Soil Nutrient Dynamics under Silviculture, Silvipasture and Hortipasture as Alternate Land-Use Systems in Semi-Arid Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Soil Sampling and Processing
2.3. Soil Analysis
2.4. Statistical Analysis
3. Results
3.1. Impact of Alternate Land-Use Systems on Soil pH and EC
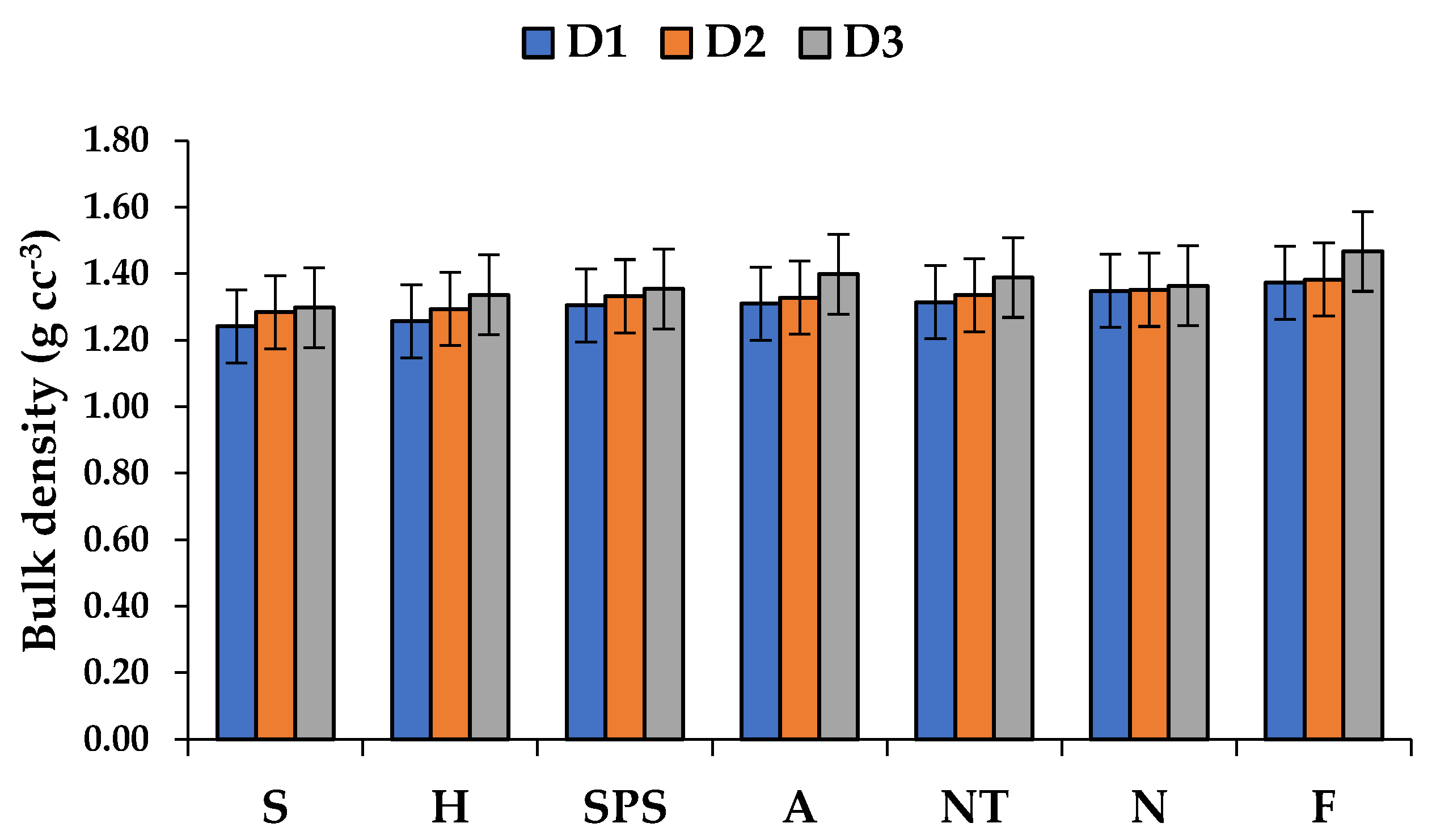
3.2. Impact of Alternate Land-Use Systems on Bulk Density (BD)
3.3. Impact of Alternate Land-Use Systems on Total Organic Carbon in Soil (TOC)
3.4. Impact of Alternate Land-Use Systems on Available Nitrogen (N)
3.5. Impact of Alternate Land-Use Systems on Available Phosphorus (P)
3.6. Impact of Alternate Land-Use Systems on Available Potassium (K)
3.7. Impact of Alternate Land-Use Systems on Iron (Fe)
3.8. Impact of Alternate Land-Use Systems on Manganese (Mn)
3.9. Impact of Alternate Land-Use Systems on Copper (Cu)
3.10. Impact of Alternate Land-Use Systems on Zinc (Zn)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Food Policy Research Institute. Fertilizer and Soil Fertility Potential in Ethiopia: Constraints and Opportunities for Enhancing the System; International Food Policy Research Institute: Washington, DC, USA, 2010; p. 42. [Google Scholar]
- Harris, J.A.; Hobbs, R.J.; Higgs, E.; Aronson, J. Ecological restoration and global climate change. Restor. Ecol. 2006, 14, 170–176. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.K.; Singh, R.; Ghosh, A.; Chaudhary, M.; Shukla, A.K.; Kumar, S.; Singh, H.V.; Ahmed, A.; Kumar, R.V. Degraded landrestoration ecological way through horti-pasture systems and soil moisture conservation to sustain productive economic viability. Land Degrad. Dev. 2019, 30, 1516–1529. [Google Scholar] [CrossRef]
- Ghosh, A.; Kumar, R.V.; Manna, M.C.; Singh, A.K.; Parihar, C.M.; Kumar, S.; Roy, A.K.; Koli, P. Eco-restoration of degraded lands through trees and grasses improves soil carbon sequestration and biological activity in tropical climates. Ecol. Eng. 2021, 162, 106176. [Google Scholar] [CrossRef]
- Dhyani, S.K.; Ram, N.; Sharma, A.R. Agroforestry: Its relation with agronomy, challenges and opportunities. Indian J. Agron. 2009, 54, 249–266. [Google Scholar]
- NRCAF. NRCAF Vision 2050; National Research Centre for Agroforestry: Jhansi, India, 2013; p. 26. [Google Scholar]
