to low fruit set in the understory, most of the fruits were collected from plants at the edges of planted and natural stands and along the sides of the roads.
2.2. Seed Collection and Treatment
Ripened fruits of glossy buckthorn were hand-harvested in September 2018 from several plants. The fruits were placed into plastic bags and kept between 3° and 5 °C for two days. Afterward, the seeds were washed and separated from the pulp. Immature or unfilled seeds were removed by the floating method [7], then clean seeds were air-dried in ambient conditions (approximately 20 °C) for 68 h. Dried seeds were then placed into sealed plastic bags and stored at 3 °C. To avoid contamination, the seeds were sterilized by soaking them in a 0.5% sodium hypochlorite solution for 10 min and rinsed multiple times under a faucet before commencing the stratification process [7,30].
2.3. Stratification
The stratification process consisted of humidifying the seeds and placing them into low-temperature conditions as part of the dormancy-breaking process [31]. To evaluate the impact of stratification on germination, seeds were placed into sealed plastic bags containing sterile sand which was humidified with distilled water and kept in a cold chamber (3 to 5 °C) for different periods (4, 8, 12, 16, and 20 weeks). At the end of each stratification period, 100 seeds were then removed from the plastic bags and placed into 9 cm Petri dishes (10 seeds per Petri dish) between two layers of humidified filter paper (Whatman Grade 1–90 mm), sealed with Para-Film, and placed into a growth chamber. At 0 weeks of stratification, the seeds were placed directly into the growth chamber without undergoing any dormancy-breaking treatment. In the growth chamber, the seeds were stored at 20 °C and exposed to a 12 h photoperiod.
2.4. Germination in a Range of Temperature
After an optimal stratification length was identified (see results), the effect of temperature on germination was assessed by placing a separate set of stratified seeds into the growth chamber with a 12 h photoperiod at 12, 14, 16, 18, and 20 °C. This set of temperatures simulated the monthly mean temperatures registered during the growing season (from May to September) [29]. Moreover, to determine the impact of higher temperatures on germination, which are common during the summer, glossy buckthorn seeds were also placed at 24 and 28 °C. One hundred seeds were used to test germination at each temperature. The seeds were divided and placed in a Petri dish between wet layers of paper filters (10 seeds per Petri dish). Seeds were then checked every 2 days for a 30-day period. Climate data used in this study were obtained from the Bromptonville meteorological station approximately 21 km away from the study area (45°29′00.000″ N; 71°57′00.000″ W, an altitude of 130 m a. s. l). Mean monthly temperature and snow values were provided based in records from 1981 to 2010 for all months of the year (Figure 1) [29].
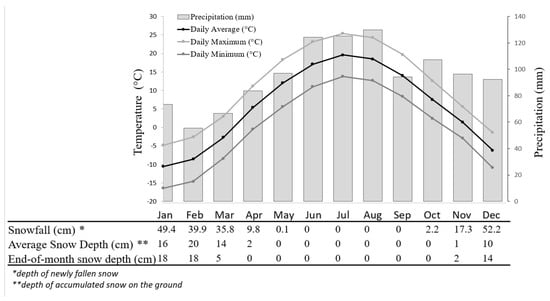
Figure 1.
Daily maximum, average, and minimum temperature, precipitation, and average snowfall and depth by month of the year registered since 1981 to 2010 at the Bromptonville station (ID station: 7020860), in Québec, Canada. Data and calculated values were provided by Environment and Climate Change Canada (graph modified from Canadian Climate Normals [29]).
2.5. Darkness and Scarification
To evaluate the effect of light and darkness on germination, a new set of stratified seeds were deposited in Petri dishes between layers of moistened filter paper, and these were then wrapped in two layers of aluminum foil to prevent the entry of light. One hundred seeds divided between 10 Petri dishes were used to test each temperature and were kept in a growing chamber at 20, 24, and 28 °C for 30 days. Every two days, to avoid seed stimulation by light, seeds were checked in the dark under a green-colored light [7].
The effect of scarification on germination was assessed by partially cutting the seed coat of stratified seeds with a scalpel. Scarified seeds were placed in Petri dishes with moistened paper filters and kept in a growing chamber at 12, 16, 20, 24, and 28 °C, with a 12 h photoperiod for 30 days. As in the other treatments, one hundred seeds were used to test each temperature and Petri dishes were examined every 2 days.
All germination experiments were performed at Université du Québec à Montréal, Canada, from November 2018 to June 2019. A total of 2100 glossy buckthorn seeds were used in this study. Seeds were kept in growth chambers for 30 days and examined every 48 h for any germinated seeds that were then counted and removed. Seeds were considered “germinated” when exhibiting a radicle length of 2 mm or more [32]. After 30 days, non-germinated seeds were gently pinched with forceps and dead seeds collapsed. The firm ones were then soaked for 24 h, cut and had their seed coat removed. The seeds were then re-soaked in a solution of 2,3,5-triphenyl tetrazolium chloride (TTC 0.1%) for 12 h at 25 °C. Seeds were analyzed under a stereoscopic microscope and red or pink uniformly stained seeds (cotyledons and embryo) were classified as viable.
