First Report on a Cliff-Nesting Pair of Black Storks (Ciconia nigra Linnaeus, 1758) and Their Nestlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Animals
2.3. Video Recording System
- Pre-hatching from 27 March to 6 May;Post-hatching from 10 May to 17 July.
2.4. Statistical Analysis
3. Results and Discussion
3.1. Exclusive Patterns in the Pre-Hatching Phase
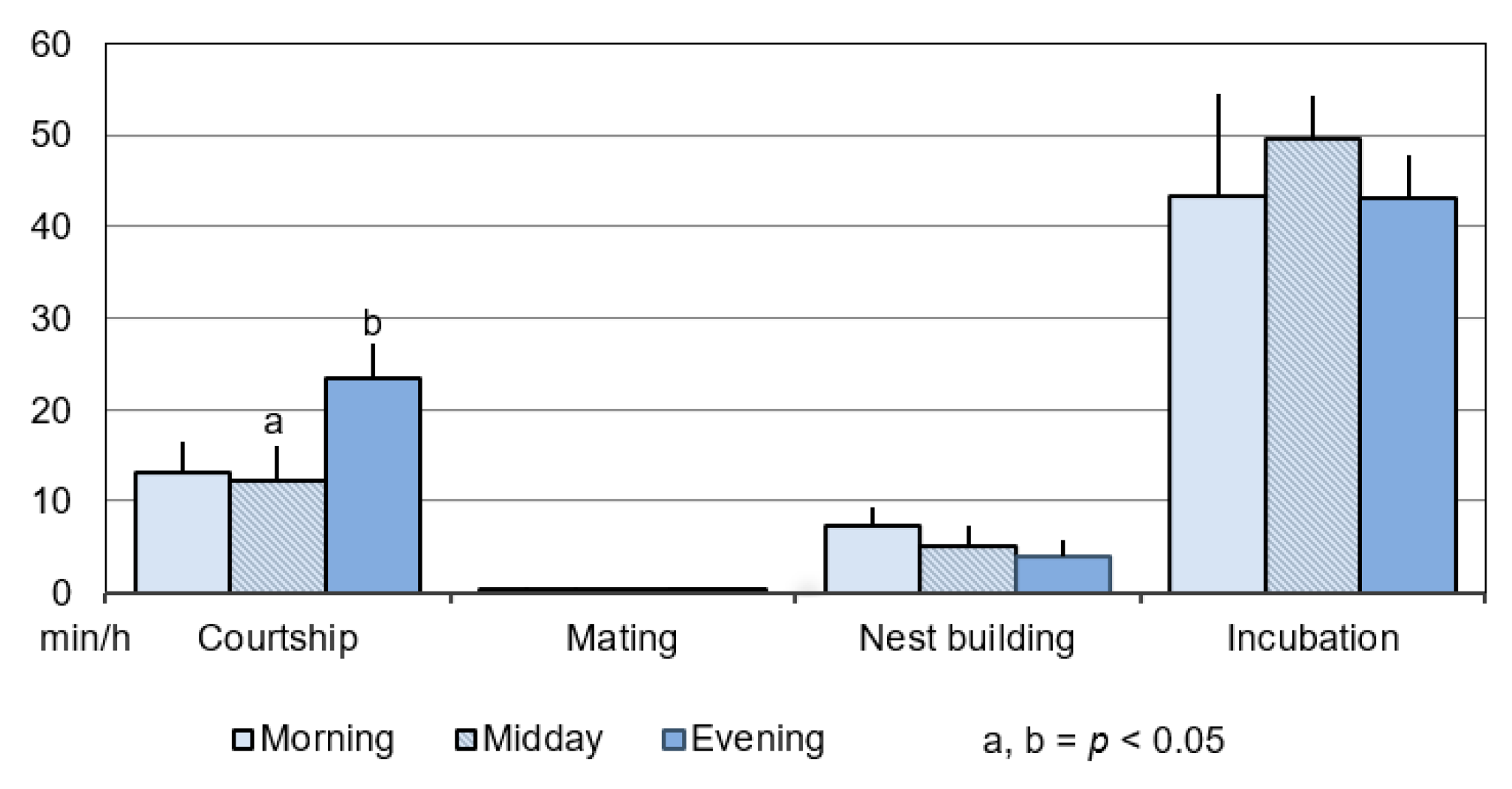
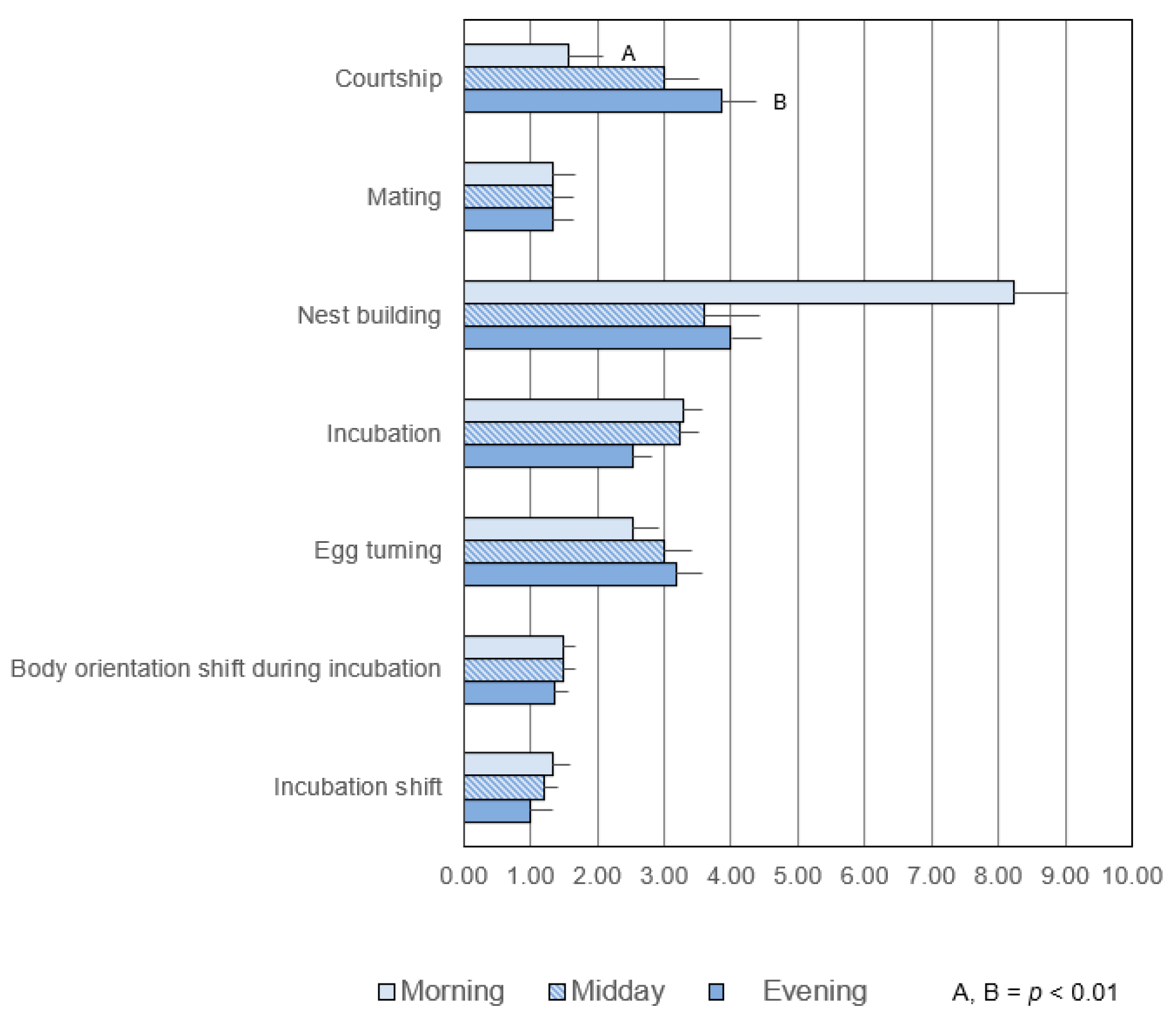
3.1.1. Duration
3.1.2. Frequency
3.2. Common Activities in the Pre- and Post-Hatching Phases
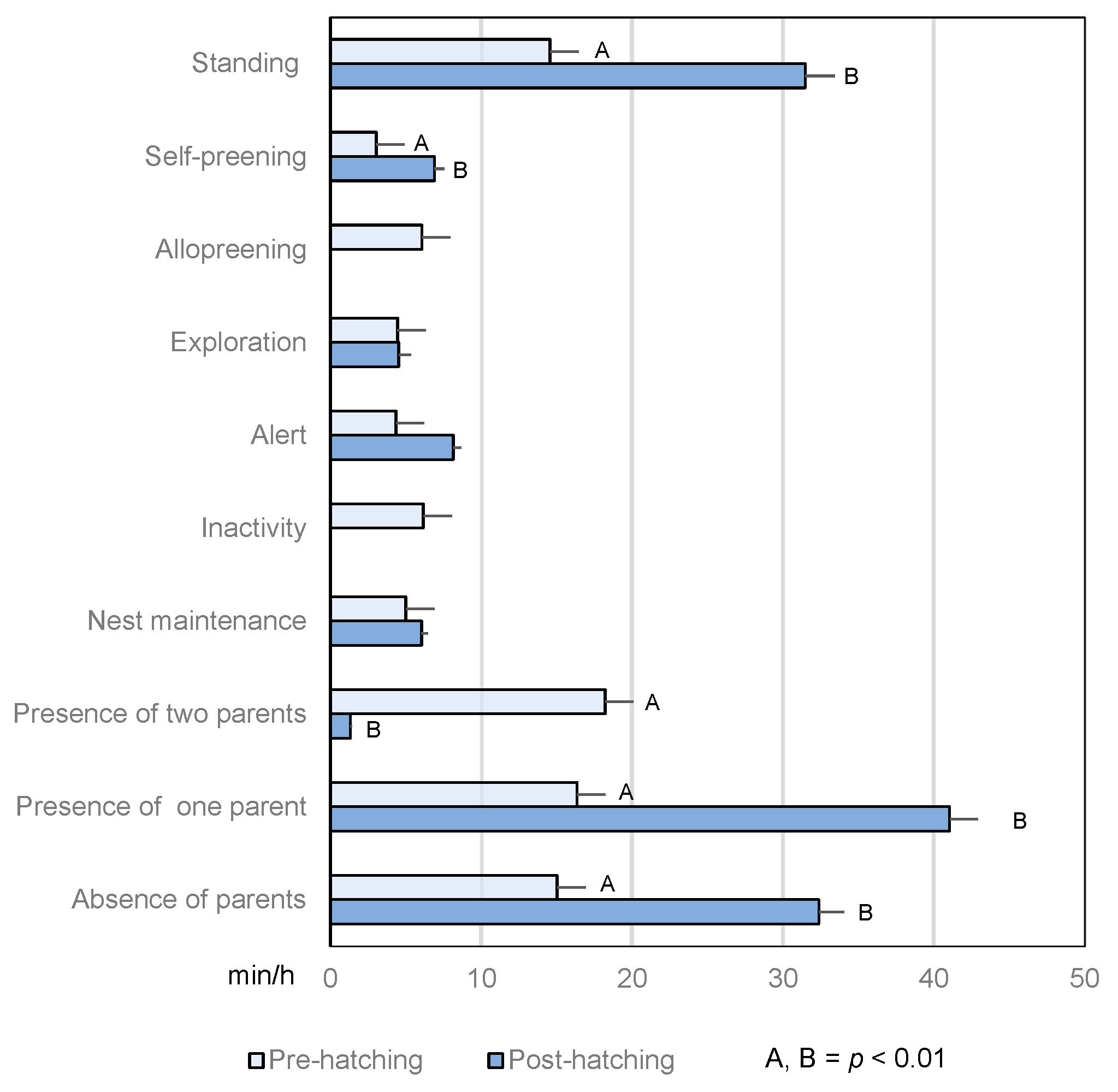
3.2.1. Duration
3.2.2. Frequency
3.3. Exclusive Activities in the Post-Hatching Phase
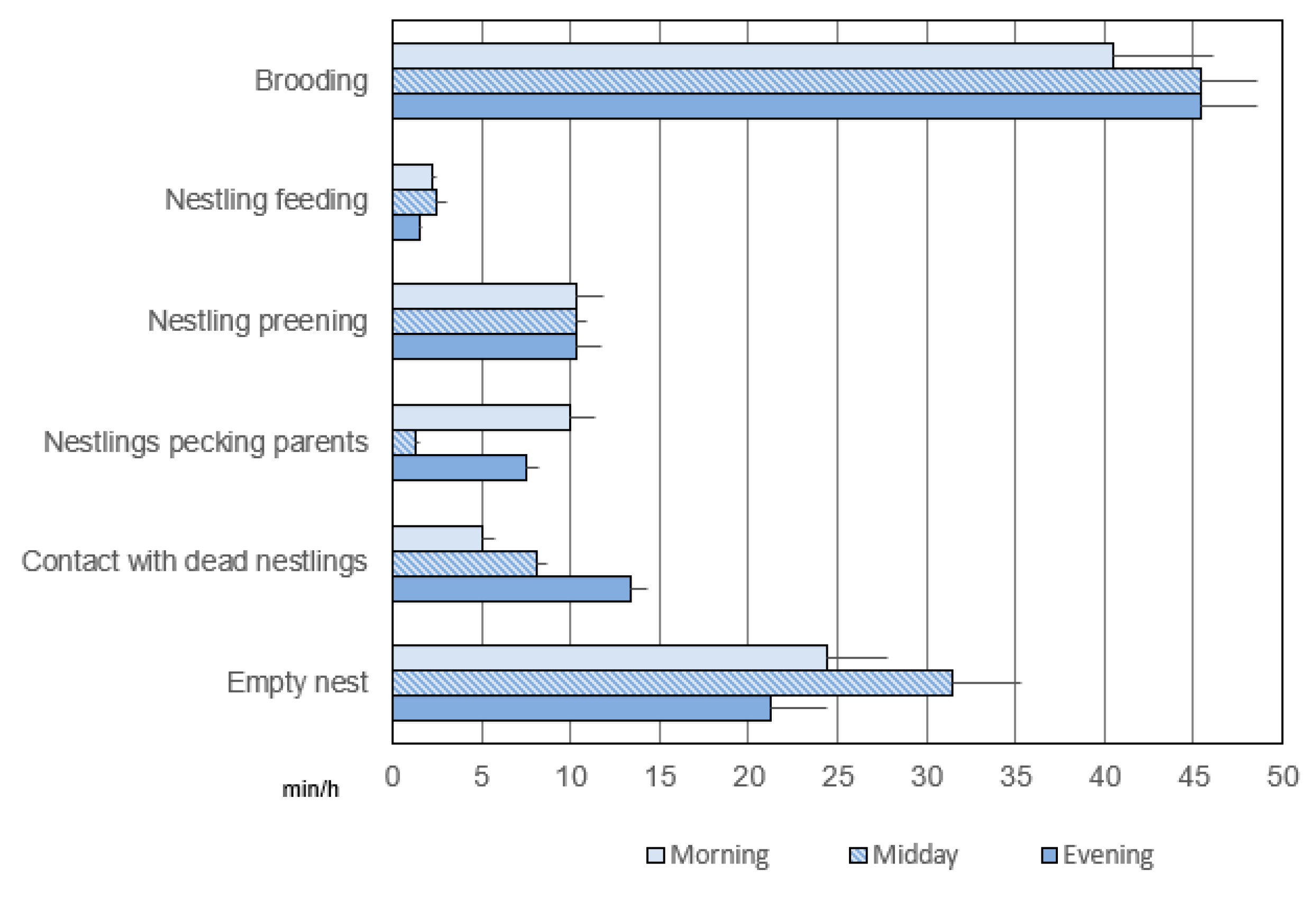
3.3.1. Duration
3.3.2. Frequency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A








References
