Effects of Oak Processionary Moth (Thaumetopoea processionea L.) Outbreaks on the Leaf Performance and Health of Urban and Forest Oak Trees (Quercus robur L.) in Brandenburg, Germany
Abstract
1. Introduction
2. Material and Methods
2.1. Test Sites
2.2. Measurements and Sampling
2.3. JIP Test
2.4. The Photosynthetic Performance
2.5. Reaction Centers’ Activity
2.6. Statistical Data Analysis
3. Results
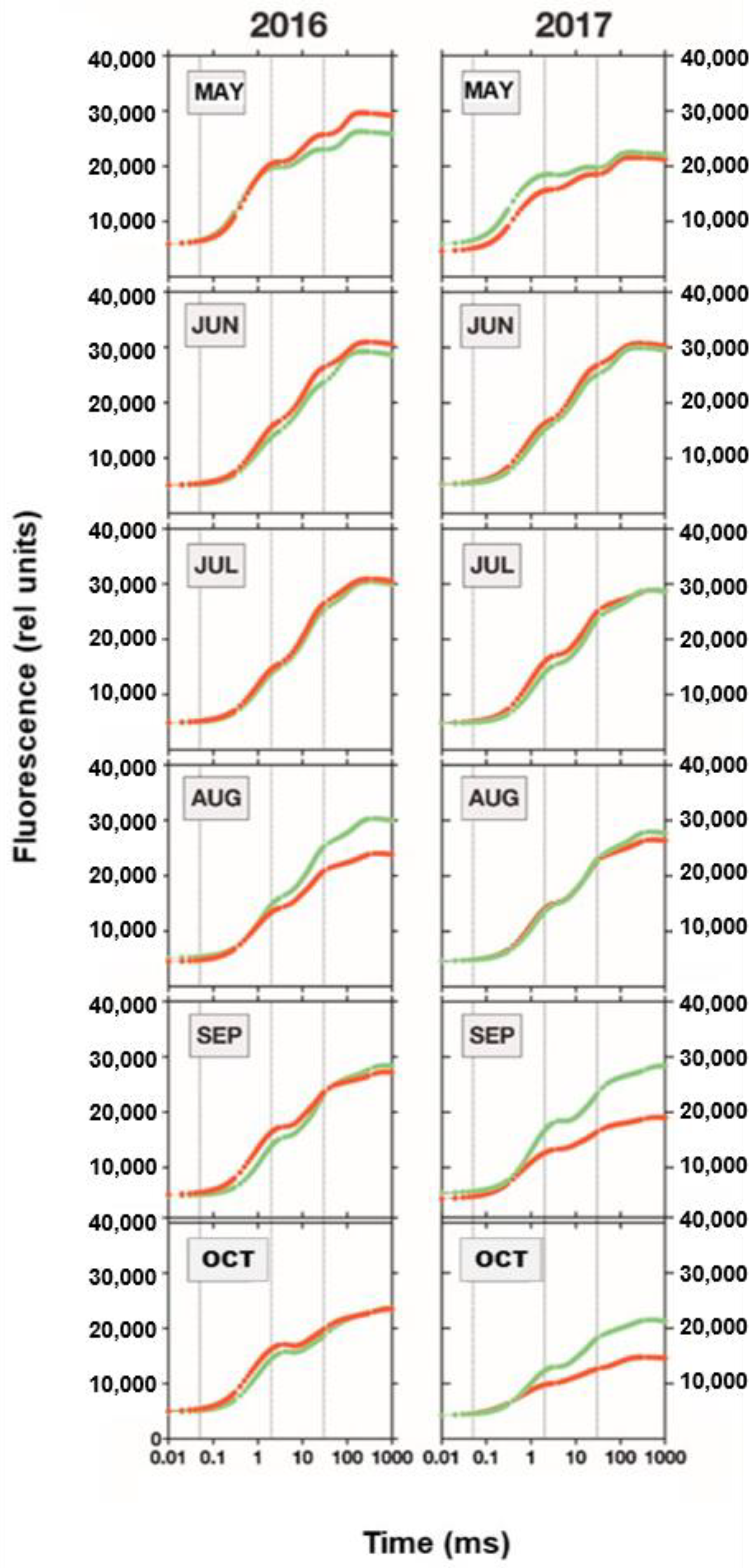
3.1. Urban Trees in Rathenow
JIP Test
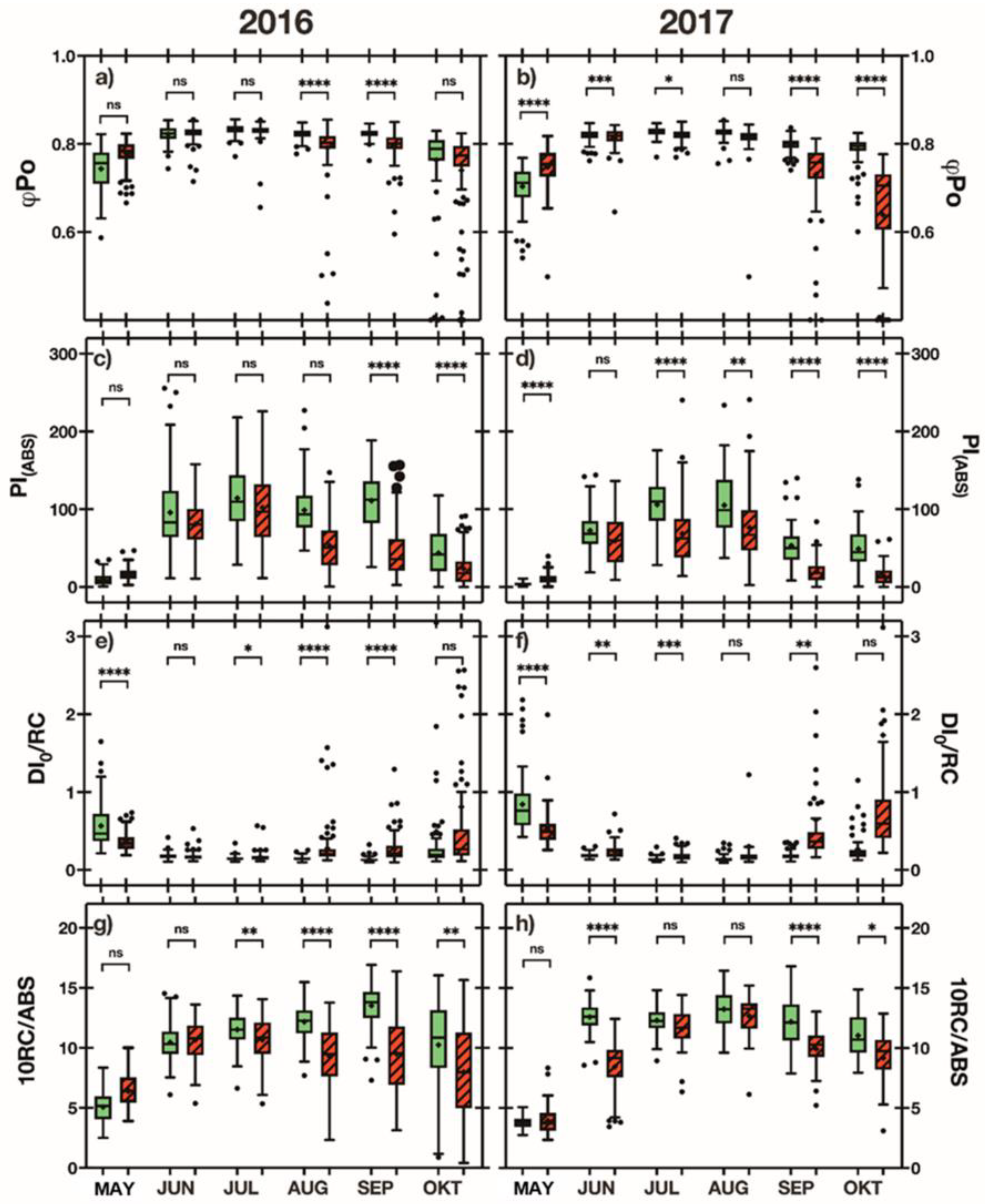
3.2. Quantum Yield of Photosystem II (PHI Po)
3.3. Performance Index PIABS
3.4. Energy Loss DIo/RC
3.5. Degree of Opening of the Reaction Centers 10RC/ABS
3.6. Forest Trees at Gollenberg
JIP Test
3.7. Quantum Yield of Photosystem II (PHI Po)
3.8. Performance Index PIABS
3.9. Energy Loss DIo/RC
3.10. Degree of Opening of the Reaction Centers 10RC/ABS
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinet, C.; Roques, A. Direct impacts of recent climate warming on insect populations. Integr. Zool. 2010, 5, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hill, J.K.; Thomas, C.D.; Descimon, H.; Huntley, B.; Kaila, L.; Kullberg, J.; Tammaru, T.; et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Townsend, M.C. Report on Survey and Control of Oak Processionary Moth Thaumetopoea processionea (Linnaeus) (Lepidoptera: Thaumetopoeidae); OPM: London/Oxford, UK, 2009. [Google Scholar]
- Stigter, H.; Van Tol, W.J.H.M.; Spijkers, H.C.P. Thaumetopea processionea in the Netherlands: Present status and management perspectives (Lepidoptera: Notodontidae). In Proceedings of the Section Experimental and Applied Entomology of the Netherlands Entomological Society; Nederlandse Entomologische Vereniging: Amsterdam, The Netherlands, 1997; Volume 8, pp. 3–16. [Google Scholar]
- Townsend, M. An outbreak of the Oak Processionary moth Thaumetopoea processionea (L.) (Lep.: Thaumetopoeidae) in south-west London. Entomol. Rec. J. Var. 2007, 118, 193. [Google Scholar]