2.6. Statistical Analysis
Our germination experiments generated an interval dataset. Life-table estimators were designed for this data type. However, according to [33], germination experiments are of the interval type but statistical methods designed for exact data should be used instead, with the condition that few seeds (less than 5%) were lost during the experiments. For these reasons, the Kaplan–Meier estimator and Cox proportional hazard model were used. Seed status was coded “1” for germinated and seeds that did not germinate by the end of the experiment were coded “0” (right-censored observation). Rotten seeds were also coded “0” and their removal time was recorded. All the variables were considered as categorical covariates, with dark and scarified treatments being coded “1” and light and non-scarified treatments being classified as “0”. The germination data were submitted for survival analysis (or time-to-event analysis) using the statistical software NCSS 12 Data Analysis (NCSS, LLC, Kaysville, UT, USA).
Nonparametric and semi-parametric methods of survival analysis are based on the distribution of germination times of each seed and are principally modelled using two related probability functions: survivor function and hazard function [33,34]. When analyzing germination data, the survival function S(t) is the probability that the germination time is greater than time (t), and the hazard function h(t) is the probability that a non-germinated seed at time t will germinate in the next instant. Survival indicates the cumulative non-germination whereas the hazard function indicates the chance of experiencing germination with dimensions of 1/time. An increase in the hazard rate will decrease the survivor function [35]. The nonparametric Kaplan–Meier method estimates the survivor function, without assuming a specific functional form according to the following formula [36]:
where is the duration of the study at point is the number of germinated seeds up to point , and is the number of seeds at risk of germination immediately before , i.e., the non-germinated, remaining seeds.
The value of S(t) is constant during an interval (i.e., initially and throughout the interval). The vertical lines in the curves represent the occurrence of germination and the vertical distances between intervals illustrate the change in the cumulative probability of not germinating in a specific time. The length of horizontal lines indicate how long the survival lasts and determine the curve steepness [34]. At time 0, the survival rate for this interval is 1 (100%), which means no seeds have germinated. In the following intervals, seeds begin germinating and the chance of not germinating drops for the ones left over the course of the experiment.
Stratification and temperature—Kaplan–Meier estimates of survivor functions were generated for each stratification period (4, 8, 12, 16, and 20 weeks) and for each temperature (12, 14, 16, 18, 20, 24, and 28 °C). Survival curves were built plotting the survival probability against time and then the median survival time was calculated. As seeds germinated independently of one another, the probability of germination was multiplied together using the Nelson–Aalen estimator to obtain the cumulative hazard function. Greenwood’s formula was used to calculate the linear pointwise confidence intervals for the Kaplan–Meier survival and cumulative hazard. Differences between treatments were assessed by comparing the survival curves, including the hazard rates (hazard ratio). Log-rank tests were performed to test the null hypothesis that the hazard rates of all treatments were equal. Most of these tests are performed by comparing the hazard rate against the expected hazard rate. The various hazard rates may be weighted equally through time or differently, with some tests applying heavier weight to early or later germination [35]. The hypothesis of hazard rate equality was rejected if this value was less than 0.05.
Darkness and scarification—Kaplan–Meier survival functions were also calculated for seeds that were submitted to continuous darkness and scarification treatments. Survival curves were built as described above and were compared to the ones from the control group (seeds germinated at light—12 h photoperiod and non-scarified seeds, respectively). The null hypothesis that the survivor curves did not differ was tested using a set of log-rank tests. To analyze the potential effects of multiple covariates (dark/light, scarification, and temperature) on germination simultaneously, Cox’s proportional hazard regression was used. Before testing, violations of the proportional hazard assumptions in Cox regression were assessed by plotting the log of the cumulative hazard functions against time and checking for parallelism [34,37]. Cox’s proportional hazard model calculates the hazard rate (chance of germination) and can be written as:
where h(t…) indicates the hazard, given the values of the m covariates (predictors or explanatory variables) for the respective cases (Z1, Z2, …, Zm), and t is the respective survival time. The term h0(t) is the baseline hazard and constitutes the hazard for the respective individual when all independent variable values are equal to zero, and β1 + β2 +…+ βm are regression coefficients capturing information about the relationship between the covariates and the hazard function [33,38]. In the Cox model, the goal is to estimate the βm and then test to see whether they are significantly different from 0. Partial likelihood provided the estimation of βm, and the Efron method was used to accommodate tied event times that are very common in germination data [33].
h((t), (Z1, Z2, …, Zm)) = h0(t) exp (β1z1 + β2z2 +…+ βmzm)
The different treatments (dark/light, scarified/non-scarified seeds by temperature) were compared using a log-rank test. The ratio of the total observed germinated seeds versus the expected ones was calculated. The hazard ratio for one group was about the other, and it was constant with regard to time. The Wald test was performed to test the significance of the regression coefficients. Although NCSS does not support random effects (commonly called frailty effects) functionality in its Cox modelling, Petri dish locations were rotated every two days to minimize differences in germination conditions [34].
3. Results
3.1. Seed Viability
Of the 2100 glossy buckthorn seeds used in this study, 1042 germinated, 9 were lost (rotten), and 1049 seeds did not germinate. The latter were tested for viability and only four were non-viable (two seeds from the stratification experiment: 4 and 8 weeks; and two seeds from the 20 °C and 28 °C, 12 h light conditions). Glossy buckthorn seeds used in the experiments had a viability of 99.8% (n = 2100 seeds).