- Tamas, E.A. Breeding and Migration of the Black Stork (Ciconia nigra), with Special Regard to a Central European Population and the Impact of Hydro-Meteorological Factors and Wetland Status. Ph.D. Thesis, University of Debrecen, Debrecen, Hungary, December 2012. [Google Scholar]
- Elliot, A. Family Ciconidae. In Handbook of the Birds of the World—Ostrich to Ducks; Del Hoyo, J., Elliot, A., Sargatal, J., Eds.; Lynx Edicions: Barcelona, Spain, 1992; Volume 1, pp. 436–465. ISBN 978-84-87334-10-8. [Google Scholar]
- BirdLife International 2012. Ciconia nigra. The IUCN Red List of Threatened Species. Available online: www.iucnredlist.org (accessed on 14 December 2022).
- Bobek, M.; Hampl, R.; Peske, L.; Pojer, F.; Simek, J.; Bures, S. African Odyssey project—Satellite tracking of black storks Ciconia nigra breeding at a migratory divide. J. Avian Biol. 2008, 39, 500–506. [Google Scholar] [CrossRef]
- Lõhmus, A.; Sellis, U. Nest trees—A limiting factor for the Black Stork (Ciconia nigra) population in Estonia. Aves 2003, 40, 84–91. [Google Scholar]