- Singh, N.; Price, C.; Downs, C.T. Aspects of the ecology and behaviour of a potential urban exploiter, the southern tree agama, Acanthocercus atricollis. Urban Ecosyst. 2021, 24, 905–914. [Google Scholar] [CrossRef]
- Adhikari, H.; Valbuena, R.; Pellikka, P.K.; Heiskanen, J. Mapping forest structural heterogeneity of tropical montane forest remnants from air borne laser scanning and Landsat time series. Ecol. Indic. 2020, 108, 105739. [Google Scholar] [CrossRef]
- Singh, M.; Marchis, A.; Capri, E. Greening, new frontiers for research and employment in the agro-food sector. Sci. Total Environ. 2014, 472, 437–443. [Google Scholar] [CrossRef]
- Murthy, I.K.; Gupta, M.; Tomar, S.; Munsi, M.; Tiwari, R.; Hegde, G.T.; Ravindranath, N.H. Carbon sequestration potential of agroforestry systems in India. J. Earth Sci. Clim. Chang. 2013, 4, 1000131. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Private Limited: New Delhi, India, 1973. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley and Sons: New York, NY, USA, 1984; p. 680. [Google Scholar]
- Muche, M.; Kokeb, A.; Molla, E. Assessing the physicochemical properties of soil under different land use types. J. Environ. Anal. Toxicol. 2015, 5, 309. [Google Scholar] [CrossRef]
- Ajami, M.; Heidari, A.; Khormali, F.; Gorji, M.; Ayoubi, S. Environmental factors controlling soil organic carbon storage in loess soils of a subhumid region, northern Iran. Geoderma 2016, 281, 1–10. [Google Scholar] [CrossRef]
- Alidoust, E.; Afyuni, M.; Hajabbasi, M.A.; Mosaddeghi, M.R. Soil carbon sequestration potential as affected by soil physical and climatic factors under different land uses in a semiarid region. Catena 2018, 171, 62–71. [Google Scholar] [CrossRef]
- Song, Q.; Gao, X.; Du, H.; Lei, J.; Li, S.; Li, S. Cultivation impacts on soil texture during oasis expansion in Xinjiang, Northwest China: Wind erosion effects. Aeolian Res. 2021, 50, 100646. [Google Scholar] [CrossRef]
- Zeraatpisheh, M.; Bakhshandeh, E.; Hosseini, M.; Alavi, S. Assessing the effects of deforestation and intensive agriculture on the soil quality through digital soil mapping. Geoderma 2020, 363, 114139. [Google Scholar] [CrossRef]
- Adams, A.M.; Gillespie, A.W.; Dhillon, G.S.; Kar, G.; Minielly, C.; Koala, S.; Ouattara, B.; Kimaro, A.A.; Bationo, A.; Schoenau, J.J.; et al. Long-term effects of integrated soil fertility management practices on soil chemical properties in the Sahel. Geoderma 2020, 366, 114207. [Google Scholar] [CrossRef]