3.2. Stratification
Glossy buckthorn seeds were unable to germinate without undergoing a period of stratification. Kaplan–Meier curves show the probability of not germinating for each stratification treatment per interval (in days) (Figure 2). Seeds stratified for 4 weeks had the highest probability of not germinating (S(t) = 0.89 ± 0.03 SE), followed by 8-week stratification with S(t) = 0.59 ± 0.04 SE. In other words, only 11% of the seeds germinated (1 − 0.89 = 11%) when stratified for 4 weeks. For seeds submitted to 8 weeks of stratification, the germination increased to 40%. Longer stratification periods (12, 16, and 20 weeks) decreased the probability of not germinating to 0.29, 0.31, and 0.27 (±0.04 SE), resulting in 71%, 69%, and 72% germination, respectively. The median germination time occurred at 14- to 16-day intervals. When comparing all the stratification periods, our results from the Mantel–Haenszel log-rank test (Table 1) show that the survival curves of 4- and 8-week stratification periods were significantly different from all the other stratification periods (p < 0.001). The 12-, 16-, and 20-week stratification periods did not differ (p > 0.1), indicating that 12 weeks is the minimum stratification time for optimal germination, and if the seeds stay longer, there is no recognizable impact on final germination outcomes.
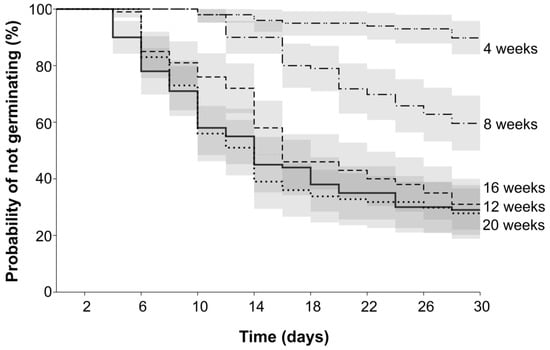
Figure 2.
Kaplan–Meier estimates of survivor functions for Frangula alnus seeds submitted to different stratification periods (in weeks). The Y-axis reflects the cumulative probability, and the time in days is indicated along the X-axis. The curves represent the probability of not germinating initially and throughout the interval until the beginning of the next interval (grey areas indicate 95% confidence intervals).

Table 1.
Mantel–Haenszel log-rank test results for comparisons between stratification periods (in weeks). The hazard ratios were calculated by comparing the hazard rate to the expected one for each treatment; hazard ratio = 1).
3.3. Temperature
Seeds at 20 °C had the lowest chance of not germinating (0.30 ± 0.04 SE), resulting in 70% germination (Figure 3). At 18 and 16 °C, seeds reached 53 and 45% germination with S(t) = 0.47 ± 0.04 SE and 0.55 ± 0.04 SE, respectively. At the coldest temperature (12 °C), germination occurred in 14% of the seeds and rose to 34% at 14 °C (S(t) = 0.86 ± 0.03 SE and 0.66 ± 0.04 SE, respectively) (Figure 3). The results of the Mantel–Haenszel log-rank test (hazard ratios) (Table 2) show that the survival functions of seeds at 12 °C are significantly different from all the other temperatures (p < 0.0001). When comparing germination at 16 °C to 14 °C and 18 °C, no significant differences were found. However, germination at 14 °C was significantly different from germination at 18 °C. At warmer temperatures (24 and 28 °C), germination reached 31% and 29%, respectively. The Kaplan–Meier curve from seeds at 20 °C was significantly different from 24 and 28 °C. However, 24 and 28 °C conditions were not significantly different from each other. When temperatures were warmer or colder than 20 °C, the probability of a seed not germinating increased; however, higher temperatures did not delay germination that started at 4–6-day intervals. At 12 and 14 °C, a delay was observed, with seeds starting to germinate later at 10–12-day intervals.
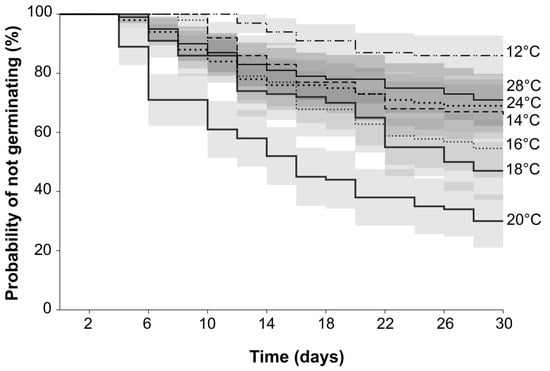
Figure 3.
Kaplan–Meier estimates of survivor functions of Frangula alnus seeds germinated at 12, 14, 16, 18, 20, 24, and 28 °C. All curves with 95% confidence intervals.

Table 2.
Results of the Mantel–Haenszel log-rank test for temperatures (°C) (see Table 1 for details).