- Zawadzki, G.; Zawadzka, D. Wybór drzew gniazdowych myszołowa, jastrzębia i kruka w Puszczy Augustowskiej. Sylwan 2017, 161, 669–676. [Google Scholar]
- Zawadzki, G.; Zawadzka, D.; Sołtys, A.; Drozdowski, S. Nest-site selection by the white-tailed eagle and black stork–implications for conservation practice. For. Ecosyst. 2020, 7, 59. [Google Scholar] [CrossRef]
- Poirazidis, K.; Bontzorlos, V. Population trends and multi-scale breeding habitat analysis for the black stork (Ciconia nigra) in Dadia-Lefkimi-Soufli National Park, north eastern Greece. Annu. Res. Rev. Biol. 2017, 19, 1–12. [Google Scholar] [CrossRef]
- Banas, J.; Zieba, S.; Bujoczek, M.; Bujoczek, L. The Impact of Different Management Scenarios on the Availability of Potential Forest Habitats for Wildlife on a Landscape Level: The Case of the Black Stork Ciconia nigra (Linnaeus, 1758). Forests 2019, 10, 362. [Google Scholar] [CrossRef]
- Vlachos, C.G.; Bakaloudis, D.E.; Alexandrou, O.G.; Bontzorlos, V.A.; Papakosta, M.A. Factors affecting the nest site selection of the black stork Ciconia nigra in the Dadia-Lefkimi-Soufli National Park, north eastern Greece. Folia Zool. 2008, 57, 2512–2557. [Google Scholar]
- Jiguet, F.; Villarubias, S. Satellite tracking of breeding black storks Ciconia nigra: New incomes for spatial conservation issues. Biol. Conserv. 2004, 120, 153–160. [Google Scholar] [CrossRef]
- Cano-Alonso, L.S.; Tellería, J.L. Breeding productivity in relation to nesting substrate and nest site accessibility to humans in the black stork Ciconia nigra. Ardeola 2013, 60, 357–363. [Google Scholar] [CrossRef]
- von Puhringer, N. Bestandserfassung des schwarzstorchs (Ciconia nigra) in Oberösterreich 2020/21 im kontext der bestandsentwicklung–von der erfolgsgeschichte zum sorgenkind? Vogelkdl. Nachr. OÖ. Naturschutz aktuell 2022, 28/29, 3–61. Available online: https://www.zobodat.at/pdf/VNO_028-029_0003-0061.pdf (accessed on 24 February 2023).