- Fathizad, H.; Hakimzadeh Ardakani, M.A.; Sodaiezadeh, H.; Kerry, R.; Taghizadeh-Mehrjardi, R. Investigation of the spatial and temporal variation of soil salinity using random forests in the central desert of Iran. Geoderma 2020, 365, 114233. [Google Scholar] [CrossRef]
- Ghosh, A.; Singh, A.K.; Das, B.; Modak, K.; Kumar, R.V.; Kumar, S.; Gautam, K.; Biswas, D.R.; Roy, A.K. Resiliencies of soil phosphorus fractions after natural summer fire are governed by microbial activity and cation availability in a semi-arid Inceptisol. Environ. Res. 2023, 216, 114583. [Google Scholar] [CrossRef]
- Baradwal, H.; Ghosh, A.; Kumar, A.; Singh, P.D.; Sannagoudar, M.S.; Ahamad, S.; Jha, P.; Singh, A.K.; Bhattacharyya, R.; Manna, M.C.; et al. Ecological restoration of degraded lands with alternate land use systems improves soil functionality in semiarid tropical India. Land Degrad. Dev. 2022, 33, 1076–1087. [Google Scholar] [CrossRef]
- Ghosh, A.; Singh, A.K.; Kumar, S.; Manna, M.C.; Bhattacharyya, R.; Agnihortri, R.; Singh Gahlaud, S.K.; Sannagoudar, M.S.; Gautam, K.; Kumar, R.V.; et al. Differentiating biological and chemical factors of top and deep soil carbon sequestration in semi-arid tropical Inceptisol: An outcome of structural equation modeling. Carbon Manag. 2020, 11, 441–453. [Google Scholar] [CrossRef]
- Ghosh, A.; Kumar, S.; Manna, M.C.; Singh, A.K.; Sharma, P.; Sarkar, A.; Saha, M.; Bhattacharyya, R.; Misra, S.; Biswas, S.S.; et al. Long-term in situ moisture conservation in horti-pasture system improves biological health of degraded land. J. Environ. Manag. 2019, 248, 109339. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Biswas, D.R.; Das, S.; Das, T.K.; Bhattacharyya, R.; Alam, K.; Rahman, M.M. Rice straw incorporation mobilizes inorganic soil phosphorus by reorienting hysteresis effect under varying hydrothermal regimes in a humid tropical Inceptisol. Soil Till. Res. 2023, 225, 105531. [Google Scholar] [CrossRef]
- Ewel, J. Designing agricultural systems for the humid tropics. Annu. Rev. Ecol. Syst. 1986, 17, 245–271. [Google Scholar] [CrossRef]
- Smith, N.; Dubois, J.; Current, D.; Lutz, E.; Clement, C. Agroforestry Experiences in the Brazilian Amazon: Constraints and Opportunities; Pilot Program to Conserve the Brazilian Rainforest: Brasilia, Brazil, 1997. [Google Scholar]