3.4. Dark and Light
Darkness had a negative impact on germination as it increased the probability of not germinating for all tested temperatures (20, 24, and 28 °C) (Kaplan–Meier curves are shown in Figure 4). Germination at 20 °C went down from 70% (at 12 h light) to 54% (in the dark), from 31% to 15% at 24 °C, and from 29% to only 7% at 28 °C. A 2-day delay in germination was also observed for seeds that germinated in the dark for all temperatures. The results of the log-rank tests demonstrated a significant difference between all treatments (Table 3). However, a test that placed higher weight on hazards at the end of the germination period (Fleming–Harrington) indicated that the survival curves for germination in light and dark at 20 °C were equal. The potential effects of light/dark and temperature (as covariates) were tested by the Cox proportional hazard model considering 20 °C and the 12 h photoperiod as reference groups. These results are shown in Table 4. All regression coefficients () are negative, indicating that the seeds tested in darkness and at warmer temperatures had a lower probability of germination than the ones in light at 20 °C. Hazard ratios are represented by exponentiated coefficients (Exp that present the effect size of covariates. A hazard ratio equal to 0.5827 (<1) indicates that the variable “darkness” alone will decrease the hazard function by 41.7%. Warmer temperatures (exp = 0.3096 for 24 °C and 0.2788 for 28 °C) and darkness decreased the hazard function even further by 69% and 72%, respectively. Moreover, the factor of light can be calculated by the reverse 1/exp (dark) and was equal to 1.71, indicating that the probability of germination increased by a factor of 1.71 due to light (p = 0.0029) when the temperature was constant. The p-value (<0.0029) further suggests a strong relationship between the covariates (darkness and temperature) and reduced chance of germination. Moreover, when the 95% confidence intervals do not overlap with the values of 1, it is further indicative of a strong relationship [34].
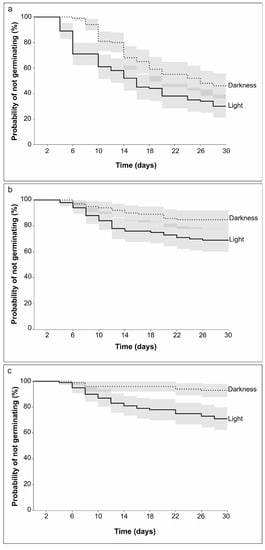
Figure 4.
Kaplan–Meier survival curves of Frangula alnus seeds germinated in light and dark conditions at (a) 20 °C; (b) 24 °C; and (c) 28 °C (95% confidence intervals).

Table 3.
Log-rank test results for survival functions between light and dark treatments at each temperature (20, 24, and 28 °C). See Jones and Crowley [39] for more details.

Table 4.
Summary table of the Cox’s PH model for Frangula alnus germination data. Darkness and temperature were selected as covariates. Seeds that germinated in light at 20 °C were selected as the reference group.
3.5. Scarification
Scarification decreased the probability of not germinating at all tested temperatures (Kaplan–Meier curves are shown in Figure 5). At 12 °C, germination increased from 14% (S(t) = 0.86 ± 0.03 SE) to 37% (S(t) = 0.63 ± 0.04 SE) when the seeds were scarified. At warmer temperatures (16 to 28 °C), germination occurred in 96 to 99% of scarified seeds. Log-rank tests showed a significant difference between scarified and non-scarified seeds at all temperatures. However, when using tests that applied heavier weighting to hazards at the end of the germination period, there was no difference between scarified and non-scarified seeds at 12 °C (Table 5).
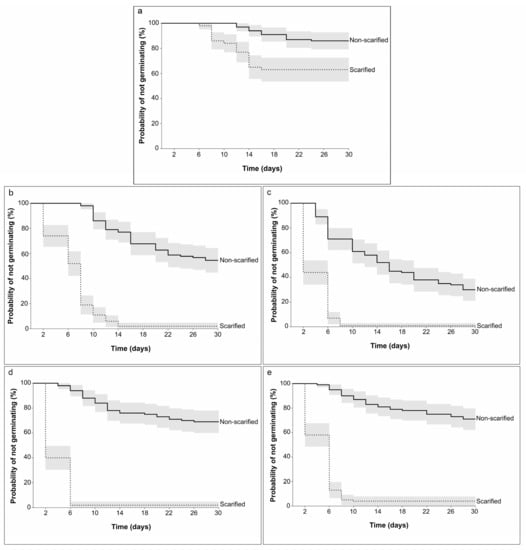
Figure 5.
Kaplan–Meier survival curves of Frangula alnus seeds that were scarified and non-scarified at different temperatures: (a) 12 °C, (b) 16 °C, (c) 20 °C, (d) 24 °C, and (e) 28 °C; 95% confidence intervals.

Table 5.
Results of log-rank tests for survival functions of Frangula alnus between scarified and non-scarified seeds at different temperatures (16, 20, 24, and 28 °C).
Results from the Cox proportional model are shown in Table 6. Scarification had a regression coefficient of βi 2.184, indicating that scarified seeds had a higher probability of germination than non-scarified ones. The regression coefficient βi for temperatures both colder and warmer than 20 °C was negative, representing a decrease in germination associated with this covariate of non-scarified seeds. The negative effect of temperature was offset by scarification, which increased the hazard function. Colder temperatures were still able to decrease germination at 12 and 16 °C. At warmer temperatures, however, scarification obscured the effect of temperature and resulted in an increase in germination. In the non-scarified seed trials, temperature decreased the hazard rate by 87% at 12 °C. When scarified seeds were submitted to the same temperature, a reduction of 64% was observed. At warmer temperatures, scarification increased the hazard function throughout the study period by 218.5% for seeds at 24 °C, and by 121.8% for seeds tested at 28 °C.