- Lee, A.T.K.; Whitecross, M.A.; Smit-Robinson, H.A.; Allan, D.G.; Van den Heever, L.; Jenkins, A.; Retief, E.F.; Colyn, R.B.; Tarboton, W.; Chetty, K.; et al. A review of the conservation status of Black Stork Ciconia nigra in South Africa, Lesotho, and Eswatini. Bird Conserv. Int. 2023, 33, e56. [Google Scholar] [CrossRef]
- Spina, F.; Volponi, S. Atlante della Migrazione degli Uccelli in Italia—Non Passeriformi; ISPRA: Rome, Italy, 2008; pp. 127–129. ISBN 9788844803780. [Google Scholar]
- Caldarella, M.; Bordignon, L.; Brunelli, M.; Cripezzi, E.; Fraissinet, M.; Mallia, E.; Marrese, M.; Norante, N.; Urso, S.; Visceglia, M. Status della Cicogna nera (Ciconia nigra) e Linee Guida per la Conservazione della Specie in Italia; Parco regionale Gallipoli Cognato–Dolomiti Lucane: Accettura, Italy, 2018. [Google Scholar]
- Fraissinet, M.; Bordignon, L.; Brunelli, M.; Caldarella, M.; Cripezzi, E.; Giustino, S.; Mallia, E.; Marrese, M.; Norante, N.; Urso, S.; et al. Breeding population of Black Stork, Ciconia nigra, in Italy between 1994 and 2016. Riv. Ital. Ornitol. 2018, 88, 15–22. [Google Scholar] [CrossRef][Green Version]
- Brunelli, M.; Bordignon, L.; Caldarella, M.; Cripezzi, E.; Dovere, B.; Fraissinet, M.; Mallia, E.; Marrese, M.; Norante, N.; Urso, S.; et al. Rapporto sulla nidificazione della Cicogna nera Ciconia nigra in Italia. Alula 2022, 29, 118–119. [Google Scholar]
- Freschi, P.; Fascetti, S.; Musto, M.; Mallia, E.; Blasi, A.C.; Cosentino, C.; Paolino, R. Diet of the Apennine hare in a southern Italy Regional Park. Eur. J. Wildl. Res. 2014, 60, 423–430. [Google Scholar] [CrossRef]
- Freschi, P.; Fascetti, S.; Musto, M.; Mallia, E.; Cosentino, C.; Paolino, R. Diet of the Italian hare (Lepus corsicanus) in a semi-natural landscape of southern Italy. Mammalia 2014, 79, 51–59. [Google Scholar] [CrossRef]
- Freschi, P.; Musto, M.; Paolino, R.; Cosentino, C. Grazing and biodiversity conservation: Highlights on a Natura 2000 Network site. In The Sustainability of Agro-Food and Natural Resource Systems in the Mediterranean Basin; Vastola, A., Ed.; Springer International Publishing AG: Basel, Switzerland, 2015; pp. 271–288. [Google Scholar] [CrossRef]
- Freschi, P.; Fascetti, S.; Musto, M.; Cosentino, C.; Paolino, R.; Valentini, V. Seasonal variation in food habits of the Italian hare in a south Apennine semi-natural landscape. Ethol. Ecol. Evol. 2016, 28, 148–162. [Google Scholar] [CrossRef]
- Freschi, P.; Fascetti, S.; Riga, F.; Cosentino, C.; Rizzardini, G.; Musto, M. Diet composition of the Italian roe deer (Capreolus capreolus italicus) (Mammalia: Cervidae) from two protected areas. Eur. Zool. J. 2017, 84, 34–42. [Google Scholar] [CrossRef]
- Freschi, P.; Fascetti, S.; Riga, F.; Rizzardini, G.; Musto, M.; Cosentino, C. Feeding Preferences of the Italian Roe Deer (Capreolus capreolus italicus Festa, 1925) in a Coastal Mediterranean Environment. Animals 2021, 11, 308. [Google Scholar] [CrossRef]
- Rizzardini, G.; Fascetti, S.; Pietri, C.; Riga, F.; Cosentino, C.; Freschi, P. Feeding preferences in dry season of the Italian hare (Lepus corsicanus) in two sites of Corsica. Eur. J. Wildl. Res. 2019, 65, 43. [Google Scholar] [CrossRef]
- Martin, P.; Bateson, P. Measuring behaviour, 4th ed.; Cambridge University Press: Cambridge, UK, 2021; pp. 79–132. ISBN 978-1-108-47831-1. [Google Scholar]
- R Core Version 3.6.1. Available online: https://www.r-project.org (accessed on 24 February 2023).