| Land-Use Systems | Area (ha) | Soil Type | Grazing Type |
|---|---|---|---|
| Leucaena leucocephala (S) | 3 | Sandy Loam | Occasional grazing |
| Hardwickia binata (H) | 2 | Sandy Loam | Occasional grazing |
| Acacia nilotica (SPS) | 1.1 | Sandy Loam | Occasional grazing |
| Emblica officinalis (A) | 1.2 | Sandy Loam | Occasional grazing |
| Natural grassland (NT) | 2 | Sandy Loam | Frequent grazing |
| Azadiracta indica (N) | 2 | Sandy Loam | Occasional grazing |
| Fallow (F) | 2 | Sandy Loam |
| LUS | pH (H2O) | EC |
|---|---|---|
| 0–15 cm | 0–15 cm | |
| S | 6.5a | 0.031 |
| H | 5.5a | 0.026 |
| SPS | 6.0a | 0.034 |
| A | 6.5a | 0.033 |
| NT | 6.5a | 0.029 |
| N | 6.0a | 0.027 |
| F | 6.5a | 0.019 |
| SE (m) | 0.39 |
| SOC | |||
| 0–15 cm | 15–30 cm | 30–45 cm | |
| S | 1.46a | 1.44a | 1.36a |
| H | 1.42a | 0.88c | 0.51d |
| SPS | 1.10b | 1.01b | 0.84bc |
| A | 0.99c | 1.01b | 0.89b |
| NT | 0.83d | 0.86c | 0.79c |
| N | 0.54e | 0.40d | 0.38e |
| F | 0.48e | 0.48d | 0.41e |
| SE (m) | 0.39 | 0.35 | 0.35 |
| N | |||
| 0–15 cm | 15–30 cm | 30–45 cm | |
| S | 1.82a | 1.80a | 1.70a |
| H | 1.77a | 1.10c | 0.63d |
| SPS | 1.38b | 1.26b | 1.05bc |
| A | 1.24b | 1.26b | 1.12b |
| NT | 1.04c | 1.08d | 0.99c |
| N | 0.67d | 0.50f | 0.48e |
| F | 0.61d | 0.60e | 0.51de |
| SE (m) | 0.48 | 0.44 | 0.43 |
| P | |||
| 0–15 cm | 15–30 cm | 30–45 cm | |
| S | 38.53a | 32.38a | 31.97a |
| H | 31.97b | 27.46b | 28.28b |
| SPS | 27.87c | 27.05b | 24.19c |
| A | 27.05c | 22.96c | 22.55de |
| NT | 25.01d | 22.96c | 21.32d |
| N | 20.50e | 19.27d | 24.60c |
| F | 18.04e | 15.58e | 20.91d |
| SE (m) | 6.89 | 5.58 | 4.01 |
| K | |||
| 0–15 cm | 15–30 cm | 30–45 cm | |
| S | 268.8b | 262.08a | 257.60b |
| H | 239.68c | 257.60ab | 230.72c |
| SPS | 238.56c | 256.48ab | 196.00d |
| A | 286.72a | 264.32a | 294.56a |
| NT | 228.48c | 240.80bc | 189.28d |
| N | 202.72d | 229.60c | 228.48c |
| F | 252bc | 244.16bc | 259.84b |
| SE (m) | 27.34 | 12.79 | 37.26 |
| Fe (ppm) | |||
| 0–15 cm | 15–30 cm | 30–45 cm | |
| S | 17.59a | 8.97a | 9.33a |
| H | 10.77b | 7.90b | 8.62b |
| SPS | 8.62c | 6.82c | 8.26b |
| A | 8.26c | 6.82c | 7.54c |
| NT | 7.90d | 5.74d | 7.18d |
| N | 6.82e | 5.38e | 6.46e |
| F | 6.46f | 5.38e | 6.10f |
| SE (m) | 3.84 | 1.35 | 1.17 |
| Mn (ppm) | |||
| 0–15 cm | 15–30 cm | 30–45 cm | |
| S | 51.32a | 43.96a | 49.24a |