Table 6.
Cox’s PH model results showing the effects of the covariates scarification and temperature on seed germination of Frangula alnus. Temperatures: 12, 16, 24, and 28 °C. Non-scarified seeds at 20 °C were used as a reference group.
4. Discussion
4.1. Breaking Dormancy and Germination Pattern
Glossy buckthorn seeds exhibit physiological dormancy (PD), and do not germinate immediately after being dispersed as they require a minimum period of cold stratification to break dormancy [40,41]. According to our results, F. alnus seeds achieved higher germination rates at 20 °C when stratified from 12 to 20 weeks. Similar results were found by [42], with a higher germination rate of 79% from seeds stratified from 16 to 20 weeks. In her research, samples stratified for 8 weeks or less resulted in minimal germination. In the study area, the stratification of glossy buckthorn seeds may start as early as mid-November, when the daily maximum temperature drops and remains below 5 °C. In the following months, temperatures decrease further, allowing glossy buckthorn seeds to stratify and for dormancy to be broken. In temperate deciduous forest, 70% of the tree and 66% of shrub species have physiological dormancy, with tree species requiring from 26 to 180 days of cold stratification and shrub species needing from 30 to 181 days of cold stratification before being able to germinate [7].
According to our findings, glossy buckthorn seeds require relatively higher temperatures to germinate (only 14% germination was observed at 12 °C), which may inhibit seedling emergence in the early spring, such as in April (daily average of 5.3 °C (±2.9 SD)). Seeds of some temperate tree and shrub species are known to germinate at lower temperatures (see table in [7]) [12]. For example, two species frequently observed in our study area, Acer rubrum and A. saccharum, both germinate at 5 °C. The transition from winter to spring in Québec has unstable conditions with frequent snowfall [29]. Therefore, this delay in beginning germination may increase the chance of survival of glossy buckthorn seedlings, giving the species a competitive advantage over earlier germinating species.
Based on our results, glossy buckthorn seeds begin germinating at the very end of spring when temperatures reach 12 °C (May). Initially and throughout the summer, germination will increase and speed as the temperature rises (14, 16, and 18 °C), reaching the highest germination rate (70%) at 20 °C. At warmer temperatures (24 and 28 °C), a decrease in germination was observed, suggesting that higher temperatures experienced in midsummer prevent most seeds from germinating and/or induce them into dormancy again (secondary dormancy). This may be a reason why the glossy buckthorn seeds do not germinate in early autumn when temperatures are very similar to the ones experienced in spring/early summer, as the seedlings would not have enough time to grow before winter (registered average daily temperature of 14.0 °C (±1.3 SD) in September and 7.5 °C (±1.3 SD) in October). A similar germination timing was observed by Eisenhaure et al. [43] while studying the impacts of different substrates on glossy buckthorn seedlings’ emergence in New Hampshire, USA. Mean temperatures from May to August in their study areas were 0.7 to 1.9 °C warmer than the ones registered for our study site [29]. The authors recorded the greatest seedling emergence at the beginning of June, with a sharp decline occurring in early July and complete cessation by mid-August and September [43]. Their findings, corroborated by ours, help to identify glossy buckthorn’s response to temperatures and outline its germination pattern.
4.2. Effects of Light and Scarification on Germination
Light is another important factor that can prompt or prevent seed germination [1,7,44,45]. Frangula alnus seeds can germinate in both light and darkness, although with less success for the latter. Early in the spring, more light can reach the soil in temperate forests when deciduous leaves are not open. According to our findings, glossy buckthorn seeds in well-lit habitats will germinate less frequently when at cold temperatures (12, 14, and 16 °C ) than seeds in shaded environments with warmer temperatures (18 and 20 °C).
Scarification has different effects on the germination of several species, including changes in light and/or darkness requirements [11] and dormancy levels [12]. In glossy buckthorn, scarified seeds could overcome limiting temperatures and germinate twice as often at colder temperatures (12 °C), and more than three times as often when submitted to warmer temperatures (24 and 28 °C), compared to non-scarified seeds. The speed of germination was also higher in scarified seeds, as germination began 2 to 8 days earlier than non-scarified seeds (see Kaplan–Meier germination curves for comparisons). However, to further understand the effect of scarification on glossy buckthorn seeds, studies comparing natural scarification caused by the seed’s passage through a frugivore’s gut (often birds) and the effect of burial on germination must be conducted, as we are not aware of any study on these subjects.
4.3. Spread and Invasion Strategies
According to Pyšek and Richardson [46], invasive species germinate earlier, more quickly, and/or over a wider range of environmental conditions than native or non-invasive ones, which provides a competitive advantage [47,48,49]. However, in volatile environments, forming a seed reservoir may be a better strategy for invasive species as the buried seeds can ensure the persistence of the species while mitigating competition [2,50,51]. Glossy buckthorn seeds require cold stratification, germinate better in light, and when scarified achieve greater germination rates at mild temperatures which are known to delay germination and promote seed burial [7,52]. Glossy buckthorn seeds are known to form seed banks that can remain viable in the soil for up to three years [18]. Our results suggest that the formation of seed banks may be the main strategy used by glossy buckthorn to invade and establish in novel habitats.