- Soler, J.J.; Moller, A.P.; Soler, M. Nest building, sexual selection and parental investment. Evol. Ecol. 1998, 12, 427–441. [Google Scholar] [CrossRef]
- Kahl, M.P. Comparative ethology of the Ciconiidae. Part 4. The “Typical” Storks (Genera Ciconia, Sphenorhynchus, Dissoura and Euxenura). Z. Tierpsychol. 1972, 30, 225–252. [Google Scholar] [CrossRef]
- Baoqing, L.; Jun, Z.; Ainan, Q. Studies on incubation and raising of oriental White Stork (Ciconia boyciana). Chin. J. Zool. 2004, 39, 45–47. [Google Scholar]
- Zbyryt, A.; Jankowia, Ł.; Jerzak, L.; Tryjanowski, P. Head and body orientation of the White Stork Ciconia ciconia during incubation: Effect of wind, apex predators and power lines. J. Ornitol. 2002, 163, 181–189. [Google Scholar] [CrossRef]
- Poussart, C.; Gauthier, G.; Larochelle, J. Incubation behaviour of greater snow geese in relation to weather conditions. Can. J. Zool. 2001, 79, 671–678. [Google Scholar] [CrossRef]
- Cotgreave, D.H.; Clayton, P. Comparative analysis of time spent grooming by birds in relation to parasite load. Behaviour 1994, 131, 171–187. [Google Scholar] [CrossRef]
- Schuz, E. Bewegungsnormen des Weißen Storchs. Z. Tierpsychol. 1942, 5, 31. [Google Scholar] [CrossRef]
- Clayton, D.H.; Cotgreave, P. Relationship of bill morphology to grooming behaviour in birds. Anim. Behav. 1994, 47, 195–201. [Google Scholar] [CrossRef]
- Goldstein, D.L. Estimates of daily energy expenditure in birds: The time-energy budget as an integrator of laboratory and field studies. Am. Zool. 1988, 28, 829–844. [Google Scholar] [CrossRef]
- Clayton, D.H.; Koop, J.A.H.; Harbison, C.W.; Moyer, B.R.; Bush, S.E. How Birds Combat Ectoparasites. Open Ornitol. J. 2010, 3, 41–71. [Google Scholar] [CrossRef]
- Ferrero, J.J.; Román, J.A. Estudio sobre la cigüeña negra en Extremadura II: Nidotópica y hábitat de nidificación. Alytes 1991, 5, 19–46. [Google Scholar]
- del Moral, J.C. Cigüeña negra Ciconia nigra. In III Atlas de las Aves en época de Reproducción en España; Molina, B., Nebreda, A., Muñoz, A.R., Seoane, J., Real, R., Bustamante, J., del Moral, J.C., Eds.; SEO/BirdLife: Madrid, Spain, 2022; pp. 1–8. Available online: https://atlasaves.seo.org/ave/ciguena-negra/ (accessed on 13 June 2023).
- Prieta, J.; Traverso, J.M. Appropriacìon de nidos de cigüeña negra por rapaces rupìcolas. Quercus 2000, 172, 24–28. [Google Scholar]
- Brambilla, M.; Rubolini, D.; Guidali, F. Rock climbing and raven Corvus corax occurrence depress breeding success of cliff-nesting peregrines Falco peregrinus. Ardeola 2004, 51, 425–430. [Google Scholar]
- Kłosowski, G.; Kłosowski, T.; Zieliński, P. A case of parental infanticide in the black stork Ciconia nigra. Avian Sci. 2002, 2, 59–62. [Google Scholar]