| H | 46.04b | 41.88ab | 46.22a |
| SPS | 43.66b | 41.82ab | 39.16b |
| A | 37.96c | 40.70b | 32.04c |
| NT | 37.56c | 38.50b | 31.80c |
| N | 34.40d | 30.96c | 28.48d |
| F | 28.48e | 28.84c | 25.04d |
| SE (m) | 7.66 | 5.86 | 9.13 |
| Cu (ppm) | |||
| 0–15 cm | 15–30 cm | 30–45 cm | |
| S | 2.17a | 2.01a | 2.25a |
| H | 2.01b | 1.97a | 1.93b |
| SPS | 1.97b | 1.74b | 1.74c |
| A | 1.78c | 1.62b | 1.74c |
| NT | 1.74cd | 1.46c | 1.70c |
| N | 1.62d | 1.39c | 1.62cd |
| F | 1.50d | 1.35c | 1.54d |
| SE (m) | 0.24 | 0.27 | 0.24 |
| Zn (ppm) | |||
| 0–15 cm | 15–30 cm | 30–45 cm | |
| S | 4.78a | 1.35a | 3.77a |
| H | 4.75a | 1.14ab | 1.30b |
| SPS | 2.07b | 1.09b | 1.16c |
| A | 1.28c | 0.99c | 1.07cd |
| NT | 1.09c | 0.99c | 1.02d |
| N | 0.97d | 0.93cd | 1.00d |
| F | 0.96d | 0.86d | 0.78e |
| SE (m) | 1.74 | 0.16 | 1.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baradwal, H.; Ghosh, A.; Singh, A.K.; Jiménez-Ballesta, R.; Yadav, R.K.; Misra, S.; Siddanagouda Sannagoudar, M.; Kumar, S.; Kumar, R.V.; Singh, S.K.; et al. Soil Nutrient Dynamics under Silviculture, Silvipasture and Hortipasture as Alternate Land-Use Systems in Semi-Arid Environment. Forests 2023, 14, 125. https://doi.org/10.3390/f14010125
Baradwal H, Ghosh A, Singh AK, Jiménez-Ballesta R, Yadav RK, Misra S, Siddanagouda Sannagoudar M, Kumar S, Kumar RV, Singh SK, et al. Soil Nutrient Dynamics under Silviculture, Silvipasture and Hortipasture as Alternate Land-Use Systems in Semi-Arid Environment. Forests. 2023; 14(1):125. https://doi.org/10.3390/f14010125
Chicago/Turabian StyleBaradwal, Hansa, Avijit Ghosh, Amit K. Singh, Raimundo Jiménez-Ballesta, Rajendra Kumar Yadav, Sukanya Misra, Manjanagouda Siddanagouda Sannagoudar, Sunil Kumar, Ram Vinod Kumar, Sanjay K. Singh, and et al. 2023. "Soil Nutrient Dynamics under Silviculture, Silvipasture and Hortipasture as Alternate Land-Use Systems in Semi-Arid Environment" Forests 14, no. 1: 125. https://doi.org/10.3390/f14010125
APA StyleBaradwal, H., Ghosh, A., Singh, A. K., Jiménez-Ballesta, R., Yadav, R. K., Misra, S., Siddanagouda Sannagoudar, M., Kumar, S., Kumar, R. V., Singh, S. K., Yadav, D. K., & Mahala, D. M. (2023). Soil Nutrient Dynamics under Silviculture, Silvipasture and Hortipasture as Alternate Land-Use Systems in Semi-Arid Environment. Forests, 14(1), 125. https://doi.org/10.3390/f14010125