- Gottschling, S.; Meyer, S. An Epidemic Airborne Disease Caused by the Oak Processionary Caterpillar. Pediatr. Dermatol. 2006, 23, 64–66. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Evaluation of a pest risk analysis on Thaumetopoea processionea L., the oak processionary moth, prepared by the UK and extension of its scope to the EU territory. EFSA J. 2009, 7, EFSA-Q-2008-711. [Google Scholar] [CrossRef]
- Bräsicke, N. Ökologische Schäden, gesundheitliche Gefahren und Maßnahmen zur Eindämmung des Eichenprozessionsspinners im Forst und im urbanen Grün. Jul. -Kühn-Arch. Nr 2012, 6, bis 07. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Black, T.A.; Curtis, P.S.; Falge, E.; Fuentes, J.D.; Granier, A.; Gu, L.; Knohl, A.; Pilegaard, K.; Schmid, H.P.; et al. Predicting the onset of net carbon uptake by deciduous forests with soil temperature and climate data: A synthesis of FLUXNET data. Int. J. Biometeorol. 2005, 49, 377–387. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.-L.; Marçais, B.; Nageleisen, L.-M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 595–610. [Google Scholar] [CrossRef]
- Sonnewald, U. Stoffwechselphysiologie. Strasburger—Lehrb. Der Pflanz. 2014, V279656, 337–446. [Google Scholar] [CrossRef]
- Vlaovic, J.; Balen, J.; Grgic, K.; Zagar, D.; Galic, V.; Simic, D. An Overview of Chlorophyll Fluorescence Measurement Process, Meters and Methods. In Proceedings of the 2020 International Conference on Smart Systems and Technologies (SST), Osijek, Croatia, 14–16 October 2020; pp. 245–250. [Google Scholar] [CrossRef]
- Sano, S.; Takemoto, T.; Ogihara, A.; Suzuki, K.; Masumura, T.; Satoh, S.; Takano, K.; Mimura, Y.; Morita, S. Stress Responses of Shade-Treated Tea Leaves to High Light Exposure after Removal of Shading. Plants 2020, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- del Pozo, A.; Méndez-Espinoza, A.M.; Romero-Bravo, S.; Garriga, M.; Estrada, F.; Alcaíno, M.; Camargo-Rodriguez, A.V.; Corke, F.M.K.; Doonan, J.H.; Lobos, G.A. Genotypic variations in leaf and whole-plant water use efficiencies are closely related in bread wheat genotypes under well-watered and water-limited conditions during grain filling. Sci. Rep. 2020, 10, 460. [Google Scholar] [CrossRef] [PubMed]