- Konarzewski, M. The evolution of clutch size and hatching asynchrony in altricial birds: The effect of environmental variability, egg failure and predation. Oikos 1993, 67, 97–106. [Google Scholar] [CrossRef]
- Mock, D.W.; Forbes, L.S. The evolution of parental optimism. Trends Ecol. Evol. 1995, 10, 130–134. [Google Scholar] [CrossRef]
- Zielinski, P. Brood reduction and parental infanticide—Are the White Stork Ciconia ciconia and the Black Stork C. nigra exceptional? Acta Ornithol. 2002, 37, 113–119. [Google Scholar] [CrossRef]
- Tortosa, F.S.; Redondo, T. Motives for parental infanticide in White Storks Ciconia ciconia. Ornis Scand. 1992, 23, 185–189. [Google Scholar] [CrossRef]



| Nest Composition | Measure | Activity/Status |
|---|---|---|
| Only parents | Pre-hatching | |
| Duration and frequency | Courtship, Mating, Nest building, Incubation | |
| Frequency | Incubation shift, Body orientation shift during incubation, Egg turning | |
| Pre- and Post-hatching | ||
| Duration and frequency | Standing, Self-preening, Allopreening, Exploration, Alert, Inactivity, Nest maintenance, Nest uncovered, Presence of two parents, Presence of one parent, Absence of parents | |
| Parents with nestlings | Post-hatching | |
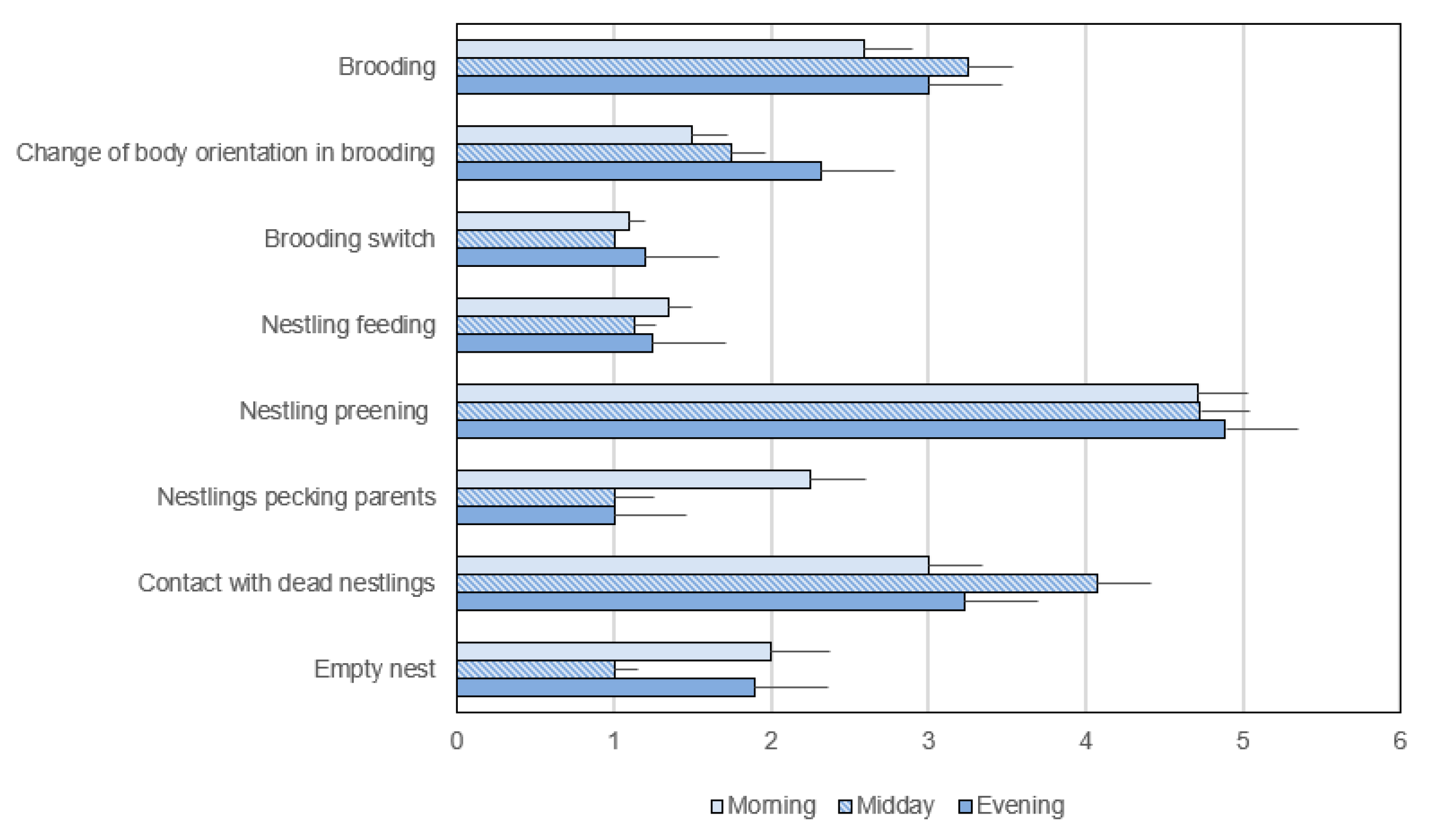
| Duration and frequency | Brooding, Nestling feeding, Nestling preening, Nestlings peck parents, Contact with dead nestlings, Empty nest | |
| Frequency | Change of body orientation in brooding, Brooding switch, Nestling feeding, Nestling pecking parents | |
| Only nestlings | Duration and frequency | Rest, Interaction among nestlings, Exploration, Self-preening, Alert, Down, Flight attempts |
| Frequency | Wings spread | |
| Activity/Status | Pre-Hatching | Post-Hatching | ||||
|---|---|---|---|---|---|---|
| Morning | Midday | Evening | Morning | Midday | Evening | |
| Self-preening | 5.27 ± 1.30 | 2.46 ± 1.30 | 2.19 ± 1.30 | 9.02 ± 1.02 a | 7.11 ± 1.15 | 5.37 ± 1.08 b |
| Allopreening | 5.23 ± 2.33 | 1.56 ± 0.16 a | 12.26 ± 3.29 b | 0.97 ± 0.10 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Exploration | 5.07 ± 1.62 | 5.30 ± 0.38 | 5.04 ± 0.32 | 7.31 ± 1.30 | 3.19 ± 1.54 | 7.59 ± 1.40 |
| Inactivity | 7.38 ± 3.02 | 8.00 ± 2.09 | 3.14 ± 0.35 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Alert | 7.06 ± 1.01 | 1.27 ± 0.11 | 0.00 ± 0.00 | 5.15 ± 1.01 | 11.37 ± 4.51 | 0.00 ± 0.00 |
| Standing | 16.39 ± 5.12 | 12.00 ± 2.03 | 16.10 ± 5.03 | 34.33 ± 3.31 | 30.03 ± 4.01 | 30.41 ± 3.34 |
| Nest maintenance | 4.48 ± 1.33 | 5.12 ± 0.88 | 4.21 ± 0.43 | 7.48 ± 1.05 Aa | 4.28 ± 0.42 B | 5.20 ± 0.42 b |
| Presence of two parents | 18.29 ± 3.14 | 17.52 ± 4.09 | 19.23 ± 4.09 | 2.48 ± 0.30 | 1.18 ± 0.15 | 0.31 ± 0.05 |