The timing and rate of germination affect the relationship between invasive and native species, as these interactions influence the ability of each species to compete for space and resources [10,53]. Glossy buckthorn fruits produced and released at the end of the growing season are dormant and will spend the least favorable season buried in soil. As most of the seeds require higher temperatures and light to germinate, germination will occur later when environmental conditions are more stable and when competition with other plants may be reduced (e.g., in open areas or recently formed canopy gaps), which can potentially increase its fitness [2,54]. Invasive species with light-sensitive seeds (photoblastic) may have an advantage during the spreading and establishment processes as most seeds will not germinate when deeply buried [2,7]. Suitable temperatures, disturbances resulting in the canopy opening, or other exposure of the seeds in the soil may promote germination during most of the growing season, which would allow seedlings to grow and become established before the temperature drops below 0 °C. When germination does not occur, the seeds are incorporated into seed banks. We believe, as an invasive species, glossy buckthorn capitalizes on these seed traits by ensuring germination at “safer” moments and spaces or otherwise amassing a persistent seed bank that promotes maintenance of the species in case of unfavorable conditions.
By 2100, eastern Canada will face warmer annual temperatures (3 to 5 °C), leading to longer growing seasons, while precipitation will decrease and increase during the summer and winter, respectively [55,56]. Shorter winters may diminish stratification periods and decrease the number of seeds germinating. However, as a 12-week stratification period may successfully break dormancy, it is unlikely that glossy buckthorn will be affected in Québec, but warmer regions of the United States may be impacted. A temperature increase will result in glossy buckthorn seeds germinating earlier, such as near the beginning of spring, granting the seeds access to a longer growing season. However, germination may also end earlier due to the high-temperature peaks during the summer, shifting the germination period. Beyond the impacts on stratification and germination, it is difficult to predict how glossy buckthorn will be affected by climate change, as the climate alters resource availability, landscapes, and impacts the dynamics among species.
5. Conclusions
This study provides us with a better understanding of how temperature, light, stratification, and scarification affect glossy buckthorn seeds and their seasonal germination pattern in North America. Glossy buckthorn seeds require a cold stratification to germinate and achieve higher germination rates at moderate temperatures and in well-lit conditions. Scarified seeds had the highest germination rates, were able to overcome limiting temperatures, and germinated faster than the non-scarified seeds. These conditions are known to delay germination and promote seed burial. Frangula alnus may rely on the formation of persistent seed banks as a strategy to colonize new habitats. Climate change will increase temperatures which may impact the seeds buried within the soil, including the length of stratification periods in some areas of North America and the timing of germination. Warmer temperatures in the spring and peaks earlier in the summer may also shift the germination period of glossy buckthorn. The experimental conditions of constant moist substrate and temperatures applied in this study are simple when compared to the complexity of reactions triggered by moisture stress and fluctuating temperatures in natural environments. For this reason, we recommend further studies and recognize the need for caution when interpreting our findings. Despite this limitation, the data show several coherent patterns that can be used to better predict the impact of climate change on glossy buckthorn germination.
Author Contributions
All three authors worked together in conceptualization, methodology, and validation. Formal analysis, T.C.; investigation, T.C. and F.G.; resources, T.C.; data curation, T.C.; writing—original draft preparation, T.C.; writing—review and editing, T.C., D.H. and F.G.; visualization, T.C.; supervision and project administration, F.G.; funding acquisition, F.G. and D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fonds de recherche du Québec—Nature et technologies through a graduate scholarship (T. Custodio) (scholarship 207620) and co-funding from Ouranos and the Mitacs Accelerate program (internship—T. Custodio) (Grant IT10586).
Data Availability Statement
Data available on demand to the corresponding author.
Acknowledgments
We thank Mario Dionne and Sylvain Dallaire for support during seed collection, treatment and experimentation, discussion, and advice. We also thank Université du Québec à Montréal (UQAM) for supplying growth chambers and laboratory access, and the landowners and the “Agence de mise en valeur de la forêt privée de l’Estrie” for supporting this study. Finally, authors are greatful to Mariana Custodio for graphical support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Fenner, M.K.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005; p. 276. [Google Scholar]