- Percival, D.; Murray, A.; Stevens, D. Drought stress dynamics of wild blueberry (Vaccinium angustifolium aiton). Acta Hortic. 2003, 618, 353–362. [Google Scholar] [CrossRef]
- Koller, S.; Holland, V.; Brüggemann, W. Effects of drought stress on the evergreen Quercus ilex L., the deciduous Q. robur L. and their hybrid Q. × turneri Willd. Photosynthetica 2013, 51, 574–582. [Google Scholar] [CrossRef]
- Percival, G. Evaluation of physiological tests as predictors of young tree establishment and growth. J. Arboric. Urban For. 2003, 30, 80–91. [Google Scholar] [CrossRef]
- Percival, G. The use of chlorophyll fluoresecence to indentify chemical and environmental stress in leaf tissue of three oaks (Quercus) species. J. Aboriculture 2005, 31, 215–227. [Google Scholar] [CrossRef]
- Yamada, M.; Hidaka, T.; Fukamachi, H. Heat tolerance in leaves of tropical fruit crops as measured by chlorophyll fluorescence. Sci. Horti. 1996, 67, 39–48. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of Photosynthesis during Abiotic Stress-Induced Photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, B.; Hincha, D.K. Chlorophyll fluorescence imaging accurately quantifies freezing damage and cold acclimation responses in Arabidopsis leaves. Plant Methods 2008, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Zulini, L.; Fischer, C.; Bertamini, M. Chlorophyll fluorescence as a tool for evaluation of viability in freeze-stressed grapevine buds. Photosynthetica 2010, 48, 317–319. [Google Scholar] [CrossRef]