| Presence of one parent | 18.13 ± 5.08 | 16.32 ± 5.19 | 13.42 ± 5.29 | 42.29 ± 3.27 | 43.16 ± 3.58 | 38.51 ± 3.24 |
| Absence of parents | 11.36 ± 10.5 | 9.50 ± 1.35 | 23.45 ± 15.26 | 17.34 ± 9.40 | 39.49 ± 7.04 | 39.07 ± 8.34 |
| Activity/Status | Pre-Hatching | Post-Hatching | ||||
|---|---|---|---|---|---|---|
| Morning | Midday | Evening | Morning | Midday | Evening | |
| Self-preening | 6.30 ± 1.09 a | 4.27 ± 1.09 b | 4.45 ± 1.09 | 2.33 ± 0.54 | 2.21 ± 1.01 | 2.34 ± 0.57 |
| Allopreening | 2.25 ± 0.53 | 2.15 ± 0.53 | 3.10 ± 1.16 | 1.00 ± 0.07 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Exploration | 5.3 ± 0.51 | 4.32 ± 1.03 | 4.26 ± 0.54 | 2.57 ± 0.41 | 1.50 ± 0.49 | 3.32 ± 0.44 |
| Inactivity | 2.38 ± 0.32 | 2.25 ± 0.37 | 2.33 ± 0.44 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Alert | 2.00 ± 1.44 | 1.00 ± 1.12 | 0.00 ± 0.00 | 3.00 ± 1.44 | 4.50 ± 1.12 | 0.00 ± 0.00 |
| Standing | 3.02 ± 0.25 A | 2.07 ± 0.26 B | 2.20 ± 0.26 C | 1.49 ± 0.17 | 2.25 ± 0.19 | 2.13 ± 0.17 |
| Nest maintenance | 6.56 ± 1.06 a | 7.18 ± 0.62 A | 4.53 ± 1.06 Bb | 3.03 ± 0.46 | 3.22 ± 0.58 | 2.30 ± 0.51 |
| Presence of two parents | 5.18 ± 1.15 A | 2.28 ± 1.27 B | 2.28 ± 1.27 B | 3.33 ± 0.36 a | 1.36 ± 0.66 | 1.00 ± 1.09 b |
| Presence of one parent | 3.50 ± 0.37 A | 1.59 ± 0.38 B | 1.59 ± 0.38 B | 2.26 ± 0.26 | 1,54 ± 0.28 | 1.32 ± 0.25 |
| Absence of parents | 3.25 ± 1.15 | 1.00 ± 1.26 | 1.50 ± 1.06 | 1.20 ± 1.07 | 2.00 ± 0.47 | 1.29 ± 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freschi, P.; Cosentino, C.; Napolitano, F.; Pacelli, C.; Manicone, D.; Mallia, E.; Ragni, M.; Paolino, R.; Braghieri, A. First Report on a Cliff-Nesting Pair of Black Storks (Ciconia nigra Linnaeus, 1758) and Their Nestlings. Forests 2023, 14, 1941. https://doi.org/10.3390/f14101941
Freschi P, Cosentino C, Napolitano F, Pacelli C, Manicone D, Mallia E, Ragni M, Paolino R, Braghieri A. First Report on a Cliff-Nesting Pair of Black Storks (Ciconia nigra Linnaeus, 1758) and Their Nestlings. Forests. 2023; 14(10):1941. https://doi.org/10.3390/f14101941
Chicago/Turabian StyleFreschi, Pierangelo, Carlo Cosentino, Fabio Napolitano, Corrado Pacelli, Danilo Manicone, Egidio Mallia, Marco Ragni, Rosanna Paolino, and Ada Braghieri. 2023. "First Report on a Cliff-Nesting Pair of Black Storks (Ciconia nigra Linnaeus, 1758) and Their Nestlings" Forests 14, no. 10: 1941. https://doi.org/10.3390/f14101941
APA StyleFreschi, P., Cosentino, C., Napolitano, F., Pacelli, C., Manicone, D., Mallia, E., Ragni, M., Paolino, R., & Braghieri, A. (2023). First Report on a Cliff-Nesting Pair of Black Storks (Ciconia nigra Linnaeus, 1758) and Their Nestlings. Forests, 14(10), 1941. https://doi.org/10.3390/f14101941