- Gioria, M.; Pyšek, P. Early bird catches the worm: Germination as a critical step in plant invasion. Biol. Invasions 2017, 19, 1055–1080. [Google Scholar] [CrossRef]
- Donohue, K.; De Casas, R.R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, Postgermination Adaptation, and Species Ecological Ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Chapter 6—The Role of Seed Ecology in Improving Weed Management Strategies in the Tropics. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2010; Volume 105, pp. 221–262. [Google Scholar]
- Brownsey, R.N.; Kyser, G.B.; DiTomaso, J.M. Seed and Germination Biology of Dittrichia graveolens (Stinkwort). Invasive Plant Sci. Manag. 2013, 6, 371–380. [Google Scholar] [CrossRef]
- Bhatt, A.; Chen, X.; Pompelli, M.F.; Jamal, A.; Mancinelli, R.; Radicetti, E. Characterization of Invasiveness, Thermotolerance and Light Requirement of Nine Invasive Species in China. Plants 2023, 12, 1192. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014; p. 680. [Google Scholar]
- Vleeshouwers, L.M.; Bouwmeester, H.J.; Karssen, C.M. Redefining Seed Dormancy: An Attempt to Integrate Physiology and Ecology. J. Ecol. 1995, 83, 1031–1037. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Change Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Benech-Arnold, R.L.; Sánchez, R.A.; Forcella, F.; Kruk, B.C.; Ghersa, C.M. Environmental control of dormancy in weed seed banks in soil. Field Crops Res. 2000, 67, 105–122. [Google Scholar] [CrossRef]
- Takaki, M.; Gama, L.H.P. The role of the seed coat in phytochrome-controlled seed germination in Lactuca sativa L. cv. Grand Rapids. Seed Sci. Technol. 1998, 26, 355–362. [Google Scholar]
- Matus-Cádiz, M.A.; Hucl, P. Rapid and Effective Germination Methods for Overcoming Seed Dormancy in Annual Canarygrass. Crop Sci. 2005, 45, 1696–1703. [Google Scholar] [CrossRef]
- Vilela, A.E.; Ravetta, D.A. The effect of seed scarification and soil-media on germination, growth, storage, and survival of seedlings of five species of Prosopis L. (Mimosaceae). J. Arid Environ. 2001, 48, 171–184. [Google Scholar] [CrossRef]
- Rees, M. Seed Dormancy. In Plant Ecology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1996; pp. 214–238. [Google Scholar]
- Mooney, H.A.; Simpson, B.B.; Solbrig, O.T. Mesquite, Its Biology in Two Desert Ecosystems; Halsted Press: Sydney, Australia, 1977; pp. 26–43. [Google Scholar]
- Catling, P.M.; Porebski, Z.S. The history of invasion and current status of glossy buckthorn, Rhamnus frangula, in southern Ontario. Can. Field Nat. 1994, 108, 305–310. [Google Scholar]
- Converse, C.K. Element Stewardship Abstract for Rhamnus cathartica, Rhamnus frangula (syn. Frangula alnus); The Nature Conservancy: Arlington, VA, USA, 1984. [Google Scholar]
- Godwin, H. Frangula alnus Miller. J. Ecol. 1943, 31, 77–92. [Google Scholar] [CrossRef]
- Fagan, M.E.; Peart, D.R. Impact of the invasive shrub glossy buckthorn (Rhamnus frangula L.) on juvenile recruitment by canopy trees. For. Ecol. Manag. 2004, 194, 95–107. [Google Scholar] [CrossRef]
- Frappier, B.; Eckert, R.T.; Lee, T.D. Potential impacts of the invasive exotic shrub Rhamnus frangula L. (glossy buckthorn) on forests of southern New Hampshire. Northeast. Nat. 2003, 10, 277–296. [Google Scholar] [CrossRef]
- Fiedler, A.K.; Landis, D.A.; Arduser, M. Rapid Shift in Pollinator Communities Following Invasive Species Removal. Restor. Ecol. 2012, 20, 593–602. [Google Scholar] [CrossRef]
- Fiedler, A.K.; Landis, D.A. Biotic and Abiotic Conditions in Michigan Prairie Fen Invaded by Glossy Buckthorn (Frangula alnus). Nat. Areas J. 2012, 32, 41–53. [Google Scholar] [CrossRef]
- Stokdyk, J.P.; Herrman, K.S. Effects of Frangula alnus on soil microbial communities and biogeochemical processes in Wisconsin forests. Plant Soil 2016, 409, 65–75. [Google Scholar] [CrossRef]
- Stokdyk, J.P.; Herrman, K.S. Short-Term Impacts of Frangula alnus Litter on Forest Soil Properties. Water Air Soil Pollut. 2014, 225, 2000. [Google Scholar] [CrossRef]
- Cunard, C.; Lee, T.D. Is patience a virtue? Succession, light, and the death of invasive glossy buckthorn (Frangula alnus). Biol. Invasions 2009, 11, 577–586. [Google Scholar] [CrossRef]
- Lanzer, N.B.; Lee, T.D.; Ducey, M.J.; Eisenhaure, S.E. Sapling white pine (Pinus strobus L.) exhibits growth response following selective release from competition with glossy buckthorn (Frangula alnus P. Mill) and associated vegetation. For. Ecol. Manag. 2017, 404, 280–288. [Google Scholar] [CrossRef]
- Lee, T.D.; Eisenhaure, S.E.; Gaudreau, I.P. Pre-logging Treatment of Invasive Glossy Buckthorn (Frangula alnus Mill.) Promotes Regeneration of Eastern White Pine (Pinus strobus L.). Forests 2017, 8, 16. [Google Scholar] [CrossRef]
- Hamelin, C.; Truax, B.; Gagnon, D. Invasive glossy buckthorn impedes growth of red oak and sugar maple under-planted in a mature hybrid poplar plantation. New For. 2016, 47, 897–911. [Google Scholar] [CrossRef]