- Aldea, M.; Hamilton, J.G.; Resti, J.P.; Zangerl, A.R.; Berenbaum, M.R.; Frank, T.D.; DeLucia, E.H. Comparison of photosynthetic damage from arthropod herbivory and pathogen infection in understory hardwood saplings. Oecologia 2006, 149, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Nabity, P.D.; Heng-Moss, T.M.; Higley, L.G. Effects of Insect Herbivory on Physiological and Biochemical (Oxidative Enzyme) Responses of the Halophyte Atriplex subspicata (Chenopodiaceae). Environ. Èntomol. 2006, 35, 1677–1689. [Google Scholar] [CrossRef]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping Plant Responses to Biotic Stress by Chlorophyll Fluorescence Imaging. Front. Plant Sci. 2019, 10, 1135. [Google Scholar] [CrossRef] [PubMed]
- El Omari, B.; Fleck, I.; Aranda, X.; Moret, A.; Nadal, M. Effect of fungal infection on leaf gas-exchange and chlorophyll fluorescence in Quercus ilex. Ann. For. Sci. 2001, 58, 165–174. [Google Scholar] [CrossRef]
- Groenen, F.; Meurisse, N. Historical distribution of the oak processionary moth Thaumetopoea processionea in Europe suggests recolonization instead of expansion. Agric. For. Èntomol. 2012, 14, 147–155. [Google Scholar] [CrossRef]
- Küpper, H.; Benedikty, Z.; Morina, F.; Andresen, E.; Mishra, A.; Trtílek, M. Analysis of OJIP Chlorophyll Fluorescence Kinetics and QA Reoxidation Kinetics by Direct Fast Imaging. Plant Physiol. 2019, 179, 369–381. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Rossini, M.; Panigada, C.; Meroni, M.; Colombo, R. Assessment of oak forest condition based on leaf biochemical variables and chlorophyll fluorescence. Tree Physiol. 2006, 26, 1487–1496. [Google Scholar] [CrossRef]
- Delb, H.; Schröter, H.; Seemann, D. Eichenprozessionsspinner—Waldschutz-Info 01/2002; Forstliche Versuchs- und Forschungsanstalt Baden-Württemberg: Freiburg-Breisgau, Germany, 2005. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanism, Regulation & Adaptation; CRC Press: Boca Raton, FL, USA, 2000; pp. 443–480. [Google Scholar]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta (BBA)—Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- R Core Team: A Language and Environment for Statistical Computing; European Environment Agency: Vienna, Austria, 2008.