- Environnement Canada. Normales et Moyennes Climatiques de la Région de l’Estrie; Environnement Canada: Ottawa, ON, Canada, 2023. [Google Scholar]
- Thomsen, K.; Diklev, S. Laboratory Manual for Basic Tree Seed Studies; DANIDA Forest Seed Centre: Humlebaek, Denmark, 2000; p. 31. [Google Scholar]
- Cavieres, L.A.; Arroyo, M.T.K. Seed germination response to cold stratification period and thermal regime in Phacelia secunda (Hydrophyllaceae)—Altitudinal variation in the mediterranean Andes of central Chile. Plant Ecol. 2000, 149, 1–8. [Google Scholar] [CrossRef]
- Mazliak, P.; Côme, D. Germination. In Physiologie Végétale II: Croissance et Développement; Hermann: Paris, France, 1982; p. 476. [Google Scholar]
- McNair, J.N.; Sunkara, A.; Frobish, D. How to analyse seed germination data using statistical time-to-event analysis: Non-parametric and semi-parametric methods. Seed Sci. Res. 2012, 22, 77–95. [Google Scholar] [CrossRef]
- Romano, A.; Stevanato, P. Germination Data Analysis by Time-to-Event Approaches. Plants 2020, 9, 617. [Google Scholar] [CrossRef]
- Klein, J.P.; Moeschberger, M.L. Survival Analysis: Techniques for Censored and Truncated Data, 2nd ed.; Springer: New York, NY, USA, 2003. [Google Scholar]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Hess, K.R. Graphical methods for assessing violations of the proportional hazards assumption in cox regression. Stat. Med. 1995, 14, 1707–1723. [Google Scholar] [CrossRef]
- Hill, T.; Lewicki, P.; Lewicki, P. Statistics: Methods and Applications: A Comprehensive Reference for Science, Industry, and Data Mining; StatSoft, Inc.: Tulsa, OK, USA, 2006; p. 854. [Google Scholar]
- J ones, M.P.; Crowley, J. A general class of nonparametric tests for survival analysis. Biometrics 1989, 45, 157–170. [Google Scholar] [CrossRef]
- Nikolaeva, M.; Razumova, M.; Gladkova, V. Spravochnik po Prorashchivaniyu Pokoyashchikhsya Semyan; Nauka: Leningrad, Russia, 1985; p. 400. [Google Scholar]
- Rosbakh, S.; Baskin, C.C.; Baskin, J.M. Nikolaeva et al.’s reference book on seed dormancy and germination. Ecology 2020, 101, e03049. [Google Scholar] [CrossRef]
- Nielsen, K.K. Dormancy in seeds from different positions on individual plants. Acta Hortic. 1988, 226, 255–262. [Google Scholar] [CrossRef]
- Eisenhaure, S.E.; McCarthy, H.C.; O’Del, J.N.; Giguere, H.; Symonds, C.J.; Lee, T.D. Effects of turf, leaf litter, and soil compaction on emergence and establishment of invasive glossy buckthorn (Frangula alnus). For. Ecol. Manag. 2021, 484, 118933. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C.; Spooner, D.M. Role of temperature, light and date: Seeds were exhumed from soil on germination of four wetland perennials. Aquat. Bot. 1989, 35, 387–394. [Google Scholar] [CrossRef]
- Yin, L.; Wang, C.; Chen, Y.; Yu, C.; Cheng, Y.; Li, W. Cold stratification, light and high seed density enhance the germination of Ottelia alismoides. Aquat. Bot. 2009, 90, 85–88. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Traits Associated with Invasiveness in Alien Plants: Where Do we Stand. In Biological Invasions; Nentwig, W., Ed.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2007; pp. 97–125. [Google Scholar]
- Gioria, M.; Jarošík, V.; Pyšek, P. Impact of invasions by alien plants on soil seed bank communities: Emerging patterns. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 132–142. [Google Scholar] [CrossRef]
- Gioria, M.; Pyšek, P.; Osborne, B.A. Timing is everything: Does early and late germination favor invasions by herbaceous alien plants? J. Plant Ecol. 2016, 11, 4–16. [Google Scholar] [CrossRef]
- Gioria, M.; Pyšek, P. The legacy of plant invasions: Changes in the soil seed bank of invaded plant communities. Bioscience 2016, 66, 40–53. [Google Scholar] [CrossRef]
- Gioria, M.; Pyšek, P.; Moravcova, L. Soil seed banks in plant invasions: Promoting species invasiveness and long-term impact on plant community dynamics. Preslia 2012, 84, 327–350. [Google Scholar]
- Thompson, K.; Grime, J.P. Seasonal Variation in the Seed Banks of Herbaceous Species in Ten Contrasting Habitats. J. Ecol. 1979, 67, 893–921. [Google Scholar] [CrossRef]
- Grime, J.P.; Mason, G.; Curtis, A.V.; Rodman, J.; Band, S.R. A Comparative Study of Germination Characteristics in a Local Flora. J. Ecol. 1981, 69, 1017–1059. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.A. Resource competition in plant invasions: Emerging patterns and research needs. Front. Plant Sci. 2014, 5, 501. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977; p. 932. [Google Scholar]
- Bush, E.; Lemmen, D.S. Canada’s Changing Climate Report; Government of Canada: Ottawa, ON, Canada, 2019; pp. 1–444. [Google Scholar]
- Dukes, J.S.; Pontius, J.; Orwig, D.; Garnas, J.R.; Rodgers, V.L.; Brazee, N.; Cooke, B.; Theoharides, K.A.; Stange, E.E.; Harrington, R.; et al. Responses of insect pests, pathogens, and invasive plant species to climate change in the forests of northeastern North America: What can we predict? Can. J. For. Res. 2009, 39, 231–248. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).