- Dinno, A. Nonparametric Pairwise Multiple Comparisons in Independent Groups using Dunn’s Test. Stata J. Promot. Commun. Stat. Stata 2015, 15, 292–300. [Google Scholar] [CrossRef]
- van Heerden, P.D.R.; Strasser, R.J.; Kruger, G.H.J. Reduction of dark chilling stress in N2-fixing soybean by nitrate as indicated by chlorophyll a fluorescence kinetics. Physiol. Plant. 2004, 121, 239–249. [Google Scholar] [CrossRef]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Dark recovery of the Chl a fluorescence transient (OJIP) after light adaptation: The qT-component of non-photochemical quenching is related to an activated photosystem I acceptor side. Biochim. Biophys. Acta (BBA)—Bioenerg. 2006, 1757, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Olsovska, K.; Slamka, P.; Galambošová, J.; Rataj, V.; Shao, H.B.; Brestic, M. Application of chlorophyll fluorescence performance indices to assess the wheat photosynthetic functions influenced by nitrogen deficiency. Plant Soil Environ. 2014, 60, 210–215. [Google Scholar] [CrossRef]
- Long, A.; Zhang, J.; Yang, L.-T.; Ye, X.; Lai, N.-W.; Tan, L.-L.; Lin, D.; Chen, L.-S. Effects of Low pH on Photosynthesis, Related Physiological Parameters, and Nutrient Profiles of Citrus. Front. Plant Sci. 2017, 8, 185. [Google Scholar] [CrossRef]
- Zhang, W.W.; Niu, J.F.; Wang, X.K.; Tian, Y.; Yao, F.F.; Feng, Z.Z. Effects of ozone exposure on growth and photosynthesis of the seedlings of Liriodendron chinense (Hemsl.) Sarg, a native tree species of subtropical China. Photosynthetica 2011, 49, 29–36. [Google Scholar] [CrossRef]
- Samborska, I.A.; Kalaji, H.M.; Sieczko, L.; Borucki, W.; Mazur, R.; Kouzmanova, M.; Goltsev, V. Can just one-second measurement of chlorophyll a fluorescence be used to predict sulphur deficiency in radish (Raphanus sativus L. sativus) plants? Curr. Plant Biol. 2019, 19, 100096. [Google Scholar] [CrossRef]
- Gomes, A.M.S.D.V.; Reis, F.D.O.; De Lemos, R.N.S.; Mondego, J.M.; Braun, H.; Araujo, J.R.G. Physiological characteristics of citrus plants infested with citrus blackfly. Rev. Bras. Èntomol. 2019, 63, 119–123. [Google Scholar] [CrossRef]
- Boshier, D.; Broadhurst, L.; Cornelius, J.; Gallo, L.; Koskela, J.; Loo, J.; Petrokofsky, G.; Clair, B.S. Is local best? Examining the evidence for local adaptation in trees and its scale. Environ. Évid. 2015, 4, 20. [Google Scholar] [CrossRef]
- Avagyan, A.B. Correlations between delayed fluorescence of chlorophyll, metabolism and yield of plants. II. Influence of moisture of leaf and temperature condition on delayed fluorescence of leaves. J. Biophys. Chem. 2010, 1, 52–57. [Google Scholar] [CrossRef]
- Sork, V.L.; Sork, K.A.; Hochwender, C. Evidence for local adaptation in closely adjacent sub-populations of northern red oak (Quercus rubra L.) expressed as resistance to leaf herbivores. Am. Nat. 1993, 142, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Callow, D.; May, P.; Johnstone, D.M. Tree Vitality Assessment in Urban Landscapes. Forests 2018, 9, 279. [Google Scholar] [CrossRef]
- Mausolf, K.; Härdtle, W.; Jansen, K.; Delory, B.M.; Hertel, D.; Leuschner, C.; Temperton, V.M.; von Oheimb, G.; Fichtner, A. Legacy effects of land-use modulate tree growth responses to climate extremes. Oecologia 2018, 187, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Marqués, L.; Peltier, D.M.P.; Camarero, J.J.; Zavala, M.A.; Madrigal-González, J.; Sangüesa-Barreda, G.; Ogle, K. Disentangling the Legacies of Climate and Management on Tree Growth. Ecosystems 2021, 25, 215–235. [Google Scholar] [CrossRef]
- Boyce, R.L.; Larson, J.R.; Sanford, J.R.L. Phosphorus and nitrogen limitations to photosynthesis in Rocky Mountain bristlecone pine (Pinus aristata) in Colorado. Tree Physiol. 2006, 26, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Alía, R.; Moro, J.R.; Denis, J.B. Performance of Pinus pinaster provenances in Spain: Interpretation of the genotype by environment interaction. Can. J. For. Res. 1997, 27, 1548–1559. [Google Scholar] [CrossRef]
- Cárdenas, A.M.; Gallardo, P. Relationship between insect damage and chlorophyll content in Mediterranean oak species. Appl. Ecol. Environ. Res. 2016, 14, 477–491. [Google Scholar] [CrossRef]
- Matsubara, S.; Chow, W.S. Populations of photoinactivated photosystem II reaction centers characterized by chlorophyll a fluorescence lifetime in vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 18234–18239. [Google Scholar] [CrossRef]
- Krause, G.H. Photoinhibition of Photosynthesis. An Evaluation of Damaging and Protective Mechanisms. In Physiologia Plantarum; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1988; Volume 74, pp. 566–574. [Google Scholar] [CrossRef]
- Strasserf, R.J.; Srivastava, A. Govindjee polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Pleban, J.R.; Guadagno, C.R.; Mackay, D.S.; Weinig, C.; Ewers, B.E. Rapid Chlorophyll a Fluorescence Light Response Curves Mechanistically Inform Photosynthesis Modeling. Plant Physiol. 2020, 183, 602–619. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ma, X.; Lv, T.; Bai, M.; Wang, Z.; Niu, J. Effects of Water Stress on Fluorescence Parameters and Photosynthetic Characteristics of Drip Irrigation in Rice. Water 2020, 12, 289. [Google Scholar] [CrossRef]
- Zhori, A.; Meco, M.; Brandl, H.; Bachofen, R. In situ chlorophyll fluorescence kinetics as a tool to quantify effects on photosynthesis in Euphorbia cyparissias by a parasitic infection of the rust fungus Uromyces pisi. BMC Res. Notes 2015, 8, 698. [Google Scholar] [CrossRef] [PubMed]
- Kjelgren, R.; Montague, T. Urban tree transpiration over turf and asphalt surfaces. Atmos. Environ. 1998, 32, 35–41. [Google Scholar] [CrossRef]
- Percival, G.; Schaffert, E.; Hailey, L. Trees in the Rural Landscape. In Horticulture: Plants for People and Places; Dixon, G., Aldous, D.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 2. [Google Scholar]


| O, J, I, P | Nomenclature of the JIP-Test; Fluorescence Values at Different Points in Time of the Fast Fluorescence Kinetics, from the Origin to the Peak with Intermediate Steps; Characteristic Points Used for Calculations to Describe the Shape of the Kinetics. |
|---|---|
| Ft | Fluorescence at time t after the onset of actinic illumination |
| F50µs = FO = F0 | Minimal fluorescence at 50 µs, the Origin of the onset of actinic illumination (F zero) |
| F2ms = FJ | Fluorescence intensity at 2 ms, at the intermediate step “J”; this marks the point of the first stabile electron acceptor within the electron transport cascade (QA) at the end of Photosystem II. |
| F30ms = FI | Fluorescence intensity at 30 ms, at the intermediate step “I”, the PSI electron acceptor side. This marks the beginning of the electron transport cascade of Photosystem I. |
| FP = FM | Maximal recorded fluorescence intensity at the peak “P” of the fast fluorescence kinetics. |
| φPo = TR0/ABS = FV/FM | Maximum quantum yield for primary photochemistry |
| ΨEo = ET0/TR0 = (1 − VJ) | Probability that a photon trapped by the PSII reaction center enters the electron transport chain further than QA-. |
| φEo = ET0/ABS = [1 − (F0/FM)] ΨEo | Quantum yield of electron transport from PS II to the electron-acceptor side of PS I. |
| M0 = 4 (F300µs − F50µs)/(FM – F0) | Initial slope (per millisecond) of the fluorescence kinetics normalized on the maximal variable fluorescence FV. |
| RC/ABS = φPo (VJ/M0) | QA reducing (active) RCs per PSII antenna Chl; measure for the activity of reaction centers. |
| 10RC/ABS | The value of RC/ABS multiplied by 10 to shift the values in a range; more reachable. |
| TR0/RC = M0 (1/VJ) | Trapped energy flux (leading to QA reduction) per reaction center. |
| DI0/RC = ABS/RC − TR0/RC = [M0(1/VJ)(1/φPo)] − [M0(1/VJ)] | Dissipated energy flux per RC; measure for energy loss. |
| PIABS = (RC/ABS)[φPo/(1 − φPo)][ΨEo/(1 − ΨEo)] | Performance index (potential) for energy conservation from photons absorbed by PSII to the reduction in intersystem electron acceptors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnold, A.L.M.; McGrath, C.; Reinhardt, A. Effects of Oak Processionary Moth (Thaumetopoea processionea L.) Outbreaks on the Leaf Performance and Health of Urban and Forest Oak Trees (Quercus robur L.) in Brandenburg, Germany. Forests 2023, 14, 124. https://doi.org/10.3390/f14010124
Arnold ALM, McGrath C, Reinhardt A. Effects of Oak Processionary Moth (Thaumetopoea processionea L.) Outbreaks on the Leaf Performance and Health of Urban and Forest Oak Trees (Quercus robur L.) in Brandenburg, Germany. Forests. 2023; 14(1):124. https://doi.org/10.3390/f14010124
Chicago/Turabian StyleArnold, Anne L. M., Conor McGrath, and Annett Reinhardt. 2023. "Effects of Oak Processionary Moth (Thaumetopoea processionea L.) Outbreaks on the Leaf Performance and Health of Urban and Forest Oak Trees (Quercus robur L.) in Brandenburg, Germany" Forests 14, no. 1: 124. https://doi.org/10.3390/f14010124
APA StyleArnold, A. L. M., McGrath, C., & Reinhardt, A. (2023). Effects of Oak Processionary Moth (Thaumetopoea processionea L.) Outbreaks on the Leaf Performance and Health of Urban and Forest Oak Trees (Quercus robur L.) in Brandenburg, Germany. Forests, 14(1), 124. https://doi.org/10.3390/f14010124







